Palmitoyl Carnitine-Anchored Nanoliposomes for Neovasculature-Specific Delivery of Gemcitabine Elaidate to Treat Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines and Culture Conditions
2.3. HPLC Analysis
2.4. Preparation of PGPLs
2.5. Characterization and Stability Study of PGPLs
2.6. In Vitro Cytotoxicity
2.7. Quantitative Cellular Uptake Assay
2.8. In Vitro Hemolysis Study
2.9. In Vitro Migration Assay
2.10. Clonogenic Assay
2.11. In Vitro Vasculogenic Mimicry Assay
2.12. Western Blot Assay
2.13. Formation and Treatment of 3D Multicellular Tumor Spheroids
2.14. Cell Viability within 3D Multicellular Tumor Spheroids
2.15. Statistical Analysis
3. Results
3.1. HPLC Analysis
3.2. Characterization and Stability of PGPLs
3.3. In Vitro Cytotoxicity Test
3.4. Quantitative Cellular Uptake Assay
3.5. In Vitro Hemolysis Study
3.6. In Vitro Migration Assay
3.7. Clonogenic Assay
3.8. In Vitro Vasculogenic Mimicry Assay
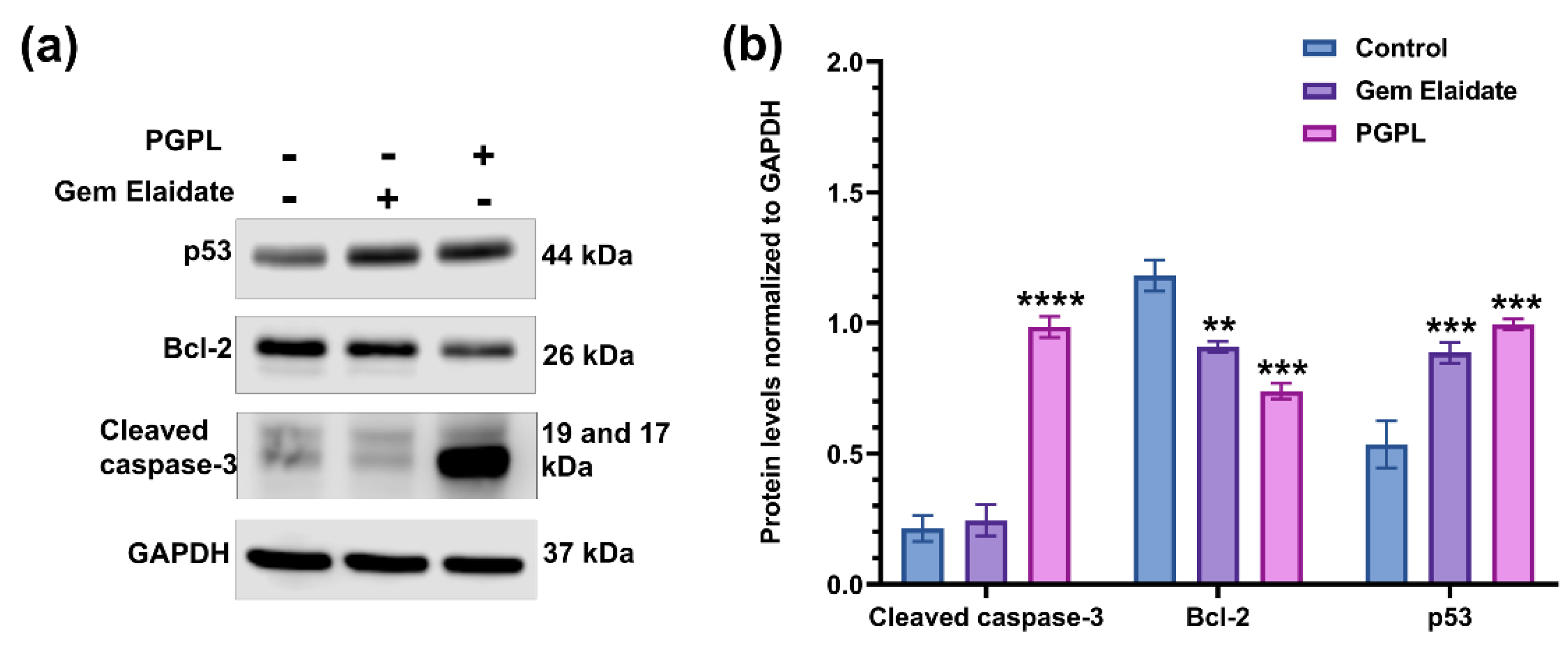
3.9. Western Blot Assay
3.10. Formation and Treatment of 3D Multicellular Tumor Spheroids
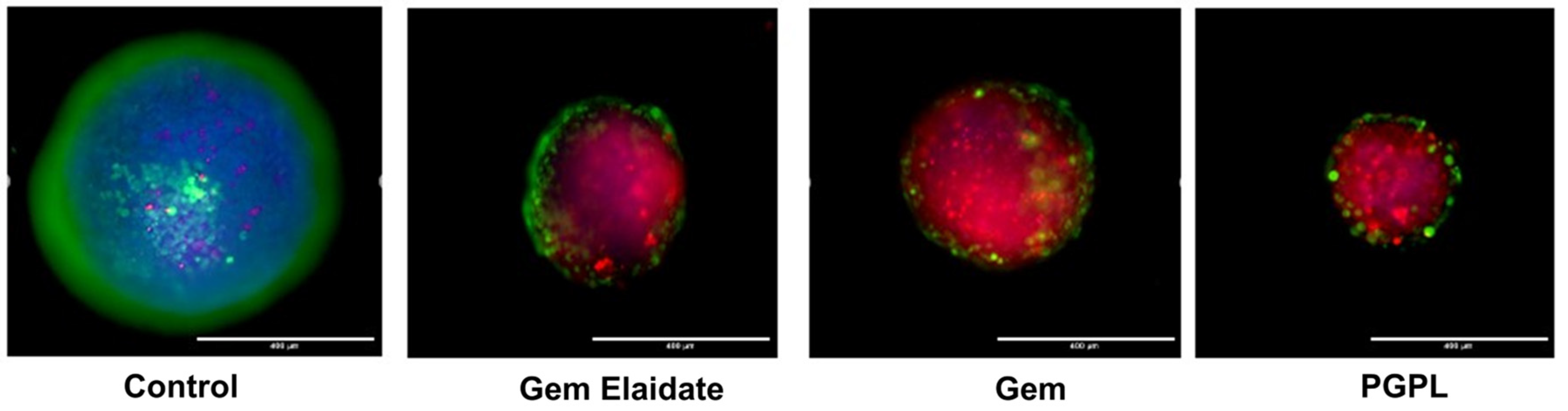
3.11. Cell Viability within 3D Multicellular Tumor Spheroids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Key Statistics for Pancreatic Cancer; American Cancer Society: Atlanta, GA, USA, 2022.
- Anderson, E.M.; Thomassian, S.; Gong, J.; Hendifar, A.; Osipov, A. Advances in Pancreatic Ductal Adenocarcinoma Treatment. Cancers 2021, 13, 5510. [Google Scholar] [CrossRef]
- Garrido-Laguna, I.; Hidalgo, M. Pancreatic cancer: From state-of-the-art treatments to promising novel therapies. Nat. Rev. Clin. Oncol. 2015, 12, 319–334. [Google Scholar] [CrossRef]
- Heinemann, V. Gemcitabine in the treatment of advanced pancreatic cancer: A comparative analysis of randomized trials. Semin. Oncol. 2002, 29, 9–16. [Google Scholar] [CrossRef]
- Matsumoto, T.; Komori, T.; Yoshino, Y.; Ioroi, T.; Kitahashi, T.; Kitahara, H.; Ono, K.; Higuchi, T.; Sakabe, M.; Kori, H.; et al. A Liposomal Gemcitabine, FF-10832, Improves Plasma Stability, Tumor Targeting, and Antitumor Efficacy of Gemcitabine in Pancreatic Cancer Xenograft Models. Pharm. Res. 2021, 38, 1093–1106. [Google Scholar] [CrossRef]
- Ciccolini, J.; Serdjebi, C.; Peters, G.J.; Giovannetti, E. Pharmacokinetics and pharmacogenetics of Gemcitabine as a mainstay in adult and pediatric oncology: An EORTC-PAMM perspective. Cancer Chemother. Pharmacol. 2016, 78, 1–12. [Google Scholar] [CrossRef]
- Nordh, S.; Ansari, D.; Andersson, R. hENT1 expression is predictive of gemcitabine outcome in pancreatic cancer: A systematic review. World J. Gastroenterol. 2014, 20, 8482–8490. [Google Scholar] [CrossRef]
- Bergman, A.M.; Adema, A.D.; Balzarini, J.; Bruheim, S.; Fichtner, I.; Noordhuis, P.; Fodstad, Ø.; Myhren, F.; Sandvold, M.L.; Hendriks, H.R. Antiproliferative activity, mechanism of action and oral antitumor activity of CP-4126, a fatty acid derivative of gemcitabine, in in vitro and in vivo tumor models. Investig. New Drugs 2011, 29, 456–466. [Google Scholar] [CrossRef]
- Spratlin, J.; Sangha, R.; Glubrecht, D.; Dabbagh, L.; Young, J.D.; Dumontet, C.; Cass, C.; Lai, R.; Mackey, J.R. The absence of human equilibrative nucleoside transporter 1 is associated with reduced survival in patients with gemcitabine-treated pancreas adenocarcinoma. Clin. Cancer Res. 2004, 10, 6956–6961. [Google Scholar] [CrossRef]
- Stuurman, F.E.; Voest, E.E.; Awada, A.; Witteveen, P.O.; Bergeland, T.; Hals, P.A.; Rasch, W.; Schellens, J.H.; Hendlisz, A. Phase I study of oral CP-4126, a gemcitabine derivative, in patients with advanced solid tumors. Investig. New Drugs 2013, 31, 959–966. [Google Scholar] [CrossRef]
- Mochly-Rosen, D.; Das, K.; Grimes, K.V. Protein kinase C, an elusive therapeutic target? Nat. Rev. Drug. Discov. 2012, 11, 937–957. [Google Scholar] [CrossRef]
- Storz, P. Targeting protein kinase C subtypes in pancreatic cancer. Expert Rev. Anticancer Ther. 2015, 15, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Spyridopoulos, I.; Luedemann, C.; Chen, D.; Kearney, M.; Chen, D.; Murohara, T.; Principe, N.; Isner, J.M.; Losordo, D.W. Divergence of angiogenic and vascular permeability signaling by VEGF: Inhibition of protein kinase C suppresses VEGF-induced angiogenesis, but promotes VEGF-induced, NO-dependent vascular permeability. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Czerwinski, P.; Hortmann, M.; Sohn, H.Y.; Forstermann, U.; Li, H. Protein kinase C alpha promotes angiogenic activity of human endothelial cells via induction of vascular endothelial growth factor. Cardiovasc. Res. 2008, 78, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Rathod, D.; Patel, K. Protein kinase C inhibitor anchored BRD4 PROTAC PEGylated nanoliposomes for the treatment of vemurafenib-resistant melanoma. Exp. Cell Res. 2020, 396, 112275. [Google Scholar] [CrossRef]
- Venugopal, B.; Awada, A.; Evans, T.R.J.; Dueland, S.; Hendlisz, A.; Rasch, W.; Hernes, K.; Hagen, S.; Aamdal, S. A first-in-human phase I and pharmacokinetic study of CP-4126 (CO-101), a nucleoside analogue, in patients with advanced solid tumours. Cancer Chemother. Pharmacol. 2015, 76, 785–792. [Google Scholar] [CrossRef]
- Campbell, R.B.; Fukumura, D.; Brown, E.B.; Mazzola, L.M.; Izumi, Y.; Jain, R.K.; Torchilin, V.P.; Munn, L.L. Cationic charge determines the distribution of liposomes between the vascular and extravascular compartments of tumors. Cancer Res. 2002, 62, 6831–6836. [Google Scholar]
- Schmitt-Sody, M.; Strieth, S.; Krasnici, S.; Sauer, B.; Schulze, B.; Teifel, M.; Michaelis, U.; Naujoks, K.; Dellian, M. Neovascular targeting therapy: Paclitaxel encapsulated in cationic liposomes improves antitumoral efficacy. Clin. Cancer Res. 2003, 9, 2335–2341. [Google Scholar]
- Saraswat, A.L.; Maher, T.J. Development and optimization of stealth liposomal system for enhanced in vitro cytotoxic effect of quercetin. J. Drug Deliv. Sci. Technol. 2020, 55, 101477. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Soteriou, K.; Fukumura, D.; Jain, R.K. Cationic nanoparticles have superior transvascular flux into solid tumors: Insights from a mathematical model. Ann. Biomed. Eng. 2013, 41, 68–77. [Google Scholar] [CrossRef]
- Patel, K.; Doddapaneni, R.; Sekar, V.; Chowdhury, N.; Singh, M. Combination approach of YSA peptide anchored docetaxel stealth liposomes with oral antifibrotic agent for the treatment of lung cancer. Mol. Pharm. 2016, 13, 2049–2058. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Rathod, D.; Abo-Ali, E.M.; Dukhande, V.V.; Patel, K. EphA2-receptor targeted PEGylated nanoliposomes for the treatment of BRAFV600E mutated parent-and vemurafenib-resistant melanoma. Pharmaceutics 2019, 11, 504. [Google Scholar] [CrossRef] [PubMed]
- Vartak, R.; Patil, S.M.; Saraswat, A.; Patki, M.; Kunda, N.K.; Patel, K. Aerosolized nanoliposomal carrier of remdesivir: An effective alternative for COVID-19 treatment in vitro. Nanomedicine 2021, 16, 1187–1202. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, A.; Vemana, H.P.; Dukhande, V.V.; Patel, K. Galactose-decorated liver tumor-specific nanoliposomes incorporating selective BRD4-targeted PROTAC for hepatocellular carcinoma therapy. Heliyon 2022, 8, e08702. [Google Scholar] [CrossRef] [PubMed]
- Vartak, R.; Saraswat, A.; Yang, Y.; Chen, Z.-S.; Patel, K. Susceptibility of Lung Carcinoma Cells to Nanostructured Lipid Carrier of ARV-825, a BRD4 Degrading Proteolysis Targeting Chimera. Pharm. Res. 2022, 39, 2745–2759. [Google Scholar] [CrossRef]
- Patki, M.; Saraswat, A.; Bhutkar, S.; Dukhande, V.; Patel, K. In vitro assessment of a synergistic combination of gemcitabine and zebularine in pancreatic cancer cells. Exp. Cell Res. 2021, 405, 112660. [Google Scholar] [CrossRef]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Huang, M.; Qiu, Q.; Xiao, Y.; Zeng, S.; Zhan, M.; Shi, M.; Zou, Y.; Ye, Y.; Liang, L.; Yang, X. BET bromodomain suppression inhibits VEGF-induced angiogenesis and vascular permeability by blocking VEGFR2-mediated activation of PAK1 and eNOS. Sci. Rep. 2016, 6, 23770. [Google Scholar] [CrossRef]
- Zhou, Q.; Kiosses, W.B.; Liu, J.; Schimmel, P. Tumor endothelial cell tube formation model for determining anti-angiogenic activity of a tRNA synthetase cytokine. Methods 2008, 44, 190–195. [Google Scholar] [CrossRef]
- Saraswat, A.; Patki, M.; Fu, Y.; Barot, S.; Dukhande, V.V.; Patel, K. Nanoformulation of PROteolysis TArgeting Chimera targeting ‘undruggable’c-Myc for the treatment of pancreatic cancer. Nanomedicine 2020, 15, 1761–1777. [Google Scholar] [CrossRef]
- Maniotis, A.J.; Folberg, R.; Hess, A.; Seftor, E.A.; Gardner, L.M.; Pe’er, J.; Trent, J.M.; Meltzer, P.S.; Hendrix, M.J. Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenic mimicry. Am. J. Pathol. 1999, 155, 739–752. [Google Scholar] [CrossRef]
- Samanta, K.; Setua, S.; Kumari, S.; Jaggi, M.; Yallapu, M.M.; Chauhan, S.C. Gemcitabine Combination Nano Therapies for Pancreatic Cancer. Pharmaceutics 2019, 11, 574. [Google Scholar] [CrossRef]
- Singh, R.R.; O’Reilly, E.M. New Treatment Strategies for Metastatic Pancreatic Ductal Adenocarcinoma. Drugs 2020, 80, 647–669. [Google Scholar] [CrossRef]
- Fu, Y.; Saraswat, A.; Vartak, R.; Patki, M.; Patel, K. Liposomal formulation: Opportunities, challenges, and industrial applicability. In Multifunctional Nanocarriers; Elsevier: Amsterdam, The Netherlands, 2022; pp. 79–102. [Google Scholar]
- Han, B.; Yang, Y.; Chen, J.; Tang, H.; Sun, Y.; Zhang, Z.; Wang, Z.; Li, Y.; Li, Y.; Luan, X.; et al. Preparation, Characterization, and Pharmacokinetic Study of a Novel Long-Acting Targeted Paclitaxel Liposome with Antitumor Activity. Int. J. Nanomed. 2020, 15, 553–571. [Google Scholar] [CrossRef]
- Yang, K.; Yu, G.; Yang, Z.; Yue, L.; Zhang, X.; Sun, C.; Wei, J.; Rao, L.; Chen, X.; Wang, R. Supramolecular Polymerization-Induced Nanoassemblies for Self-Augmented Cascade Chemotherapy and Chemodynamic Therapy of Tumor. Angew. Chem. Int. Ed. Engl. 2021, 60, 17570–17578. [Google Scholar] [CrossRef]
- Alavi, M.; Hamidi, M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. Ther. 2019, 34, 20180032. [Google Scholar] [CrossRef]
- Primassin, S.; Ter Veld, F.; Mayatepek, E.; Spiekerkoetter, U. Carnitine supplementation induces acylcarnitine production in tissues of very long-chain acyl-CoA dehydrogenase-deficient mice, without replenishing low free carnitine. Pediatr. Res. 2008, 63, 632–637. [Google Scholar] [CrossRef]
- Saffari, M.; Hoseini Shirazi, F.; Moghimi, H.R. Terpene-loaded Liposomes and Isopropyl Myristate as Chemical Permeation Enhancers Toward Liposomal Gene Delivery in Lung Cancer cells; A Comparative Study. Iran. J. Pharm. Res. 2016, 15, 261–267. [Google Scholar]
- Winter, E.; Dal Pizzol, C.; Locatelli, C.; Crezkynski-Pasa, T.B. Development and Evaluation of Lipid Nanoparticles for Drug Delivery: Study of Toxicity In, Vitro and In Vivo. J. Nanosci. Nanotechnol. 2016, 16, 1321–1330. [Google Scholar] [CrossRef]
- Mourtas, S.; Michanetzis, G.P.; Missirlis, Y.F.; Antimisiaris, S.G. Haemolytic activity of liposomes: Effect of vesicle size, lipid concentration and polyethylene glycol-lipid or arsonolipid incorporation. J. Biomed. Nanotechnol. 2009, 5, 409–415. [Google Scholar] [CrossRef]
- Mendonsa, A.M.; Chalfant, M.C.; Gorden, L.D.; VanSaun, M.N. Modulation of the leptin receptor mediates tumor growth and migration of pancreatic cancer cells. PLoS ONE 2015, 10, e0126686. [Google Scholar] [CrossRef]
- Han, H.; Du, L.; Cao, Z.; Zhang, B.; Zhou, Q. Triptonide potently suppresses pancreatic cancer cell-mediated vasculogenic mimicry by inhibiting expression of VE-cadherin and chemokine ligand 2 genes. Eur. J. Pharmacol. 2018, 818, 593–603. [Google Scholar] [CrossRef]
- Scotti, M.L.; Bamlet, W.R.; Smyrk, T.C.; Fields, A.P.; Murray, N.R. Protein kinase Ciota is required for pancreatic cancer cell transformed growth and tumorigenesis. Cancer Res. 2010, 70, 2064–2074. [Google Scholar] [CrossRef]
- Fu, Y.; Saraswat, A.; Wei, Z.; Agrawal, M.Y.; Dukhande, V.V.; Reznik, S.E.; Patel, K. Development of Dual ARV-825 and Nintedanib-Loaded PEGylated Nano-Liposomes for Synergistic Efficacy in Vemurafnib-Resistant Melanoma. Pharmaceutics 2021, 13, 1005. [Google Scholar] [CrossRef]
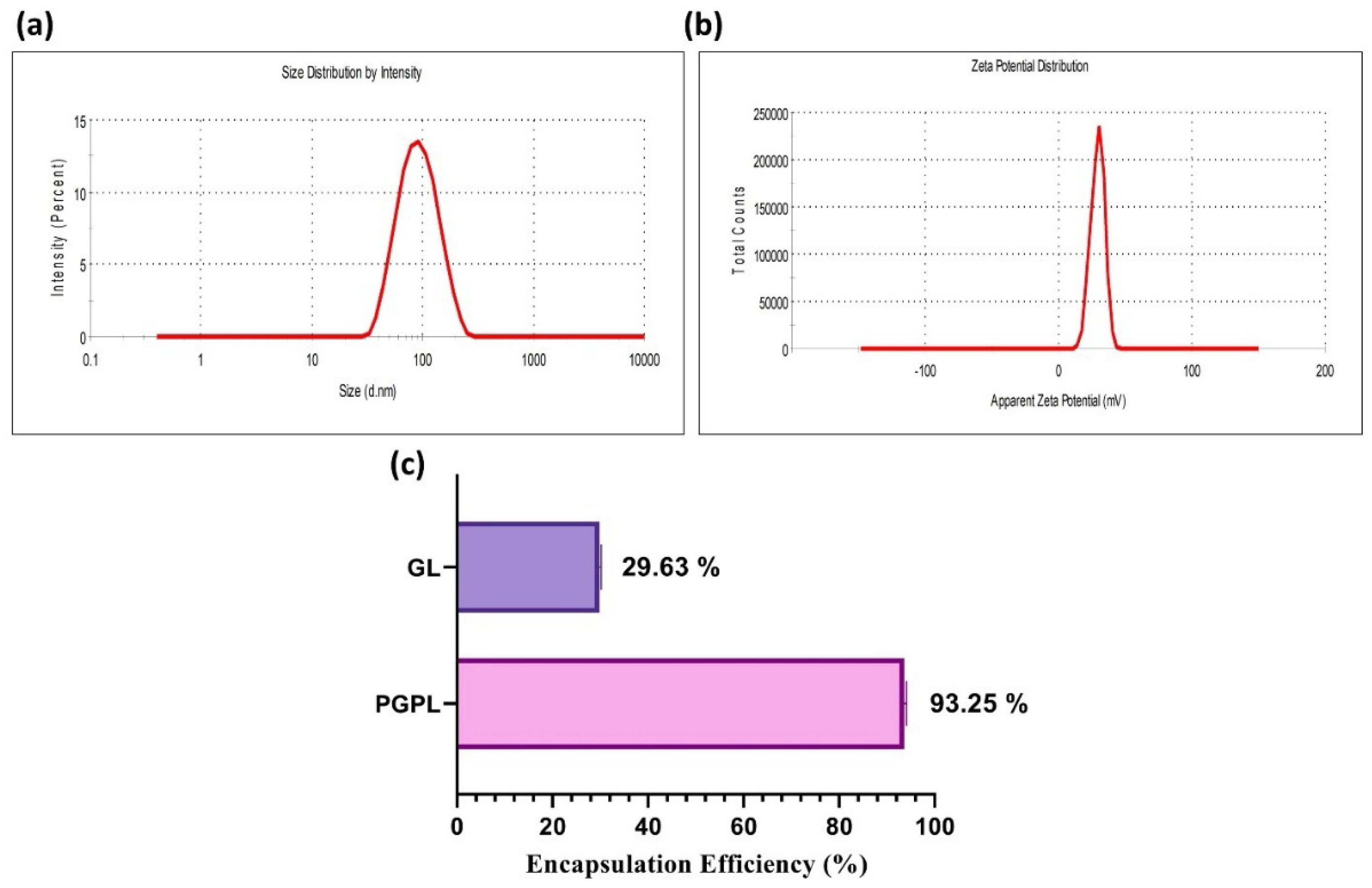
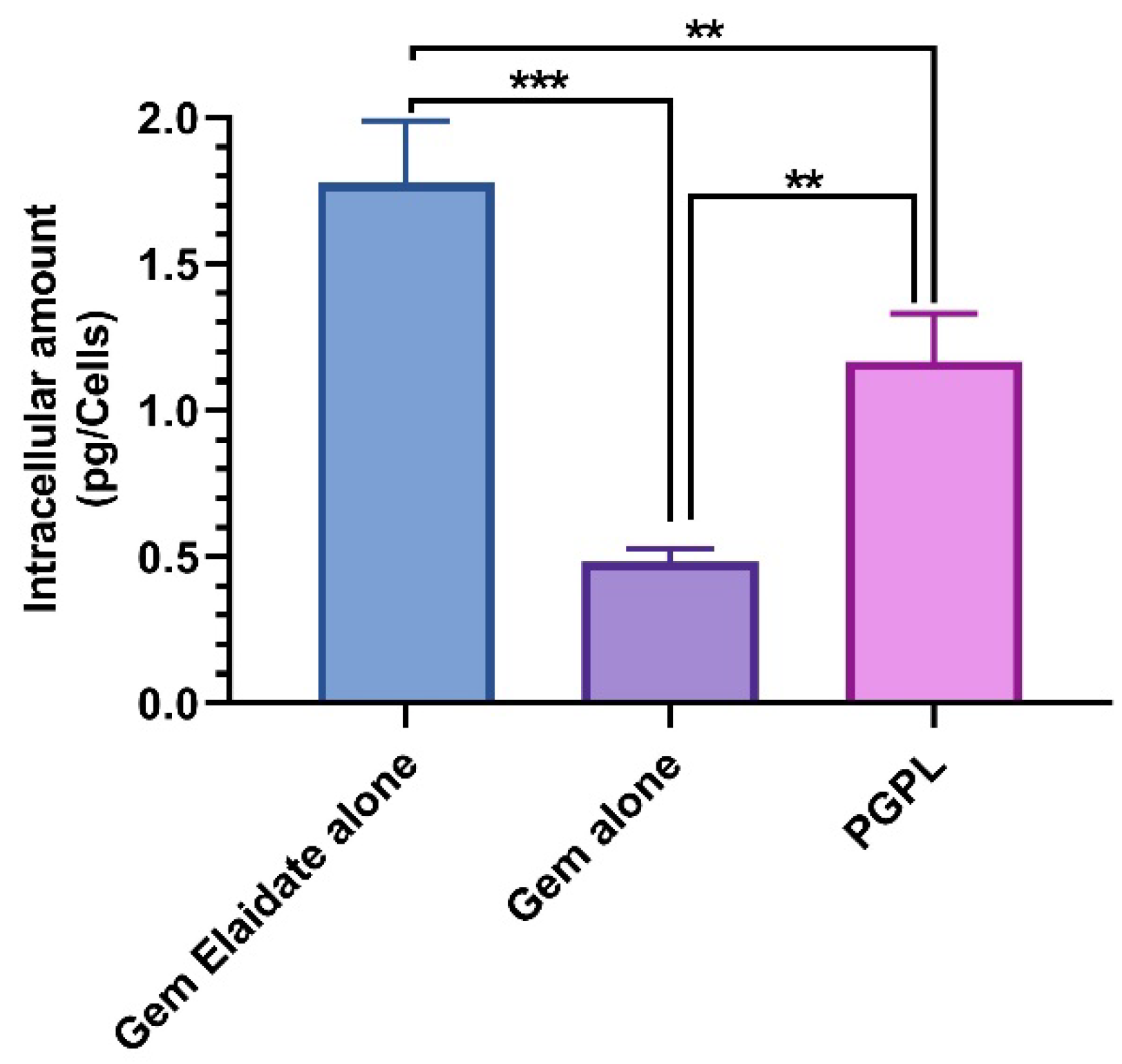


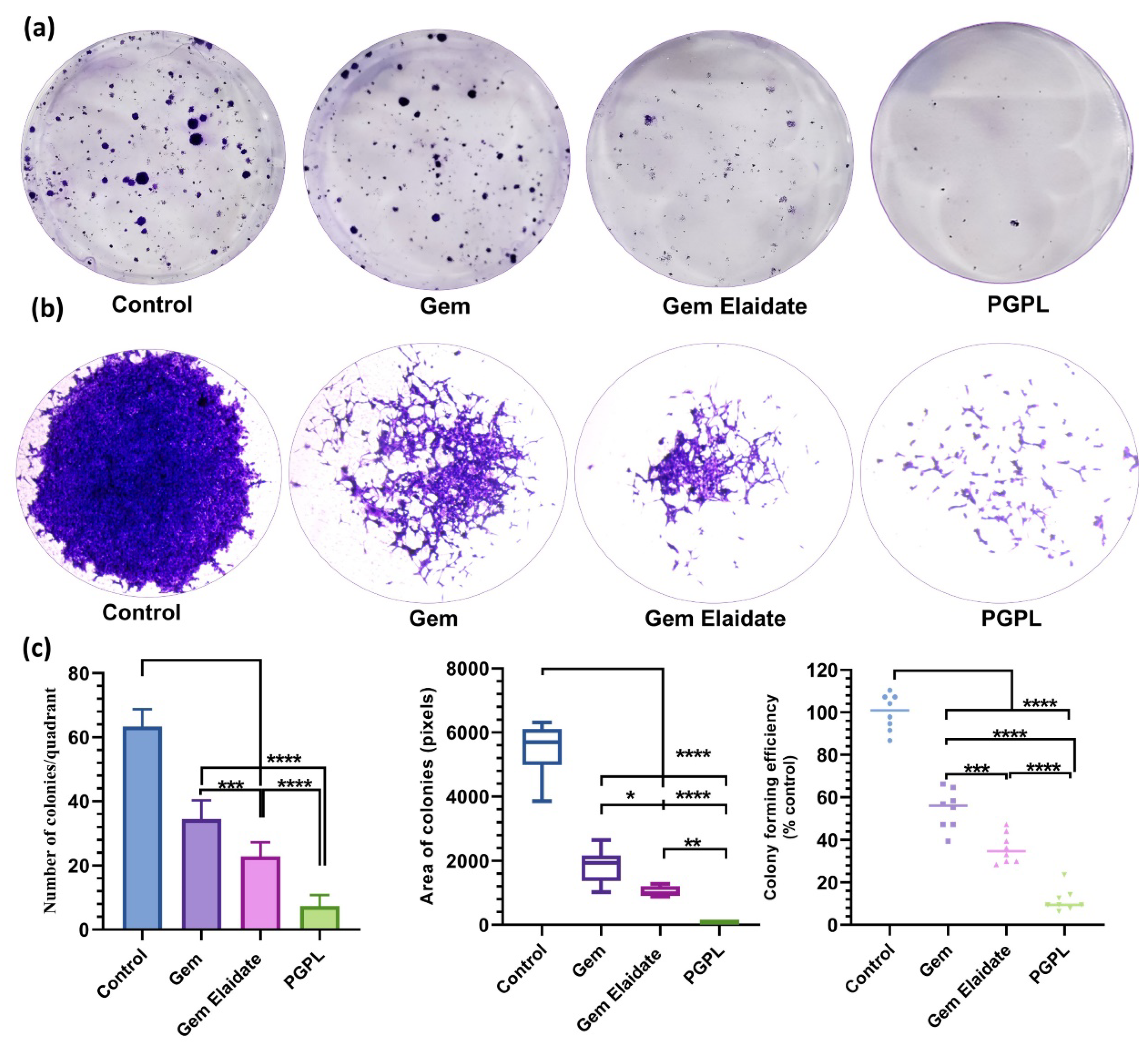
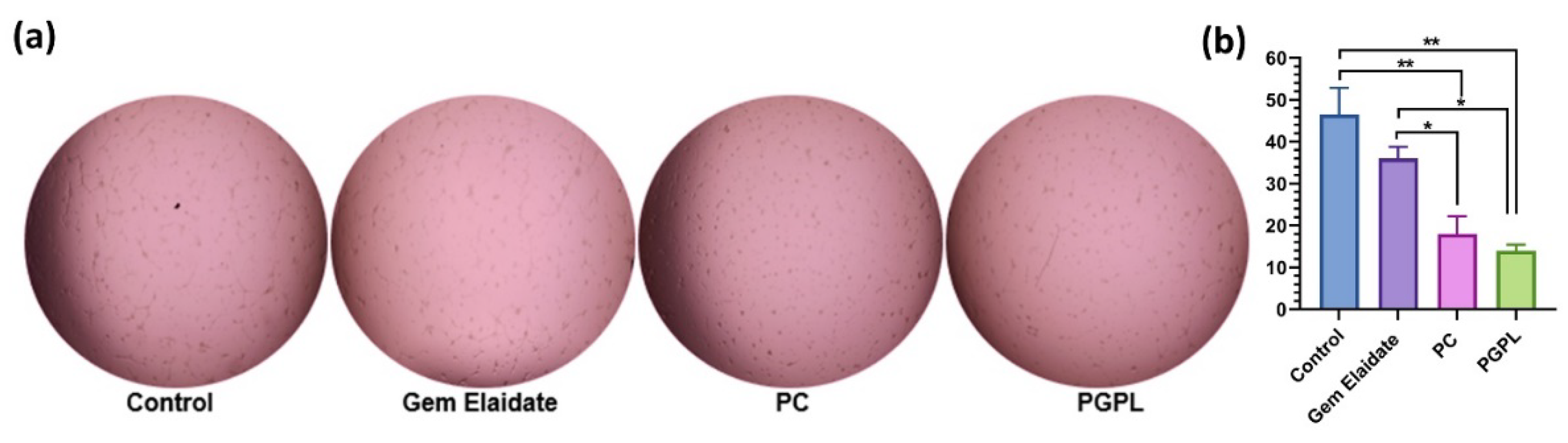

| Time | Particle Size (nm) | Polydispersity Index | Zeta Potential (mV) |
|---|---|---|---|
| 0 day | 81.78 ± 12.34 | 0.150 ± 0.012 | +31.6 ± 1.21 |
| 7 days | 80.12 ± 10.98 | 0.154 ± 0.034 | +31.9 ± 0.98 |
| 14 days | 83.96 ± 10.12 | 0.154 ± 0.021 | +32.1 ± 2.02 |
| 21 days | 83.12 ± 14.56 | 0.159 ± 0.067 | +32.7 ± 1.34 |
| 1 month | 87.01 ± 10.09 | 0.163 ± 0.014 | +32.6 ± 2.17 |
| 2 months | 88.12 ± 9.10 | 0.169± 0.019 | +31.8 ± 1.97 |
| IC50 (µM) | MIA PaCa-2 | BxPC-3 |
|---|---|---|
| Gem | 0.550 ± 0.101 | 0.614 ± 0.07 |
| Gem Elaidate | 0.164 ± 0.08 | 0.193 ± 0.06 |
| PGPL | 0.152 ± 0.05 | 0.129 ± 0.08 |
| PC | 36.62 ± 3.895 | 31.79 ± 4.161 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, A.; Saraswat, A.; Patel, H.; Chen, Z.-S.; Patel, K. Palmitoyl Carnitine-Anchored Nanoliposomes for Neovasculature-Specific Delivery of Gemcitabine Elaidate to Treat Pancreatic Cancer. Cancers 2023, 15, 182. https://doi.org/10.3390/cancers15010182
Patel A, Saraswat A, Patel H, Chen Z-S, Patel K. Palmitoyl Carnitine-Anchored Nanoliposomes for Neovasculature-Specific Delivery of Gemcitabine Elaidate to Treat Pancreatic Cancer. Cancers. 2023; 15(1):182. https://doi.org/10.3390/cancers15010182
Chicago/Turabian StylePatel, Akanksha, Aishwarya Saraswat, Harsh Patel, Zhe-Sheng Chen, and Ketan Patel. 2023. "Palmitoyl Carnitine-Anchored Nanoliposomes for Neovasculature-Specific Delivery of Gemcitabine Elaidate to Treat Pancreatic Cancer" Cancers 15, no. 1: 182. https://doi.org/10.3390/cancers15010182
APA StylePatel, A., Saraswat, A., Patel, H., Chen, Z.-S., & Patel, K. (2023). Palmitoyl Carnitine-Anchored Nanoliposomes for Neovasculature-Specific Delivery of Gemcitabine Elaidate to Treat Pancreatic Cancer. Cancers, 15(1), 182. https://doi.org/10.3390/cancers15010182










