Effect of Perioperative Blood Transfusions and Infectious Complications on Inflammatory Activation and Long-Term Survival Following Gastric Cancer Resection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Outcomes and Definitions
2.4. Statistical Analysis
3. Results
3.1. Baseline Variables
3.2. Dynamic Changes of Inflammatory Markers
3.3. Effect of Postoperative Infectious Complications and Blood Transfusion on DFS
3.4. Effect of Inflammatory Changes on DFS
| Clinicopathological Features | Univariate Analysis HR (95% CI) | p Ratio | Multivariate Analysis HR (95% CI) | p Ratio |
|---|---|---|---|---|
| Perioperative classification, (%): | ||||
| 1: Trans(−)/Inf(−) | Ref. | Ref. | Ref. | Ref. |
| 2: Trans(+)/Inf(−) | 1.36 [0.70; 2.67] | 0.364 | 0.80 [0.40; 1.61] | 0.540 |
| 3: Trans(−)/Inf(+) | 1.51 [0.94; 2.42] | 0.086 | 1.42 [0.88; 2.30] | 0.148 |
| 4: Trans(+)/Inf(+) | 2.85 [1.64; 4.95] | <0.001 | 1.77 [1.01–3.11] | 0.046 |
| NLR difference | ||||
| <2.6 | Ref. | Ref. | Ref. | Ref. |
| ≥2.6 | 1.55 [1.06; 2.26] | 0.025 | 1.67 [1.14; 2.46] | 0.009 |
| Weight loss | ||||
| 0–5% | Ref. | Ref. (34.1%) | ||
| 6–10% | 1.66 [1.08; 2.55] | 0.022 | ||
| >10% | 1.99 [1.20; 3.30] | 0.008 (55.6%) | ||
| Radicality | ||||
| R0 | Ref. | Ref. (37.6%) | Ref. | Ref. |
| R1–R2 | 2.32 [1.38; 3.89] | 0.001(70.8%) | 2.15 [1.26; 3.69] | 0.005 |
| pN a | ||||
| N0 | Ref. | Ref. (19.7%) | Ref. | Ref. |
| ≥N1 | 3.41 [2.17; 5.36] | <0.001 (56.2%) | 2.89 [1.75; 4.76] | <0.001 |
| pT a | ||||
| ≤T2 | Ref. | Ref. (51.6%) | Ref. | Ref. |
| >T3 | 0.47 [0.31; 0.70] | <0.001 (25.6%) | 0.87 [0.54; 1.40] | 0.561 |
| ECOG b | ||||
| ECOG 0 | Ref. | Ref. (25.8%) | Ref. | Ref. |
| ECOG ≥ 1 | 2.49 [1.59; 3.92] | <0.001 (48.1%) | 2.04 [1.29; 3.24] | 0.002 |
| ASA | ||||
| ASA I/II | Ref. | Ref. (39.0%) | ||
| ASA III/IV | 1.25 [0.86; 1.81] | 0.242 (41.8%) |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Machlowska, J.; Baj, J.; Sitarz, M.; Sitarz, R.M. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef] [PubMed]
- Baiocchi, G.L.; Giacopuzzi, S.; Reim, D.; Piessen, G.; da Costa, P.M.; Reynolds, J.V.M.; Meyer, H.-J.; Morgagni, P.; Gockel, I.M.; Santos, L.L.; et al. Incidence and Grading of Complications After Gastrectomy for Cancer Using the GASTRODATA Registry: A European Retrospective Observational Study. Ann. Surg. 2020, 272, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Pirker, R.; Wiesenberger, K.; Minar, W. Cancer-related anemia. Clinical relevance and treatment strategies. Am. J. Cancer 2005, 4, 233–245. [Google Scholar] [CrossRef]
- Xiao, H.; Quan, H.; Pan, S.; Yin, B.; Luo, W.; Huang, G.; Ouyang, Y. Impact of peri-operative blood transfusion on post-operative infections after radical gastrectomy for gastric cancer: A propensity score matching analysis focusing on the timing, amount of transfusion and role of leukocyte depletion. J. Cancer Res. Clin. Oncol. 2018, 144, 1143–1154. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Y.; Yao, H.; Hu, Z. Allogeneic blood transfusion and the prognosis of gastric cancer patients: Systematic review and meta-analysis. Int. J. Surg. 2015, 13, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Squires, M.H.; Kooby, D.A.; Poultsides, G.A.; Weber, S.M.; Bloomston, M.; Fields, R.C.; Pawlik, T.M.; Votanopoulos, K.I.; Schmidt, C.R.; Ejaz, A.; et al. Effect of Perioperative Transfusion on Recurrence and Survival after Gastric Cancer Resection: A 7-Institution Analysis of 765 Patients from the US Gastric Cancer Collaborative. J. Am. Coll. Surg. 2015, 221, 767–777. [Google Scholar] [CrossRef]
- Yuan, P.; Wu, Z.; Li, Z.; Bu, Z.; Wu, A.; Wu, X.; Zhang, L.; Shi, J.; Ji, J. Impact of postoperative major complications on long-term survival after radical resection of gastric cancer. BMC Cancer 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Snyder, G.L.; Greenberg, S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br. J. Anaesth. 2010, 105, 106–115. [Google Scholar] [CrossRef]
- Cata, J.; Wang, H.; Gottumukkala, V.; Reuben, J.; Sessler, D. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br. J. Anaesth. 2013, 110, 690–701. [Google Scholar] [CrossRef]
- Hirahara, N.; Matsubara, T.; Mizota, Y.; Ishibashi, S.; Tajima, Y. Prognostic value of preoperative inflammatory response biomarkers in patients with esophageal cancer who undergo a curative thoracoscopic esophagectomy. BMC Surg. 2016, 16, 1–12. [Google Scholar] [CrossRef]
- Qu, J.; Qu, X.; Li, Z.; Zhang, J.; Teng, Y.; Jin, B.; Zhao, M.; Yu, P.; Wang, Z.; Liu, Y. Role of patient-, tumor- and systemic inflammatory response–related factors in predicting survival of patients with node-negative gastric cancer. Tumor Biol. 2017, 39, 101042831769837. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chang, Q.; Meng, X.; Gao, N.; Wang, W. Prognostic value of Systemic immune-inflammation index in cancer: A meta-analysis. J. Cancer 2018, 9, 3295–3302. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zou, K.; Yang, C.; Chen, F.; Guo, T.; Xiong, B. Inflammation-based indexes and clinicopathologic features are strong predictive values of preoperative circulating tumor cell detection in gastric cancer patients. Clin. Transl. Oncol. 2017, 19, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huang, Y.; Feng, J.; Liu, J. Prognostic significance of neutrophil-to-lymphocyte ratio in esophageal cancer: A meta-analysis. OncoTargets Ther. 2015, 8, 789–794. [Google Scholar] [CrossRef]
- Sun, X.; Liu, X.; Liu, J.; Chen, S.; Xu, D.; Li, W.; Zhan, Y.; Li, Y.; Chen, Y.; Zhou, Z. Preoperative neutrophil-to-lymphocyte ratio plus platelet-to-lymphocyte ratio in predicting survival for patients with stage I–II gastric cancer. Chin. J. Cancer 2016, 35, 57. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Lee, J.W.; Yoo, H.M.; Park, C.H.; Song, K.Y. The Platelet-to-Lymphocyte Ratio Versus Neutrophil-to-Lymphocyte Ratio: Which is Better as a Prognostic Factor in Gastric Cancer? Ann. Surg. Oncol. 2015, 22, 4363–4370. [Google Scholar] [CrossRef]
- Pan, Q.-X.; Su, Z.-J.; Zhang, J.-H.; Wang, C.-R.; Ke, S.-Y. A comparison of the prognostic value of preoperative inflammation-based scores and TNM stage in patients with gastric cancer. OncoTargets Ther. 2015, 8, 1375–1385. [Google Scholar] [CrossRef]
- Min, K.-W.; Kwon, M.J.; Kim, D.-H.; Son, B.K.; Kim, E.-K.; Oh, Y.H.; Wi, Y.C. Persistent elevation of postoperative neutrophil-to-lymphocyte ratio: A better predictor of survival in gastric cancer than elevated preoperative neutrophil-to-lymphocyte ratio. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Lin, J.-X.; Wang, Z.-K.; Huang, Y.-Q.; Xie, J.-W.; Wang, J.-B.; Lu, J.; Chen, Q.-Y.; Lin, M.; Tu, R.-H.; Huang, Z.-N.; et al. Dynamic Changes in Pre- and Postoperative Levels of Inflammatory Markers and Their Effects on the Prognosis of Patients with Gastric Cancer. J. Gastrointest. Surg. 2020, 25, 387–396. [Google Scholar] [CrossRef]
- Kim, E.Y.; Song, K.Y. The preoperative and the postoperative neutrophil-to-lymphocyte ratios both predict prognosis in gastric cancer patients. World J. Surg. Oncol. 2020, 18, 1–8. [Google Scholar] [CrossRef]
- Thomson, A.; Farmer, S.; Hofmann, A.; Isbister, J.; Shander, A. Patient blood management—A new paradigm for transfusion medicine? ISBT Sci. Ser. 2009, 4, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Shander, A.; Hofmann, A.; Isbister, J.; Van Aken, H. Patient blood management—The new frontier. Best Pract. Res. Clin. Anaesthesiol. 2013, 27, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.; Jericó, C.; Miranda, C.; Santamaría, M.; Artigau, E.; Galofré, G.; Garsot, E.; Luna, A.; Puértolas, N.; Aldeano, A.; et al. Improved postoperative outcomes and reduced transfusion rates after implementation of a Patient Blood Management program in gastric cancer surgery. Eur. J. Surg. Oncol. (EJSO) 2020, 47, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Agha, R.; Abdall-Razak, A.; Crossley, E.; Dowlut, N.; Iosifidis, C.; Mathew, G.; Beamishaj; Bashashati, M.; Millham, F.H.; Orgill, D.P.; et al. STROCSS 2019 Guideline: Strengthening the reporting of cohort studies in surgery. Int. J. Surg. 2019, 72, 156–165. [Google Scholar] [CrossRef]
- Cero, M.D.; Rodríguez-Santiago, J.; Miró, M.; Castro, S.; Miranda, C.; Santamaría, M.; Gobbini, Y.; Garsot, E.; Pujadas, M.; Luna, A.; et al. Evaluation of data quality in the Spanish EURECCA Esophagogastric Cancer Registry. Eur. J. Surg. Oncol. (EJSO) 2021, 47, 3081–3087. [Google Scholar] [CrossRef]
- Kelly, C.M.; Shahrokni, A. Moving beyond Karnofsky and ECOG Performance Status Assessments with New Technologies. J. Oncol. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- In, H.; Solsky, I.; Palis, B.; Langdon-Embry, M.; A Ajani, J.; Sano, T. Validation of the 8th Edition of the AJCC TNM Staging System for Gastric Cancer using the National Cancer Database. Ann. Surg. Oncol. 2017, 24, 3683–3691. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017, 20, 1–19. [Google Scholar] [CrossRef]
- Baiocchi, G.L.; Giacopuzzi, S.; Marrelli, D.; Reim, D.; Piessen, G.; da Costa, P.M.; Reynolds, J.V.; Meyer, H.-J.; Morgagni, P.; Gockel, I.; et al. International consensus on a complications list after gastrectomy for cancer. Gastric Cancer 2018, 22, 172–189. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, H.; Ichikura, T.; Ono, S.; Sugasawa, H.; Hiraki, S.; Sakamoto, N.; Yaguchi, Y.; Yoshida, K.; Matsumoto, Y.; Hase, K. Impact of Postoperative Infection on Long-Term Survival After Potentially Curative Resection for Gastric Cancer. Ann. Surg. Oncol. 2008, 16, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.M.; Lee, H.H.; Shim, J.H.; Jeon, H.M.; Park, C.H.; Song, K.Y. Negative impact of leakage on survival of patients undergoing curative resection for advanced gastric cancer. J. Surg. Oncol. 2011, 104, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-G. Impact of postoperative complications on long-term survival after radical resection for gastric cancer. World J. Gastroenterol. 2013, 19, 4060–4065. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, M.; Kurokawa, Y.; Machida, R.; Sato, Y.; Takiguchi, S.; Doki, Y.; Yabusaki, H.; Watanabe, M.; Hato, S.; Nakamori, M.; et al. Impact of postoperative complications on survival outcomes in patients with gastric cancer: Exploratory analysis of a randomized controlled JCOG1001 trial. Gastric Cancer 2020, 24, 214–223. [Google Scholar] [CrossRef]
- Climent, M.; Hidalgo, N.; Vidal, Ó.; Puig, S.; Iglesias, M.; Cuatrecasas, M.; Ramón, J.; García-Albéniz, X.; Grande, L.; Pera, M. Postoperative complications do not impact on recurrence and survival after curative resection of gastric cancer. Eur. J. Surg. Oncol. (EJSO) 2015, 42, 132–139. [Google Scholar] [CrossRef][Green Version]
- Nakanishi, K.; Kanda, M.; Kodera, Y. Long-lasting discussion: Adverse effects of intraoperative blood loss and allogeneic transfusion on prognosis of patients with gastric cancer. World J. Gastroenterol. 2019, 25, 2743–2751. [Google Scholar] [CrossRef]
- Mizuno, A.; Kanda, M.; Kobayashi, D.; Tanaka, C.; Iwata, N.; Yamada, S.; Fujii, T.; Nakayama, G.; Sugimoto, H.; Koike, M.; et al. Adverse Effects of Intraoperative Blood Loss on Long-Term Outcomes after Curative Gastrectomy of Patients with Stage II/III Gastric Cancer. Dig. Surg. 2016, 33, 121–128. [Google Scholar] [CrossRef]
- Ito, Y.; Kanda, M.; Ito, S.; Mochizuki, Y.; Teramoto, H.; Ishigure, K.; Murai, T.; Asada, T.; Ishiyama, A.; Matsushita, H.; et al. Intraoperative Blood Loss is Associated with Shortened Postoperative Survival of Patients with Stage II/III Gastric Cancer: Analysis of a Multi-institutional Dataset. World J. Surg. 2018, 43, 870–877. [Google Scholar] [CrossRef]
- Hsu, F.-K.; Chang, W.-K.; Lin, K.-J.; Liu, C.-Y.; Fang, W.-L.; Chang, K.-Y. The Associations between Perioperative Blood Transfusion and Long-Term Outcomes after Stomach Cancer Surgery. Cancers 2021, 13, 5438. [Google Scholar] [CrossRef]
- Li, L.; Zhu, D.; Chen, X.; Huang, Y.; Ouyang, M.; Zhang, W. Perioperative Allogenenic Blood Transfusion is Associated With Worse Clinical Outcome for Patients Undergoing Gastric Carcinoma Surgery. Medicine 2015, 94, e1574. [Google Scholar] [CrossRef] [PubMed]
- Agnes, A.; Lirosi, M.C.; Panunzi, S.; Santocchi, P.; Persiani, R.; D’Ugo, D. The prognostic role of perioperative allogeneic blood transfusions in gastric cancer patients undergoing curative resection: A systematic review and meta-analysis of non-randomized, adjusted studies. Eur. J. Surg. Oncol. (EJSO) 2018, 44, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, J.; Coburn, N.; Callum, J.; Mahar, A.L.; Acuña, S.A.; Guttman, M.P.; Zuk, V.; Lin, Y.; Turgeon, A.F.; Martel, G.; et al. Association of perioperative red blood cell transfusions with all-cause and cancer-specific death in patients undergoing surgery for gastrointestinal cancer: Long-term outcomes from a population-based cohort. Surgery 2021, 170, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Ghidini, M.; Ghidini, A.; Sgroi, G.; Vavassori, I.; Petrò, D.; Cabiddu, M.; Aiolfi, A.; Bonitta, G.; Zaniboni, A.; et al. Red blood cell transfusions and the survival in patients with cancer undergoing curative surgery: A systematic review and meta-analysis. Surg. Today 2021, 51, 1535–1557. [Google Scholar] [CrossRef]
- Liang, Y.; Guo, H.; Deng, J.; Wang, B.; Ding, X.; Wang, X.; Zhang, L.; Liang, H. Impact of intraoperative blood loss on survival after curative resection for gastric cancer. World J. Gastroenterol. 2013, 19, 5542–5550. [Google Scholar] [CrossRef]
- Pacelli, F.; Rosa, F.; Marrelli, D.; Pedrazzani, C.; Bossola, M.; Zoccali, M.B.; Marchet, A.; Di Cosmo, M.; Roata, C.; Graziosi, L.; et al. Do Perioperative Blood Transfusions Influence Prognosis of Gastric Cancer Patients? Analysis of 927 Patients and Interactions with Splenectomy. Ann. Surg. Oncol. 2011, 18, 1615–1623. [Google Scholar] [CrossRef]
- Rausei, S.; Ruspi, L.; Galli, F.; Tirotta, F.; Inversini, D.; Frattini, F.; Chiappa, C.; Rovera, F.; Boni, L.; Dionigi, G.; et al. Peri-operative blood transfusion in gastric cancer surgery: Prognostic or confounding factor? Int. J. Surg. 2013, 11, S100–S103. [Google Scholar] [CrossRef][Green Version]
- Danish Ranx05 Colorectal Cancer Study Group; Mynster, T.; Christensen, I.J.; Moesgaard, F.; Nielsen, H.J. Effects of the combination of blood transfusion and postoperative infectious complications on prognosis after surgery for colorectal cancer. Br. J. Surg. 2000, 87, 1553–1562. [Google Scholar] [CrossRef]
- Xiao, H.; Xiao, Y.; Chen, P.; Quan, H.; Luo, J.; Huang, G. Association Among Blood Transfusion, Postoperative Infectious Complications, and Cancer-Specific Survival in Patients with Stage II/III Gastric Cancer After Radical Gastrectomy: Emphasizing Benefit from Adjuvant Chemotherapy. Ann. Surg. Oncol. 2020, 28, 2394–2404. [Google Scholar] [CrossRef]
- Jericó, C.; Osorio, J.; García-Erce, J.A.; Pera, M. Patient Blood Management strategies for iron deficiency anemia management in gastric cancer. Eur. J. Gastroenterol. Hepatol. 2019, 31, 547–548. [Google Scholar] [CrossRef]
- Mungan, I.; Dicle, B.; Bektaş, Ş.; Sarı, S.; Yamanyar, S.; Çavuş, M.; Turan, S.; Bostancı, E.B. Does the preoperative platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio predict morbidity after gastrectomy for gastric cancer? Mil. Med. Res. 2020, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Inaoka, K.; Kanda, M.; Uda, H.; Tanaka, Y.; Tanaka, C.; Kobayashi, D.; Takami, H.; Iwata, N.; Hayashi, M.; Niwa, Y.; et al. Clinical utility of the platelet-lymphocyte ratio as a predictor of postoperative complications after radical gastrectomy for clinical T2-4 gastric cancer. World J. Gastroenterol. 2017, 23, 2519–2526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, J.-J.; Du, Y.-P.; Feng, C.-X.; Wang, L.-Q.; Chen, M.-B. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in gastric cancer. Medicine 2018, 97, e0144. [Google Scholar] [CrossRef] [PubMed]
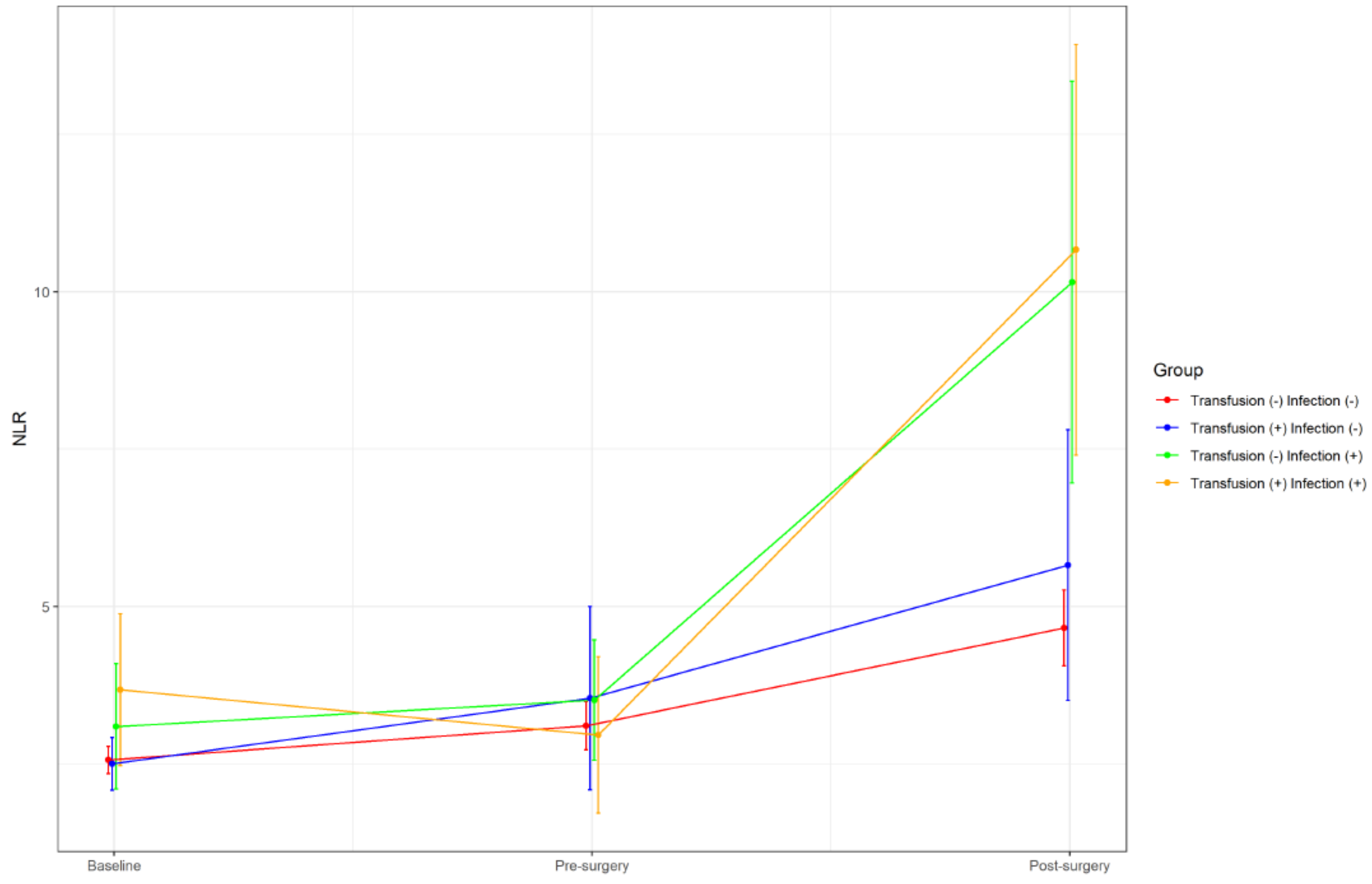
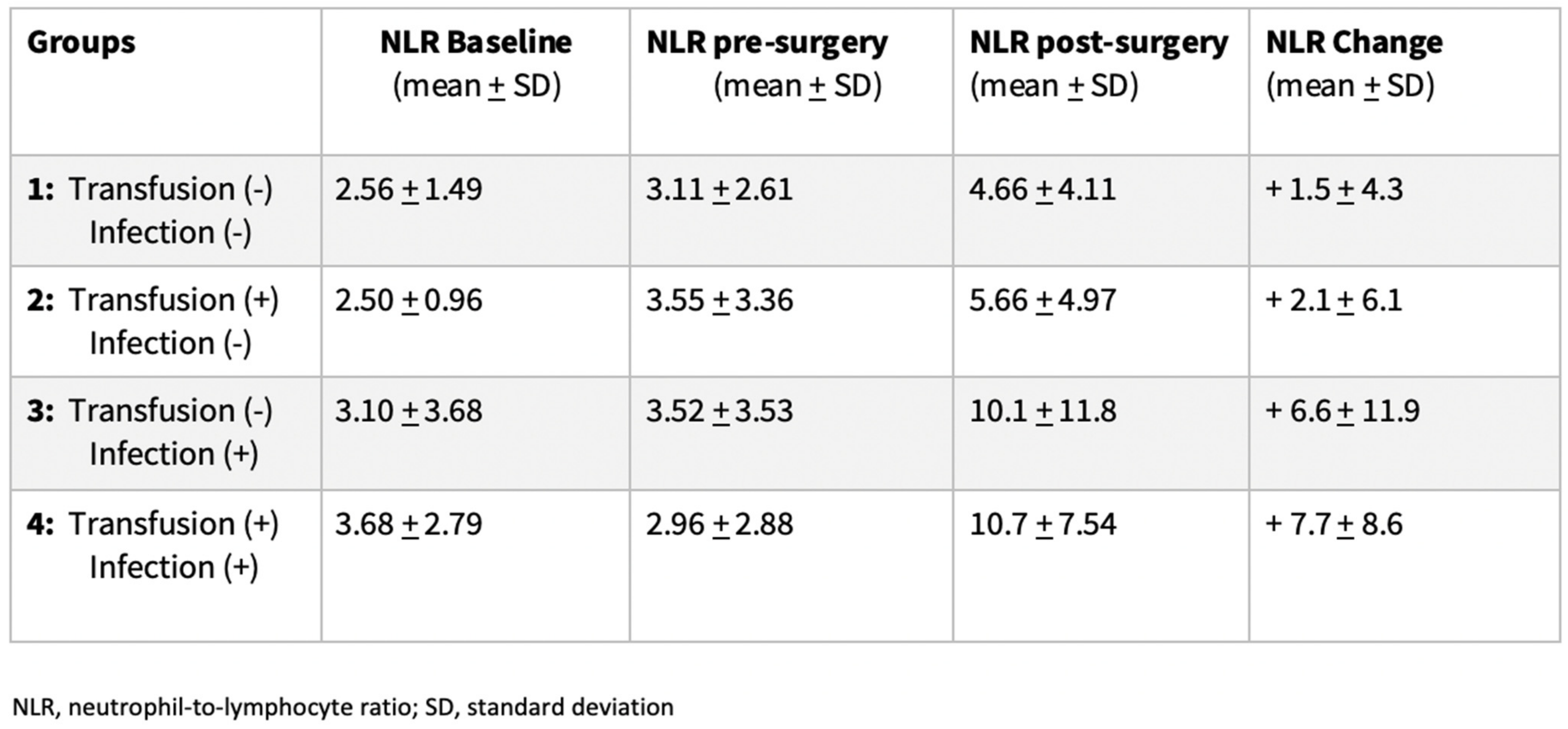
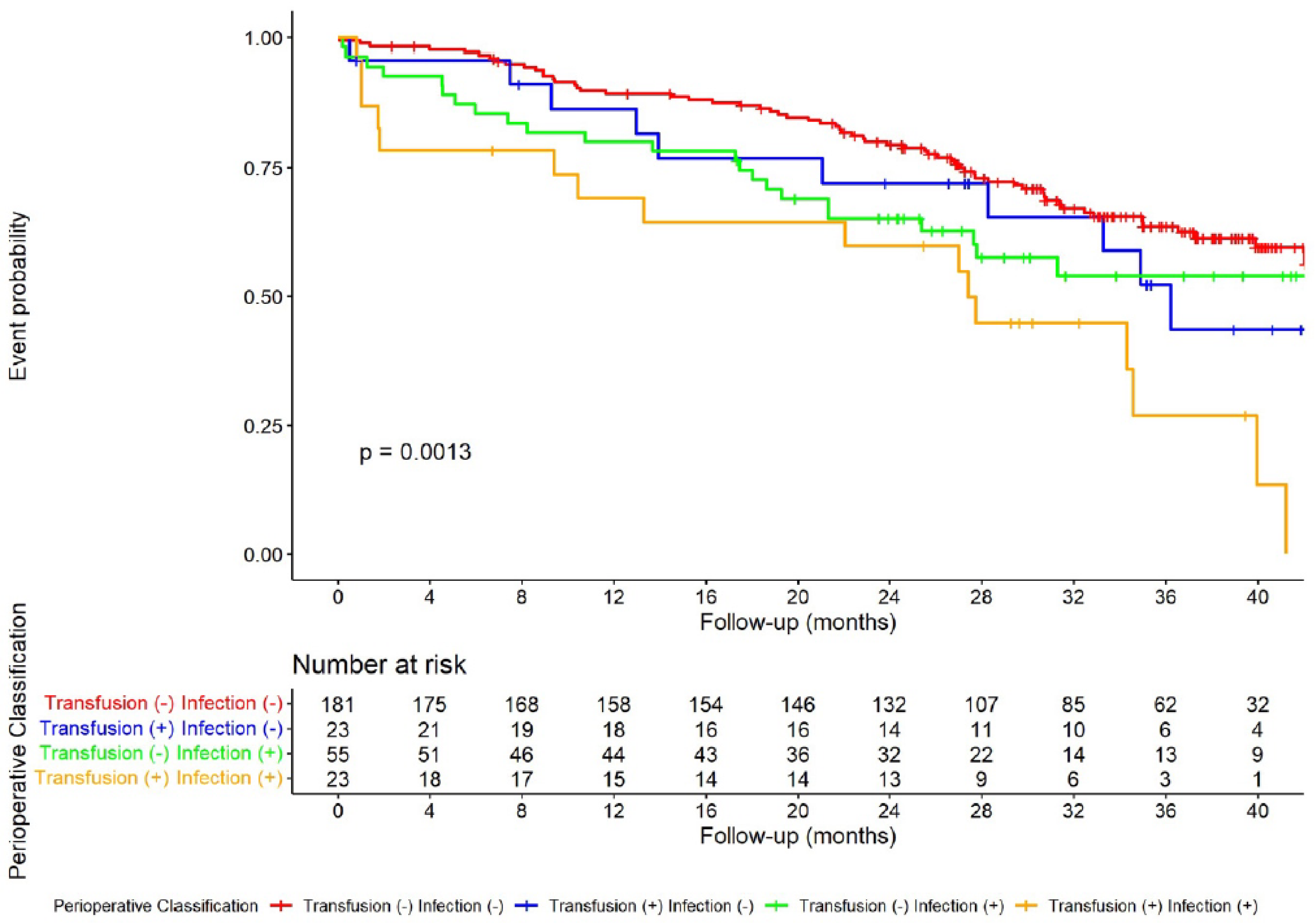

| Variables | Transfusion (−) Infection (−) | Transfusion (+) Infection (−) | Transfusion (−) Infection (+) | Transfusion (+) Infection (+) | p Values |
|---|---|---|---|---|---|
| N = 181 | N = 23 | N = 55 | N = 23 | ||
| Age, Mean (SD) | 68.4 (11.5) | 76.0(9.32) | 70.8 (12.3) | 74.4(8.27) | 0.004 |
| Sex, N (%): | 0.059 | ||||
| Male | 118 (65.2%) | 9 (39.1%) | 39 (70.9%) | 15 (65.2%) | |
| Female | 63 (34.8%) | 14(60.9%) | 16 (29.1%) | 8 (34.8%) | |
| ASA score, N (%): | 0.237 | ||||
| ASA I | 7 (3.87%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | |
| ASA II | 85 (47.0%) | 7 (30.4%) | 28 (50.9%) | 9 (39.1%) | |
| ASA III | 85 (47.0%) | 15(65.2%) | 27 (49.1%) | 12 (52.2%) | |
| ASA IV | 4 (2.21%) | 1 (4.35%) | 0 (0.00%) | 2 (8.70%) | |
| ECOG c, N (%): | 0.027 | ||||
| ECOG 0 | 70 (38.7%) | 4 (17.4%) | 20 (36.4%) | 3 (13.0%) | |
| ECOG ≥ 1 | 111 (61.3%) | 19(82.6%) | 35 (63.6%) | 20 (87.0%) | |
| Charlson score a, N (%): | 0.376 | ||||
| 0–2 | 98 (54.1%) | 10(43.5%) | 25 (45.5%) | 9 (39.1%) | |
| ≥3 | 83 (45.9%) | 13(56.5%) | 30 (54.5%) | 14 (60.9%) | |
| Ischemic heart, N (%): disease, N (%) | 6 (3.31%) | 3 (13.0%) | 1 (1.82%) | 2 (8.70%) | 0.076 |
| CHF, N (%) | 5 (2.76%) | 3 (13.0%) | 1 (1.82%) | 3 (13.0%) | 0.017 |
| DM complic., N (%) | 26 (14.4%) | 4 (17.4%) | 13 (23.6%) | 7 (30.4%) | 0.138 |
| DM no complic., N (%) | 12 (6.63%) | 2 (8.70%) | 3 (5.45%) | 2 (8.70%) | 0.840 |
| COPD, N (%) | 22 (12.2%) | 3 (13.0%) | 12 (21.8%) | 4 (17.4%) | 0.298 |
| Renal failure, N (%) | 5 (2.76%) | 1 (4.35%) | 1 (1.82%) | 4 (17.4%) | 0.019 |
| Vasc. Dis., N (%) | 20 (11.0%) | 5 (21.7%) | 9 (16.4%) | 2 (8.70%) | 0.350 |
| Weight loss, N (%): | 0.174 | ||||
| 0–5% | 120 (66.3%) | 17(73.9%) | 36 (65.5%) | 12 (52.2%) | |
| 6–10% | 41 (22.7%) | 1 (4.35%) | 11 (20.0%) | 8 (34.8%) | |
| >10% | 20 (11.0%) | 5 (21.7%) | 8 (14.5%) | 3 (13.0%) | |
| Hb (g/dL), Mean (SD) | 12.0 (2.63) | 10.4(2.26) | 11.8 (2.57) | 10.8 (2.99) | 0.012 |
| Tumor location, N (%): | 0.060 | ||||
| Upper third | 15 (8.29%) | 1 (4.35%) | 10 (18.2%) | 4 (17.4%) | |
| Middle third | 67 (37.0%) | 6 (26.1%) | 21 (38.2%) | 7 (30.4%) | |
| Lower third | 97 (53.6%) | 16 (69.6%) | 23 (41.8%) | 12 (52.2%) | |
| Entire | 2 (1.10%) | 0 (0.00%) | 1 (1.82%) | 0 (0.00%) | |
| Neoadjuvancy, N (%) | 70 (38.7%) | 4 (17.4%) | 25 (45.5%) | 10 (43.5%) | 0.128 |
| Gastrectomy, N (%): | 0.006 | ||||
| Distal subtotal | 117 (64.6%) | 18(78.3%) | 23 (41.8%) | 13 (56.5%) | |
| Total | 64 (35.4%) | 5 (21.7%) | 32 (58.2%) | 10 (43.5%) | |
| Access, N (%): | 0.269 | ||||
| Open | 88 (48.6%) | 14(60.9%) | 23 (41.8%) | 8 (34.8%) | |
| Laparoscopic | 93 (51.4%) | 9 (39.1%) | 32 (58.2%) | 15 (65.2%) | |
| pT b, N (%): | 0.179 | ||||
| ≤T2 | 98 (54.1%) | 17(73.9%) | 30 (54.5%) | 16 (69.6%) | |
| >T3 | 83 (45.9%) | 6 (26.1%) | 25 (45.5%) | 7 (30.4%) | |
| pN b, N (%): | 0.008 | ||||
| N0 | 91 (50.3%) | 6 (26.1%) | 20 (36.4%) | 5 (21.7%) | |
| ≥N1 | 90 (49.7%) | 17(73.9%) | 35 (63.6%) | 18 (78.3%) | |
| Node count, Mean (SD) | 27.6 (15.8) | 27.7(16.3) | 26.5 (13.5) | 27.7 (9.69) | 0.972 |
| Radicality, N (%): | 0.881 | ||||
| R0 | 164 (90.6%) | 21(91.3%) | 52 (94.5%) | 21 (91.3%) | |
| R1–R2 | 17 (9.39%) | 2 (8.70%) | 3 (5.45%) | 2 (8.70%) |
| lnfectious Complications a | n (%) b |
|---|---|
| Gastrointestinal complications | |
| Anastomotic leak | 24 (8.3%) |
| Duodenal stump leak | 7 (2.4%) |
| Pancreatic fistula | 2 (0.7%) |
| Abdominal collection | 17 (5.5%) |
| Clostridium infection | 1 (0.3%) |
| Wound infection | 7 (2.4%) |
| Pneumonia | 13 (4.5%) |
| Catheter infection | 10 (3.4%) |
| Urinary infection | 7 (2.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puértolas, N.; Osorio, J.; Jericó, C.; Miranda, C.; Santamaría, M.; Artigau, E.; Galofré, G.; Garsot, E.; Luna, A.; Aldeano, A.; et al. Effect of Perioperative Blood Transfusions and Infectious Complications on Inflammatory Activation and Long-Term Survival Following Gastric Cancer Resection. Cancers 2023, 15, 144. https://doi.org/10.3390/cancers15010144
Puértolas N, Osorio J, Jericó C, Miranda C, Santamaría M, Artigau E, Galofré G, Garsot E, Luna A, Aldeano A, et al. Effect of Perioperative Blood Transfusions and Infectious Complications on Inflammatory Activation and Long-Term Survival Following Gastric Cancer Resection. Cancers. 2023; 15(1):144. https://doi.org/10.3390/cancers15010144
Chicago/Turabian StylePuértolas, Noelia, Javier Osorio, Carlos Jericó, Coro Miranda, Maite Santamaría, Eva Artigau, Gonzalo Galofré, Elisenda Garsot, Alexis Luna, Aurora Aldeano, and et al. 2023. "Effect of Perioperative Blood Transfusions and Infectious Complications on Inflammatory Activation and Long-Term Survival Following Gastric Cancer Resection" Cancers 15, no. 1: 144. https://doi.org/10.3390/cancers15010144
APA StylePuértolas, N., Osorio, J., Jericó, C., Miranda, C., Santamaría, M., Artigau, E., Galofré, G., Garsot, E., Luna, A., Aldeano, A., Olona, C., Molinas, J., Pulido, L., Gimeno, M., & Pera, M. (2023). Effect of Perioperative Blood Transfusions and Infectious Complications on Inflammatory Activation and Long-Term Survival Following Gastric Cancer Resection. Cancers, 15(1), 144. https://doi.org/10.3390/cancers15010144







