Effects of Hormones on Breast Development and Breast Cancer Risk in Transgender Women
Abstract
:Simple Summary
Abstract
1. Introduction
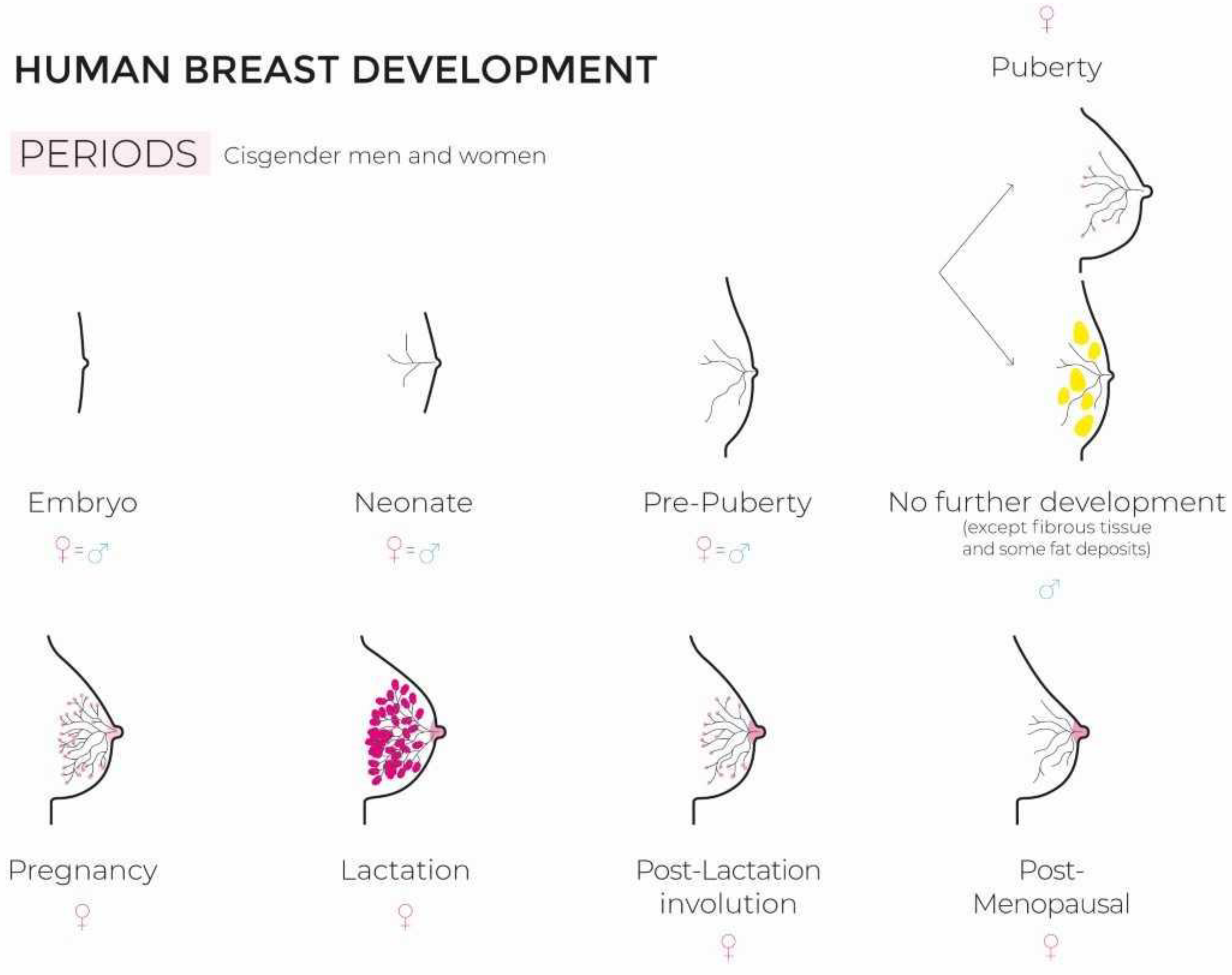
2. Breast Development
2.1. In Cisgender Women
2.2. In Transgender Women
3. Hormone Therapy in Transwomen
- -
- An increased thromboembolic risk [55];
- -
- Liver disorders;
- -
- Lipid abnormalities;
- -
- -
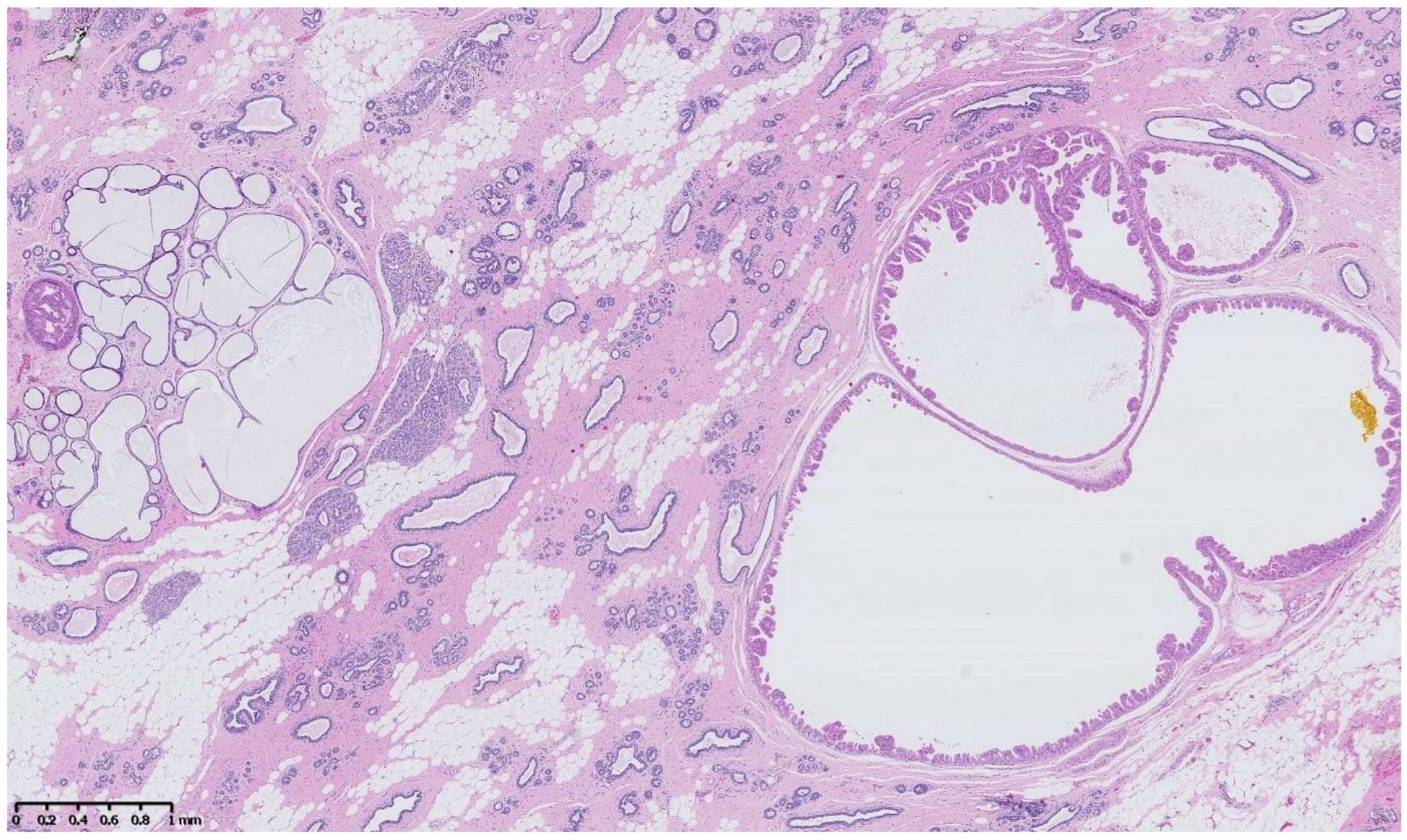
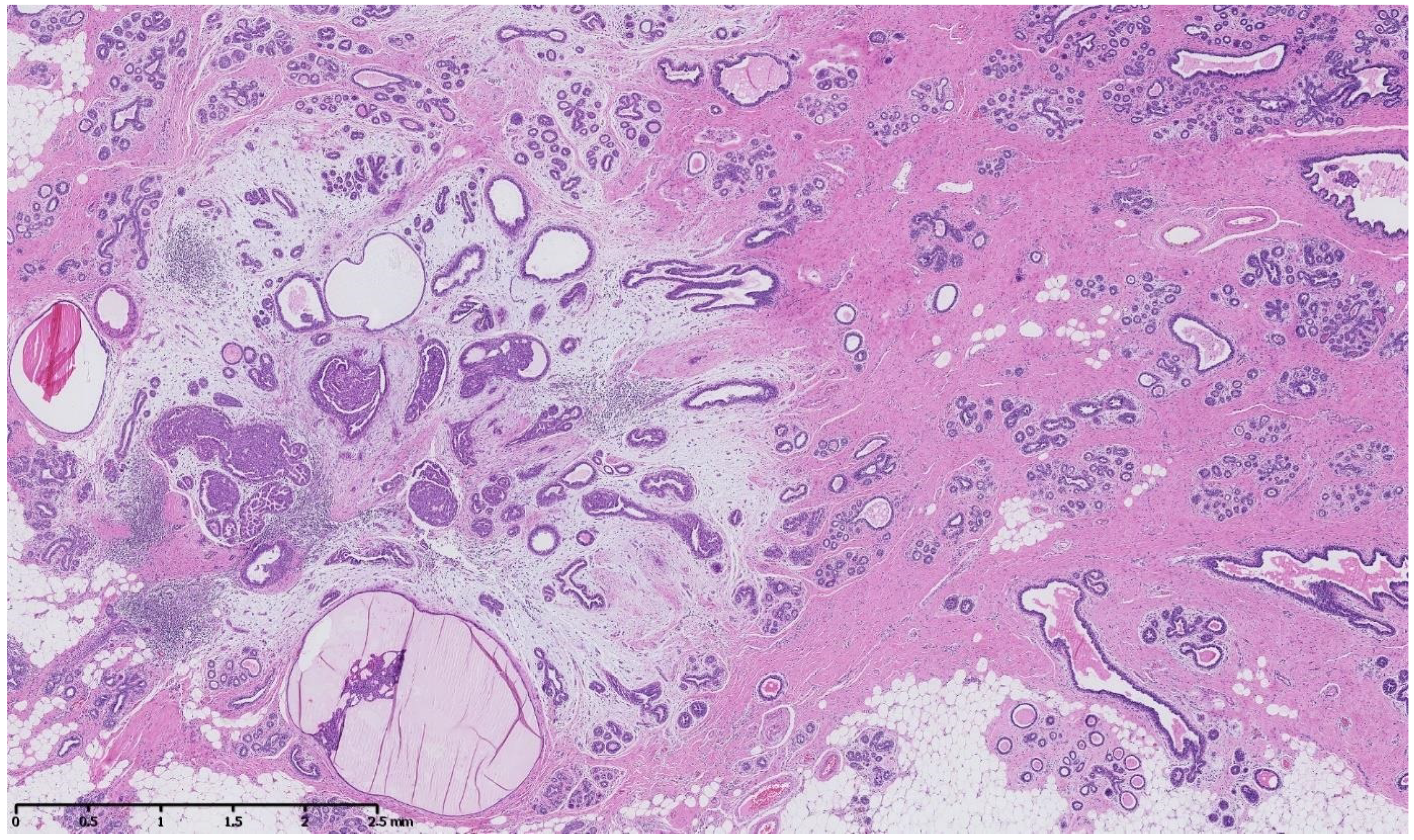
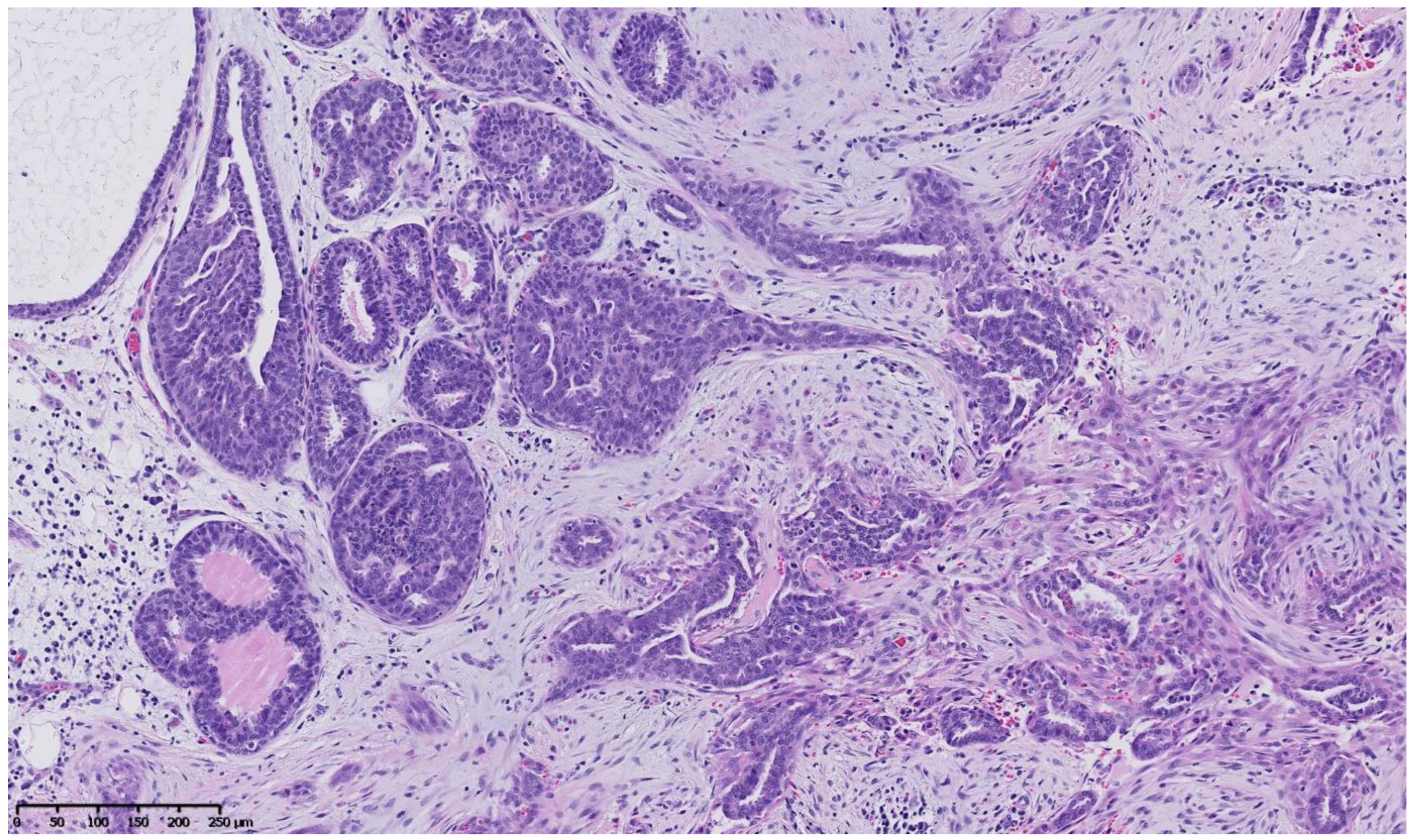
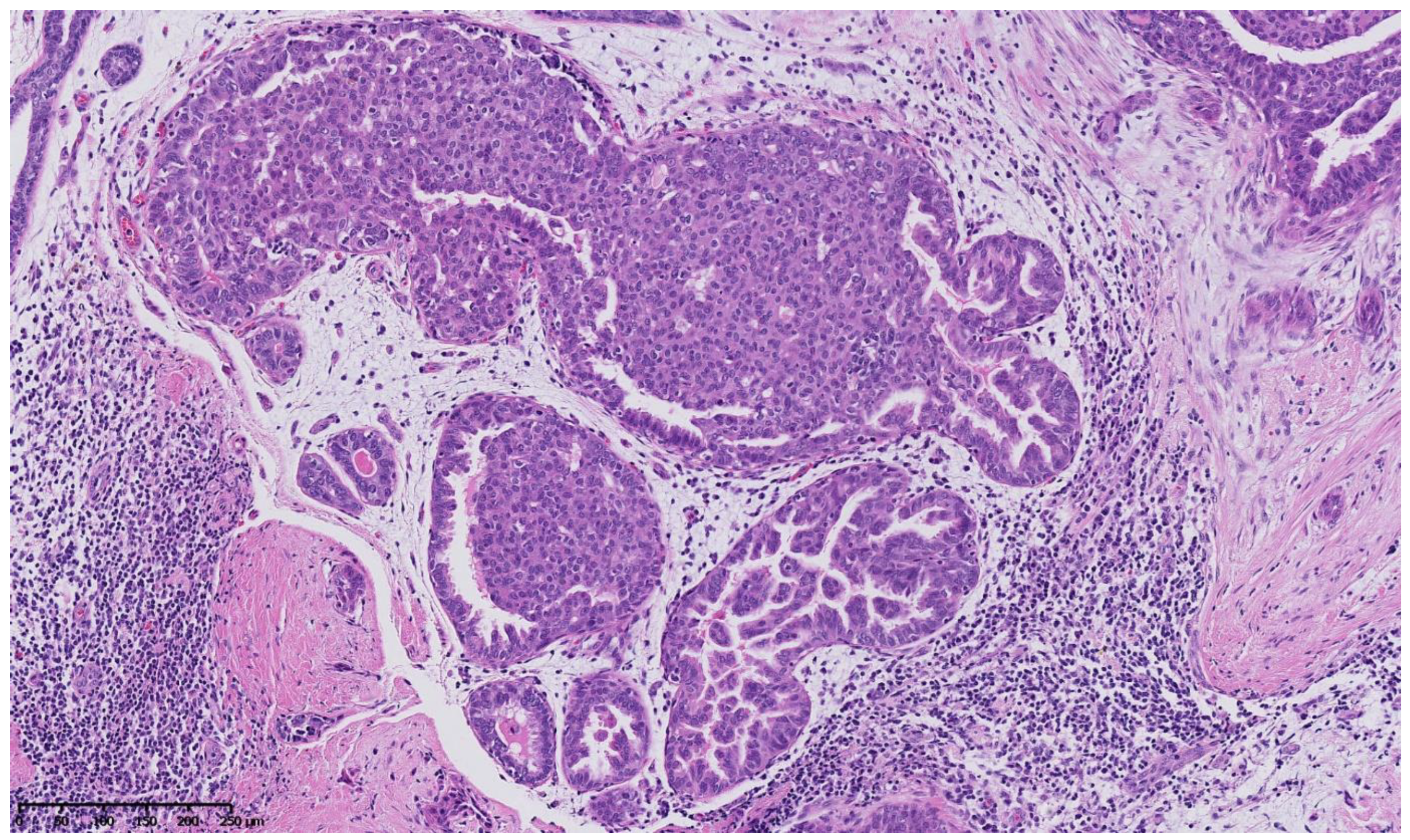
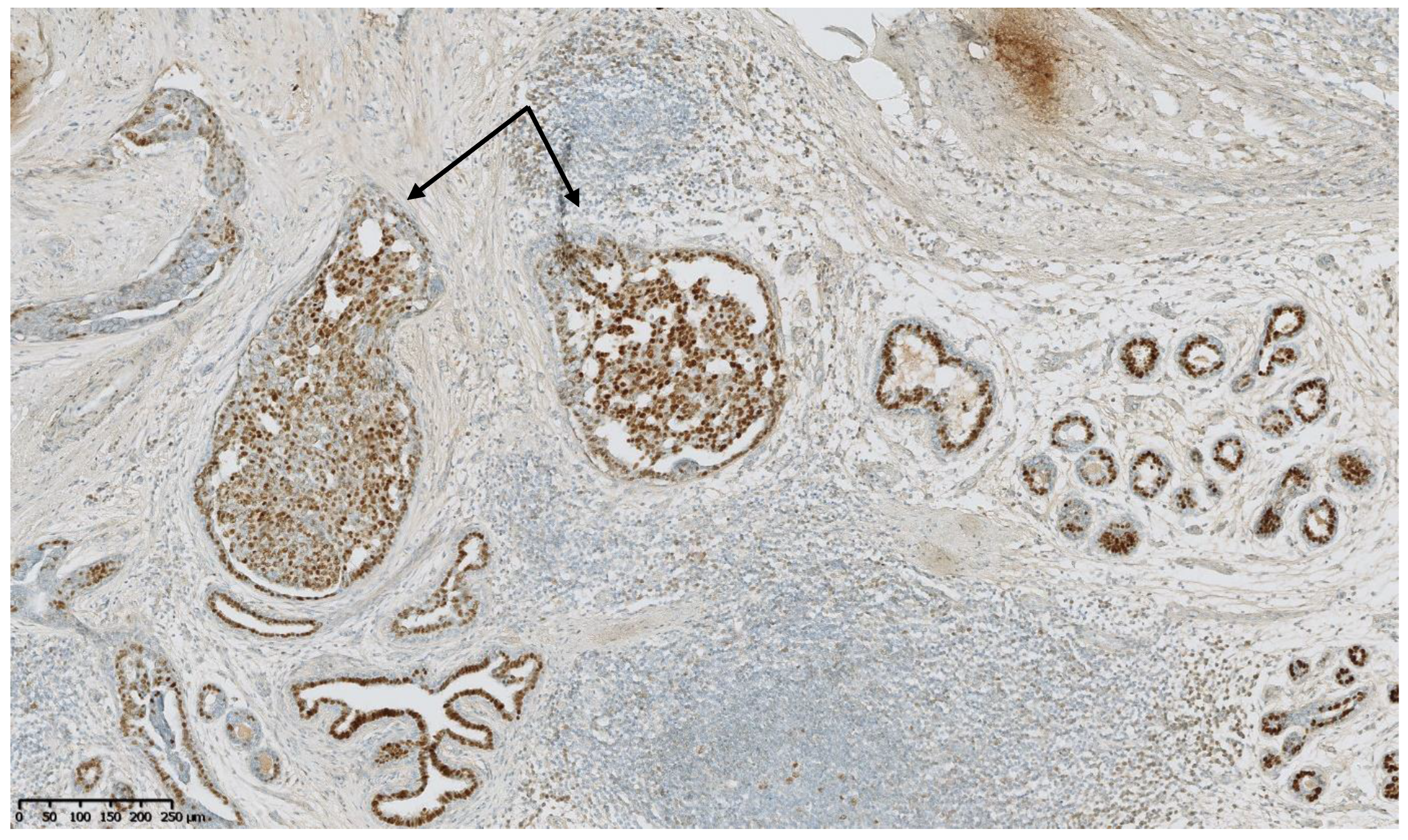
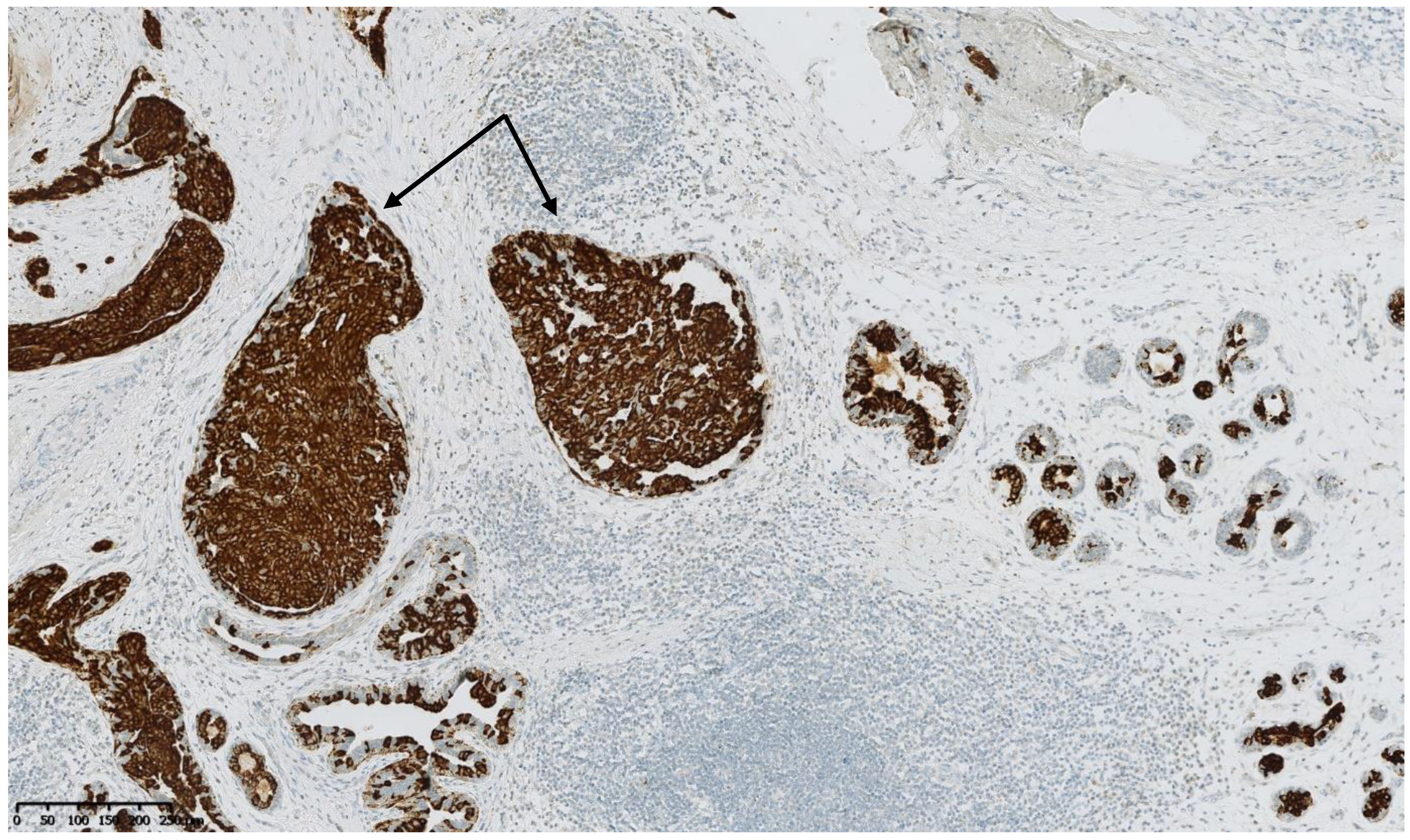
4. Benign Breast Lesions in Transwomen
5. Risk of Breast Cancer: What Do We Know?
- -
- -
- -
- As a general rule, the duration of GAHT in transwomen will far exceed the recommended duration of HRT in ciswomen, and this duration criteria needs to be taken into account and analyzed.
6. Transgender Screening Recommendations for Breast Cancer
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MTF | Male-To-Female |
| ERa | Estrogen Receptor alpha |
| GH | Growth Hormone |
| IGF-1 | Insulin Growth Factor-1 |
| GAHT | Gender-Affirming Hormone Therapy |
| HRT | Hormone Replacement Therapy |
| GnRH | Gonadotropin-Releasing Hormone |
| IM | Intramuscular |
| SC | Subcutaneous |
| WPATH | World Professional Association for Transgender Health |
| WHI | Women’s Health Initiative |
References
- Shumer, D.E.; Nokoff, N.J.; Spack, N.P. Advances in the Care of Transgender Children and Adolescents. Adv. Pediatr. 2016, 63, 79–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen-Kettenis, P.T.; Klink, D. Adolescents with gender dysphoria. Best. Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Safer, J.D.; Tangpricha, V. Care of the Transgender Patient. Ann. Intern. Med. 2019, 171, ITC1–ITC16. [Google Scholar] [CrossRef] [PubMed]
- Puckett, J.A.; Cleary, P.; Rossman, K.; Newcomb, M.E.; Mustanski, B. Barriers to Gender-Affirming Care for Transgender and Gender Nonconforming Individuals. Sex. Res. Soc. Policy 2018, 15, 48–59. [Google Scholar] [CrossRef] [PubMed]
- McNamara, M.C.; Ng, H. Best practices in LGBT care: A guide for primary care physicians. Clevel. Clin. J. Med. 2016, 83, 531–541. [Google Scholar] [CrossRef]
- de Blok, C.J.M.; Wiepjes, C.M.; Nota, N.M.; van Engelen, K.; Adank, M.A.; Dreijerink, K.M.A.; Barbé, E.; Konings, I.R.H.M.; den Heijer, M. Breast cancer risk in transgender people receiving hormone treatment: Nationwide cohort study in the Netherlands. BMJ 2019, 365, l1652. [Google Scholar] [CrossRef] [Green Version]
- Wiepjes, C.M.; Nota, N.M.; de Blok, C.J.M.; Klaver, M.; de Vries, A.L.C.; Wensing-Kruger, S.A.; de Jongh, R.T.; Bouman, M.B.; Steesma, T.D.; Cohen-Kettenis, P.; et al. The Amsterdam Cohort of Gender Dysphoria Study (1972–2015): Trends in Prevalence, Treatment, and Regrets. J. Sex. Med. 2018, 15, 582–590. [Google Scholar] [CrossRef]
- Dowshen, N.; Nguyen, G.T.; Gilbert, K.; Feiler, A.; Margo, K.L. Improving Transgender Health Education for Future Doctors. Am. J. Public Health 2014, 104, e5–e6. [Google Scholar] [CrossRef]
- Joseph, A.; Cliffe, C.; Hillyard, M.; Majeed, A. Gender identity and the management of the transgender patient: A guide for non-specialists. J. R. Soc. Med. 2017, 110, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Collin, L.; Reisner, S.L.; Tangpricha, V.; Goodman, M. Prevalence of Transgender Depends on the “Case” Definition: A Systematic Review. J. Sex. Med. 2016, 13, 613–626. [Google Scholar] [CrossRef]
- Tangpricha, V. Health disparities in transgender people. Lancet Diabetes Endocrinol. 2021, 9, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Murad, M.H.; Elamin, M.B.; Garcia, M.Z.; Mullan, R.J.; Murad, A.; Erwin, P.J.; Montori, V. Hormonal therapy and sex reassignment: A systematic review and meta-analysis of quality of life and psychosocial outcomes. Clin. Endocrinol. 2010, 72, 214–231. [Google Scholar] [CrossRef] [PubMed]
- Asscheman, H.; Giltay, E.J.; Megens, J.A.J.; de Ronde, W.P.; van Trotsenburg, M.A.A.; Gooren, L.J.G. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur. J. Endocrinol. 2011, 164, 635–642. [Google Scholar] [CrossRef] [Green Version]
- Medina-Martínez, J.; Saus-Ortega, C.; Sánchez-Lorente, M.M.; Sosa-Palanca, E.M.; García-Martínez, P.; Mármol-López, M.I. Health Inequities in LGBT People and Nursing Interventions to Reduce Them: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 11801. [Google Scholar] [CrossRef] [PubMed]
- Brisken, C.; O’Malley, B. Hormone Action in the Mammary Gland. Cold Spring Harb. Perspect. Biol. 2010, 2, a003178. [Google Scholar] [CrossRef]
- Safer, J.D. Research gaps in medical treatment of transgender/nonbinary people. J. Clin. Investig. 2021, 131, 142029. [Google Scholar] [CrossRef]
- Nguyen, H.B.; Chavez, A.M.; Lipner, E.; Hantsoo, L.; Kornfield, S.L.; Davies, R.D.; Epperson, C.N. Gender-Affirming Hormone Use in Transgender Individuals: Impact on Behavioral Health and Cognition. Curr. Psychiatry Rep. 2018, 20, 110. [Google Scholar] [CrossRef]
- Glintborg, D.; T’Sjoen, G.; Ravn, P.; Andersen, M.S. Management of endocrine disease: Optimal feminizing hormone treatment in transgender people. Eur. J. Endocrinol. 2021, 185, R49–R63. [Google Scholar] [CrossRef]
- Weinand, J.D.; Safer, J.D. Hormone therapy in transgender adults is safe with provider supervision; A review of hormone therapy sequelae for transgender individuals. J. Clin. Transl. Endocrinol. 2015, 2, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Mahase, E. Hormone therapy increases breast cancer risk in transgender women, study finds. BMJ 2019, 365, l2221. [Google Scholar] [CrossRef]
- Sutherland, N.; Espinel, W.; Grotzke, M.; Colonna, S. Unanswered Questions: Hereditary breast and gynecological cancer risk assessment in transgender adolescents and young adults. J. Genet. Couns. 2020, 29, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Lienhoop, T.; Green, L. Breast imaging in transgender women: A review. Clin. Imaging 2021, 80, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Maglione, K.D.; Margolies, L.; Jaffer, S.; Szabo, J.; Schmidt, H.; Weltz, C.; Sonnenblick, E.B. Breast Cancer in Male-to-Female Transsexuals: Use of Breast Imaging for Detection. Am. J. Roentgenol. 2014, 203, W735–W740. [Google Scholar] [CrossRef] [PubMed]
- Macias, H.; Hinck, L. Mammary Gland Development. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 533. [Google Scholar] [CrossRef] [Green Version]
- Laurence, D.J.; Monaghan, P.; Gusterson, B.A. The development of the normal human breast. Oxf. Rev. Reprod. Biol. 1991, 13, 149–174. [Google Scholar]
- Lteif, A.; Javed, A. Development of the Human Breast. Semin. Plast. Surg. 2013, 27, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Rosen, J.M. On Hormone Action in the Mammary Gland. Cold Spring Harb. Perspect. Biol. 2012, 4, a013086. [Google Scholar] [CrossRef] [Green Version]
- Weber, R.J.; Desai, T.A.; Gartner, Z.J. Non-autonomous cell proliferation in the mammary gland and cancer. Curr. Opin. Cell Biol. 2017, 45, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.K.; Banerjee, S.; Baker, G.W.; Kuo, C.-Y.; Chowdhury, I. The Mammary Gland: Basic Structure and Molecular Signaling during Development. Int. J. Mol. Sci. 2022, 23, 3883. [Google Scholar] [CrossRef]
- Anderson, S.M.; Rudolph, M.C.; McManaman, J.L.; Neville, M.C. Key stages in mammary gland development. Secretory activation in the mammary gland: It’s not just about milk protein synthesis! Breast Cancer Res. BCR 2007, 9, 204. [Google Scholar] [CrossRef]
- Hinck, L.; Silberstein, G.B. Key stages in mammary gland development: The mammary end bud as a motile organ. Breast Cancer Res. BCR 2005, 7, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D. Key stages in mammary gland development: The cues that regulate ductal branching morphogenesis. Breast Cancer Res. BCR 2005, 8, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNally, S.; Martin, F. Molecular regulators of pubertal mammary gland development. Ann. Med. 2011, 43, 212–234. [Google Scholar] [CrossRef] [PubMed]
- Hilton, H.N.; Clarke, C.L.; Graham, J.D. Estrogen and progesterone signalling in the normal breast and its implications for cancer development. Mol. Cell. Endocrinol. 2018, 466, 2–14. [Google Scholar] [CrossRef]
- Arendt, L.M.; Rudnick, J.A.; Keller, P.J.; Kuperwasser, C. Stroma in breast development and disease. Semin. Cell Dev. Biol. 2010, 21, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Anderson, E. Progesterone receptors—Animal models and cell signaling in breast cancer: The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res. BCR 2002, 4, 197–201. [Google Scholar] [CrossRef] [Green Version]
- Siegel, P.M.; Muller, W.J. Transcription factor regulatory networks in mammary epithelial development and tumorigenesis. Oncogene 2010, 29, 2753–2759. [Google Scholar] [CrossRef] [Green Version]
- McCready, J.; Arendt, L.M.; Rudnick, J.A.; Kuperwasser, C. The contribution of dynamic stromal remodeling during mammary development to breast carcinogenesis. Breast Cancer Res. BCR 2010, 12, 205. [Google Scholar] [CrossRef] [Green Version]
- Taraborrelli, S. Physiology, production and action of progesterone. Acta Obstet. Gynecol. Scand. 2015, 94 (Suppl. S161), 8–16. [Google Scholar] [CrossRef]
- Ruan, W.; Monaco, M.E.; Kleinberg, D.L. Progesterone stimulates mammary gland ductal morphogenesis by synergizing with and enhancing insulin-like growth factor-I Action. Endocrinology 2005, 146, 1170–1178. [Google Scholar] [CrossRef]
- Grimm, S.L.; Hartig, S.M.; Edwards, D.P. Progesterone Receptor Signaling Mechanisms. J. Mol. Biol. 2016, 428, 3831–3849. [Google Scholar] [CrossRef] [PubMed]
- Oakes, S.R.; Rogers, R.L.; Naylor, M.J.; Ormandy, C.J. Prolactin regulation of mammary gland development. J. Mammary Gland Biol. Neoplasia 2008, 13, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.; Clarke, R. Steroid Receptors and Cell Cycle in Normal Mammary Epithelium. J. Mammary Gland. Biol. Neoplasia 2004, 9, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, S.; Jena, M.K.; Anand, V.; Jaswal, A.; Kancharla, S.; Kolli, P.; Mandadapu, G.; Kumar, S.; Mohanty, A.K. Critical Review on Physiolgical and Molecular Features during Bovine Mammary Gland Development: Recent Advances. Cells 2022, 11, 3325. [Google Scholar] [CrossRef]
- T’Sjoen, G.; Arcelus, J.; Gooren, L.; Klink, D.T.; Tangpricha, V. Endocrinology of Transgender Medicine. Endocr. Rev. 2019, 40, 97–117. [Google Scholar] [CrossRef] [Green Version]
- de Blok, C.J.M.; Dijkman, B.A.M.; Wiepjes, C.M.; Staphorsius, A.S.; Timmermans, F.W.; Smit, J.M.; Dreijerink, K.M.A.; den Heijer, M. Sustained Breast Development and Breast Anthropometric Changes in 3 Years of Gender-Affirming Hormone Treatment. J. Clin. Endocrinol. Metab. 2021, 106, e782–e790. [Google Scholar] [CrossRef]
- Wierckx, K.; Van Caenegem, E.; Schreiner, T.; Haraldsen, I.; Fisher, A.; Toye, K.; Kaufman, J.M.; T’Sjoen, G. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: Results from the European network for the investigation of gender incongruence. J. Sex. Med. 2014, 11, 1999–2011. [Google Scholar] [CrossRef]
- Prior, J.C. Progesterone Is Important for Transgender Women’s Therapy—Applying Evidence for the Benefits of Progesterone in Ciswomen. J. Clin. Endocrinol. Metab. 2019, 104, 1181–1186. [Google Scholar] [CrossRef] [Green Version]
- Tollinche, L.; Walters, C.B.; Radix, A.; Long, M.; Galante, L.; Goldstein, Z.G.; Kapinos, Y.; Yeoh, C. The Perioperative Care of the Transgender Patient. Obstet. Anesthesia Dig. 2018, 127, 359–366. [Google Scholar] [CrossRef]
- Zurada, A.; Salandy, S.; Roberts, W.; Gielecki, J.; Schober, J.; Loukas, M. The evolution of transgender surgery. Clin. Anat. 2018, 31, 878–886. [Google Scholar] [CrossRef]
- Tangpricha, V.; den Heijer, M. Oestrogen and anti-androgen therapy for transgender women. Lancet Diabetes Endocrinol. 2017, 5, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, Z.; Patel, S.; Guo, B.; Nixon, E.; Bouman, W.P.; Witcomb, G.L.; Arcelus, J. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: A prospective cohort study. Andrology 2020, 9, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Nota, N.M.; den Heijer, M.; Gooren, L.J.; Feingold, K.R.; Anawalt, B.; Boyce, A.; Chrousos, G.; de Herder, W.W.; Dhatariya, K.; Dungan, K.; et al. Evaluation and Treatment of Gender-Dysphoric/Gender Incongruent Adults. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: http://www.ncbi.nlm.nih.gov/books/NBK544426/ (accessed on 27 February 2022).
- Hembree, W.C.; Cohen-Kettenis, P.; Delemarre-van de Waal, H.A.; Gooren, L.J.; Meyer, W.J., 3rd; Spack, N.P.; Tangpricha, V.; Montori, V.M. Endocrine Treatment of Transsexual Persons:An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2009, 94, 3132–3154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Defreyne, J.; Van de Bruaene, L.D.L.; Rietzschel, E.; Van Schuylenbergh, J.; T’Sjoen, G.G.R. Effects of Gender-Affirming Hormones on Lipid, Metabolic, and Cardiac Surrogate Blood Markers in Transgender Persons. Clin Chem. 2019, 65, 119–134. [Google Scholar] [CrossRef] [Green Version]
- Patel, H.; Arruarana, V.; Yao, L.; Cui, X.; Ray, E. Effects of hormones and hormone therapy on breast tissue in transgender patients: A concise review. Endocrine 2020, 68, 6–15. [Google Scholar] [CrossRef]
- Fisher, A.D.; Castellini, G.; Ristori, J.; Casale, H.; Cassioli, E.; Sensi, C.; Fanni, E.; Amato, A.M.; Bettini, E.; Mosconi, M.; et al. Cross-Sex Hormone Treatment and Psychobiological Changes in Transsexual Persons: Two-Year Follow-Up Data. J. Clin. Endocrinol. Metab. 2016, 101, 4260–4269. [Google Scholar] [CrossRef] [Green Version]
- Radix, A. Hormone Therapy for Transgender Adults. Urol. Clin. N. Am. 2019, 46, 467–473. [Google Scholar] [CrossRef]
- Haupt, C.; Henke, M.; Kutschmar, A.; Hauser, B.; Baldinger, S.; Saenz, S.R.; Schreiber, G. Antiandrogen or estradiol treatment or both during hormone therapy in transitioning transgender women. Cochrane Database Syst. Rev. 2020, 2020, CD013138. [Google Scholar] [CrossRef]
- Burinkul, S.; Panyakhamlerd, K.; Suwan, A.; Tuntiviriyapun, P.; Wainipitapong, S. Anti-Androgenic Effects Comparison Between Cyproterone Acetate and Spironolactone in Transgender Women: A Randomized Controlled Trial. J. Sex. Med. 2021, 18, 1299–1307. [Google Scholar] [CrossRef]
- Dittrich, R.; Binder, H.; Cupisti, S.; Hoffmann, I.; Beckmann, M.W.; Mueller, A. Endocrine Treatment of Male-to-Female Transsexuals Using Gonadotropin-Releasing Hormone Agonist. Exp. Clin. Endocrinol. Diabetes 2005, 113, 586–592. [Google Scholar] [CrossRef]
- de Vries, A.L.C.; Steensma, T.D.; Doreleijers, T.A.H.; Cohen-Kettenis, P.T. Puberty suppression in adolescents with gender identity disorder: A prospective follow-up study. J. Sex. Med. 2011, 8, 2276–2283. [Google Scholar] [CrossRef] [PubMed]
- Salas-Humara, C.; Sequeira, G.M.; Rossi, W.; Dhar, C.P. Gender affirming medical care of transgender youth. Curr. Probl. Pediatr. Adolesc. Health Care 2019, 49, 100683. [Google Scholar] [CrossRef] [PubMed]
- Weigert, R.; Frison, E.; Sessiecq, Q.; Al Mutairi, K.; Casoli, V. Patient satisfaction with breasts and psychosocial, sexual, and physical well-being after breast augmentation in male-to-female transsexuals. Plast. Reconstr. Surg. 2013, 132, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Azeem, R.; Bin Zubair, U.; Jalil, A.; Kamal, A.; Nizami, A.; Minhas, F. Prevalence of Suicide Ideation and its Relationship with Depression among Transgender Population. J. Coll. Physicians Surg. Pak. 2019, 29, 349–352. [Google Scholar] [CrossRef]
- Tucker, R.P. Suicide in Transgender Veterans: Prevalence, Prevention, and Implications of Current Policy. Perspect. Psychol. Sci. 2019, 14, 452–468. [Google Scholar] [CrossRef]
- Brown, G.R. Breast Cancer in Transgender Veterans: A Ten-Case Series. LGBT Health 2015, 2, 77–80. [Google Scholar] [CrossRef]
- de Blok, C.J.M.; Dijkman BAm Wiepjes, C.M.; Konings, I.R.H.M.; Dreijerink, K.M.A.; Barbé, E.; den Heijer, M. Frequency and outcomes of benign breast biopsies in trans women: A nationwide cohort study. Breast Off. J. Eur. Soc. Mastology 2021, 57, 118–122. [Google Scholar] [CrossRef]
- Richards, S.M.; Pine-Twaddell, E.D.; Ioffe, O.B.; Bellavance, E.C. A Case of Benign Phyllodes Tumor in a Transgender Woman Receiving Cross-Sex Hormones. Int. J. Surg. Pathol. 2017, 26, 356–359. [Google Scholar] [CrossRef]
- O’Bryan, J.; Wolf-Gould, C.; Matsuo, Y. Mammary Myofibroblastoma in a Transgender Patient on Feminizing Hormones: Literature Review and Case Report. Transgender Health 2018, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Yu, Q.; Chen, R.; Seeger, H.; Fehm, T.; Cahill, M.A.; Mueck, A.O.; Neubauer, H. Medroxyprogesterone acetate-driven increase in breast cancer risk might be mediated via cross-talk with growth factors in the presence of progesterone receptor membrane component-1. Maturitas 2013, 76, 129–133. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Anderson, G.; Manson, J.E.; Pettinger, M.; Yasmeen, S.; Lane, D.; Langer, R.D.; Hubbell, F.A.; McTiernan, A.; Hendrix, S.; et al. Estrogen Alone in Postmenopausal Women and Breast Cancer Detection by Means of Mammography and Breast Biopsy. J. Clin. Oncol. 2010, 28, 2690–2697. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Anderson, G.L.; Aragaki, A.K.; Manson, J.E.; Stefanick, M.L.; Pan, K.; Barrington, W.; Kuller, L.H.; Simon, M.S.; Lane, D.; et al. Association of Menopausal Hormone Therapy With Breast Cancer Incidence and Mortality During Long-term Follow-up of the Women’s Health Initiative Randomized Clinical Trials. JAMA 2020, 324, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Rohan, T.E.; Manson, J.E.; Aragaki, A.K.; Kaunitz, A.; Stefanick, M.L.; Simon, M.S.; Johnson, K.C.; Wactawski-Wende, J.; O’Sullivan, M.J.; et al. Breast Cancer After Use of Estrogen Plus Progestin and Estrogen Alone: Analyses of Data From 2 Women’s Health Initiative Randomized Clinical Trials. JAMA Oncol. 2015, 1, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Hendrix, S.L.; Langer, R.D.; Stefanick, M.L.; Gass, M.; Lane, D.; Rodabough, R.J.; Gilligan, M.A.; Cyr, M.G.; Thomson, C.A.; et al. Influence of Estrogen Plus Progestin on Breast Cancer and Mammography in Healthy Postmenopausal Women: The Women’s Health Initiative Randomized Trial. Obstet. Gynecol. Surv. 2003, 58, 735–737. [Google Scholar] [CrossRef]
- Geller, M.L.; Chlebowski, R.T. HT and breast cancer risk. Fertil. Steril. 2003, 80 (Suppl. S4), 5–9, quiz 54–55. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.; Binder, H.; Cupisti, S.; Hoffmann, I.; Beckmann, M.; Dittrich, R. Effects on the Male Endocrine System of Long-term Treatment with Gonadotropin-releasing Hormone Agonists and Estrogens in Male-to-Female Transsexuals. Horm. Metab. Res. 2006, 38, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pino, I.; Puig, T.; Quintana, M.J.; Solà, J.; Bonfill, X. [Prevalence of use of hormone replacement therapy in women participating in a breast cancer screening program]. Med. Clin. (Barc) 2010, 134, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Stuedal, A.; Ma, H.; Bjørndal, H.; Ursin, G. Postmenopausal hormone therapy with estradiol and norethisterone acetate and mammographic density: Findings from a cross-sectional study among Norwegian women. Climacteric 2009, 12, 248–258. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Anderson, G.L. Menopausal hormone therapy and cancer: Changing clinical observations of target site specificity. Steroids 2014, 90, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: Individual participant meta-analysis of the worldwide epidemiological evidence. Lancet Lond. Engl. 2019, 394, 1159–1168. [Google Scholar] [CrossRef]
- Brown, G.R.; Jones, K.T. Incidence of breast cancer in a cohort of 5,135 transgender veterans. Breast Cancer Res. Treat. 2014, 149, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Pattison, S.; McLaren, B.R. Triple negative breast cancer in a male-to-female transsexual. Intern. Med. J. 2013, 43, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Feldman, J.; Brown, G.R.; Deutsch, M.B.; Hembree, W.; Meyer, W.; Meyer-Bahlburg, H.F.; Tangpricha, V.; T’Sjoen, G.; Safer, J.D. Priorities for transgender medical and healthcare research. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 180–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hines, L.M.; Risendal, B.; Slattery, M.L.; Baumgartner, K.B.; Giuliano, A.R.; Sweeney, C.; Rollison, D.E.; Byers, T. Comparative analysis of breast cancer risk factors among hispanic and non-hispanic white women. Cancer 2010, 116, 3215–3223. [Google Scholar] [CrossRef] [Green Version]
- Baral, S.D.; Poteat, T.; Strömdahl, S.; Wirtz, A.L.; Guadamuz, T.E.; Beyrer, C. Worldwide burden of HIV in transgender women: A systematic review and meta-analysis. Lancet Infect. Dis. 2013, 13, 214–222. [Google Scholar] [CrossRef]
- White Hughto, J.M.; Reisner, S.L.; Pachankis, J.E. Transgender stigma and health: A critical review of stigma determinants, mechanisms, and interventions. Soc. Sci. Med. 2015, 147, 222–231. [Google Scholar] [CrossRef] [Green Version]
- Guss, C.; Shumer, D.; Katz-Wise, S.L. Transgender and gender nonconforming adolescent care: Psychosocial and medical considerations. Curr. Opin. Pediatr. 2015, 27, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Mepham, N.; Bouman, W.P.; Arcelus, J.; Hayter, M.; Wylie, K.R. People with gender dysphoria who self-prescribe cross-sex hormones: Prevalence, sources, and side effects knowledge. J. Sex. Med. 2014, 11, 2995–3001. [Google Scholar] [CrossRef]
- Restar, A.J.; Jin, H.; Ogunbajo, A.; Goedel, W.C.; Millett, G.; Sherwood, J.; Kuhns, L.; Reisner, S.L.; Garofalo, R.; Mimiaga, M.J. Prevalence and Risk Factors of Nonmedical Prescription Opioid Use Among Transgender Girls and Young Women. JAMA Netw. Open 2020, 3, e201015. [Google Scholar] [CrossRef]
- Moseson, H.; Zazanis, N.; Goldberg, E.; Fix, L.; Durden, M.; Stoeffler, A.; Hastings, J.; Cudlitz, L.; Lesser-Lee, B.; Letcher, L.; et al. The Imperative for Transgender and Gender Nonbinary Inclusion: Beyond Women’s Health. Obstet Gynecol. 2020, 135, 1059–1068. [Google Scholar] [CrossRef]
- Connolly, M.D.; Zervos, M.J.; Barone, C.J.; Johnson, C.C.; Joseph, C.L. The Mental Health of Transgender Youth: Advances in Understanding. J. Adolesc. Health 2016, 59, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Skuban, T.; Orzechowski, M.; Steger, F. Restriction of Access to Healthcare and Discrimination of Individuals of Sexual and Gender Minority: An Analysis of Judgments of the European Court of Human Rights from an Ethical Perspective. Int. J. Environ. Res. Public Health 2022, 19, 2650. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, S.J.; Grimstad, F.; Irwig, M.S.; Rothman, M.S. Routine Screening for Transgender and Gender Diverse Adults Taking Gender-Affirming Hormone Therapy: A Narrative Review. J. Gen. Intern. Med. 2021, 36, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Mandelblatt, J.S.; Stout, N.; Trentham-Dietz, A. To screen or not to screen women in their 40s for breast cancer: Is personalized risk-based screening the answer? Ann. Intern. Med. 2011, 155, 58–60. [Google Scholar] [CrossRef]
- Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: An independent review. Lancet Lond. Engl. 2012, 380, 1778–1786. [Google Scholar] [CrossRef]
- Gräwingholt, A. The role of artificial intelligence in breast cancer screening: How can it improve detection? Expert Rev. Mol. Diagn. 2020, 20, 1161–1162. [Google Scholar] [CrossRef]
- Seely, J.M.; Alhassan, T. Screening for breast cancer in 2018-what should we be doing today? Curr. Oncol. 2018, 25 (Suppl. S1), S115–S124. [Google Scholar] [CrossRef] [Green Version]
- Cirrincione, L.R.; Huang, K.J. Sex and Gender Differences in Clinical Pharmacology: Implications for Transgender Medicine. Clin. Pharmacol. Ther. 2021, 110, 897–908. [Google Scholar] [CrossRef]
- Gooren, L.J. Clinical practice. Care of transsexual persons. N. Engl. J. Med. 2011, 364, 1251–1257. [Google Scholar] [CrossRef]
- Gooren, L.J.; Giltay, E.J.; Bunck, M.C. Long-Term Treatment of Transsexuals with Cross-Sex Hormones: Extensive Personal Experience. J. Clin. Endocrinol. Metab. 2008, 93, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Hartley, R.L.; Stone, J.P.; Temple-Oberle, C. Breast cancer in transgender patients: A systematic review. Part 1: Male to female. Eur. J. Surg. Oncol. 2018, 44, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Loshak, H. Primary Care Initiated Gender-Affirming Therapy for Gender Dysphoria: A Review of Evidence Based Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2020. Available online: http://www.ncbi.nlm.nih.gov/books/NBK563451/ (accessed on 22 August 2022).
- Dhejne, C.; Lichtenstein, P.; Boman, M.; Johansson, A.; Långström, N.; Landén, M. Long-Term Follow-Up of Transsexual Persons Undergoing Sex Reassignment Surgery: Cohort Study in Sweden. PLoS ONE 2011, 6, e16885. [Google Scholar] [CrossRef] [Green Version]
- Reisner, S.L.; Vetters, R.; Leclerc, M.; Zaslow, S.; Wolfrum, S.; Shumer, D.; Mimiaga, M.J. Mental Health of Transgender Youth in Care at an Adolescent Urban Community Health Center: A Matched Retrospective Cohort Study. J. Adolesc. Health 2015, 56, 274–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becasen, J.S.; Denard, C.L.; Mullins, M.M.; Higa, D.H.; Sipe, T.A. Estimating the Prevalence of HIV and Sexual Behaviors Among the US Transgender Population: A Systematic Review and Meta-Analysis, 2006–2017. Am. J. Public Health 2019, 109, e1–e8. [Google Scholar] [CrossRef]
- Hughto, J.M.W.; Quinn, E.K.; Dunbar, M.S.; Rose, A.J.; Shireman, T.I.; Jasuja, G.K. Prevalence and Co-occurrence of Alcohol, Nicotine, and Other Substance Use Disorder Diagnoses Among US Transgender and Cisgender Adults. JAMA Netw. Open 2021, 4, e2036512. [Google Scholar] [CrossRef] [PubMed]
| Drugs | Cisgender Women | Transgender Women |
|---|---|---|
| Androgens lowering drugs | ||
| Cyproterone acetate | Low doses of cyproterone acetate in some hormonal associations (no longer recommended) | 50–100 mg/daily Widely used in the past (T’Sjoen—Patel) Decreased use because of side effects:
|
| Spironolactone (T’Sjoen—Patel) | / | 100–200 mg orally dailyMax 400 mg |
| Peripheral androgen receptors blockers (Finasteride) | / | 1.5 mg orally daily |
| GnRH analogs gonadotropin-Releasing hormone) | / | 3.75 mg IM (intramuscular) or SC (subcutaneous)/month |
| Estrogens In the past: oral conjugated estrogens | 0.625 mg daily | 2.5–7.5 mg daily |
| Currently: Estradiol valerate | ||
| 0.5–1 mg/daily | 4 mg daily (2–6 mg) |
| 10–20 mg IM/4 weeks | 20 mg IM every 2 weeks |
| 0.0375–0.05 mg daily (patch changed weekly) | Patch 0.1–0.5 mg/3 days |
| Daily gel (0.8–1.5 mg daily) | Daily gel (0.8–3 mg daily) 2 times/day |
| Authors | Screening YES/NO | Frequency/Beginning |
|---|---|---|
| Maglione et al., 2014 [23] | Yes | Based on risk factors |
| Goren et al., 2011 [98] | No | / |
| Brown et al., 2015 [67,82] | Yes | Mammography every 2 years from 50 years old |
| Hartley et al., 2018 [102] | No recommendation | No recommendation |
| De Blok et al., 2019 [6] | Yes | Every 2 years = similar as ciswomen |
| T’Sjoen, 2019 [45] | Yes | After 5 years of hormonal treatment |
| Canadian Society of Cancer, 2022 Chen [103] | Yes | After 5 years of hormonal treatment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berliere, M.; Coche, M.; Lacroix, C.; Riggi, J.; Coyette, M.; Coulie, J.; Galant, C.; Fellah, L.; Leconte, I.; Maiter, D.; et al. Effects of Hormones on Breast Development and Breast Cancer Risk in Transgender Women. Cancers 2023, 15, 245. https://doi.org/10.3390/cancers15010245
Berliere M, Coche M, Lacroix C, Riggi J, Coyette M, Coulie J, Galant C, Fellah L, Leconte I, Maiter D, et al. Effects of Hormones on Breast Development and Breast Cancer Risk in Transgender Women. Cancers. 2023; 15(1):245. https://doi.org/10.3390/cancers15010245
Chicago/Turabian StyleBerliere, Martine, Maximilienne Coche, Camille Lacroix, Julia Riggi, Maude Coyette, Julien Coulie, Christine Galant, Latifa Fellah, Isabelle Leconte, Dominique Maiter, and et al. 2023. "Effects of Hormones on Breast Development and Breast Cancer Risk in Transgender Women" Cancers 15, no. 1: 245. https://doi.org/10.3390/cancers15010245





