Margin Free Resection Achieves Excellent Long Term Outcomes in Parathyroid Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- -
- Local excision (LE) only, i.e. pericapsular excision of the parathyroid lesion such as that usually employed for benign lesions.
- -
- En bloc excision; this includes en bloc and oncological resection. En bloc describes excision of the parathyroid with circumferential soft tissue as minimal criterion; in this process the tumour capsule must not be laid open at any point; oncological resection additionally includes ipsilateral thyroid lobectomy, centro-cervical lymphadenectomy of level VI lymph nodes and/or further locoregional excision.Histological diagnosis was defined by WHO criteria [26].Resection Margins were defined as per WHO criteria [26].
- -
- R0: no cancer cells at the margins.
- -
- R1: cancer cells at the edge of the histological specimen or resection within less than 1 mm of the edge.
- -
- Low risk: Capsular invasion combined with invasion of surrounding tissue only.
- -
- High risk: Vascular invasion and/or lymph node metastases and/or invasion of vital organs and/or distant metastases.
2.1. Literature Review
2.2. Eligibility Criteria
3. Results
3.1. Surgical Procedures, Margin Status and Nodal Status
3.2. Outcomes of Follow-Up
3.2.1. Overall Survival (OS)
3.2.2. Recurrence-Free Survival (RFS), Distant Metastasis, and Disease-Specific Survival (DSS)
3.3. Recurrence and Treatment Outcomes after Recurrence
3.4. Surgery as Driver of Margin Status and Recurrence
3.5. Locoregional Outcomes following Adjuvant Radiotherapy
3.6. Survivorship of Patients with Parathyroid Cancer
3.7. Benchmarking Outcomes
3.7.1. Survival Outcomes
3.7.2. Outcomes following Adjuvant Radiotherapy
4. Discussion
4.1. A High Index of Suspicion Drives Intra-Operative Decision-Making and Promotes Clear Margins
4.2. The Limited Role of Second-Line Management
4.3. External Beam Radiation Therapy as Adjunct to Surgery?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lo, W.M.; Good, M.L.; Nilubol, N.; Perrier, N.D.; Patel, D.T. Tumor Size and Presence of Metastatic Disease at Diagnosis are Associated with Disease-Specific Survival in Parathyroid Carcinoma. Ann. Surg. Oncol. 2018, 25, 2535–2540. [Google Scholar] [CrossRef]
- Cetani, F.; Marcocci, C. Parathyroid Carcinoma. In The Parathyroids: Basic and Clinical Concepts, 3rd ed.; Elsevier: Oxford, UK, 2015; pp. 409–421. [Google Scholar]
- Schulte, K.M.; Talat, N. Diagnosis and management of parathyroid cancer. Nat. Rev. Endocrinol. 2012, 8, 612–622. [Google Scholar] [CrossRef]
- Cetani, F.; Pardi, E.; Marcocci, C. Update on parathyroid carcinoma. J. Endocrinol. Investig. 2016, 39, 595–606. [Google Scholar] [CrossRef]
- Talat, N.; Schulte, K.M. Clinical presentation, staging and long-term evolution of parathyroid cancer. Ann. Surg. Oncol. 2010, 17, 2156–2174. [Google Scholar] [CrossRef]
- Tsai, W.H.; Zeng, Y.H.; Lee, C.C.; Tsai, M.C. Mortality factors in recurrent parathyroid cancer: A pooled analysis. J. Bone Miner. Metab. 2022, 40, 508–517. [Google Scholar] [CrossRef]
- Asare, E.A.; Sturgeon, C.; Winchester, D.J.; Liu, L.; Palis, B.; Perrier, N.D.; Evans, D.B.; Winchester, D.P.; Wang, T.S. Parathyroid Carcinoma: An Update on Treatment Outcomes and Prognostic Factors from the National Cancer Data Base (NCDB). Ann. Surg. Oncol. 2015, 22, 3990–3995. [Google Scholar] [CrossRef]
- Ryhänen, E.M.; Leijon, H.; Metso, S.; Eloranta, E.; Korsoff, P.; Ahtiainen, P.; Kekäläinen, P.; Tamminen, M.; Ristamäki, R.; Knutar, O.; et al. A nationwide study on parathyroid carcinoma. Acta Oncol. 2017, 56, 991–1003. [Google Scholar] [CrossRef]
- Harari, A.; Waring, A.; Fernandez-Ranvier, G.; Hwang, J.; Suh, I.; Mitmaker, E.; Shen, W.; Gosnell, J.; Duh, Q.Y.; Clark, O. Parathyroid carcinoma: A 43-year outcome and survival analysis. J. Clin. Endocrinol. Metab. 2011, 96, 3679–3686. [Google Scholar] [CrossRef]
- Sadler, C.; Gow, K.W.; Beierle, E.A.; Doski, J.J.; Langer, M.; Nuchtern, J.G.; Vasudevan, S.A.; Goldfarb, M. Parathyroid carcinoma in more than 1,000 patients: A population-level analysis. Surgery 2014, 156, 1622–1629. [Google Scholar] [CrossRef]
- Ullah, A.; Khan, J.; Waheed, A.; Sharma, N.; Pryor, E.K.; Stumpe, T.R.; Zarate, L.V.; Cason, F.D.; Kumar, S.; Misra, S.; et al. Parathyroid Carcinoma: Incidence, Survival Analysis, and Management: A Study from the SEER Database and Insights into Future Therapeutic Perspectives. Cancers 2022, 14, 1426. [Google Scholar] [CrossRef]
- Dood, R.L.; Zhao, Y.; Armbruster, S.D.; Coleman, R.L.; Tworoger, S.; Sood, A.K.; Baggerly, K.A. Defining survivorship trajectories across patients with solid tumors: An evidence-based approach. JAMA Oncol. 2018, 4, 1519–1526. [Google Scholar] [CrossRef]
- Zhang, K.; Su, A.; Wang, X.; Zhao, W.; He, L.; Wei, T.; Li, Z.; Zhu, J.; Chen, Y.W. Non-Linear Correlation Between Tumor Size and Survival Outcomes for Parathyroid Carcinoma: A SEER Population-Based Cohort Study. Front. Endocrinol. 2022, 13, 882579. [Google Scholar] [CrossRef]
- Nana, M.; Morgan, H.; Shrikrishnapalasuriyar, N.; Kalhan, A. Primary hyperparathyroidism: Comparing cardiovascular morbidity and mortality in patients treated with parathyroidectomy versus conservative management. J. Endocrinol. Metab. 2019, 9, 95–107. [Google Scholar] [CrossRef]
- Wermers, R.A.; Griebeler, M.L.; Thapa, P.; Hathcock, M.A.; Kearns, A.E. Survival in primary hyperparathyroidism over five decades (1965–2010) a population-based retrospective study. Bone 2021, 152, 116099. [Google Scholar] [CrossRef]
- Ioachimescu, A.G. Hypercalcemia and its Multiple Facets. Endocrinol. Metab. Clin. N. Am. 2021, 50, xiii–xiv. [Google Scholar] [CrossRef]
- Scheen, M.; Nowak, G.; Sanchez, B.; Teta, D. Fine-tuned continuous renal replacement therapy with calcium-free dialysate to manage severe hypercalcemia refractory to medical and intermittent hemodialysis. Eur. J. Med. Res. 2022, 27, 89. [Google Scholar] [CrossRef]
- O’Callaghan, S.; Yau, H. Treatment of malignancy-associated hypercalcemia with cinacalcet: A paradigm shift. Endocr. Connect. 2021, 10, 13–24. [Google Scholar] [CrossRef]
- Wong, H.K.G.; Shipman, K.; Allan, K.; Ghabbour, A.; Borumandi, F. Giant parathyroid tumours in primary hyperparathyroidism: A systematic review. Langenbeck’s Arch. Surg. 2022, 407, 501–516. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Wang, T.S.; Ruan, D.T.; Lee, J.A.; Asa, S.L.; Duh, Q.Y.; Doherty, G.M.; Herrera, M.F.; Pasieka, J.L.; Perrier, N.D.; et al. The American association of endocrine surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg. 2016, 151, 959–968. [Google Scholar] [CrossRef]
- Fingeret, A.L. Contemporary Evaluation and Management of Parathyroid Carcinoma. JCO Oncol. Pract. 2021, 17, 17–21. [Google Scholar] [CrossRef]
- Fackelmayer, O.J.; Harari, A. Parathyroid Carcinoma: Surgical Resection and Therapies Beyond the Scalpel. JCO Oncol. Pract. 2021, 17, 128–129. [Google Scholar] [CrossRef]
- Schulte, K.M.; Talat, N.; Galata, G.; Gilbert, J.; Miell, J.; Hofbauer, L.C.; Barthel, A.; Diaz-Cano, S.; Bornstein, S.R. Oncologic resection achieving r0 margins improves disease-free survival in parathyroid cancer. Ann. Surg. Oncol. 2014, 21, 1891–1897. [Google Scholar] [CrossRef]
- Schulte, K.M.; Talat, N.; Miell, J.; Moniz, C.; Sinha, P.; Diaz-Cano, S. Lymph node involvement and surgical approach in parathyroid cancer. World J. Surg. 2010, 34, 2611–2620. [Google Scholar] [CrossRef]
- Schulte, K.M.; Gill, A.J.; Barczynski, M.; Karakas, E.; Miyauchi, A.; Knoefel, W.T.; Lombardi, C.P.; Talat, N.; Diaz-Cano, S.; Grant, C.S. Classification of parathyroid cancer. Ann. Surg. Oncol. 2012, 19, 2620–2628. [Google Scholar] [CrossRef]
- DeLellis, R.A. Pathology and Genetics of Tumours of Endocrine Organs; IARC: Lyon, France, 2004; Volume 8. [Google Scholar]
- Robbins, K.T.; Shaha, A.R.; Medina, J.E.; Califano, J.A.; Wolf, G.T.; Ferlito, A.; Som, P.M.; Day, T.A. Consensus statement on the classification and terminology of neck dissection. Arch. Otolaryngol. Head Neck Surg. 2008, 134, 536–538. [Google Scholar] [CrossRef]
- Wynne, A.G.; Van Heerden, J.; Aidan Carney, J.; Fitzpatrick, L.A. Parathyroid carcinoma: Clinical and pathologic features in 43 patients. Medicine 1992, 71, 197–205. [Google Scholar] [CrossRef]
- Chow, E.; Tsang, R.W.; Brierley, J.D.; Filice, S. Parathyroid carcinoma–The Princess Margaret Hospital experience. Int. J. Radiat. Oncol. Biol. Phys. 1998, 41, 569–572. [Google Scholar] [CrossRef]
- Dotzenrath, C.; Goretzki, P.E.; Sarbia, M.; Cupisti, K.; Feldkamp, J.; Röher, H.D. Parathyroid carcinoma: Problems in diagnosis and the need for radical surgery even in recurrent disease. Eur. J. Surg. Oncol. 2001, 27, 383–389. [Google Scholar] [CrossRef]
- Munson, N.D.; Foote, R.L.; Northcutt, R.C.; Tiegs, R.D.; Fitzpatrick, L.A.; Grant, C.S.; van Heerden, J.A.; Thompson, G.B.; Lloyd, R.V. Parathyroid carcinoma: Is there a role for adjuvant radiation therapy? Cancer 2003, 98, 2378–2384. [Google Scholar] [CrossRef]
- Busaidy, N.L.; Jimenez, C.; Habra, M.A.; Schultz, P.N.; El-Naggar, A.K.; Clayman, G.L.; Asper, J.A.; Diaz Jr, E.M.; Evans, D.B.; Gagel, R.F.; et al. Parathyroid carcinoma: A 22-year experience. Head Neck 2004, 26, 716–726. [Google Scholar] [CrossRef]
- Iihara, M.; Okamoto, T.; Suzuki, R.; Kawamata, A.; Nishikawa, T.; Kobayashi, M.; Obara, T. Functional parathyroid carcinoma: Long-term treatment outcome and risk factor analysis. Surgery 2007, 142, 936–943.e931. [Google Scholar] [CrossRef] [PubMed]
- Karakas, E.; Müller, H.H.; Lyadov, V.K.; Luz, S.; Schneider, R.; Rothmund, M.; Bartsch, D.K.; Schlosser, K. Development of a formula to predict parathyroid carcinoma in patients with primary hyperparathyroidism. World J. Surg. 2012, 36, 2605–2611. [Google Scholar] [CrossRef] [PubMed]
- Silva-Figueroa, A.M.; Hess, K.R.; Williams, M.D.; Clarke, C.N.; Christakis, I.; Graham, P.H.; Grubbs, E.G.; Lee, J.E.; Busaidy, N.L.; Perrier, N.D. Prognostic Scoring System to Risk Stratify Parathyroid Carcinoma. J. Am. Coll. Surg. 2017, 224, 980–987. [Google Scholar] [CrossRef]
- Basceken, S.I.; Genc, V.; Ersoz, S.; Sevim, Y.; Celik, S.U.; Bayram, I.K. Is local resection sufficient for parathyroid carcinoma? Clinics 2015, 70, 247–249. [Google Scholar] [CrossRef]
- Xue, S.; Chen, H.; Lv, C.; Shen, X.; Ding, J.; Liu, J.; Chen, X. Preoperative diagnosis and prognosis in 40 Parathyroid Carcinoma Patients. Clin. Endocrinol. 2016, 85, 29–36. [Google Scholar] [CrossRef]
- Libánský, P.; Adámek, S.; Broulík, P.; Fialová, M.; Kubinyi, J.; Lischke, R.; Naňka, O.; Pafko, P.; Šedý, J.; Bobek, V. Parathyroid carcinoma in patients that have undergone surgery for primary hyperparathyroidism. In Vivo 2017, 31, 925–930. [Google Scholar] [CrossRef][Green Version]
- Asare, E.A.; Silva-Figueroa, A.; Hess, K.R.; Busaidy, N.; Graham, P.H.; Grubbs, E.G.; Lee, J.E.; Williams, M.D.; Perrier, N.D. Risk of Distant Metastasis in Parathyroid Carcinoma and Its Effect on Survival: A Retrospective Review from a High-Volume Center. Ann. Surg. Oncol. 2019, 26, 3593–3599. [Google Scholar] [CrossRef]
- Zheng, Y.; Wen, J.; Lin, W.; Lin, L.; Chen, G. Approach to parathyroid carcinoma (PC): Seven cases and a review of the literature. Transl. Cancer Res. 2020, 9, 4982–4987. [Google Scholar] [CrossRef]
- De Pasquale, L.; Bulfamante, A.M.; Felisati, G.; Castellani, L.; Ghilardi, G.; Saibene, A.M. Management and Outcome of Parathyroid Carcinoma-Induced Primary Hyperparathyroidism: A Single-Centre Experience. Int. J. Endocrinol. 2021, 2021, 5397941. [Google Scholar] [CrossRef]
- Sali, A.P.; Motghare, P.; Bal, M.; Mittal, N.; Rane, S.; Kane, S.; Patil, A. Parathyroid Carcinoma: A Single-Institution Experience with an Emphasis on Histopathological Features. Head Neck Pathol. 2021, 15, 544–554. [Google Scholar] [CrossRef]
- Cunha, C.; Pinheiro, S.L.; Donato, S.; Tavares Bello, C.; Simões, H.; Nunes Silva, T.; Prazeres, S.; Doutel, D.; Cavaco, B.M.; Leite, V. Parathyroid carcinoma: Single centre experience. Clin. Endocrinol. 2022, 97, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Zhao, T.; Shen, H.; Jin, M.; Zhou, Q.; Liu, X.; Wang, J.; Wang, Q. Extended En Bloc Reoperation for Recurrent or Persistent Parathyroid Carcinoma: Analysis of 31 Cases in a Single Institute Experience. Ann. Surg. Oncol. 2022, 29, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Hundahl, S.A.; Fleming, I.D.; Fremgen, A.M.; Menck, H.R. Two hundred eighty-six cases of parathyroid carcinoma treated in the U.S. between 1985-1995: A National Cancer Data Base report. Cancer 1999, 86, 538–544. [Google Scholar] [CrossRef]
- Schaapveld, M.; Jorna, F.H.; Aben, K.K.H.; Haak, H.R.; Plukker, J.T.M.; Links, T.P. Incidence and prognosis of parathyroid gland carcinoma: A population-based study in the Netherlands estimating the preoperative diagnosis. Am. J. Surg. 2011, 202, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.T.; Sippel, R.S.; Chen, H.; Schneider, D.F. Is central lymph node dissection necessary for parathyroid carcinoma? Surgery 2014, 156, 1336–1341. [Google Scholar] [CrossRef]
- Villar Del Moral, J.; Jiménez-García, A.; Salvador-Egea, P.; Martos-Martínez, J.M.; Nuño-Vázquez-Garza, J.M.; Serradilla-Martín, M.; Gómez-Palacios, A.; Moreno-Llorente, P.; Ortega-Serrano, J.; De La Quintana-Basarrate, A. Prognostic factors and staging systems in parathyroid cancer: A multicenter cohort study. Surgery 2014, 156, 1132–1144. [Google Scholar] [CrossRef]
- Christakis, I.; Silva, A.M.; Kwatampora, L.J.; Warneke, C.L.; Clarke, C.N.; Williams, M.D.; Grubbs, E.G.; Lee, J.E.; Busaidy, N.L.; Perrier, N.D. Oncologic progress for the treatment of parathyroid carcinoma is needed. J. Surg. Oncol. 2016, 114, 708–713. [Google Scholar] [CrossRef]
- Young, S.; Wu, J.X.; Li, N.; Yeh, M.W.; Livhits, M.J. More Extensive Surgery May Not Improve Survival Over Parathyroidectomy Alone in Parathyroid Carcinoma. Ann. Surg. Oncol. 2016, 23, 2898–2904. [Google Scholar] [CrossRef]
- Wang, P.; Xue, S.; Wang, S.; Lv, Z.; Meng, X.; Wang, G.; Meng, W.; Liu, J.; Chen, G. Clinical characteristics and treatment outcomes of parathyroid carcinoma: A retrospective review of 234 cases. Oncol. Lett. 2017, 14, 7276–7282. [Google Scholar] [CrossRef]
- Kong, S.H.; Kim, J.H.; Park, M.Y.; Kim, S.W.; Shin, C.S. Epidemiology and prognosis of parathyroid carcinoma: Real-world data using nationwide cohort. J. Cancer Res. Clin. Oncol. 2021, 147, 3091–3097. [Google Scholar] [CrossRef]
- Qian, B.; Qian, Y.; Hu, L.; Zhang, S.; Mei, L.; Qu, X. Prognostic Analysis for Patients With Parathyroid Carcinoma: A Population-Based Study. Front. Neurosci. 2022, 16, 784599. [Google Scholar] [CrossRef] [PubMed]
- Erovic, B.M.; Goldstein, D.P.; Kim, D.; Mete, O.; Brierley, J.; Tsang, R.; Freeman, J.L.; Asa, S.L.; Rotstein, L.; Irish, J.C. Parathyroid cancer: Outcome analysis of 16 patients treated at the princess margaret hospital. Head Neck 2013, 35, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Selvan, B.; Paul, M.J.; Seshadri, M.S.; Thomas, N.; Paul, T.; Abraham, D.; Oommen, R.; Shandhly, N.; John, S.; Rajaratnam, S.; et al. High index of clinical suspicion with optimal surgical techniques and adjuvant radiotherapy is critical to reduce locoregional disease progression in parathyroid carcinoma. Am. J. Clin. Oncol. 2013, 36, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Christakis, I.; Silva, A.M.; Williams, M.D.; Garden, A.; Grubbs, E.G.; Busaidy, N.L.; Lee, J.E.; Perrier, N.D.; Zafereo, M. Postoperative local-regional radiation therapy in the treatment of parathyroid carcinoma: The MD Anderson experience of 35 years. Pract. Radiat. Oncol. 2017, 7, e463–e470. [Google Scholar] [CrossRef] [PubMed]
- Akirov, A.; Asa, S.L.; Larouche, V.; Mete, O.; Sawka, A.M.; Jang, R.; Ezzat, S. The Clinicopathological Spectrum of Parathyroid Carcinoma. Front. Endocrinol. 2019, 10, 00731. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Arya, A.K.; Bhadada, S.K.; Saikia, U.N.; Sood, A.; Dahiya, D.; Behera, A. Parathyroid Carcinoma: An Experience from the Indian Primary Hyperparathyroidism Registry. Endocr. Pract. 2021, 27, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Lenschow, C.; Schrägle, S.; Kircher, S.; Lorenz, K.; Machens, A.; Dralle, H.; Riss, P.; Scheuba, C.; Pfestroff, A.; Spitzweg, C.; et al. Clinical Presentation, Treatment, and Outcome of Parathyroid Carcinoma: Results of the NEKAR Retrospective International Multicenter Study. Ann. Surg. 2022, 275, E479–E487. [Google Scholar] [CrossRef]
- Sun, X.M.; Pang, F.; Zhuang, S.M.; Xie, L.E.; Zhong, Q.Y.; Liu, T.R. Tumor size rather than the thyroid invasion affects the prognosis of parathyroid carcinoma without lymph node or distant metastasis. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 4587–4594. [Google Scholar] [CrossRef]
- Tao, M.; Luo, S.; Wang, X.; Jia, M.; Lu, X. A Nomogram Predicting the Overall Survival and Cancer-Specific Survival in Patients with Parathyroid Cancer: A Retrospective Study. Front. Endocrinol. 2022, 13, 850457. [Google Scholar] [CrossRef]
- Bollerslev, J.; Schalin-Jäntti, C.; Rejnmark, L.; Siggelkow, H.; Morreau, H.; Thakker, R.; Sitges-Serra, A.; Cetani, F.; Marcocci, C.; Guistina, A.; et al. Unmet therapeutic, educational and scientific needs in parathyroid disorders: Consensus statement from the first European Society of Endocrinology Workshop (PARAT). Eur. J. Endocrinol. 2019, 181, P1–P19. [Google Scholar] [CrossRef]
- Cetani, F.; Pardi, E.; Marcocci, C. Parathyroid Carcinoma and Ectopic Secretion of Parathyroid hormone. Endocrinol. Metab. Clin. N. Am. 2021, 50, 683–709. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Pettinga, D.; Schubert, A.D.; Ladenson, P.W.; Ball, D.W.; Chung, J.H.; Schrock, A.B.; Madison, R.; Frampton, G.M.; Stephens, P.J.; et al. Genomic Profiling of Parathyroid Carcinoma Reveals Genomic Alterations Suggesting Benefit from Therapy. Oncologist 2019, 24, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Pandya, C.; Uzilov, A.V.; Bellizzi, J.; Lau, C.Y.; Moe, A.S.; Strahl, M.; Hamou, W.; Newman, L.C.; Fink, M.Y.; Antipin, Y.; et al. Genomic profiling reveals mutational landscape in parathyroid carcinomas. JCI Insight 2017, 2, e92061. [Google Scholar] [CrossRef] [PubMed]
- Wielogórska, M.; Podgórska, B.; Niemira, M.; Szelachowska, M.; Krętowski, A.; Siewko, K. MicroRNA Profile Alterations in Parathyroid Carcinoma: Latest Updates and Perspectives. Cancers 2022, 14, 876. [Google Scholar] [CrossRef] [PubMed]
- Juhlin, C.C.; Erickson, L.A. Genomics and Epigenomics in Parathyroid Neoplasia: From Bench to Surgical Pathology Practice. Endocr. Pathol. 2021, 32, 17–34. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Talat, N.; Patel, P.; Mulholland, N.J.; Schulte, K.M. Ultrasound features of malignancy in the preoperative diagnosis of parathyroid cancer: A retrospective analysis of parathyroid tumours larger than 15 mm. Eur. Radiol. 2011, 21, 1865–1873. [Google Scholar] [CrossRef]
- Fang, C.; Konstantatou, E.; Mulholland, N.J.; Baroncini, S.; Husainy, M.A.; Schulte, K.M.; Sidhu, P.S. A retrospective review of the role of B-mode and color Doppler ultrasonography in the investigation of primary hyperparathyroidism: Features that differentiate benign from malignant lesions. Ultrasound 2018, 26, 110–117. [Google Scholar] [CrossRef]
- Van Der Tuin, K.; Tops, C.M.J.; Adank, M.A.; Cobben, J.M.; Hamdy, N.A.T.; Jongmans, M.C.; Menko, F.H.; Van Nesselrooij, B.P.M.; Netea-Maier, R.T.; Oosterwijk, J.C.; et al. CDC73-related disorders: Clinical manifestations and case detection in primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 2017, 102, 4534–4540. [Google Scholar] [CrossRef]
- Marx, S.J.; Lourenco, D.M. Familial Hyperparathyroidism–Disorders of Growth and Secretion in Hormone-Secretory Tissue. Horm. Metab. Res. 2017, 49, 805–815. [Google Scholar] [CrossRef]
- Yin, H.; Shi, H. Re: Hyperparathyroidism Due to Concurrent Parathyroid Carcinoma and Parathyroid Adenoma. Clin. Nucl. Med. 2022, 47, E380. [Google Scholar] [CrossRef]
- Dorgeloh, J.R. Parathyroid Carcinoma Causing Hyperparathyroidism. Calif. Med. 1948, 69, 138–140. [Google Scholar] [PubMed]
- Pelizzo, M.R.; Piotto, A.; Bergamasco, A.; Rubello, D.; Casara, D. Parathyroid carcinoma. Therapeutic strategies derived from 20 years of experience. Minerva Endocrinol. 2001, 26, 23–29. [Google Scholar] [PubMed]
- Lee, P.K.; Jarosek, S.L.; Virnig, B.A.; Evasovich, M.; Tuttle, T.M. Trends in the incidence and treatment of parathyroid cancer in the United States. Cancer 2007, 109, 1736–1741. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.J.; Chan, C.; Symons, J.; Learoyd, D.L.; Sidhu, S.B.; Delbridge, L.W.; Gill, A.; Sywak, M.S. Parathyroid carcinoma encountered after minimally invasive focused parathyroidectomy may not require further radical surgery. World J. Surg. 2011, 35, 147–153. [Google Scholar] [CrossRef]
- Medas, F.; Erdas, E.; Loi, G.; Podda, F.; Pisano, G.; Nicolosi, A.; Calo, P.G. Controversies in the management of parathyroid carcinoma: A case series and review of the literature. Int. J. Surg. 2016, 28 (Suppl. 1), S94–S98. [Google Scholar] [CrossRef]
- Lo, W.M.; Patel, D.T. ASO Author Reflections: Parathyroid Carcinoma–Setting the Stage for Prognosis. Ann. Surg. Oncol. 2018, 25, 864–865. [Google Scholar] [CrossRef]
- Leonard-Murali, S.; Ivanics, T.; Kwon, D.S.; Han, X.; Steffes, C.P.; Shah, R. Local resection versus radical surgery for parathyroid carcinoma: A National Cancer Database analysis. Eur. J. Surg. Oncol. 2021, 47, 2768–2773. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, Y.; Zeng, W.; Chen, S.; Zhou, W.; Wang, M.; Wei, W.; Zhang, C.; Huang, J.; Liu, Z.; et al. Surgical Disparities of Parathyroid Carcinoma: Long-Term Outcomes and Deep Excavation Based on a Large Database. J. Oncol. 2021, 2021, 8898926. [Google Scholar] [CrossRef]
- Gray, W.K.; Navaratnam, A.V.; Day, J.; Wass, J.A.H.; Briggs, T.W.R.; Lansdown, M. Volume-Outcome Associations for Parathyroid Surgery in England: Analysis of an Administrative Data Set for the Getting It Right First Time Program. JAMA Surg. 2022, 157, 581–588. [Google Scholar] [CrossRef]
- Krishnan, S.; Suresh, D.; Foote, R.L. Radiation treatment of endocrine tumors. In Endocrine Pathology: Differential Diagnosis and Molecular Advances; Springer: New York, NY, USA; Heidelberg, Germany; London, UK, 2010; pp. 567–579. [Google Scholar]
- Goel, M.K.; Khanna, P.; Kishore, J. Understanding survival analysis: Kaplan-Meier estimate. Int. J. Ayurveda Res. 2010, 1, 274–278. [Google Scholar] [CrossRef]
- Winchester, D.P.; Stewart, A.K.; Bura, C.; Jones, R.S. The National Cancer Data Base: A Clinical Surveillance and Quality Improvement Tool. J. Surg. Oncol. 2004, 85, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Limberg, J.; Stefanova, D.; Ullmann, T.M.; Thiesmeyer, J.W.; Bains, S.; Beninato, T.; Zarnegar, R.; Fahey, T.J., III; Finnerty, B.M. The Use and Benefit of Adjuvant Radiotherapy in Parathyroid Carcinoma: A National Cancer Database Analysis. Ann. Surg. Oncol. 2021, 28, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Vaz-Luis, I.; Masiero, M.; Cavaletti, G.; Cervantes, A.; Chlebowski, R.T.; Curigliano, G.; Felip, E.; Ferreira, A.R.; Ganz, P.A.; Hegarty, J.; et al. ESMO Expert Consensus Statements on Cancer Survivorship: Promoting high-quality survivorship care and research in Europe. Ann. Oncol. 2022, 33, 1119–1133. [Google Scholar] [CrossRef] [PubMed]
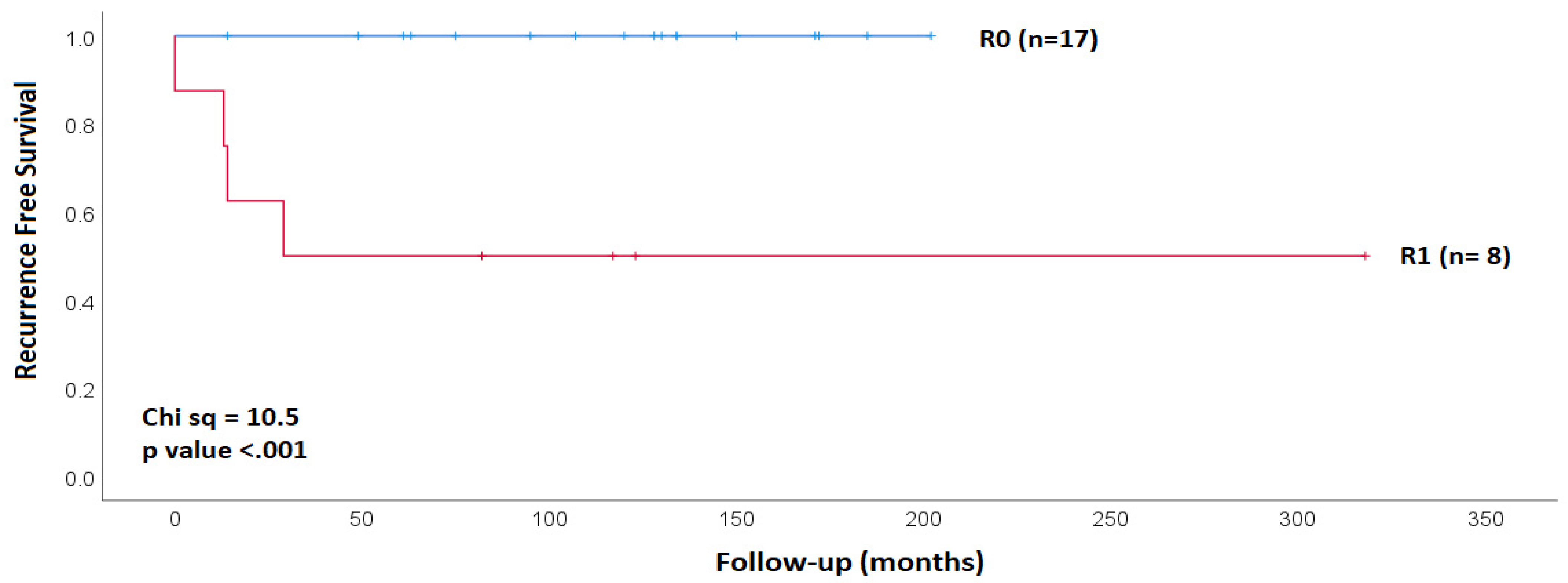
| Clinical Characteristics | R0 | R1 | Chi Square |
|---|---|---|---|
| n = 17 | n = 8 | p Value | |
| gender | |||
| male/female | 5/12 | 5/3 | n.s. |
| age (years) | |||
| mean ± SD | 54.4 ± 14.5 | 62.9 ± 11.9 | n.s. |
| median | 58.0 | 63.5 | |
| range | 33–82 | 43–81 | |
| PTH a | |||
| mean ± SD | 9.3 ± 7.2 | 7.1 ± 4.6 | n.s. |
| median | 6.1 | 6.7 | |
| range | 1.5–22.3 | 1.3–13.7 | |
| corrected calcium (mmol/L) | |||
| mean ± SD | 3.1 ± 0.5 | 3.1 ± 0.3 | n.s. |
| median | 2.8 | 3.0 | |
| range | 2.8–4.7 | 2.7–3.6 | |
| size of lesion (mm) | |||
| mean ± SD | 38.4 ± 15.3 | 31.4 ± 14.1 | n.s. |
| median | 35.0 | 25.0 | |
| range | 15–65 | 20–56 | |
| lymph node metastasis | |||
| yes/no | 0/14 b | 1/3 c | n.s. |
| distant metastasis | |||
| yes/no | 0/17 | 0/8 | n.s. |
| histology | |||
| low risk/high risk | 9/8 | 5/3 | n.s. |
| Clinical Characteristics | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| RFS | No RFS | Chi Square | Chi Square | |
| n = 21 | n = 4 | p Value | p Value | |
| gender | ||||
| male/female | 5/16 | 3/1 | n.s. | n.s. |
| age (years) | ||||
| mean ± SD | 59.9 ± 14.0 | 67.0 ± 10.0 | n.s. | n.s. |
| median | 59.0 | 65.0 | ||
| range | 33–82 | 57–81 | ||
| PTH a | ||||
| mean ± SD | 9.0 ± 6.8 | 6.7 ± 4.5 | n.s. | n.s. |
| median | 6.7 | 6.6 | ||
| range | 1.5–22.3 | 1.3–22.3 | ||
| corrected calcium (mmol/L) | ||||
| mean ± SD | 3.1 ± 0.5 | 3.1 ± 0.4 | n.s. | n.s. |
| median | 2.9 | 3.1 | ||
| range | 2.8–4.7 | 2.7–3.6 | ||
| size of lesion (mm) | ||||
| mean ± SD | 36.9 ± 12.7 | 32.8 ± 12.7 | n.s. | n.s. |
| median | 33.0 | 30.5 | ||
| range | 15–65 | 20–50 | ||
| margin status | 17/4 | 0/4 | 10.1 | 19.5 |
| R0/R1 | <0.01 | <0.001 |
| Surgery | All | Margin | Recurrence | ||||
|---|---|---|---|---|---|---|---|
| R1 Positive | R0 Negative | Positive Margin | Yes | No | Risk of Recurrence | ||
| all | 25 | 8 | 17 | 32.0% | 4 | 21 | 8.0% |
| en bloc resection | 17 | 3 | 14 | 17.7% | 1 | 16 | 5.9% |
| local excision | 8 | 5 | 3 | 62.5% | 3 | 5 | 37.5% |
| relative risk (RR) | 3.54 | 6.38 | |||||
| 95% CI | (1.11–11.28) | (0.78–52.1) | |||||
| significance | p = 0.03 | p = 0.08 | |||||
| Margin | All | Recurrence | Recurrence | Risk of Recurrence |
|---|---|---|---|---|
| Yes | No | |||
| positive | 8 | 4 | 4 | 50.0% |
| negative | 17 | 0 | 17 | 0% |
| relative risk | 18.0 | |||
| 95% CI | 1.1–299.0 | |||
| significance, two-sided | p = 0.04 |
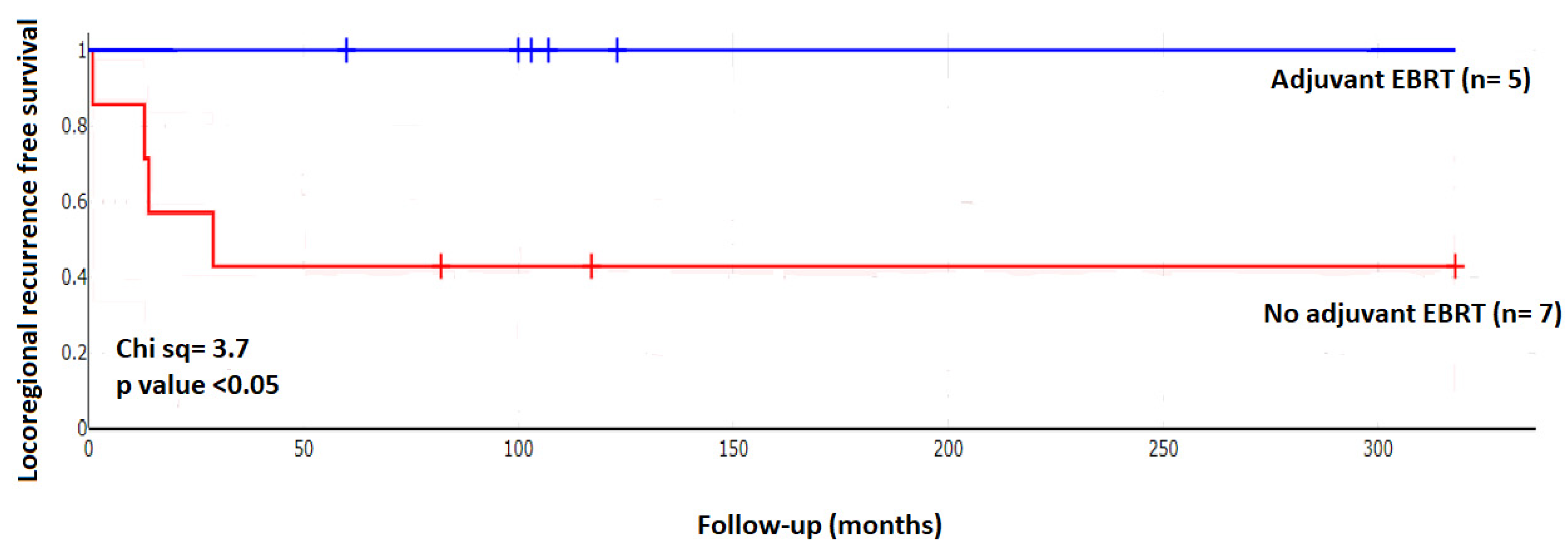
| Recurrence | Recurrence | Risk of Recurrence with EBRT | ||
|---|---|---|---|---|
| Yes | No | |||
| all | 12 | 4 | 8 | 33.3% |
| adjuvant EBRT | 5 | 0 | 5 | 0.0% |
| no EBRT | 7 | 4 | 3 | 57.1% |
| relative risk | 0.18 | |||
| 95% CI | 0.01–2.65 | |||
| significance, two-sided | p = 0.20 | |||
| Odd’s ratio | 0.09 | |||
| 95% CI | 0.003–2.203 | |||
| significance, two-sided | p = 0.13 | |||
| Number needed to treat NNT to achieve a benefit | 2.2 | |||
| 95% CI | 1.1–51.4 |
| Parameter | R0 Resection | R1 Resection | Difference |
|---|---|---|---|
| n = 17 | n = 8 | ||
| redo surgery | 0 | 4 | RR 18.0 |
| 95% CI 1.1–299.0 | |||
| p = 0.04 | |||
| adjuvant EBRT | 0 | 5 | RR 22.0 |
| 95% CI 1.4–355.5 | |||
| p = 0.03 | |||
| recurrent nerve palsy (following nerve resection) | 3 | 1 | RR 1.41 |
| 95% CI 0.2–11.6 | |||
| p = 0.75 | |||
| days of all inpatient admissions (mean ± SD) a | 0 | 8 (1.0 ± 1.1) | not calculated |
| days of outpatient admissions (mean ± SD) b | 4 (0.3 ± 1.0) | 176 (22.0 ± 23.7) | Chi sq = 979.5 p < 0.001 |
“days of worry”; i.e., days before either
| 0 | 1369 (171.1 ± 302.5) | Chi sq = 11.3 p = 0.003 |
| Source | n | Overall Survival | Recurrence Free Survival | Disease-Specific Survival | |||
|---|---|---|---|---|---|---|---|
| OS | RFS | DSS | |||||
| 5-Year | 10-Year | 5-Year | 10-Year | 5-Year | 10-Year | ||
| case series | |||||||
| range | 484 | 50.0–100% | 43.0–100% | 33.2–88.9% | 29.4–83.0% | 80.0–100% | 72.0–100% |
| median | 79.2% | 68.0% | 62.0% | 51.0% | 90.9% | 83.3% | |
| register data | |||||||
| range | 1703 | 78.5–90.6% | 49.1–72.9% | 59.6–79.0% | 51.5–70.8% | 82.5–94.1% | 67.0–92.1% |
| median | 82.6% | 68.3% | 73.9% | 69.1% | 87.0% | 78.9% | |
| our cohort | 25 | 95.8% | 84.2% | 82.6% | 79.0% | 100% | 100% |
| R0 or R1 + EBRT | 19 | 94.7% | 89.4% | 100% | 100% | 100% | 100% |
| R1, no EBRT | 6 | 100% | 66.7% | 33.3% | 33.3% | 100% | 100% |
| Year | Author | n | EBRT | No EBRT |
|---|---|---|---|---|
| Recurrence | Recurrence | |||
| Yes/No | Yes/No | |||
| 1998 | Chow [29] | 10 | 0/6 | 3/1 |
| 2003 | Munson [31] | 4 | 0/4 | 0 |
| 2004 | Busaidy [32] | 26 | 1/5 | 9/11 |
| 2011 | Schaapveld [46] | 10 | 3/1 | 5/1 |
| 2013 | Erovic [54] | 16 | 3/2 | 2/9 |
| 2013 | Selvan [55] | 9 | 0/6 | 3/0 |
| 2017 | Christakis [56] | 8 | 1/4 | 3/0 |
| 2019 | Akirov [57] | 7 | 4/0 | 2/1 |
| 2021 | Sali [42] | 16 | 2/7 | 0/7 |
| 2021 | Cunha [43] | 17 | 3/0 | 5/9 |
| 1999–2014 | Total | 123 | 17/35 | 32/39 |
| 32.7% | 45.1% | |||
| relative risk of recurrence with EBRT | 0.7 | |||
| 95% CI | 0.45–1.16 | |||
| significance | p = 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulte, K.-M.; Talat, N.; Galatá, G. Margin Free Resection Achieves Excellent Long Term Outcomes in Parathyroid Cancer. Cancers 2023, 15, 199. https://doi.org/10.3390/cancers15010199
Schulte K-M, Talat N, Galatá G. Margin Free Resection Achieves Excellent Long Term Outcomes in Parathyroid Cancer. Cancers. 2023; 15(1):199. https://doi.org/10.3390/cancers15010199
Chicago/Turabian StyleSchulte, Klaus-Martin, Nadia Talat, and Gabriele Galatá. 2023. "Margin Free Resection Achieves Excellent Long Term Outcomes in Parathyroid Cancer" Cancers 15, no. 1: 199. https://doi.org/10.3390/cancers15010199
APA StyleSchulte, K.-M., Talat, N., & Galatá, G. (2023). Margin Free Resection Achieves Excellent Long Term Outcomes in Parathyroid Cancer. Cancers, 15(1), 199. https://doi.org/10.3390/cancers15010199







