A Multidisciplinary Approach Establishes a Link between Transglutaminase 2 and the Kv10.1 Voltage-Dependent K+ Channel in Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Growth and Treatments
2.2. Detection of Apoptosis
2.3. Quantification of Gene Expression by RT-qPCR
2.4. Patch-Clamp Technique
2.5. Immunoprecipitation and Immunochemical Analysis
2.6. Samples’ Preparation for Metabolomic Analysis of Cell Supernatants
3. Results
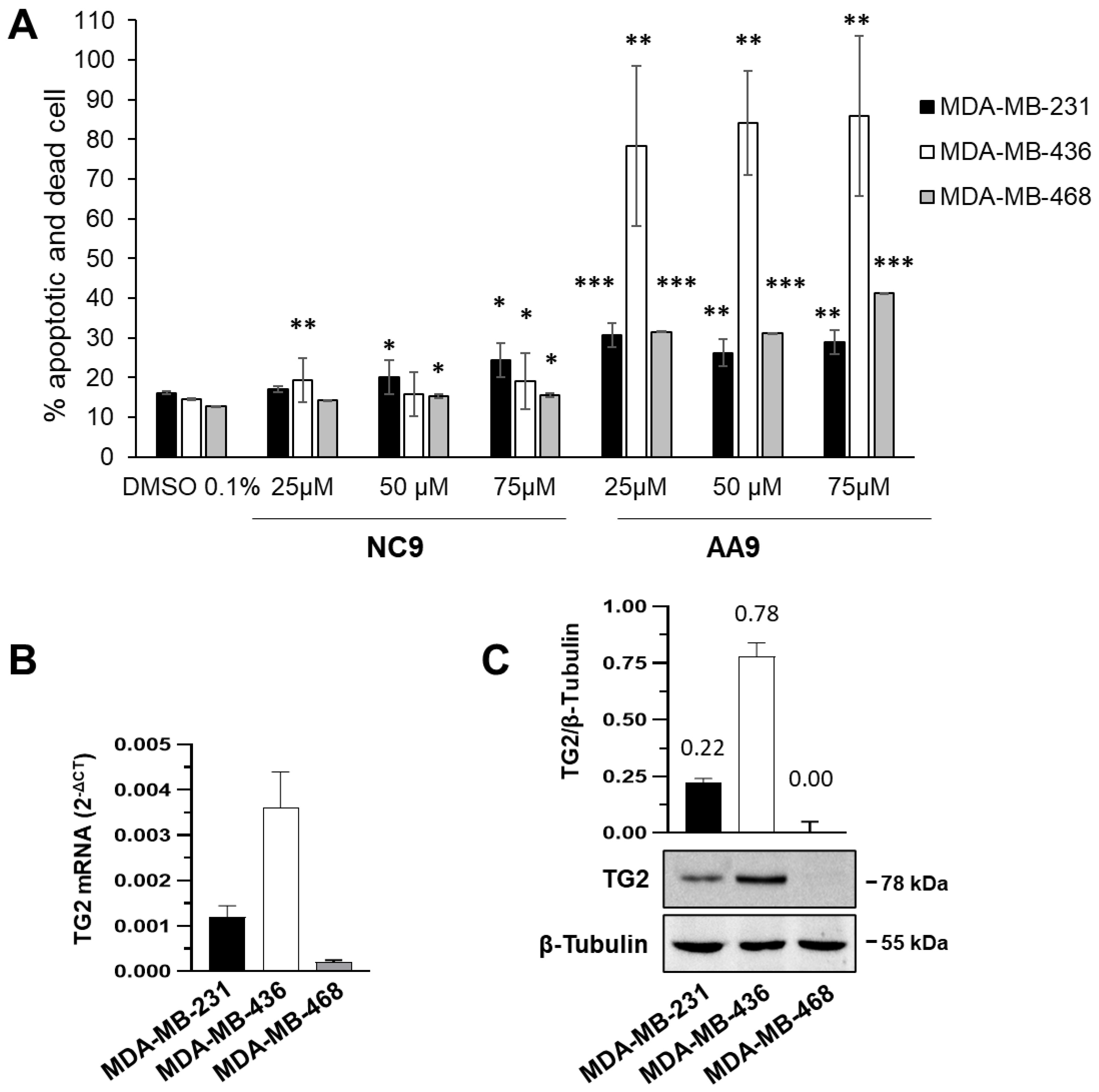
3.1. Comparison between Apoptotic Effects of TG2 Inhibitors
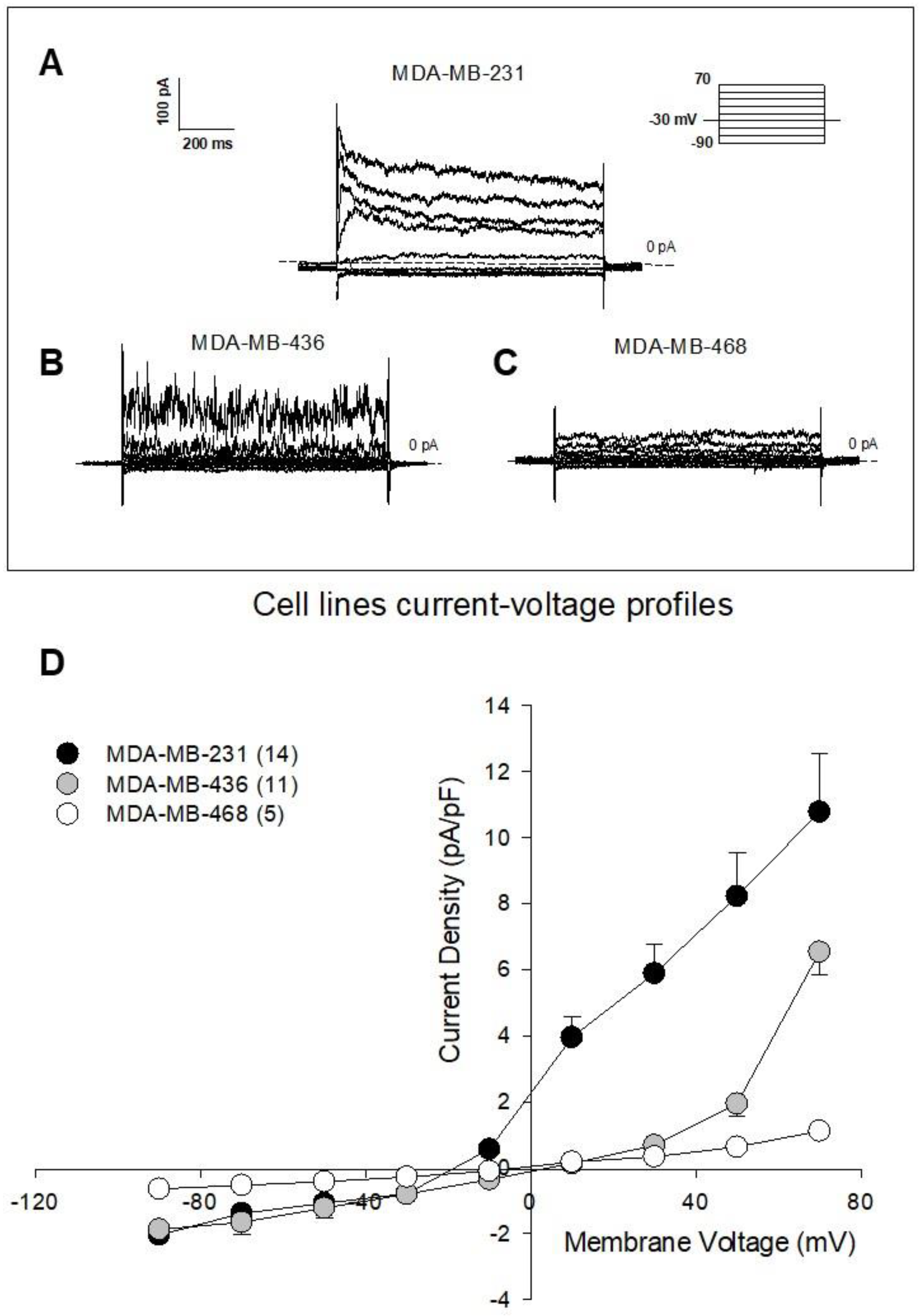
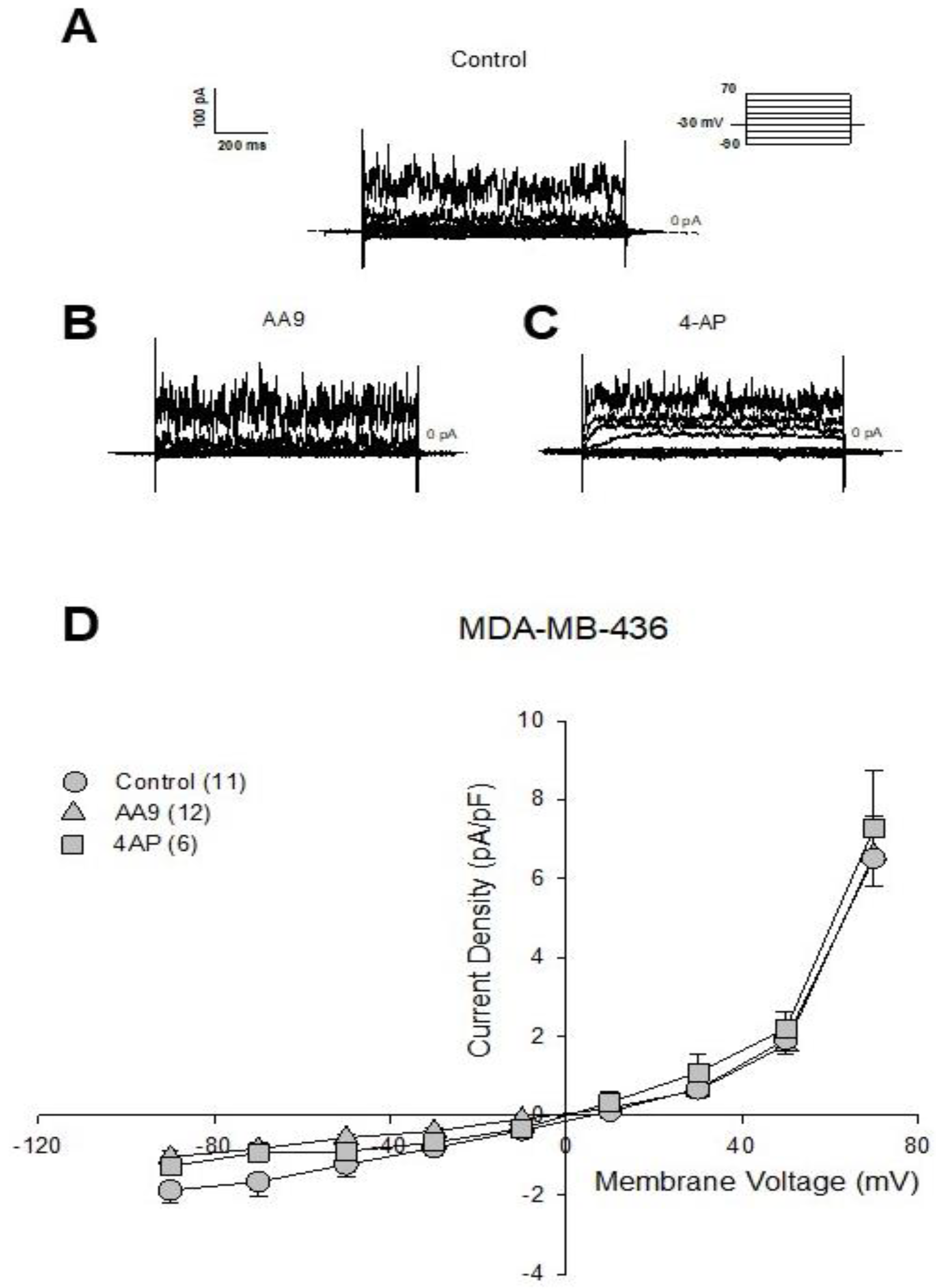
3.2. The AA9 Inhibitor of TG2 Leads to a Significant Decrease in the Membrane Current
3.3. Identification of the Voltage-Dependent Kv10.1 K+ Channel as an Interaction Partner of TG2
3.4. Analysis of the Metabolites Secreted after AA9 Treatment to Find Cell-Response Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beninati, S.; Piacentini, M.; Bergamini, C.M. Transglutaminase 2, a double face enzyme. Amino Acids. 2017, 49, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.S.; Lin, C.J.; Greenberg, C.S. Role of tissue transglutaminase-2 (TG2)-mediated aminylation in biological processes. Amino Acids. 2017, 49, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Nurminskaya, M.V.; Belkin, A.M. Cellular functions of tissue transglutaminase. Int. Rev. Cell Mol. Biol. 2012, 294, 1–97. [Google Scholar] [CrossRef] [PubMed]
- Kitakaze, T.; Yoshikawa, M.; Kobayashi, Y.; Kimura, N.; Goshima, N.; Ishikawa, T.; Ogata, Y.; Yamashita, Y.; Ashida, H.; Harada, N.; et al. Extracellular transglutaminase 2 induces myotube hypertrophy through G protein-coupled receptor 56. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118563–118574. [Google Scholar] [CrossRef] [PubMed]
- Soluri, M.F.; Boccafoschi, F.; Cotella, D.; Moro, L.; Forestieri, G.; Autiero, I.; Cavallo, L.; Oliva, R.; Griffin, M.; Wang, Z.; et al. Mapping the minimum domain of the fibronectin binding site on transglutaminase 2 (TG2) and its importance in mediating signaling, adhesion, and migration in TG2-expressing cells. FASEB J. 2019, 33, 2327–2342. [Google Scholar] [CrossRef]
- Cardoso, I.; Østerlund, E.C.; Stamnaes, J.; Iversen, R.; Andersen, J.T.; Jørgensen, T.J.; Sollid, L.M. Dissecting the interaction between transglutaminase 2 and fibronectin. Amino Acids. 2017, 49, 489–500. [Google Scholar] [CrossRef]
- Faye, C.; Inforzato, A.; Bignon, M.; Hartmann, D.J.; Muller, L.; Ballut, L.; Olsen, B.R.; Day, A.J.; Ricard-Blum, S. Transglutaminase-2: A new endostatin partner in the extracellular matrix of endothelial cells. Biochem. J. 2010, 427, 467–475. [Google Scholar] [CrossRef]
- Wang, Z.; Collighan, R.J.; Gross, S.R.; Danen, E.H.; Orend, G.; Telci, D.; Griffin, M. RGD-independent cell adhesion via a tissue transglutaminase-fibronectin matrix promotes fibronectin fibril deposition and requires syndecan-4/2 α5β1 integrin co-signaling. J. Biol. Chem. 2010, 285, 40212–40229. [Google Scholar] [CrossRef]
- Jeong, E.M.; Lee, K.B.; Kim, G.E.; Kim, C.M.; Lee, J.H.; Kim, H.J.; Shin, J.W.; Kwon, M.A.; Park, H.H.; Kim, I.G. Competitive binding of magnesium to calcium binding sites reciprocally regulates transamidase and GTP hydrolysis activity of Transglutaminase 2. Int. J. Mol. Sci. 2020, 21, 791. [Google Scholar] [CrossRef]
- Feng, J.F.; Rhee, S.G.; Im, M.J. Evidence that phospholipase delta1 is the effector in the Gh (transglutaminase II)-mediated signaling. J. Biol. Chem. 1996, 271, 16451–16454. [Google Scholar] [CrossRef]
- Rossin, F.; Costa, R.; Bordi, M.; D’Eletto, M.; Occhigrossi, L.; Farrace, M.G.; Barlev, N.; Ciccosanti, F.; Muccioli, S.; Chieregato, L.; et al. Transglutaminase Type 2 regulates the Wnt/β-catenin pathway in vertebrates. Cell Death Dis. 2021, 12, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Adhikary, G.; Shrestha, S.; Xu, W.; Keillor, J.W.; Naselsky, W.; Eckert, R.L. Transglutaminase 2 maintains hepatocyte growth factor signaling to enhance the cancer cell phenotype. Mol. Cancer Res. 2021, 19, 2026–2035. [Google Scholar] [CrossRef] [PubMed]
- Almami, I.; Dickenson, J.M.; Hargreaves, A.J.; Bonner, P.L. Modulation of transglutaminase 2 activity in H9c2 cells by PKC and PKA signalling: A role for transglutaminase 2 in cytoprotection. Br. J. Pharmacol. 2014, 171, 3946–3960. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, N.; Brugnoli, F.; Grassilli, S.; Bourgeois, K.; Keillor, J.W.; Bergamini, C.M.; Aguiari, G.; Volinia, S.; Bertagnolo, V. The motility and mesenchymal features of breast cancer cells correlate with the levels and intracellular localization of Transglutaminase type 2. Cells 2021, 10, 3059. [Google Scholar] [CrossRef]
- Song, Y.; Kirkpatrick, L.L.; Schilling, A.B.; Helseth, D.L.; Chabot, N.; Keillor, J.W.; Johnson, G.V.; Brady, S.T. Transglutaminase and polyamination of tubulin: Posttranslational modification for stabilizing axonal microtubules. Neuron 2013, 78, 109–123. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H.; Cho, S.Y.; Jeon, J.H.; Kim, I.G. Genes Genomics. Transglutaminase 2 mediates transcriptional regulation through BAF250a polyamination. Genes Genomics. 2021, 43, 333–342. [Google Scholar] [CrossRef]
- Farrelly, L.A.; Thompson, R.E.; Zhao, S.; Lepack, A.E.; Lyu, Y.; Bhanu, N.V.; Zhang, B.; Loh, Y.E.; Ramakrishnan, A.; Vadodaria, K.C.; et al. Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3. Nature 2019, 567, 535–539. [Google Scholar] [CrossRef]
- Tatsukawa, H.; Fukaya, Y.; Frampton, G.; Martinez-Fuentes, A.; Suzuki, K.; Kuo, T.F.; Nagatsuma, K.; Shimokado, K.; Okuno, M.; Wu, J.; et al. Role of transglutaminase 2 in liver injury via cross-linking and silencing of transcription factor Sp1. Gastroenterology 2009, 136, 1783–1795.e10. [Google Scholar] [CrossRef]
- Brown, K.D. Transglutaminase 2 and NF-κB: An odd couple that shapes breast cancer phenotype. Breast Cancer Res Treat. 2013, 137, 329–336. [Google Scholar] [CrossRef]
- D’Eletto, M.; Rossin, F.; Occhigrossi, L.; Farrace, M.G.; Faccenda, D.; Desai, R.; Marchi, S.; Refolo, G.; Falasca, L.; Antonioli, M.; et al. Transglutaminase type 2 regulates ER-mitochondria contact sites by interacting with GRP75. Cell Rep. 2018, 25, 3573–3581.e4. [Google Scholar] [CrossRef]
- Iwai, K.; Shibukawa, Y.; Yamazaki, N.; Wada, Y. Transglutaminase 2-dependent deamidation of glyceraldehyde-3-phosphate dehydrogenase promotes trophoblastic cell fusion. J. Biol. Chem. 2014, 289, 4989–4999. [Google Scholar] [CrossRef] [PubMed]
- Ku, B.M.; Lee, C.H.; Lee, S.H.; Kim, S.Y. Increased expression of transglutaminase 2 drives glycolytic metabolism in renal carcinoma cells. Amino Acids. 2014, 46, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, R.; Khosla, C. Substrates, inhibitors, and probes of mammalian transglutaminase 2. Anal. Biochem. 2020, 591, 113560–113574. [Google Scholar] [CrossRef] [PubMed]
- Keillor, J.W.; Apperley, K.Y.; Akbar, A. Inhibitors of tissue transglutaminase. Trends Pharmacol. Sci. 2015, 36, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, E.; Comerma-Steffensen, S.; Prat-Duran, J.; Rivera, L.; Matchkov, V.V.; Buus, N.H.; Simonsen, U. Transglutaminase 2 inhibitor LDN 27219 age-dependently lowers blood pressure and improves endothelium-dependent vasodilation in resistance arteries. Hypertension 2021, 77, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Jeitner, T.M.; Pinto, J.T.; Cooper, A.J.L. Cystamine and cysteamine as inhibitors of transglutaminase activity in vivo. Biosci. Rep. 2018, 38, BSR20180691. [Google Scholar] [CrossRef]
- Wang, Z.; Stuckey, D.J.; Murdoch, C.E.; Camelliti, P.; Lip, G.Y.H.; Griffin, M. Cardiac fibrosis can be attenuated by blocking the activity of transglutaminase 2 using a selective small-molecule inhibitor. Cell Death Dis. 2018, 9, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Huaying, S.; Dong, Y.; Chihong, Z.; Xiaoqian, Q.; Danying, W.; Jianguo, F. Transglutaminase 2 Inhibitor KCC009 Induces p53-independent radiosensitization in lung adenocarcinoma cells. Med. Sci. Monit. 2016, 22, 5041–5048. [Google Scholar] [CrossRef]
- Ku, B.M.; Kim, S.J.; Kim, N.; Hong, D.; Choi, Y.B.; Lee, S.H.; Gong, Y.D.; Kim, S.Y. Transglutaminase 2 inhibitor abrogates renal cell carcinoma in xenograft models. J. Cancer Res. Clin. Oncol. 2014, 140, 757–767. [Google Scholar] [CrossRef]
- Yuan, L.; Siegel, M.; Choi, K.; Khosla, C.; Miller, C.R.; Jackson, E.N.; Piwnica-Worms, D.; Rich, K.M. Transglutaminase 2 inhibitor, KCC009, disrupts fibronectin assembly in the extracellular matrix and sensitizes orthotopic glioblastomas to chemotherapy. Oncogene 2007, 26, 2563–2573. [Google Scholar] [CrossRef]
- Aguiari, G.; Crudele, F.; Taccioli, C.; Minotti, L.; Corrà, F.; Keillor, J.W.; Grassilli, S.; Cervellati, C.; Volinia, S.; Bergamini, C.M.; et al. Dysregulation of Transglutaminase type 2 through GATA3 defines aggressiveness and Doxorubicin sensitivity in breast cancer. Int. J. Biol. Sci. 2022, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Park, K.S.; Kim, S.Y. Silencing of TGase 2 sensitizes breast cancer cells to apoptosis by regulation of survival factors. Front. Biosci. 2009, 14, 2514–2521. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, D.S.; Park, S.S.; Nam, B.H.; Kim, I.H.; Kim, S.Y. Reversal of drug resistance in breast cancer cells by transglutaminase 2 inhibition and nuclear factor-kappaB inactivation. Cancer Res. 2006, 66, 10936–10943. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.L. Transglutaminase 2 takes center stage as a cancer cell survival factor and therapy target. Mol. Carcinog. 2019, 58, 837–853. [Google Scholar] [CrossRef]
- Akbar, A.; McNeil, N.M.R.; Albert, M.R.; Ta, V.; Adhikary, G.; Bourgeois, K.; Eckert, R.L.; Keillor, J.W. Structure-activity relationships of potent, targeted covalent inhibitors that abolish both the transamidation and GTP binding activities of human tissue Transglutaminase. J. Med. Chem. 2017, 60, 7910–7927. [Google Scholar] [CrossRef] [PubMed]
- Tonoli, E.; Verduci, I.; Gabrielli, M.; Prada, I.; Forcaia, G.; Coveney, C.; Savoca, M.P.; Boocock, D.J.; Sancini, G.; Mazzanti, M.; et al. Extracellular transglutaminase-2, nude or associated with astrocytic extracellular vesicles, modulates neuronal calcium homeostasis. Prog. Neurobiol. 2022, 216–233, 102313. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.A.; Iliev, I.G.; Schwalke, M.A.; Gonzalez, E.; Marler, K.C.; Flanagan, C.A. Association between cell membrane potential and breast cancer. Tumour. Biol. 1994, 15, 82–89. [Google Scholar] [CrossRef]
- Fnu, G.; Weber, G.F. Alterations of ion homeostasis in cancer metastasis: Implications for treatment. Front. Oncol. 2021, 11, 765329–765352. [Google Scholar] [CrossRef]
- Restrepo-Angulo, I.; Bañuelos, C.; Camacho, J. Ion channel regulation by Sex steroid hormones and vitamin D in cancer: A potential opportunity for cancer diagnosis and therapy. Front. Pharmacol. 2020, 11, 152–165. [Google Scholar] [CrossRef]
- Lastraioli, E. Focus on triple-negative breast cancer: Potassium channel expression and clinical correlates. Front. Pharmacol. 2020, 11, 725–733. [Google Scholar] [CrossRef]
- Toplak, Ž.; Hendrickx, L.A.; Abdelaziz, R.; Shi, X.; Peigneur, S.; Tomašič, T.; Tytgat, J.; Peterlin-Mašič, L.; Pardo, L.A. Overcoming challenges of HERG potassium channel liability through rational design: Eag1 inhibitors for cancer treatment. Med. Res. Rev. 2022, 42, 183–226. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, K.; Gong, X.; Vasilescu, S.; Sun, Y.; Hong, K.; Li, H.; Li, L.; Shan, Y. Inducing polyclonal Eag1-specific antibodies by vaccination with a linear epitope immunogen and its relation to breast tumorigenesis. Pathol. Oncol. Res. 2017, 23, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Chung, S.; Bang, H.W.; Baek, K.J.; Uhm, D. Modulation of large conductance Ca2+-activated K+ channel by Galphah (transglutaminase II) in the vascular smooth muscle cell. Pflugers. Arch. 1997, 433, 671–673. [Google Scholar] [CrossRef]
- Engholm, M.; Pinilla, E.; Mogensen, S.; Matchkov, V.; Hedegaard, E.R.; Chen, H.; Mulvany, M.J.; Simonsen, U. Involvement of transglutaminase 2 and voltage-gated potassium channels in cystamine vasodilatation in rat mesenteric small arteries. Br. J. Pharmacol. 2016, 173, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Mauro, T.; Dixon, D.B.; Komuves, L.; Hanley, K.; Pappone, P.A. Keratinocyte K+ channels mediate Ca2+-induced differentiation. J. Investig. Dermatol. 1997, 108, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Nagata, Y. Effects of depolarizing agents on transglutaminase activity, Ca2+ influx, and protein synthesis in superior cervical and nodose ganglia excised from rats. Mol. Chem. Neuropathol. 1993, 19, 121–135. [Google Scholar] [CrossRef]
- Kerr, C.; Szmacinski, H.; Fisher, M.L.; Nance, B.; Lakowicz, J.R.; Akbar, A.; Keillor, J.W.; Lok Wong, T.; Godoy-Ruiz, R.; Toth, E.A.; et al. Transamidase site-targeted agents alter the conformation of the transglutaminase cancer stem cell survival protein to reduce GTP binding activity and cancer stem cell survival. Oncogene 2017, 36, 2981–2990. [Google Scholar] [CrossRef]
- Jambrovics, K.; Uray, I.P.; Keillor, J.W.; Fésüs, L.; Balajthy, Z. Benefits of combined all-trans retinoic acid and arsenic trioxide treatment of acute promyelocytic leukemia cells and further enhancement by inhibition of atypically expressed Transglutaminase 2. Cancers 2020, 12, 648. [Google Scholar] [CrossRef]
- Jambrovics, K.; Uray, I.P.; Keresztessy, Z.; Keillor, J.W.; Fésüs, L.; Balajthy, Z. Transglutaminase 2 programs differentiating acute promyelocytic leukemia cells in all-trans retinoic acid treatment to inflammatory stage through NF-κB activation. Haematologica 2019, 104, 505–515. [Google Scholar] [CrossRef]
- Sakmann, B.; Neher, E. Patch clamp techniques for studying ionic channels in excitable membranes. Annu Rev. Physiol. 1984, 46, 455–472. [Google Scholar] [CrossRef]
- Khammy, M.M.; Kim, S.; Bentzen, B.H.; Lee, S.; Choi, I.; Aalkjaer, C.; Jepps, T.A. 4-Aminopyridine: A pan voltage-gated potassium channel inhibitor that enhances Kv 7.4 currents and inhibits noradrenaline-mediated contraction of rat mesenteric small arteries. Br. J. Pharmacol. 2018, 175, 501–516. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.G.; Liu, W.C.; Dong, S.; Du, C.; Wang, X.J.; Li, J.S.; Xie, X.P.; Wu, L.; Ma, D.C.; Yu, Z.B.; et al. Activation of BK(Ca) channels in zoledronic acid-induced apoptosis of MDA-MB-231 breast cancer cells. PLoS ONE 2012, 7, e37451. [Google Scholar] [CrossRef]
- Valdés-Abadía, B.; Morán-Zendejas, R.; Rangel-Flores, J.M.; Rodríguez-Menchaca, A.A. Chloroquine inhibits tumor-related Kv10.1 channel and decreases migration of MDA-MB-231 breast cancer cells in vitro. Eur. J. Pharmacol. 2019, 855, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Franzese, O.; Minotti, L.; Aguiari, G.; Corrà, F.; Cervellati, C.; Ferrari, C.; Volinia, S.; Bergamini, C.M.; Bianchi, N. Involvement of non-coding RNAs and transcription factors in the induction of Transglutaminase isoforms by ATRA. Amino Acids. 2019, 51, 1273–1288. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.J.; Eggenton, J. Membrane potentials of epithelial cells in rat small intestine. J. Physiol. 1972, 227, 201–216. [Google Scholar] [CrossRef]
- Stein, M.A.; Mathers, D.A.; Yan, H.; Baimbridge, K.G.; Finlay, B.B. Enteropathogenic Escherichia coli markedly decreases the resting membrane potential of Caco-2 and HeLa human epithelial cells. Infect. Immun. 1996, 64, 4820–4825. [Google Scholar] [CrossRef]
- Brugnoli, F.; Grassilli, S.; Al-Qassab, Y.; Capitani, S.; Bertagnolo, V. PLC-β2 is modulated by low oxygen availability in breast tumor cells and plays a phenotype dependent role in their hypoxia-related malignant potential. Mol. Carcinog 2016, 55, 2210–2221. [Google Scholar] [CrossRef]
- Al-Qassab, Y.; Grassilli, S.; Brugnoli, F.; Vezzali, S.; Capitani, S.; Bertagnolo, V. Protective role of all-trans retinoic acid (ATRA) against hypoxia-induced malignant potential of non-invasive breast tumor derived cells. BMC Cancer 2018, 18, 11941–11954. [Google Scholar] [CrossRef]
- Gallo, M.; Giovati, L.; Magliani, W.; Pertinhez, T.A.; Conti, S.; Ferrari, E.; Spisni, A.; Ciociola, T. Metabolic plasticity of Candida albicans in response to different environmental conditions. J. Fungi 2022, 8, 723. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic. Acids. Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Sun, H.; Kaartinen, M.T. Transglutaminase activity regulates differentiation, migration and fusion of osteoclasts via affecting actin dynamics. J. Cell Physiol. 2018, 233, 7497–7513. [Google Scholar] [CrossRef] [PubMed]
- Movsisyan, N.; Pardo, L.A. Kv10.1 Regulates microtubule dynamics during mitosis. Cancers 2020, 12, 2409. [Google Scholar] [CrossRef] [PubMed]
- Breuer, E.-K.; Fukushiro-Lopes, D.; Dalheim, A.; Burnette, M.; Zartman, J.; Kaja, S.; Wells, C.; Campo, L.; Curtis, K.J.; Romero-Moreno, R.; et al. Potassium channel activity controls breast cancer metastasis by affecting β-catenin signaling. Cell Death Dis. 2019, 10, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Hatlapatka, K.; Willenborg, M.; Rustenbeck, I. Plasma membrane depolarization as a determinant of the first phase of insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E315–E322. [Google Scholar] [CrossRef]
- Tolon, R.M.; Sanchez Franco, F.; de los Frailes, M.T.; Lorenzo, M.J.; Cacicedo, L. Effect of potassium-induced depolarization on somatostatin gene expression in cultured fetal rat cerebrocortical cells. J. Neurosci. 1994, 14, 1053–1059. [Google Scholar] [CrossRef]
- Willenborg, M.; Ghaly, H.; Hatlapatka, K.; Urban, K.; Panten, U.; Rustenbeck, I. The signalling role of action potential depolarization in insulin secretion: Metabolism-dependent dissociation between action potential increase and secretion increase by TEA. Biochem. Pharmacol. 2010, 80, 104–112. [Google Scholar] [CrossRef]
- Belz, M.; Willenborg, M.; Görgler, N.; Hamada, A.; Schumacher, K.; Rustenbeck, I. Insulinotropic effect of high potassium concentration beyond plasma membrane depolarization. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E697–E706. [Google Scholar] [CrossRef]
- Fraij, B.M.; Birckbichler, P.J.; Patterson, M.K., Jr.; Lee, K.N.R.; Gonzales, A. A retinoic acid-inducible mRNA from human erythroleukemia cells encodes a novel tissue transglutaminase homologue. J. Biol. Chem. 1992, 267, 22616–22623. [Google Scholar] [CrossRef]
- Phatak, V.M.; Croft, S.M.; Rameshaiah Setty, S.G.; Scarpellini, A.; Hughes, D.C.; Rees, R.; McArdle, S.; Verderio, E.A. Expression of transglutaminase-2 isoforms in normal human tissues and cancer cell lines: Dysregulation of alternative splicing in cancer. Amino Acids. 2013, 44, 33–44. [Google Scholar] [CrossRef]
- Lai, T.-S.; Greenberg, C.S. TGM2 and implications for human disease: Role of alternative splicing. Front. Biosci. 2013, 18, 504–519. [Google Scholar] [CrossRef]
- Phan, N.N.; Wang, C.Y.; Chen, C.F.; Sun, Z.; Lai, M.D.; Lin, Y.C. Voltage-gated calcium channels: Novel targets for cancer therapy. Oncol. Lett. 2017, 14, 2059–2074. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, P.E.; Wheeler, M.B. Voltage-dependent K(+) channels in pancreatic beta cells: Role, regulation and potential as therapeutic targets. Diabetologia 2003, 46, 1046–1062. [Google Scholar] [CrossRef] [PubMed]
- Bungay, P.J.; Owen, R.A.; Coutts, I.C.; Griffin, M. A role for transglutaminase in glucose-stimulated insulin release from the pancreatic beta-cell. Biochem. J. 1986, 235, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Marsella, C.; Nardo, G.; Massignan, T.; Alessio, M.; Piermarini, E.; La Rosa, S.; Finzi, G.; Bonetto, V.; Bertuzzi, F.; et al. Transglutaminase 2 transamidation activity during first-phase insulin secretion: Natural substrates in INS-1E. Acta Diabetol. 2013, 50, 61–72. [Google Scholar] [CrossRef]
- Hemmerlein, B.; Weseloh, R.M.; Mello de Queiroz, F.; Knötgen, H.; Sánchez, A.; Rubio, M.E.; Martin, S.; Schliephacke, T.; Jenke, M.; Heinz-Joachim-Radzun; et al. Overexpression of Eag1 potassium channels in clinical tumours. Mol. Cancer 2006, 5, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Hammadi, M.; Chopin, V.; Matifat, F.; Dhennin-Duthille, I.; Chasseraud, M.; Sevestre, H.; Ouadid-Ahidouch, H. Human ether à-gogo K(+) channel 1 (hEag1) regulates MDA-MB-231 breast cancer cell migration through Orai1-dependent calcium entry. J. Cell Physiol. 2012, 227, 3837–3846. [Google Scholar] [CrossRef] [PubMed]
- Badaoui, M.; Mimsy-Julienne, C.; Saby, C.; Van Gulick, L.; Peretti, M.; Jeannesson, P.; Morjani, H.; Ouadid-Ahidouch, H. Collagen type 1 promotes survival of human breast cancer cells by overexpressing Kv10.1 potassium and Orai1 calcium channels through DDR1-dependent pathway. Oncotarget 2017, 9, 24653–24671. [Google Scholar] [CrossRef]
- Zúñiga, L.; Cayo, A.; González, W.; Vilos, C.; Zúñiga, R. Potassium Channels as a Target for Cancer Therapy: Current Perspectives. Onco Targets Ther. 2022, 15, 783–797. [Google Scholar] [CrossRef]
- Sher, E.; Biancardi, E.; Passafaro, M.; Clementi, F. Physiopathology of neuronal voltage-operated calcium channels. FASEB J. 1991, 5, 2677–2683. [Google Scholar] [CrossRef]
- Sener, A.; Gomis, R.; Lebrun, P.; Herchuelz, A.; Malaisse-Lagae, F.; Malaisse, W.J. Methylamine and islet function: Possible relationship to Ca2+-sensitive transglutaminase. Mol. Cell Endocrinol. 1984, 36, 175–180. [Google Scholar] [CrossRef]
- Lebrun, P.; Gomis, R.; Deleers, M.; Billaudel, B.; Mathias, P.C.; Herchuelz, A.; Malaisse-Lagae, F.; Sener, A.; Malaisse, W.J. Methylamines and islet function: Cationic aspects. J. Endocrinol. Investig. 1984, 7, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.N.; Lomasney, J.W.; Mak, E.C.; Lorand, L. Interactions of G(h)/transglutaminase with phospholipase Cdelta1 and with GTP. Proc. Natl. Acad. Sci. USA 1999, 96, 11815–11819. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.J.; Kang, S.; Damron, D.; Im, M. Phospholipase Cdelta1 is a guanine nucleotide exchanging factor for transglutaminase II (Galpha h) and promotes alpha 1B-adrenoreceptor-mediated GTP binding and intracellular calcium release. J. Biol. Chem. 2001, 276, 5591–5597. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Kim, D.K.; Damron, D.S.; Baek, K.J.; Im, M.J. Modulation of intracellular Ca(2+) via alpha(1B)-adrenoreceptor signaling molecules, G alpha(h) (transglutaminase II) and phospholipase C-delta 1. Biochem. Biophys. Res. Commun. 2002, 293, 383–390. [Google Scholar] [CrossRef]
- Ali, U.; Lu, S.; Fadlalla, T.; Iqbal, S.; Yue, H.; Yang, B.; Hong, Y.; Wang, X.; Guo, L. The functions of phospholipases and their hydrolysis products in plant growth, development and stress responses. Prog. Lipid Res. 2022, 86, 101158–101176. [Google Scholar] [CrossRef]
- Iorio, E.; Caramujo, M.J.; Cecchetti, S.; Spadaro, F.; Carpinelli, G.; Canese, R.; Podo, F. Key players in choline metabolic reprograming in triple-negative breast cancer. Front. Oncol. 2016, 6, 205–213. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics for investigating physiological and pathophysiological processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef]
- Loo, R.L.; Chan, Q.; Nicholson, J.K.; Holmes, E. Balancing the equation: A natural history of trimethylamine and trimethylamine-N-oxide. J. Proteome Res. 2022, 21, 560–589. [Google Scholar] [CrossRef]
- Zeisel, S.H.; DaCosta, K.A.; Fox, J.G. Endogenous formation of dimethylamine. Biochem. J. 1985, 232, 403–408. [Google Scholar] [CrossRef]
- Lin, H.Y.; Kuei, C.H.; Lee, H.H.; Lin, C.H.; Zheng, J.Q.; Chiu, H.W.; Chen, C.L.; Lin, Y.F. The Gαh/phospholipase C-δ1 interaction promotes autophagosome degradation by activating the Akt/mTORC1 pathway in metastatic triple-negative breast cancer. Aging 2020, 12, 13023–13037. [Google Scholar] [CrossRef]
- Altamura, C.; Gavazzo, P.; Pusch, M.; Desaphy, J.F. Ion Channel Involvement in Tumor Drug Resistance. J. Pers. Med. 2022, 12, 210. [Google Scholar] [CrossRef] [PubMed]
- García-Quiroz, J.; González-González, M.E.; Díaz, L.; Ordaz-Rosado, D.; Segovia-Mendoza, M.; Prado-García, H.; Larrea, F.; García-Becerra, R. Astemizole, an inhibitor of Ether-à-go-go potassium channel, increases the activity of the tyrosine kinase inhibitor Gefitinib in breast cancer cells. Rev. Investig. Clin. 2019, 71, 186–194. [Google Scholar] [CrossRef] [PubMed]
- García-Quiroz, J.; García-Becerra, R.; Santos-Martínez, N.; Barrera, D.; Ordaz-Rosado, D.; Avila, E.; Halhali, A.; Villanueva, O.; Ibarra-Sánchez, M.J.; Esparza-López, J.; et al. In vivo dual targeting of the oncogenic Ether-à-go-go-1 potassium channel by calcitriol and astemizole results in enhanced antineoplastic effects in breast tumors. BMC Cancer 2014, 14, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Luis, E.; Anaya-Hernández, A.; León-Sánchez, P.; Durán-Pastén, M.L. The Kv10.1 Channel: A Promising Target in Cancer. Int. J. Mol. Sci. 2022, 23, 8458. [Google Scholar] [CrossRef]
- Lin, H.; Li, Z.; Chen, C.; Luo, X.; Xiao, J.; Dong, D.; Lu, Y.; Yang, B.; Wang, Z. Transcriptional and post-transcriptional mechanisms for oncogenic overexpression of ether à go-go K+ channel. PLoS ONE 2011, 6, e20362. [Google Scholar] [CrossRef]
- Assi, J.; Srivastava, G.; Matta, A.; Chang, M.C.; Walfish, P.G.; Ralhan, R. Transglutaminase 2 overexpression in tumor stroma identifies invasive ductal carcinomas of breast at high risk of recurrence. PLoS ONE 2013, 8, e74437. [Google Scholar] [CrossRef]
- Hartung, F.; Krüwel, T.; Shi, X.; Pfizenmaier, K.; Kontermann, R.; Chames, P.; Alves, F.; Pardo, L.A. A Novel Anti-Kv10.1 Nanobody Fused to Single-Chain TRAIL Enhances Apoptosis Induction in Cancer Cells. Front. Pharmacol. 2020, 11, 686–697. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canella, R.; Brugnoli, F.; Gallo, M.; Keillor, J.W.; Terrazzan, A.; Ferrari, E.; Grassilli, S.; Gates, E.W.J.; Volinia, S.; Bertagnolo, V.; et al. A Multidisciplinary Approach Establishes a Link between Transglutaminase 2 and the Kv10.1 Voltage-Dependent K+ Channel in Breast Cancer. Cancers 2023, 15, 178. https://doi.org/10.3390/cancers15010178
Canella R, Brugnoli F, Gallo M, Keillor JW, Terrazzan A, Ferrari E, Grassilli S, Gates EWJ, Volinia S, Bertagnolo V, et al. A Multidisciplinary Approach Establishes a Link between Transglutaminase 2 and the Kv10.1 Voltage-Dependent K+ Channel in Breast Cancer. Cancers. 2023; 15(1):178. https://doi.org/10.3390/cancers15010178
Chicago/Turabian StyleCanella, Rita, Federica Brugnoli, Mariana Gallo, Jeffrey W. Keillor, Anna Terrazzan, Elena Ferrari, Silvia Grassilli, Eric W. J. Gates, Stefano Volinia, Valeria Bertagnolo, and et al. 2023. "A Multidisciplinary Approach Establishes a Link between Transglutaminase 2 and the Kv10.1 Voltage-Dependent K+ Channel in Breast Cancer" Cancers 15, no. 1: 178. https://doi.org/10.3390/cancers15010178
APA StyleCanella, R., Brugnoli, F., Gallo, M., Keillor, J. W., Terrazzan, A., Ferrari, E., Grassilli, S., Gates, E. W. J., Volinia, S., Bertagnolo, V., Bianchi, N., & Bergamini, C. M. (2023). A Multidisciplinary Approach Establishes a Link between Transglutaminase 2 and the Kv10.1 Voltage-Dependent K+ Channel in Breast Cancer. Cancers, 15(1), 178. https://doi.org/10.3390/cancers15010178











