GISTs with NTRK Gene Fusions: A Clinicopathological, Immunophenotypic, and Molecular Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
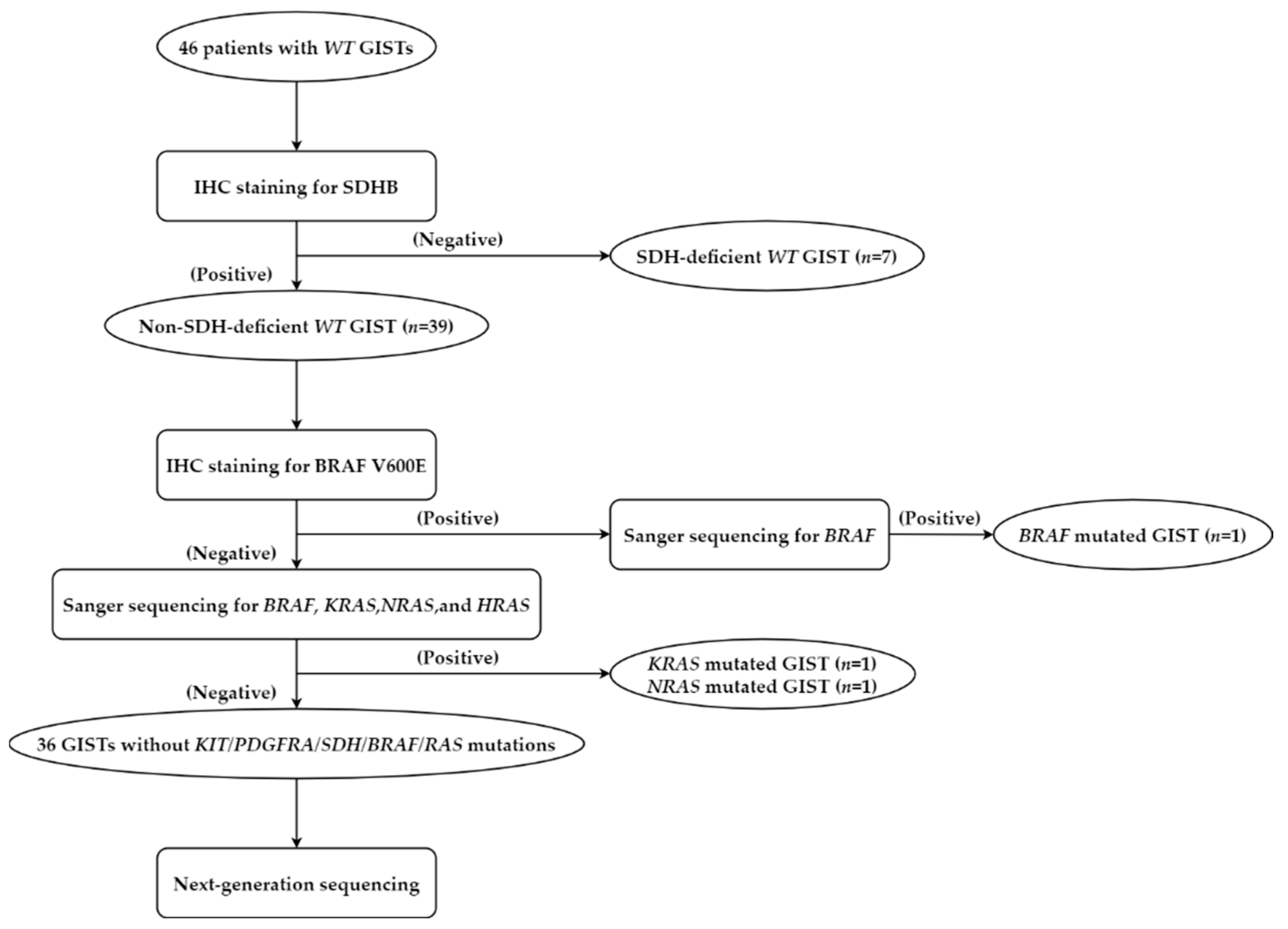
2.1. Patient Selection
2.2. Immunohistochemistry
2.3. Sanger Sequencing
2.4. Next-Generation Target Sequencing (DNA and RNA)
2.5. Fluorescence In Situ Hybridization
3. Results
3.1. Preliminary Testing of WT GISTs
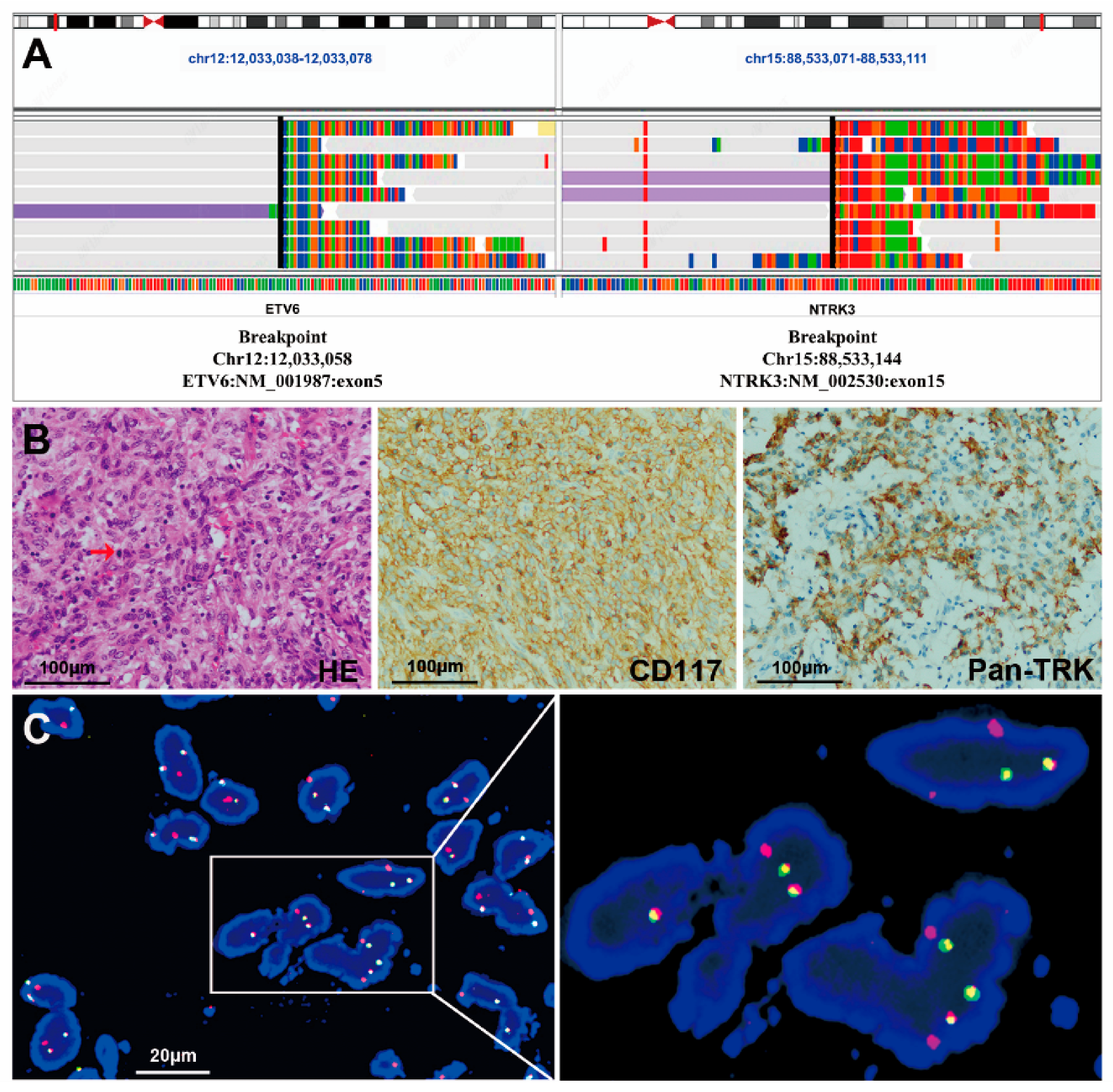
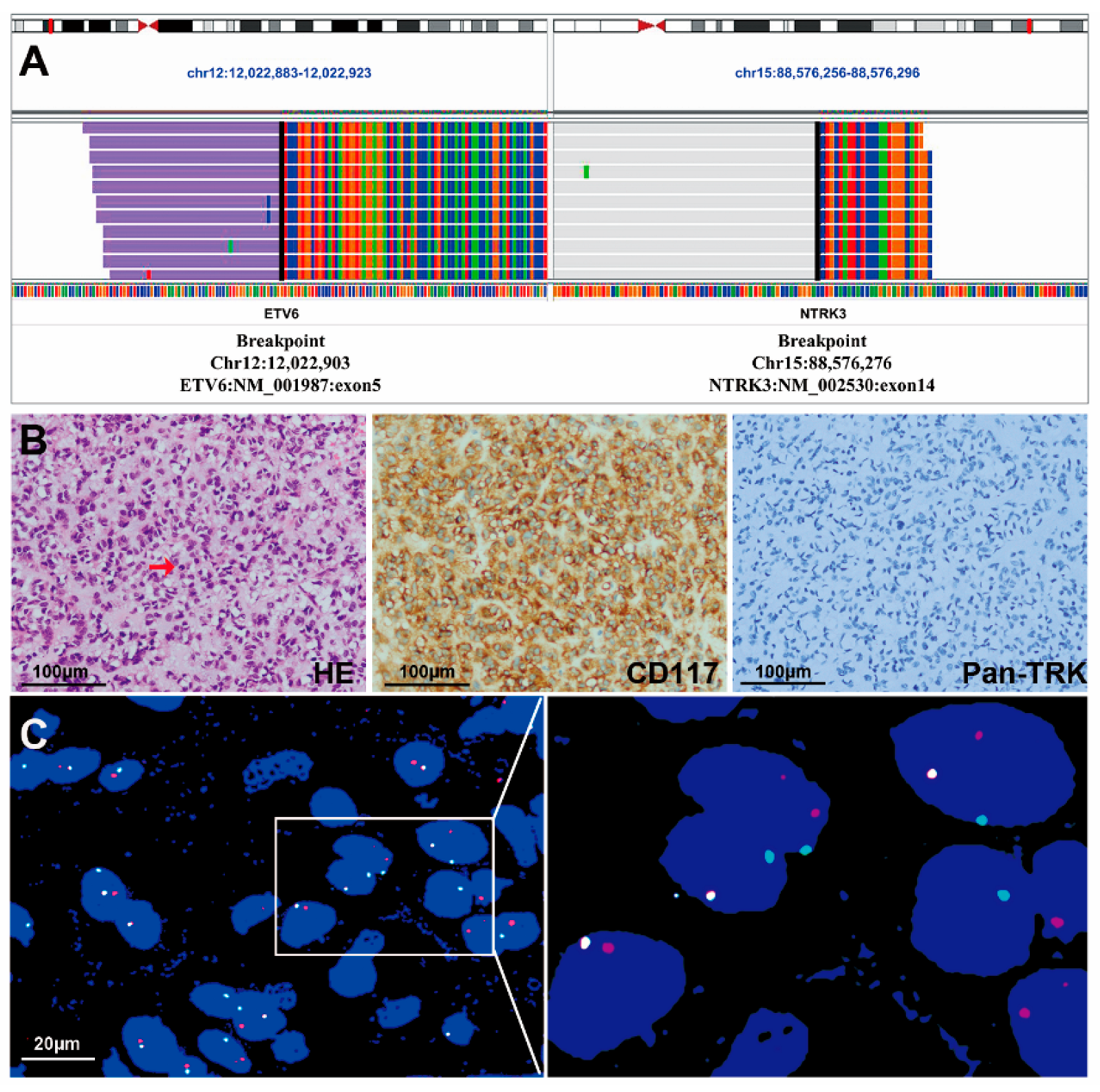
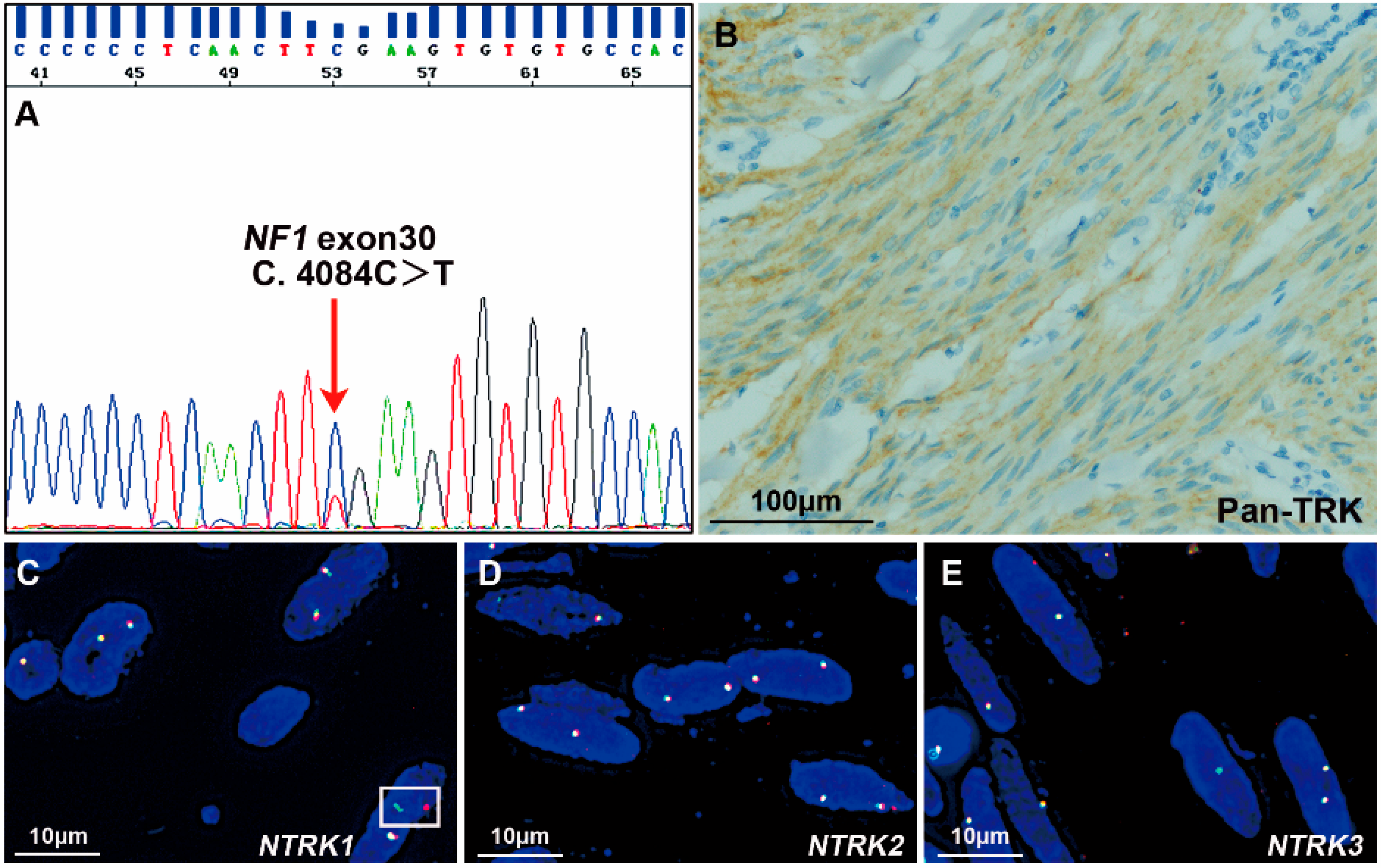
3.2. NTRK Fusions Identified with NGS
3.3. IHC Staining of Pan-TRK
3.4. FISH Assessments
3.5. Comparisons among the Results of NGS, FISH, and IHC
3.6. The Clinicopathological Features of the Two GISTs with the ETV6-NTRK3 Fusion
3.7. The Clinicopathological and Genetic Features of 12 GISTs with NTRK Fusions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Søreide, K.; Sandvik, O.M.; Søreide, J.A.; Giljaca, V.; Jureckova, A.; Bulusu, V.R. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016, 40, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Verschoor, A.J.; Bovée, J.; Overbeek, L.; PALGA group; Hogendoorn, P.; Gelderblom, H. The incidence, mutational status, risk classification and referral pattern of gastro-intestinal stromal tumours in the Netherlands: A nationwide pathology registry (PALGA) study. Virchows Arch. 2018, 472, 221–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, K.M.; Ward, S.M.; Koh, S.D. Interstitial cells: Regulators of smooth muscle function. Physiol. Rev. 2014, 94, 859–907. [Google Scholar] [CrossRef] [PubMed]
- Parab, T.M.; DeRogatis, M.J.; Boaz, A.M.; Grasso, S.A.; Issack, P.S.; Duarte, D.A.; Urayeneza, O.; Vahdat, S.; Qiao, J.H.; Hinika, G.S. Gastrointestinal stromal tumors: A comprehensive review. J. Gastrointest. Oncol. 2019, 10, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Corless, C.L.; Barnett, C.M.; Heinrich, M.C. Gastrointestinal stromal tumours: Origin and molecular oncology. Nat. Rev. Cancer. 2011, 11, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Settas, N.; Faucz, F.R.; Stratakis, C.A. Succinate dehydrogenase (SDH) deficiency, Carney triad and the epigenome. Mol. Cell. Endocrinol. 2018, 469, 107–111. [Google Scholar] [CrossRef]
- Ibrahim, A.; Chopra, S. Succinate Dehydrogenase-Deficient Gastrointestinal Stromal Tumors. Arch. Pathol. Lab. Med. 2020, 144, 655–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hostein, I.; Faur, N.; Primois, C.; Boury, F.; Denard, J.; Emile, J.F.; Bringuier, P.P.; Scoazec, J.Y.; Coindre, J.M. BRAF mutation status in gastrointestinal stromal tumors. Am. J. Clin. Pathol. 2010, 133, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Huss, S.; Pasternack, H.; Ihle, M.A.; Merkelbach-Bruse, S.; Heitkötter, B.; Hartmann, W.; Trautmann, M.; Gevensleben, H.; Büttner, R.; Schildhaus, H.U.; et al. Clinicopathological and molecular features of a large cohort of gastrointestinal stromal tumors (GISTs) and review of the literature: BRAF mutations in KIT/PDGFRA wild-type GISTs are rare events. Hum. Pathol. 2017, 62, 206–214. [Google Scholar] [CrossRef]
- Miranda, C.; Nucifora, M.; Molinari, F.; Conca, E.; Anania, M.C.; Bordoni, A.; Saletti, P.; Mazzucchelli, L.; Pilotti, S.; Pierotti, M.; et al. KRAS and BRAF mutations predict primary resistance to imatinib in gastrointestinal stromal tumors. Clin. Cancer Res. 2012, 18, 1769–1776. [Google Scholar] [CrossRef]
- Pantaleo, M.A.; Nannini, M.; Corless, C.L.; Heinrich, M.C. Quadruple wild-type (WT) GIST: Defining the subset of GIST that lacks abnormalities of KIT, PDGFRA, SDH, or RAS signaling pathways. Cancer Med. 2015, 4, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Brenca, M.; Rossi, S.; Polano, M.; Gasparotto, D.; Zanatta, L.; Racanelli, D.; Valori, L.; Lamon, S.; Dei Tos, A.P.; Maestro, R. Transcriptome sequencing identifies ETV6-NTRK3 as a gene fusion involved in GIST. J. Pathol. 2016, 238, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Castillon, M.; Kammerer-Jacquet, S.F.; Cariou, M.; Costa, S.; Conq, G.; Samaison, L.; Douet-Guilbert, N.; Marcorelles, P.; Doucet, L.; Uguen, A. Fluorescent In Situ Hybridization Must be Preferred to pan-TRK Immunohistochemistry to Diagnose NTRK3-rearranged Gastrointestinal Stromal Tumors (GIST). Appl. Immunohistochem. Mol. Morphol. 2021, 29, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Shi, E.; Chmielecki, J.; Tang, C.M.; Wang, K.; Heinrich, M.C.; Kang, G.; Corless, C.L.; Hong, D.; Fero, K.E.; Murphy, J.D.; et al. FGFR1 and NTRK3 actionable alterations in “Wild-Type” gastrointestinal stromal tumors. J. Transl. Med. 2016, 14, 339. [Google Scholar] [CrossRef] [Green Version]
- D’Alpino Peixoto, R.; Medeiros, B.A.; Cronemberger, E.H. Resected High-Risk Rectal GIST Harboring NTRK1 Fusion: A Case Report and Review of the Literature. J. Gastrointest. Cancer 2021, 52, 316–319. [Google Scholar] [CrossRef]
- Nannini, M.; Urbini, M.; Astolfi, A.; Biasco, G.; Pantaleo, M.A. The progressive fragmentation of the KIT/PDGFRA wild-type (WT) gastrointestinal stromal tumors (GIST). J. Transl. Med. 2017, 15, 113. [Google Scholar] [CrossRef]
- Pantaleo, M.A.; Urbini, M.; Indio, V.; Ravegnini, G.; Nannini, M.; De Luca, M.; Tarantino, G.; Angelini, S.; Gronchi, A.; Vincenzi, B.; et al. Genome-Wide Analysis Identifies MEN1 and MAX Mutations and a Neuroendocrine-Like Molecular Heterogeneity in Quadruple WT GIST. Mol. Cancer Res. 2017, 15, 553–562. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Yuan, W.; Ren, L.; Xu, C.; Luo, R.; Zhou, Y.; Lu, W.; Hao, Q.; Xu, M.; Hou, Y. A novel fusion between CDC42BPB and ALK in a patient with quadruple wild-type gastrointestinal stromal tumor. Mol. Genet. Genom. Med. 2022, 10, e1881. [Google Scholar] [CrossRef]
- Knezevich, S.R.; McFadden, D.E.; Tao, W.; Lim, J.F.; Sorensen, P.H. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat. Genet. 1998, 18, 184–187. [Google Scholar] [CrossRef]
- Rubin, B.P.; Chen, C.J.; Morgan, T.W.; Xiao, S.; Grier, H.E.; Kozakewich, H.P.; Perez-Atayde, A.R.; Fletcher, J.A. Congenital mesoblastic nephroma t(12;15) is associated with ETV6-NTRK3 gene fusion: Cytogenetic and molecular relationship to congenital (infantile) fibrosarcoma. Am. J. Pathol. 1998, 153, 1451–1458. [Google Scholar] [CrossRef]
- Eguchi, M.; Eguchi-Ishimae, M.; Tojo, A.; Morishita, K.; Suzuki, K.; Sato, Y.; Kudoh, S.; Tanaka, K.; Setoyama, M.; Nagamura, F.; et al. Fusion of ETV6 to neurotrophin-3 receptor TRKC in acute myeloid leukemia with t(12;15)(p13;q25). Blood 1999, 93, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Forghieri, F.; Morselli, M.; Potenza, L.; Maccaferri, M.; Pedrazzi, L.; Paolini, A.; Bonacorsi, G.; Artusi, T.; Giacobbi, F.; Corradini, G.; et al. Chronic eosinophilic leukaemia with ETV6-NTRK3 fusion transcript in an elderly patient affected with pancreatic carcinoma. Eur. J. Haematol. 2011, 86, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Tognon, C.; Knezevich, S.R.; Huntsman, D.; Roskelley, C.D.; Melnyk, N.; Mathers, J.A.; Becker, L.; Carneiro, F.; MacPherson, N.; Horsman, D.; et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell 2002, 2, 367–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skálová, A.; Vanecek, T.; Sima, R.; Laco, J.; Weinreb, I.; Perez-Ordonez, B.; Starek, I.; Geierova, M.; Simpson, R.H.; Passador-Santos, F.; et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: A hitherto undescribed salivary gland tumor entity. Am. J. Surg. Pathol. 2010, 34, 599–608. [Google Scholar] [CrossRef]
- Leeman-Neill, R.J.; Kelly, L.M.; Liu, P.; Brenner, A.V.; Little, M.P.; Bogdanova, T.I.; Evdokimova, V.N.; Hatch, M.; Zurnadzy, L.Y.; Nikiforova, M.N.; et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer 2014, 120, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Alassiri, A.H.; Ali, R.H.; Shen, Y.; Lum, A.; Strahlendorf, C.; Deyell, R.; Rassekh, R.; Sorensen, P.H.; Laskin, J.; Marra, M.; et al. ETV6-NTRK3 Is Expressed in a Subset of ALK-Negative Inflammatory Myofibroblastic Tumors. Am. J. Surg. Pathol. 2016, 40, 1051–1061. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, S.J.; Choe, E.A.; Kim, J.; Hyung, W.J.; Kim, H.S.; Jung, M.; Beom, S.H.; Kim, T.I.; Ahn, J.B.; et al. Tropomyosin-Related Kinase Fusions in Gastrointestinal Stromal Tumors. Cancers 2022, 14, 2659. [Google Scholar] [CrossRef]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Laetsch, T.W.; DuBois, S.G.; Mascarenhas, L.; Turpin, B.; Federman, N.; Albert, C.M.; Nagasubramanian, R.; Davis, J.L.; Rudzinski, E.; Feraco, A.M.; et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: Phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018, 19, 705–714. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.P.; Hechtman, J.F. Detection of NTRK Fusions: Merits and Limitations of Current Diagnostic Platforms. Cancer Res. 2019, 79, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Marchiò, C.; Scaltriti, M.; Ladanyi, M.; Iafrate, A.J.; Bibeau, F.; Dietel, M.; Hechtman, J.F.; Troiani, T.; López-Rios, F.; Douillard, J.Y.; et al. ESMO recommendations on the standard methods to detect NTRK fusions in daily practice and clinical research. Ann. Oncol. 2019, 30, 1417–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hechtman, J.F. NTRK insights: Best practices for pathologists. Mod. Pathol. 2022, 35, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Ferrarotto, R.; Liang, L.; Goepfert, R.P.; Li, J.; Ning, J.; Broaddus, R.; Weber, R.S.; El-Naggar, A.K. Pan-Trk immunohistochemistry reliably identifies ETV6-NTRK3 fusion in secretory carcinoma of the salivary gland. Virchows Arch. 2020, 476, 295–305. [Google Scholar] [CrossRef]
- Hechtman, J.F.; Benayed, R.; Hyman, D.M.; Drilon, A.; Zehir, A.; Frosina, D.; Arcila, M.E.; Dogan, S.; Klimstra, D.S.; Ladanyi, M.; et al. Pan-Trk Immunohistochemistry Is an Efficient and Reliable Screen for the Detection of NTRK Fusions. Am. J. Surg. Pathol. 2017, 41, 1547–1551. [Google Scholar] [CrossRef]
- Cao, J.; Chen, L.; Li, H.; Chen, H.; Yao, J.; Mu, S.; Liu, W.; Zhang, P.; Cheng, Y.; Liu, B.; et al. An Accurate and Comprehensive Clinical Sequencing Assay for Cancer Targeted and Immunotherapies. Oncologist 2019, 24, e1294–e1302. [Google Scholar] [CrossRef] [Green Version]
- Haas, B.J.; Dobin, A.; Li, B.; Stransky, N.; Pochet, N.; Regev, A. Accuracy assessment of fusion transcript detection via read-mapping and de novo fusion transcript assembly-based methods. Genome Biol. 2019, 20, 213. [Google Scholar] [CrossRef] [Green Version]
- Moes-Sosnowska, J.; Skupinska, M.; Lechowicz, U.; Szczepulska-Wojcik, E.; Skronska, P.; Rozy, A.; Stepniewska, A.; Langfort, R.; Rudzinski, P.; Orlowski, T.; et al. FGFR1-4 RNA-Based Gene Alteration and Expression Analysis in Squamous Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2022, 23, 10506. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Pan, G.; Li, S.; Wu, Y.; Liu, L. Pure secretory carcinoma in situ: A case report and literature review. Diagn. Pathol. 2019, 14, 95. [Google Scholar] [CrossRef]
- Chen, L.; Yang, F.; Feng, T.; Wu, S.; Li, K.; Pang, J.; Shi, X.; Liang, Z. PD-L1, Mismatch Repair Protein, and NTRK Immunohistochemical Expression in Cervical Small Cell Neuroendocrine Carcinoma. Front. Oncol. 2021, 11, 752453. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum. Pathol. 2008, 39, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Vaishnavi, A.; Le, A.T.; Doebele, R.C. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov. 2015, 5, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klug, L.R.; Khosroyani, H.M.; Kent, J.D.; Heinrich, M.C. New treatment strategies for advanced-stage gastrointestinal stromal tumours. Nat. Rev. Clin. Oncol. 2022, 19, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Al-Salama, Z.T.; Keam, S.J. Entrectinib: First Global Approval. Drugs 2019, 79, 1477–1483. [Google Scholar] [CrossRef]
- Scott, L.J. Larotrectinib: First Global Approval. Drugs 2019, 79, 201–206. [Google Scholar] [CrossRef]
- Drilon, A.; Siena, S.; Ou, S.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef] [Green Version]
- Awada, A.; Berghmans, T.; Clement, P.M.; Cuppens, K.; De Wilde, B.; Machiels, J.P.; Pauwels, P.; Peeters, M.; Rottey, S.; Van Cutsem, E. Belgian expert consensus for tumor-agnostic treatment of NTRK gene fusion-driven solid tumors with larotrectinib. Crit. Rev. Oncol. Hematol. 2022, 169, 103564. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology. Gastrointestinal Stromal Tumors (GISTs), Version 1.2021. 2021. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx#gist (accessed on 23 January 2021).
- Qian, H.; Yan, N.; Hu, X.; Jiang, J.; Cao, Z.; Shen, D. Molecular Portrait of GISTs Associated with Clinicopathological Features: A Retrospective Study with Molecular Analysis by a Custom 9-Gene Targeted Next-Generation Sequencing Panel. Front. Genet. 2022, 13, 864499. [Google Scholar] [CrossRef]
- Mavroeidis, L.; Metaxa-Mariatou, V.; Papoudou-Bai, A.; Lampraki, A.M.; Kostadima, L.; Tsinokou, I.; Zarkavelis, G.; Papadaki, A.; Petrakis, D.; Gκoura, S.; et al. Comprehensive molecular screening by next generation sequencing reveals a distinctive mutational profile of KIT/PDGFRA genes and novel genomic alterations: Results from a 20-year cohort of patients with GIST from north-western Greece. ESMO Open 2018, 3, e000335. [Google Scholar] [CrossRef]
- Unk, M.; Bombač, A.; Jezeršek Novaković, B.; Stegel, V.; Šetrajčič Dragoš, V.; Blatnik, O.; Klančar, G.; Novaković, S. Correlation of treatment outcome in sanger/RT-qPCR KIT/PDGFRA wild-type metastatic gastrointestinal stromal tumors with next-generation sequencing results: A single-center report. Oncol. Rep. 2022, 48, 167. [Google Scholar] [CrossRef] [PubMed]
- Suurmeijer, A.J.; Dickson, B.C.; Swanson, D.; Zhang, L.; Sung, Y.S.; Huang, H.Y.; Fletcher, C.D.; Antonescu, C.R. The histologic spectrum of soft tissue spindle cell tumors with NTRK3 gene rearrangements. Genes Chromosomes Cancer 2019, 58, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Atiq, M.A.; Davis, J.L.; Hornick, J.L.; Dickson, B.C.; Fletcher, C.D.M.; Fletcher, J.A.; Folpe, A.L.; Mariño-Enríquez, A. Mesenchymal tumors of the gastrointestinal tract with NTRK rearrangements: A clinicopathological, immunophenotypic, and molecular study of eight cases, emphasizing their distinction from gastrointestinal stromal tumor (GIST). Mod. Pathol. 2021, 34, 95–103. [Google Scholar] [CrossRef]
- Rudzinski, E.R.; Lockwood, C.M.; Stohr, B.A.; Vargas, S.O.; Sheridan, R.; Black, J.O.; Rajaram, V.; Laetsch, T.W.; Davis, J.L. Pan-Trk Immunohistochemistry Identifies NTRK Rearrangements in Pediatric Mesenchymal Tumors. Am. J. Surg. Pathol. 2018, 42, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ye, Y.; Wang, J.; Zhang, B.; Qin, S.; Shi, Y.; He, Y.; Liang, X.; Liu, X.; Zhou, Y.; et al. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin. J. Cancer Res. 2017, 29, 281–293. [Google Scholar] [CrossRef]
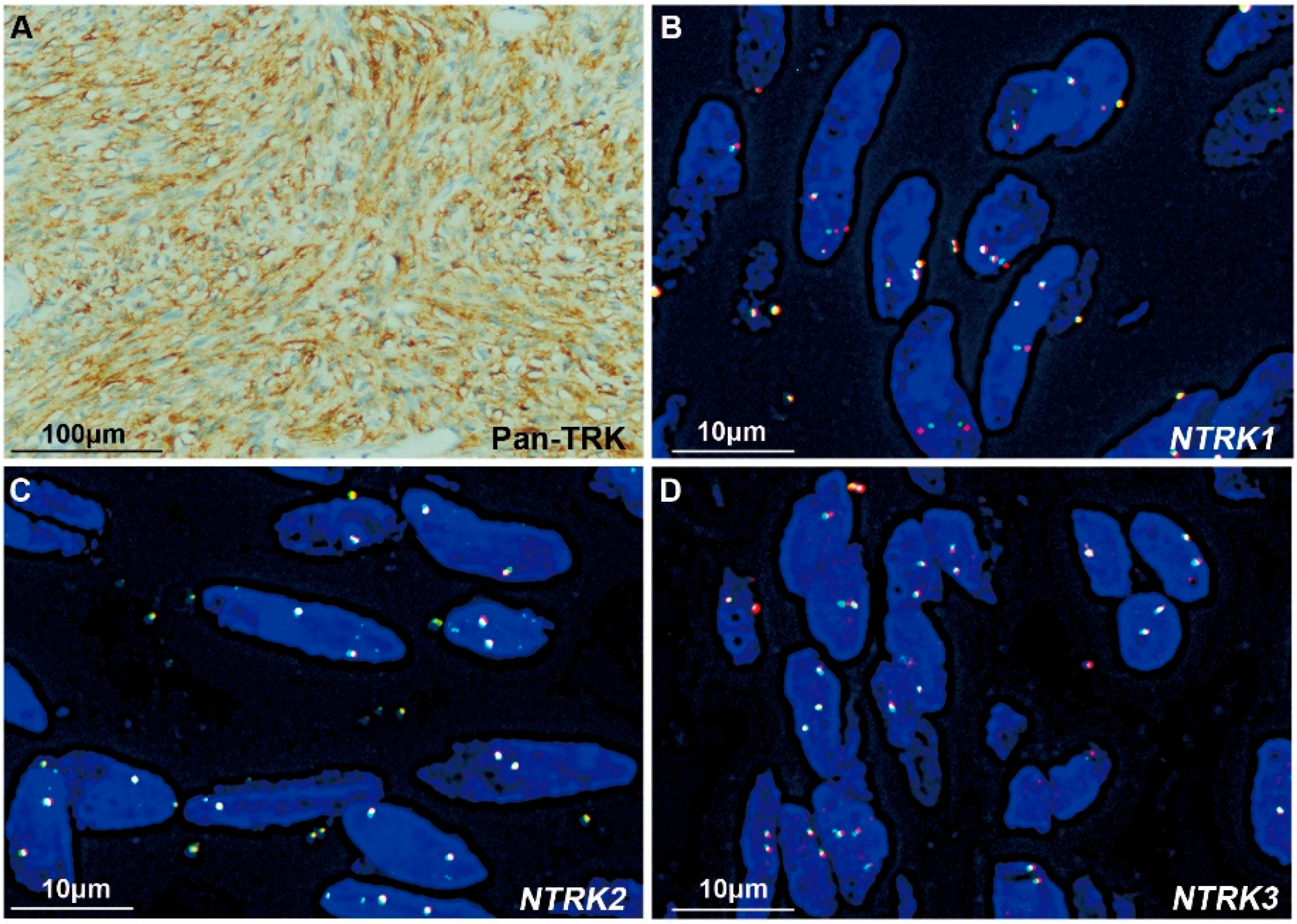
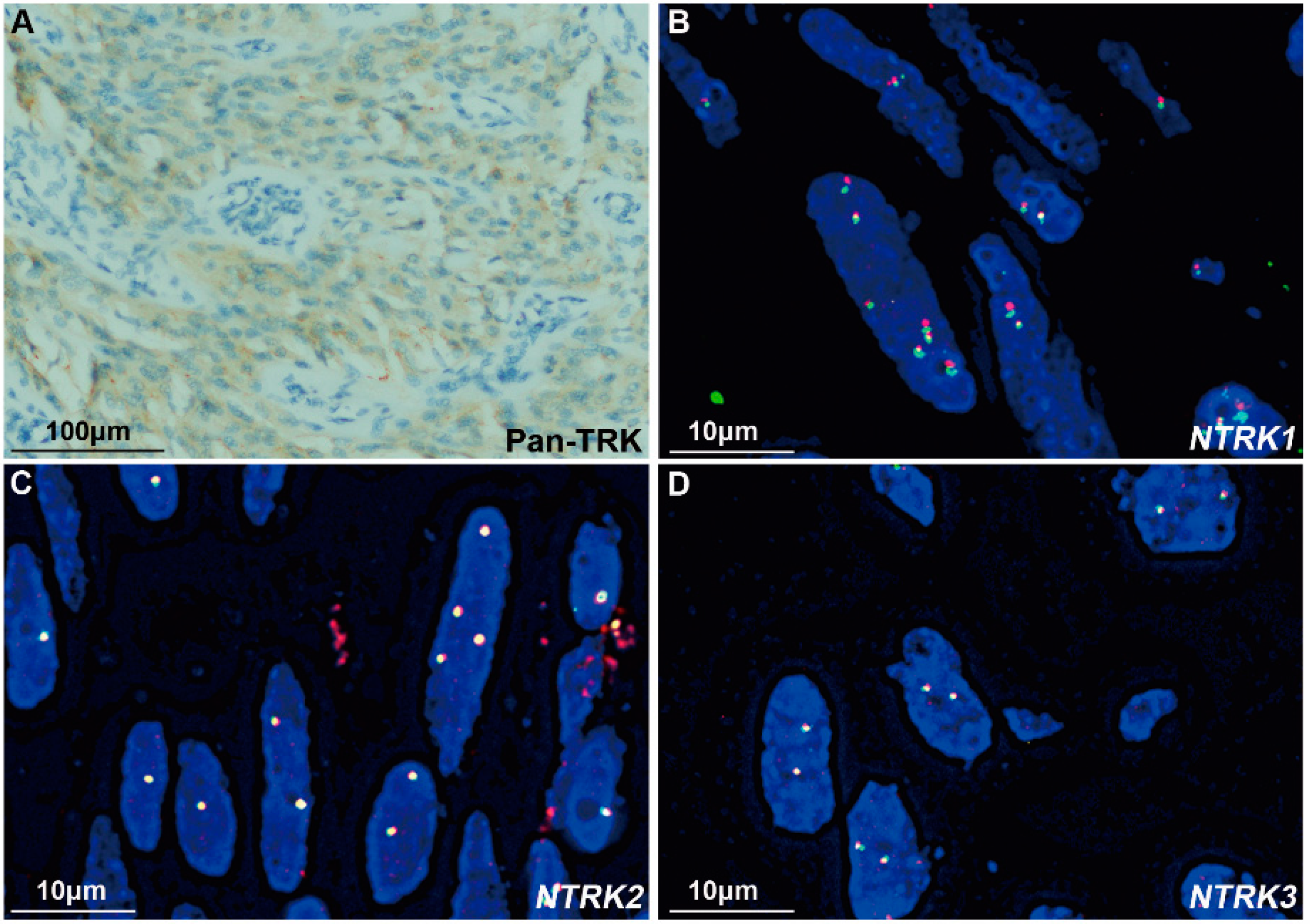
| Case | IHC for Pan-TRK | NGS | FISH | ||||
|---|---|---|---|---|---|---|---|
| Intensity | Positive Proportion | NTRK Fusions | ETV6-NTRK3 | NTRK3 | NTRK2 | NTRK1 | |
| #1 | Strong | 50% | ETV6-NTRK3 | Positive | Positive | Negative | Negative |
| #2 | Negative | 0 | ETV6-NTRK3 | Positive | NP | Negative | Negative |
| #3 | Weak-moderate | 70% | No a | NP | Negative | Negative | Negative |
| #4 | Strong | 80% | No | NP | Negative | Negative | Negative |
| #5 | Strong | 50% | No | NP | NA b | NA b | NA b |
| #6 | Weak-moderate | 30% | No | NP | Negative | Negative | Negative |
| #7 | Weak-moderate | 30% | No | NP | Negative | Negative | Negative |
| Case | Age (Years), Sex | Location/ Localized or Metastatic | Size (cm) | Morphology | MI (/5 mm²) | Immunohistochemistry | KIT/ PDGFRA Mutation | NTRK Fusion | Surgery | Drug Treatment | Progression/PFS (Months) | Status/OS (Months) | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD117 | DOG-1 | SDHB | PTRK | Type | NGS | FISH | ||||||||||||
| #1 | 52, F | Mesentery/ Localized | 10 | S | 8 | P | P | P | P | No | ETV6-NTRK3 | DNA-based | ETV6-NTRK3 | Yes | No | Yes/11 | Dead/11 | this study |
| #2 | 56, M | Stomach/ Localized | 16 | E | 3 | P | P | P | N | No | ETV6-NTRK3 | RNA-based | ETV6-NTRK3 | Yes | Imatinib | No/58 | Alive/58 | this study |
| #8 | 44, M | Rectum/NA | 5 | E | 34 | P | P | P | NA | No | ETV6-NTRK3 | RNA-based | ETV6 | Yes | No | No/44 | Alive/44 | [12] |
| #9 | 55, M | Small bowel/Metastatic | NA | NA | NA | NA | NA | P | NA | No | ETV6-NTRK3 | DNA-based | NA | No | Imatinib | Yes/NA a | Alive/159 | [14] |
| Sunitinib | ||||||||||||||||||
| Sorafenib | ||||||||||||||||||
| Nilotinib | ||||||||||||||||||
| Regorafenib Larotrectinib | ||||||||||||||||||
| #10 | 54, M | Colon/NA | NA | NA | NA | NA | NA | P | NA | No | ETV6-NTRK3 | DNA-based | NA | No | Imatinib Sunitinib Sorafenib Linsitinib | Yes/NA b | Alive/12 | [14] |
| #11 | 20, M | Rectum/ Localized | 7 | S,E | 10 | N | P | NA | NA | No | LMNA-NTRK1 | DNA-based | NA | Yes | No | No/7 | Alive/7 | [15] |
| #12 | 59, M | NA/NA | NA | E | NA | P | P | NA | N | No | ETV6-NTRK3 | No | NTRK3 and ETV6, respectively | No | No | No/0 | Dead/0 | [13] |
| #13 | 44, F | Rectum/NA | 2.8 | NA | 17 | P | NA | NA | Weakly positive in only one of the following five patients | No | NTRK1 | No | NTRK1 | Yes | No | Yes/108 | Dead/132 | [27] |
| #14 | 45, M | Duodenum/NA | 1.7 | NA | 1 | P | NA | NA | No | NTRK3 | No | NTRK3 | Yes | No | No/72 | Alive/72 | [27] | |
| #15 | 65, F | Stomach/NA | 17 | NA | 70 | P | NA | NA | No | NTRK1 | No | NTRK1 | Yes | Imatinib | Yes/26 | Dead/96 | [27] | |
| #16 | 61, F | Jejunum/NA | 3.9 | NA | 12 | P | NA | NA | No | NTRK1 | No | NTRK1 | Yes | Imatinib | No/48 | Alive/48 | [27] | |
| #17 | 43, M | Rectum/NA | 11 | NA | 0 | P | NA | NA | No | ETV6-NTRK3 | RNA-based | NTRK3 | Yes | No | No/36 | Alive/36 | [27] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Li, J.; Sun, L.; Xu, Z.; Ke, Y.; Shao, B.; Guo, Y.; Sun, Y. GISTs with NTRK Gene Fusions: A Clinicopathological, Immunophenotypic, and Molecular Study. Cancers 2023, 15, 105. https://doi.org/10.3390/cancers15010105
Cao Z, Li J, Sun L, Xu Z, Ke Y, Shao B, Guo Y, Sun Y. GISTs with NTRK Gene Fusions: A Clinicopathological, Immunophenotypic, and Molecular Study. Cancers. 2023; 15(1):105. https://doi.org/10.3390/cancers15010105
Chicago/Turabian StyleCao, Zi, Jiaxin Li, Lin Sun, Zanmei Xu, Yan Ke, Bing Shao, Yuhong Guo, and Yan Sun. 2023. "GISTs with NTRK Gene Fusions: A Clinicopathological, Immunophenotypic, and Molecular Study" Cancers 15, no. 1: 105. https://doi.org/10.3390/cancers15010105
APA StyleCao, Z., Li, J., Sun, L., Xu, Z., Ke, Y., Shao, B., Guo, Y., & Sun, Y. (2023). GISTs with NTRK Gene Fusions: A Clinicopathological, Immunophenotypic, and Molecular Study. Cancers, 15(1), 105. https://doi.org/10.3390/cancers15010105





