Simple Summary
The tumor microenvironment (TME) is the complex and heterogenous ecosystem of solid tumors known to influence their growth and their progression. Besides tumor cells, the TME comprises a variety of host-derived cell types, ranging from endothelial cells to fibroblasts and immune cells. Clinical and experimental data are converging to indicate that platelets, originally known for their fundamental hemostatic function, also participate in tumor development and shaping of the TME. Considering the abundance of antiplatelet drugs, understanding if and how platelets contribute to the TME may lead to new therapeutic tools for improved cancer prevention and treatments.
Abstract
The tumor microenvironment (TME) has gained considerable interest because of its decisive impact on cancer progression, response to treatment, and disease recurrence. The TME can favor the proliferation, dissemination, and immune evasion of cancer cells. Likewise, there is accumulating evidence that intratumoral platelets could favor the development and aggressiveness of solid tumors, notably by influencing tumor cell phenotype and shaping the vascular and immune TME components. Yet, in contrast to other tumor-associated cell types like macrophages and fibroblasts, platelets are still often overlooked as components of the TME. This might be due, in part, to a deficit in investigating and reporting the presence of platelets in the TME and its relationships with cancer characteristics. This review summarizes available evidence from clinical and animal studies supporting the notion that tumor-associated platelets are not incidental bystanders but instead integral and active components of the TME. A particular emphasis is given to the description of intratumoral platelets, as well as to the functional consequences and possible mechanisms of intratumoral platelet accumulation.
1. Introduction
The local ecosystem of tumors or tumor microenvironment (TME) has gained considerable interest because of its decisive role in maintaining a supportive niche for cancer cells. Among its numerous effects, the TME can indeed favor the proliferation, dissemination, and immune evasion of cancer cells, and can therefore impact therapy and disease recurrence. Components of the TME are highly heterogeneous and vary greatly with the cancer location, type, and stage, and can also be affected by chemotherapy [1,2]. The TME comprises an extracellular matrix, matricellular proteins, cytokines, and growth factors, as well as many different cell types, ranging from cancer cells themselves to a variety of non-malignant cells. Non-malignant cells of the TME include both nearby endogenous cells normally present in the affected tissue, such as fibroblasts and adipocytes, and cells recruited from distant sites, including immune cells and mesenchymal stem cells. Recent studies indicate that the TME also contains non-dividing supportive cells arising directly from cancer stem cells [3,4]. Tumor-associated macrophages (TAM), cancer-associated fibroblasts (CAFs), and vascular endothelial cells, together with their supporting pericytes, have been arguably among the most prominent and intensively scrutinized stromal cells of the TME [5,6,7]. Lymphocytes and adipocytes have also had their rightful share of attention [2,5,7,8]. On the contrary, although they have long been recognized to support hematogenous metastatic dissemination [9], platelets have been largely omitted from the TME equation.
Platelets are the second most numerous of the circulating blood cells. Historically known as the primary effector cells of haemostasis and thrombosis due to their ability to aggregate and promote coagulation, platelets are also recognized for their pivotal role in other processes, such as innate and adaptive immune responses [10]. Platelets are anucleated cells arising from the cytoplasmic fragmentation of megakaryocytes whose diameter of ~2–3 μm is considerably smaller than those of other circulating blood cells. Despite their small size and lack of nucleus, platelets are actually highly versatile cells whose functions far exceed the formation of blood clots. It is now well established that platelets regulate leukocyte recruitment and activation [11,12] and participate in angiogenesis [13,14,15], lymphangiogenesis [16,17,18], and tissue remodeling in general, as in wound healing [16,19,20], which all contribute to TME formation. The ability of platelets to exert such a wide range of effects is due to the fact that, in addition to their multiple receptors and interaction partners, platelets release numerous bioactive molecules stored in their secretion granules [21]. The lifespan of platelets is approximately 10 days in humans and 5 days in mice. In view of the time required for cancer to develop and spread representing years, this lifespan appears incompatible with a lengthy presence of platelets at the individual level in the tumor stroma. However, the continuous supply and renewal of platelets at a daily rate of 1011 platelets a day [22] is likely sufficient to enable a persistent presence of platelets in tumors. In the present review, we summarize and discuss available evidence that tumor-associated platelets (TAP) exist and are not incidental bystanders but instead represent integral and active components of the TME.
2. Clinical Evidence That Platelets Impact Tumor Progression and Response to Therapy
Evidence that cancers can impact platelets came very early and even preceded the discovery that platelets mediate thrombus formation, with the observation by Armand Trousseau in 1865 that patients with late-stage cancer frequently developed thrombotic complications [23]. Since then, this observation has been largely confirmed, with thrombosis representing the second leading cause of death in cancer patients, just behind infections [24]. Moreover, and more importantly with respect to the focus of the current review, it has become clear that influences between platelets and cancers go in both directions. Various correlations between platelet parameters and cancer prognosis have indeed been reported. Thrombocytosis, which is defined as a platelet count greater than 400 × 103 platelets/µL blood, has been found to be associated with shorter survival and poor prognosis in a variety of solid cancers, including lung [25], breast [26], kidney [27], glioblastoma [28], pancreatic [29], ovarian [30], and gastrointestinal [31] cancer. Remarkably, in all of these examples, the correlation of thrombocytosis with an adverse outcome was maintained in multivariate analyses, including adjustment for the clinical stage, thus making of an elevated platelet count an independent predictor of shorter survival. It is also important to note that a worsening of cancer prognosis in patients with thrombocytosis was not associated with an increased incidence of thromboembolism.
There is emerging evidence that, like platelet count, the plateletcrit (PCT, the volume occupied by platelets in blood) and the mean platelet volume (MPV) may also bear a prognostic value in some cancers. For instance, a more elevated preoperative plateletcrit was recently found to be an independent marker of poor prognosis in patients with resectable non-small cell lung cancer [32]. Regarding MPV, there are conflicting reports of either greater or lesser MPV being associated with cancer prognosis. While lower MPV was found to predict poor prognosis in renal cell carcinoma [33], pancreatic cancer [34], and bladder cancer [35], quite the opposite was found in colorectal [36] and gastric cancer [37], with elevated MPV instead being associated with decreased overall survival. These inconsistencies might be related to the type of cancer but also to the fact that MPV remains a controversial marker due to its lack of specificity. Indeed, variations in MPV can reflect different aspects of platelet biology, including platelet reactivity and activation status, but also age and turnover rate [38].
Several studies have indicated that, in addition to overall cancer progression and outcome, platelets can provide information on treatment response and recurrence risk. For instance, normalization of platelet counts following chemotherapy was found to be indicative of a good treatment response in patients with lung [39] or ovarian [40] cancer, while lack of platelet count normalization was associated with an increased risk of recurrence. Co-culture experiments of platelets with various human cancer cell lines have shown that by providing proliferative and survival signals, platelets can directly counteract the antiproliferative and cytotoxic action of several chemotherapeutic drugs, including gemcitabine [41], cisplatin [39], and docetaxel [30,40]. Thus, platelets may not just be markers of a response to chemotherapy but may directly impair it as well. Interestingly, results from recent studies suggest that platelets may participate in immune checkpoint interactions, whose inhibition has become a new standard adjuvant treatment for several cancers. Programmed death-ligand 1 (PD-L1), a prominent immune checkpoint protein expressed by antigen-presenting cells and by a variety of tumor cells, was detected on platelets of patients with PD-L1-positive tumors, but not on platelets from healthy individuals or from patients with PD-L1-negative tumors [42,43,44]. PD-L1-expressing platelets were found both in the circulation and within tumors, and possessed the ability to inhibit CD4 and CD8 T-cells [44], suggesting that they might favor PD-L1-mediated tumor immune evasion. Because levels of platelet PD-L1 were found to mirror PD-L1 expression in tumors [44], it was proposed that PD-L1 on platelets could help to identify patients who would benefit from inhibitors of the PD-1/PD-L1 axis, the main target of current checkpoint therapies. In support of this notion, high levels of platelet PD-L1 were found to predict a good response to anti-PD-1 antibodies in patients with PD-L1-positive lung cancer [44]. Besides its potential prognostic value, the expression of PD-L1 on platelets raises several questions. First, could anti-PD-L1 therapy cause thrombocytopenia or thrombosis via elimination or activation of PD-L1-positive platelets? With reports of up to more than 50% PD-L1-positive platelets in patients with head and neck squamous cell carcinoma or lung cancer [43,44], these issues may warrant attention when assessing the adverse effects of anti-immune checkpoint therapies. Another concern is whether PD-L1 on platelets could act as a decoy for binding of anti-PD-L1 therapeutic antibodies intended to target cancer cells.
Another line of evidence pointing to a contribution of platelets to tumor development and progression comes from the anticancer effects of antiplatelet drugs. Daily low dose acetylsalicylic acid (aspirin), an irreversible inhibitor of cyclooxygenases (COX-1 and COX-2), has been recommended since 2007 for primary prevention of colorectal cancer by the US Preventive Services Task Force [45]. Moreover, several studies suggest that another antiplatelet drug, clopidogrel, an antagonist of the purinergic P2Y12 receptor, also reduces the incidence of colorectal cancer [46]. Nevertheless, COX and P2Y12 are not specific to platelets and can also be expressed by other cell types, including various cancer cell types [47,48,49,50,51,52]. Therefore, one cannot exclude that part of the anticancer effects of antiplatelet drugs may be independent of platelets. However, there are strong arguments (reviewed in [53,54]) indicating that aspirin and clopidogrel do exert their anticancer effects through platelet inhibition. In particular, with respect to aspirin, its short half-life (20 min), combined with the fact that its anticancer effects have been observed at a low-dose sufficient to irreversibly and completely inhibit the activity of COX-1 in platelets but not that of renewable COX-2 in cancer cells or other nucleated cells, make a significant contribution due to non-platelet cells being unlikely.
3. Tumor Cell-Platelet Interactions: Mechanisms and Functional Consequences
The clinical evidence that platelets play a role in the thrombotic complications and progression of cancers has stimulated intensive research on the interactions and mechanisms that could underlie these effects. It is now well established that platelets and tumor cells are able to interact through paracrine and direct physical contacts [55]. Various ligand/receptor couples have been reported to mediate these contacts. For example, ADAM9 was identified as a counter receptor for platelet integrin α6β1 on two different types of mouse tumor cells [56], and galectin-3 and podoplanin as counter-receptors for platelet glycoprotein VI (GPVI) [57] and CLEC-2 [58], respectively. Cadherin-6, a marker and mediator of epithelial-mesenchymal transition (EMT), is also expressed by platelets [59] and was recently shown to support contacts between platelets and tumor cells via homophilic and heterotypic interactions [60]. Other platelet receptors such as GPIIb/IIIa [55], α2β1 integrin [61], and P-Selectin [62] have also been shown to support direct platelet-tumor cell interactions, but their exact ligands on tumor cells have not been identified to date.
Platelet-tumor cell interactions have multiple and bidirectional functional consequences. With respect to platelet function, these interactions have long been known to cause platelet activation and aggregation [63,64], an effect with obvious relevance not only to the adhesion and dissemination potential of cancer cells, but also to the risk of cancer-associated thrombosis initially described by Trousseau [23]. Nevertheless, it is worth noting that tumor-induced platelet responses do not univocally ultimately result in platelet aggregation. Recent data by Plantureux et al. indeed indicate that contacts between tumor cells and individual platelets can trigger microvesicle production by platelets without causing aggregation [60]. Likewise, in a much earlier electron microscopy study depicting the attachment of tumor cells to circulating platelets and vessel walls in vivo, Warren et al. reported that “the contact between platelets and tumor cells did not elicit a wave of platelet-platelet adhesion such as occurs when a white thrombus is formed” [65].
Many different non-exclusive pathways have been reported to participate in cancer-induced platelet activation. These pathways include both cell contact-dependent and -independent mechanisms. Excellent comprehensive reviews on this matter can be found elsewhere [66,67] and thus just a brief overview will be given here. Cancer-induced platelet activation can occur via the generation of small amounts of thrombin by tumor cells- or tumor microvesicle-expressed tissue factor [68,69,70], the secretion of ADP [68,70] or cathepsin B [71] by tumor cells, tumor cell-induced generation of neutrophil extracellular traps (NETs) [72,73], tumor cell-induced release of ADP and thromboxane A2 by platelets [63,70], or via direct engagement of platelet receptors with signaling properties such as CLEC-2 [58] and cadherin 6 [60] by tumor cell ligands.
As indicated by clinical observations, in addition to platelet activation, cancers can also enhance thrombopoiesis and influence platelet phenotype. To date, cancers have been shown to promote thrombopoiesis by at least 3 mechanisms: the production by tumor cells of interleukin 6 [74,75], that of granulocyte- and granulocyte-macrophage colony stimulating factors [76], and, possibly, of thrombopoietin [77,78]. One mechanism by which modifications of platelet phenotype occurs in cancers is the active and selective sequestration of cancer-derived secreted proteins such as VEGF-A and other growth factors by circulating platelets [14,79]. Transfer of proteins from cancer cells to platelets can also occur during transient direct cell-cell contacts, as was recently shown for the acquisition of functional PD-L1 by platelets from PD-L1-expressing tumor cells [44]. In addition to protein content, cancers have been shown to impact platelet mRNA content. Cancers can transfer mRNA to platelets through the emission of microvesicles by tumor cells [80,81] and might also alter the platelet transcriptome through modulation of platelet pre-mRNA splicing [81,82]. Interestingly, cancer-induced phenotypic alterations of platelets are not solely considered across the spectrum of their possible functional consequences, but also with respect to their prognostic potential as biomarkers of the presence of cancer or its progression [43,44,80,83].
While cancer can alter thrombopoiesis quantitatively and modify the cargo of mature circulating platelets, there is evidence that cancer can also alter the platelet transcriptome and proteome by acting at the megakaryocyte level. For instance, in 2010, Zaslavsky et al. showed that thrombospondin-1 mRNA levels were up-regulated in megakaryocytes of tumor-bearing mice [84]. Interestingly, thrombospondin-1 has emerged as a potential regulator of the TME that would play a role in tumor vessel growth and function, as well as in escape from innate and adaptive antitumor immunity [85]. Its upregulation in platelets in cancer thus resonates particularly well with a platelet contribution to the shaping of the TME. As discussed further below, there is mounting evidence that, like inflammation [86], solid cancers might trigger megakaryopoiesis programs distinct from the traditional ones.
With respect to cancer cells, the most reported and scrutinized consequence of their interactions with platelets is arguably the increased metastatic potential these interactions induce. Since the seminal study by Gasic et al. [9], numerous experimental studies in rats and mice using a variety of tumor cell lines have confirmed the ability of platelets to promote tumor foci formation in the lungs [55,56,57,58,62,87,88,89] and have extended this observation to other organs such as the liver [62,88,90] and kidneys [62]. Because the pro-metastatic potential of platelets was mostly demonstrated using models of hematogenous metastases relying on the injection of tumor cells directly into the bloodstream, it is noteworthy that it was also observed in models of spontaneous metastasis [56,57,89,90]. Platelets interacting with cancer cells promote their metastatic potential by stimulating their epithelial-mesenchymal transition (EMT) [60,61,91,92], by protecting them from NK cell antitumor immunity [93,94], and by enhancing their adhesiveness to the vessel wall and subsequent extravasation [57,65,95]. Apart from enhancing the metastatic potential of cancer cells, platelets can also influence cancer cell survival and proliferation via direct interactions and paracrine signaling. Platelets have been shown to promote the proliferation of various human and mouse cancer cell lines in vitro [96,97,98,99,100,101], an effect attributed in part to the release of growth factors such as transforming growth factor-β (TGF-β) by activated platelets [96,98,101]. Not all studies agree on the pro-proliferation effect of platelets on cancer cells. Several studies have described that, on the contrary, platelets can exert anti-proliferative and even cytotoxic effects on cancer cells in vitro [100,102,103,104,105]. These discrepancies regarding the impact of platelets on cancer cell proliferation and survival in vitro also apply to in vivo studies, as platelets were found to have no impact [87,99,106,107], a positive impact [74,96,108,109,110,111], or a negative [60,105,112] impact on primary tumor growth in mouse models of solid cancers.
Collectively, these data show that a large number of mechanisms can support platelet-cancer cell interactions, and that the resulting biological effects can vary greatly. In this context, it is worth stressing that the type of cancer cell [89,113,114], as well as the localization of platelet-cancer cell interactions (i.e., in the bloodstream or locally at the primary tumor site) [60], have both been identified as important determinants of the mechanisms and functional consequences of platelet-cancer cell interactions.
4. Intratumoral Platelets: Occurrence and Possible Origins
Intriguingly, although many studies have highlighted the possible mechanisms and consequences of direct contacts and paracrine communication between platelets and cancer cells, it remains unclear if and how such interactions occur within primary tumors and their microenvironment. There is little if not no doubt that platelets and cancer cells interact closely together once cancer cells have entered the bloodstream, with the formation of tumor cell/platelet aggregates having been detected in models of experimental metastasis [65,115,116]. The lack of reports of such interactions in cancer patients can be easily explained by the scarcity of circulating tumor cells and the technical difficulties involved in detecting and isolating them [117].
In contrast to interactions in the bloodstream, evidence of interactions between platelets and cancer cells at the primary tumor site is scarcer. Yet, over the last 10 years, several experimental and clinical studies have provided data on tumor-infiltrating platelets (a list of studies describing intratumor platelets is given in Table 1).

Table 1.
Clinical and preclinical reports of intratumoral platelet occurrence.
In 2012, Stone et al. reported the presence of platelets in the tumor perivascular and extravascular compartments in a mouse model of ovarian cancer [74]. Further observations of intravascular, perivascular, and extravascular platelets within tumors have since been made in mouse models of colorectal and brain cancer [60,110,130]. In humans, intravascular and extravascular platelets have also been found in the stroma of various types of solid tumors, including breast, lung, pancreatic, gastric, and colorectal adenocarcinoma [118,119,122,127,128,129] (Figure 1).
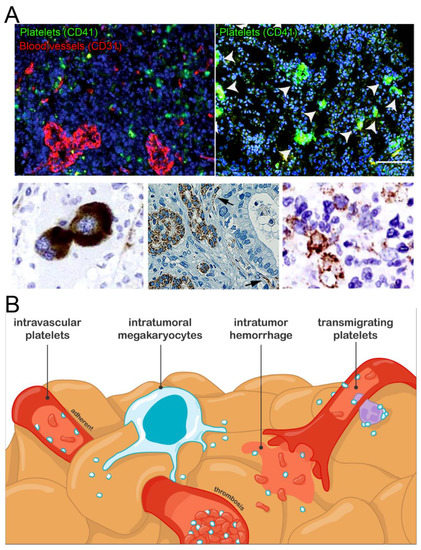
Figure 1.
Intratumoral platelets: occurrence and possible origins. (A). Images of the different types of intratumoral platelet localization and interactions reported: perivascular aggregates (upper left panel, mouse ovarian cancer), aggregates with tumor cells (upper right panel: mouse glioma, platelet aggregates are indicated by white arrows, scale bar = 100 µm; bottom right panel: colorectal cancer), intravascular platelets (black arrows, lower middle panel: human pancreatic cancer), and intratumor megakaryocytes (lower left panel: human renal cell carcinoma). Adapted with permission from Refs. [109,119,126,130,131]. Copyright 2016, American Society for Clinical Investigation; Copyright 2018, Society of Surgical Oncology; Copyright 2021, UICC; Copyright 2019, American Society of Hematology; Copyright 2014, Elsevier. (B). Schematic representation of the possible mechanisms supporting intratumoral platelets accumulation.
Although the studies and information on tumor-infiltrating platelets and their impact on and relation to cancer progression in humans are still limited, currently available data suggest that the presence of platelets in the tumor stroma is unlikely to be incidental. In fact, tumor-infiltrating platelets have been shown to be associated with the advanced stage of colorectal cancer [127], expression of EMT markers in pancreatic, and breast cancer [118,128], as wells as with chemoresistance and poor overall survival in breast, gastric, and pancreatic cancer [119,120,122,128]. These data, together with the observation that platelets are preferentially localized at the invasive front of pancreatic and breast tumors [118,128], suggest that, like cancer-associated thrombocytosis, the occurrence of tumor-infiltrating platelets may be indicative of an aggressive cancer phenotype.
How do platelets end up in the extravascular tumor microenvironment? One mechanism by which platelets can reach the extravascular space and interact directly with cancer cells is through the occurrence of intratumoral bleeding. Indeed, spontaneous intratumoral bleeding, related to tumor angiogenesis or to tumoral invasion, occurs in a variety of cancers [132,133,134,135,136]. Whether sporadic intratumoral bleeding events are sufficient to ensure a continuous presence of platelets in the tumor stroma is, however, uncertain. Nonetheless, even if they are spatially scattered and episodic during the course of cancer progression, platelet-cancer cell interactions subsequent to intratumoral bleeding may be sufficient to cause consequential phenotypic changes in cancer cells.
Reports of extravasated platelets found in the absence of bleeding in various inflamed organs [137], including experimental tumors [60,109,124,130], suggest that platelets may also access the extravascular tumor stroma via transmigration, either directly, or through the association with transmigrating leukocytes. The fact that platelet-specific deficiency in focal adhesion kinase [109], a protein known for its role in cell adhesion and migration, or deficiency in P-Selectin [124], a protein central to platelet-leukocyte interactions, is associated with reduced platelet deposition within the microenvironment of experimental tumors argues in favor of this possibility. However, it should be noted that, although the ability of platelets to migrate within the intravascular compartment was recently demonstrated [138,139], direct observation of active transmigration or of platelets migrating in the extravascular space has yet to be provided.
It has become clear over the last years that adaptative megakaryopoiesis programs can be triggered in inflammatory conditions [86,140]. In addition, extramedullary hematopoiesis has been shown to occur in various solid tumors [131,141,142,143,144]. Therefore, apart from blood-borne platelets, a subset of intratumoral platelets may also originate from local production programs, as suggested by the detection of megakaryocytes within the tumor stroma of patients with brain, hepatic, renal, or breast cancer [131,142,143,144] (Figure 1).
Along with platelets infiltrating the extravascular tumor stroma, platelets interacting with the tumor vasculature likely account for a substantial fraction of intratumoral platelets. Indeed, inflammation and angiogenesis are constitutive features of solid cancers, and platelets are now known to continuously interact with and accumulate in blood vessels at sites of inflammation [138,145] and angiogenesis [13]. Furthermore, platelet accumulation in the tumor vasculature can also occur through intratumoral thrombosis [58,146,147]. Finally, circulating platelets may also interact directly with cancer cells at sites of vascular mimicry, which corresponds to areas where cancer cells organize themselves into vascular channels to supply blood independently of endothelial cells [148].
5. Shaping of the Tumor Microenvironment by Platelets
5.1. Platelets and Tumor Angiogenesis and Vascular Integrity
Studies in animal models of solid tumors strongly indicate that the functional relevance of intratumoral platelets exceeds the regulation of cancer cell phenotype, proliferation, or survival. There is converging data in favor of a role of intratumoral platelets in shaping the TME, in particular its vascular compartment. Platelets are well-known for containing a variety of angiogenic factors in their alpha granules and have been shown to participate in angiogenesis in a variety of experimental settings, including models of solid tumors [13,74,107,149,150]. The involvement of platelets in tumor angiogenesis ranges from stimulating the proliferation of endothelial cells [99,150], promoting the recruitment of pericytes [74,88,107] and that of bone marrow-derived cells [149], to maintaining tumor vessel function and integrity [88,107,108,151,152]. As a result of these activities, depletion of platelets or targeting of their activation receptors has been shown to result in reduced tumor vessel density [74,107,149], maturation [74,88,107], and functionality [58,88,107,108,151,152]. Thus, experimental studies indicate that platelets regulate tumor angiogenesis not only quantitatively but also qualitatively, notably by continuously preventing tumor vessel leakage and bleeding [88,107,108,151,152]. The latter supportive functions of platelets towards tumor vessel integrity emphasize the importance of the physical presence of platelets within tumors for regulating the TME. Indeed, the stabilization of both angiogenic and inflamed vessels by platelets requires direct contacts between platelets and such vessels [13,145]. Interestingly, several studies in mouse models of solid cancers are converging to suggest that targeting the vasculoprotective function of platelets in tumors can enhance the intratumor delivery and antitumor effects of chemotherapeutic drugs such as paclitaxel via the induction of tumor vascular leakiness [106,108,153].
Despite evidence from experimental models, if and how tumor platelet content correlates with tumor angiogenesis in cancer patients has not been investigated. Additionally, it is worth mentioning that platelet depletion had no impact on tumor vessel density in a mouse model of glioblastoma [99], which suggests that the contribution of platelets to tumor angiogenesis may vary with the cancer type.
5.2. Platelets and Tumor Lymphangiogenesis
Platelets are now well-established actors of lymphangiogenesis. They ensure proper separation of blood and lymphatic vessels during development, notably by regulating the proliferation, migration, and tube formation of lymphatic endothelial cells (LECs) through engagement of their CLEC-2 receptor by podoplanin on LECs [17,125]. Platelet CLEC-2 is also required for the development and maintenance of lymph nodes [154]. Remarkably, platelets are not required for maintaining the mature blood and lymphatic systems separated in the absence of challenges post-development [155]. However, recent studies in mice have indicated that, upon vascular remodeling, such as during wound-healing or in the TME, platelets again intervene in lymphangiogenesis by stimulating lymphatic growth via the secretion of VEGF-C [16] and by preventing the mixing of lymphatic and blood circulations through the engagement of CLEC-2 [155]. Considering that lymphatics provide routes for the dissemination of cancer cells, these data suggest that platelets may promote metastasis not only via their direct interactions with cancer cells but also through shaping the TME and giving cancer cells access to regional lymph nodes. The description of a positive correlation between intratumoral platelet content and both lymphatic vessel density and lymphovascular invasion in human esophageal cancer supports this hypothesis [125].
5.3. Platelets and the Tumor Immune Microenvironment
In addition to their role in tumor blood and lymphatic network formation, platelets may potentially also participate in setting up the immune component of the TME. Platelets indeed regulate the infiltration and functions of leukocytes in many inflammatory diseases and conditions [12,156]. Furthermore, platelets were found to recruit neutrophils to form early metastatic niches in the lungs of mice injected intravenously with various types of tumor cells [157]. Intriguingly, however, there are very few reports linking platelets to tumor immune cell content. Among the sparse available data, induction of acute thrombocytopenia was shown to have no significant impact on neutrophil and macrophage infiltration into the stroma of subcutaneously implanted Lewis lung carcinoma tumors in mice [152]. In contrast to these results, there is evidence supporting the role of platelets in controlling intratumor T cell infiltration. As mentioned above, functional PD-L1 was found on platelets of patients with PD-L1-positive lung cancers [44]. Interestingly, high levels of PD-L1 on platelets were associated with lower numbers of infiltrating T cells in the TME [44]. In agreement with these results, in mice, platelet depletion caused an increase in T cells in the TME of a colon adenocarcinoma model, an effect that could be reverted by transfusion of PD-L1-positive but not PD-L1-negative platelets [111]. Thus, together, these clinical and experimental results suggest a possible role of platelet PD-L1 in tumor immune evasion [111].
Finally, the observation of platelets interacting with podoplanin-expressing CAF in pancreatic cancer [121] or in peritoneal metastasis of gastric cancer [123], suggests that platelets may influence the activities of other major actors of the TME.
6. Conclusions
Despite accumulating evidence that platelets can impact the development and aggressiveness of solid tumors, a more systematic assessment of their presence in the TME and of how it relates to cancer characteristics and other TME components appears necessary. Such investigations could help to improve our fundamental understanding of the platelet-cancer crosstalk and enable the development of new diagnostic, prognostic, and therapeutic tools for solid cancers. In addition, they should allow a better appreciation of the cancer type-specific aspects of the platelet actions towards solid tumors, as hinted by earlier reports on mouse models. Yet, despite the current blind spots in our knowledge of intratumoral platelets, they should now be recognized as integral components of the TME, at least for their contribution to tumor vasculature formation and stabilization.
Author Contributions
O.L.C. and B.H.-T.-N. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank Mary Osborne-Pellegrin for her critical reading and help in editing this manuscript.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Runa, F.; Hamalian, S.; Meade, K.; Shisgal, P.; Gray, P.C.; Kelber, J.A. Tumor Microenvironment Heterogeneity: Challenges and Opportunities. Curr. Mol. Biol. Rep. 2017, 3, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Ruffell, B.; Au, A.; Rugo, H.S.; Esserman, L.J.; Hwang, E.S.; Coussens, L.M. Leukocyte Composition of Human Breast Cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 2796–2801. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Sanchez-Rivera, F.J.; Cetinbas, N.M.; Wu, K.; Joshi, N.S.; Helenius, K.; Park, Y.; Azimi, R.; Kerper, N.R.; Wesselhoeft, R.A.; et al. A Wnt-Producing Niche Drives Proliferative Potential and Progression in Lung Adenocarcinoma. Nature 2017, 545, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Ibaseta, A.; Fischer, M.M.; Cancilla, B.; O’Young, G.; Cristea, S.; Luca, V.C.; Yang, D.; Jahchan, N.S.; Hamard, C.; et al. Intratumoural Heterogeneity Generated by Notch Signalling Promotes Small-Cell Lung Cancer. Nature 2017, 545, 360–364. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- López de Andrés, J.; Griñán-Lisón, C.; Jiménez, G.; Marchal, J.A. Cancer Stem Cell Secretome in the Tumor Microenvironment: A Key Point for an Effective Personalized Cancer Treatment. J. Hematol. Oncol. 2020, 13, 136. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef] [PubMed]
- Pallegar, N.K.; Christian, S.L. Adipocytes in the Tumour Microenvironment. Adv. Exp. Med. Biol. 2020, 1234, 1–13. [Google Scholar] [CrossRef]
- Gasic, G.J.; Gasic, T.B.; Stewart, C.C. Antimetastatic Effects Associated with Platelet Reduction. Proc. Natl. Acad. Sci. USA 1968, 61, 46–52. [Google Scholar] [CrossRef]
- Xu, X.R.; Zhang, D.; Oswald, B.E.; Carrim, N.; Wang, X.; Hou, Y.; Zhang, Q.; Lavalle, C.; McKeown, T.; Marshall, A.H.; et al. Platelets Are Versatile Cells: New Discoveries in Hemostasis, Thrombosis, Immune Responses, Tumor Metastasis and Beyond. Crit. Rev. Clin. Lab. Sci. 2016, 53, 409–430. [Google Scholar] [CrossRef]
- Bourne, J.H.; Beristain-Covarrubias, N.; Zuidscherwoude, M.; Campos, J.; Di, Y.; Garlick, E.; Colicchia, M.; Terry, L.V.; Thomas, S.G.; Brill, A.; et al. CLEC-2 Prevents Accumulation and Retention of Inflammatory Macrophages During Murine Peritonitis. Front. Immunol. 2021, 12, 693974. [Google Scholar] [CrossRef] [PubMed]
- Gros, A.; Ollivier, V.; Ho-Tin-Noé, B. Platelets in Inflammation: Regulation of Leukocyte Activities and Vascular Repair. Front. Immunol. 2015, 6, 678. [Google Scholar] [CrossRef] [PubMed]
- Kisucka, J.; Butterfield, C.E.; Duda, D.G.; Eichenberger, S.C.; Saffaripour, S.; Ware, J.; Ruggeri, Z.M.; Jain, R.K.; Folkman, J.; Wagner, D.D. Platelets and Platelet Adhesion Support Angiogenesis While Preventing Excessive Hemorrhage. Proc. Natl. Acad. Sci. USA 2006, 103, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Klement, G.L.; Yip, T.T.; Cassiola, F.; Kikuchi, L.; Cervi, D.; Podust, V.; Italiano, J.E.; Wheatley, E.; Abou-Slaybi, A.; Bender, E.; et al. Platelets Actively Sequester Angiogenesis Regulators. Blood 2009, 113, 2835–2842. [Google Scholar] [CrossRef]
- Walsh, T.G.; Metharom, P.; Berndt, M.C. The Functional Role of Platelets in the Regulation of Angiogenesis. Platelets 2015, 26, 199–211. [Google Scholar] [CrossRef]
- Lim, L.; Bui, H.; Farrelly, O.; Yang, J.; Li, L.; Enis, D.; Ma, W.; Chen, M.; Oliver, G.; Welsh, J.D.; et al. Hemostasis Stimulates Lymphangiogenesis through Release and Activation of VEGFC. Blood 2019, 134, 1764–1775. [Google Scholar] [CrossRef]
- Osada, M.; Inoue, O.; Ding, G.; Shirai, T.; Ichise, H.; Hirayama, K.; Takano, K.; Yatomi, Y.; Hirashima, M.; Fujii, H.; et al. Platelet Activation Receptor CLEC-2 Regulates Blood/Lymphatic Vessel Separation by Inhibiting Proliferation, Migration, and Tube Formation of Lymphatic Endothelial Cells. J. Biol. Chem. 2012, 287, 22241–22252. [Google Scholar] [CrossRef]
- Bertozzi, C.C.; Hess, P.R.; Kahn, M.L. Platelets: Covert Regulators of Lymphatic Development. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2368–2371. [Google Scholar] [CrossRef]
- Wichaiyo, S.; Lax, S.; Montague, S.J.; Li, Z.; Grygielska, B.; Pike, J.A.; Haining, E.J.; Brill, A.; Watson, S.P.; Rayes, J. Platelet Glycoprotein VI and C-Type Lectin-like Receptor 2 Deficiency Accelerates Wound Healing by Impairing Vascular Integrity in Mice. Haematologica 2019, 104, 1648–1660. [Google Scholar] [CrossRef]
- Levoux, J.; Prola, A.; Lafuste, P.; Gervais, M.; Chevallier, N.; Koumaiha, Z.; Kefi, K.; Braud, L.; Schmitt, A.; Yacia, A.; et al. Platelets Facilitate the Wound-Healing Capability of Mesenchymal Stem Cells by Mitochondrial Transfer and Metabolic Reprogramming. Cell Metab. 2021, 33, 283–299.e9. [Google Scholar] [CrossRef]
- Manne, B.K.; Xiang, S.C.; Rondina, M.T. Platelet Secretion in Inflammatory and Infectious Diseases. Platelets 2017, 28, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Giannini, S.; Falet, H.; Hoffmeister, K. Platelet Glycobiology and the Control of Platelet Function and Lifespan. In Platelets; Academic Press: Cambridge, UK, 2019; pp. 79–97. ISBN 9780128134566. [Google Scholar]
- Trousseau, A. Phlegmatia Alba Dolens. In Clinique Médicale de l’Hotel-Dieu de Paris; J.-B. Baillière et Fils: Paris, France, 1865; Volume 3, pp. 654–712. [Google Scholar]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Thromboembolism Is a Leading Cause of Death in Cancer Patients Receiving Outpatient Chemotherapy. J. Thromb. Haemost. 2007, 5, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Møller Pedersen, L.; Milman, N. Prognostic Significance of Thrombocytosis in Patients with Primary Lung Cancer. Eur. Respir. J. 1996, 9, 1826–1830. [Google Scholar] [CrossRef] [PubMed]
- Taucher, S.; Salat, A.; Gnant, M.; Kwasny, W.; Mlineritsch, B.; Menzel, R.C.; Schmid, M.; Smola, M.G.; Stierer, M.; Tausch, C.; et al. Impact of Pretreatment Thrombocytosis on Survival in Primary Breast Cancer. Thromb. Haemost. 2003, 89, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Symbas, N.P.; Townsend, M.F.; El-Galley, R.; Keane, T.E.; Graham, S.D.; Petros, J.A. Poor Prognosis Associated with Thrombocytosis in Patients with Renal Cell Carcinoma. BJU Int. 2000, 86, 203–207. [Google Scholar] [CrossRef]
- Brockmann, M.A.; Giese, A.; Mueller, K.; Kaba, F.J.; Lohr, F.; Weiss, C.; Gottschalk, S.; Nolte, I.; Leppert, J.; Tuettenberg, J.; et al. Preoperative Thrombocytosis Predicts Poor Survival in Patients with Glioblastoma. Neuro. Oncol. 2007, 9, 335–342. [Google Scholar] [CrossRef]
- Sarma, D.; Kim, S.Y.; Henry, D.H. Assessing a Prognostic Model for Predicting VTE Occurrence in Cancer Patients. J. Clin. Oncol. 2012, 30, 1577. [Google Scholar] [CrossRef]
- Bottsford-Miller, J.; Choi, H.J.; Dalton, H.J.; Stone, R.L.; Cho, M.S.; Haemmerle, M.; Nick, A.M.; Pradeep, S.; Zand, B.; Previs, R.A.; et al. Differential Platelet Levels Affect Response to Taxane-Based Therapy in Ovarian Cancer. Clin. Cancer Res. 2015, 21, 602–610. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. Thrombocytosis as a Prognostic Marker in Gastrointestinal Cancers. World J. Gastrointest. Oncol. 2014, 6, 34–40. [Google Scholar] [CrossRef]
- Hur, J.Y.; Lee, H.Y.; Chang, H.J.; Choi, C.W.; Kim, D.H.; Eo, W.K. Preoperative Plateletcrit Is a Prognostic Biomarker for Survival in Patients with Non-Small Cell Lung Cancer. J. Cancer 2020, 11, 2800–2807. [Google Scholar] [CrossRef]
- Yun, Z.Y.; Zhang, X.; Liu, Y.S.; Liu, T.; Liu, Z.P.; Wang, R.T.; Yu, K.J. Lower Mean Platelet Volume Predicts Poor Prognosis in Renal Cell Carcinoma. Sci. Rep. 2017, 7, 6700. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Ge, X.X.; Wu, J.; Gong, F.R.; Wu, M.Y.; Xu, M.D.; Lian, L.; Wang, W.J.; Li, W.; Tao, M. Prognostic Evaluation of Resectable Colorectal Cancer Using Platelet-Associated Indicators. Oncol. Lett. 2019, 18, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cui, M.M.; Xu, Y.; Liu, L.; Niu, Y.; Liu, T.; Liu, Z.P.; Wang, R.T.; Yu, K.J. Decreased Mean Platelet Volume Predicts Poor Prognosis in Invasive Bladder Cancer. Oncotarget 2017, 8, 68115–68122. [Google Scholar] [CrossRef]
- Li, N.; Yu, Z.; Zhang, X.; Liu, T.; Sun, Y.X.; Wang, R.T.; Yu, K.J. Elevated Mean Platelet Volume Predicts Poor Prognosis in Colorectal Cancer. Sci. Rep. 2017, 7, 10261. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.M.; Xia, Y.Y.; Lian, L.; Zhou, C.; Li, X.L.; Han, S.G.; Zheng, Y.; Gong, F.R.; Tao, M.; Mao, Z.Q.; et al. Mean Platelet Volume Provides Beneficial Diagnostic and Prognostic Information for Patients with Resectable Gastric Cancer. Oncol. Lett. 2016, 12, 2501–2506. [Google Scholar] [CrossRef]
- Handtke, S.; Thiele, T. Large and Small Platelets—(When) Do They Differ? J. Thromb. Haemost. 2020, 18, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fang, M.; Li, J.; Yang, R.; Du, J.; Luo, Y. High Platelet Levels Attenuate the Efficacy of Platinum-Based Treatment in Non-Small Cell Lung Cancer. Cell. Physiol. Biochem. 2018, 48, 2456–2469. [Google Scholar] [CrossRef]
- Hu, Q.; Hada, A.; Han, L. Platelet Count as a Biomarker for Monitoring Treatment Response and Disease Recurrence in Recurrent Epithelial Ovarian Cancer. J. Ovarian Res. 2020, 13, 78. [Google Scholar] [CrossRef]
- Elaskalani, O.; Falasca, M.; Moran, N.; Berndt, M.C.; Metharom, P. The Role of Platelet-Derived ADP and ATP in Promoting Pancreatic Cancer Cell Survival and Gemcitabine Resistance. Cancers 2017, 9, 142. [Google Scholar] [CrossRef]
- Darga, E.P.; Dolce, E.M.; Fang, F.; Kidwell, K.M.; Gersch, C.L.; Kregel, S.; Thomas, D.G.; Gill, A.; Brown, M.E.; Gross, S.; et al. PD-L1 Expression on Circulating Tumor Cells and Platelets in Patients with Metastatic Breast Cancer. PLoS ONE 2021, 16, e0260124. [Google Scholar] [CrossRef]
- Rolfes, V.; Idel, C.; Pries, R.; Plötze-Martin, K.; Habermann, J.; Gemoll, T.; Bohnet, S.; Latz, E.; Ribbat-Idel, J.; Franklin, B.S.; et al. PD-L1 Is Expressed on Human Platelets and Is Affected by Immune Checkpoint Therapy. Oncotarget 2018, 9, 27460–27470. [Google Scholar] [CrossRef] [PubMed]
- Hinterleitner, C.; Strähle, J.; Malenke, E.; Hinterleitner, M.; Henning, M.; Seehawer, M.; Bilich, T.; Heitmann, J.; Lutz, M.; Mattern, S.; et al. Platelet PD-L1 Reflects Collective Intratumoral PD-L1 Expression and Predicts Immunotherapy Response in Non-Small Cell Lung Cancer. Nat. Commun. 2021, 12, 7005. [Google Scholar] [CrossRef] [PubMed]
- Chubak, J.; Whitlock, E.P.; Williams, S.B.; Kamineni, A.; Burda, B.U.; Buist, D.S.M.; Anderson, M.L. Aspirin for the Prevention of Cancer Incidence and Mortality: Systematic Evidence Reviews for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2016, 164, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Miguel, A.; García-Rodríguez, L.A.; Gil, M.; Montoya, H.; Rodríguez-Martín, S.; de Abajo, F.J. Clopidogrel and Low-Dose Aspirin, Alone or Together, Reduce Risk of Colorectal Cancer. Clin. Gastroenterol. Hepatol. 2019, 17, 2024–2033.e2. [Google Scholar] [CrossRef] [PubMed]
- Elaskalani, O.; Domenchini, A.; Razak, N.B.A.; Dye, D.E.; Falasca, M.; Metharom, P. Antiplatelet Drug Ticagrelor Enhances Chemotherapeutic Efficacy by Targeting the Novel P2Y12-AKT Pathway in Pancreatic Cancer Cells. Cancers 2020, 12, 250. [Google Scholar] [CrossRef]
- Ballerini, P.; Dovizio, M.; Bruno, A.; Tacconelli, S.; Patrignani, P. P2Y12 Receptors in Tumorigenesis and Metastasis. Front. Pharmacol. 2018, 9, 66. [Google Scholar] [CrossRef]
- Pannunzio, A.; Coluccia, M. Cyclooxygenase-1 (COX-1) and COX-1 Inhibitors in Cancer: A Review of Oncology and Medicinal Chemistry Literature. Pharmaceuticals 2018, 11, 101. [Google Scholar] [CrossRef]
- Hashemi Goradel, N.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in Cancer: A Review. J. Cell. Physiol. 2019, 234, 5683–5699. [Google Scholar] [CrossRef]
- Czajkowski, R.; Lei, L.; Saba, P.; Barańska, J. ADP-Evoked Phospholipase C Stimulation and Adenylyl Cyclase Inhibition in Glioma C6 Cells Occur through Two Distinct Nucleotide Receptors, P2Y1 and P2Y12. FEBS Lett. 2002, 513, 179–183. [Google Scholar] [CrossRef]
- Jin, J.; Tomlinson, W.; Kirk, I.P.; Kim, Y.B.; Humphries, R.G.; Kunapuli, S.P. The C6-2B Glioma Cell P2Y(AC) Receptor Is Pharmacologically and Molecularly Identical to the Platelet P2Y(12) Receptor. Br. J. Pharmacol. 2001, 133, 521–528. [Google Scholar] [CrossRef]
- Xu, X.R.; Yousef, G.M.; Ni, H. Cancer and Platelet Crosstalk: Opportunities and Challenges of Aspirin and Other Antiplatelet Agents. Blood 2018, 131, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberger, L.M.; Vijayan, K.V. Are Platelets the Primary Target of Aspirin’s Remarkable Anticancer Activity? Cancer Res. 2019, 79, 3820–3823. [Google Scholar] [CrossRef] [PubMed]
- Karpatkin, S.; Pearlstein, E.; Ambrogio, C.; Coller, B.S. Role of Adhesive Proteins in Platelet Tumor Interaction in Vitro and Metastasis Formation in Vivo. J. Clin. Investig. 1988, 81, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Mammadova-Bach, E.; Zigrino, P.; Brucker, C.; Bourdon, C.; Freund, M.; De Arcangelis, A.; Abrams, S.I.; Orend, G.; Gachet, C.; Mangin, P.H. Platelet Integrin A6β1 Controls Lung Metastasis through Direct Binding to Cancer Cell–Derived ADAM9. JCI Insight 2016, 1, e88245. [Google Scholar] [CrossRef]
- Mammadova-Bach, E.; Gil-Pulido, J.; Sarukhanyan, E.; Burkard, P.; Shityakov, S.; Schonhart, C.; Stegner, D.; Remer, K.; Nurden, P.; Nurden, A.T.; et al. Platelet Glycoprotein VI Promotes Metastasis through Interaction with Cancer Cell–Derived Galectin-3. Blood 2020, 135, 1146–1160. [Google Scholar] [CrossRef]
- Shirai, T.; Inoue, O.; Tamura, S.; Tsukiji, N.; Sasaki, T.; Endo, H.; Satoh, K.; Osada, M.; Sato-Uchida, H.; Fujii, H.; et al. C-Type Lectin-like Receptor 2 Promotes Hematogenous Tumor Metastasis and Prothrombotic State in Tumor-Bearing Mice. J. Thromb. Haemost. 2017, 15, 513–525. [Google Scholar] [CrossRef]
- Dunne, E.; Spring, C.M.; Reheman, A.; Jin, W.; Berndt, M.C.; Newman, D.K.; Newman, P.J.; Ni, H.; Kenny, D. Cadherin 6 Has a Functional Role in Platelet Aggregation and Thrombus Formation. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1724–1731. [Google Scholar] [CrossRef]
- Plantureux, L.; Mege, D.; Crescence, L.; Carminita, E.; Robert, S.; Cointe, S.; Brouilly, N.; Ezzedine, W.; Dignat-George, F.; Dubois, C.; et al. The Interaction of Platelets with Colorectal Cancer Cells Inhibits Tumor Growth but Promotes Metastasis. Cancer Res. 2020, 80, 291–303. [Google Scholar] [CrossRef]
- Zuo, X.X.; Yang, Y.; Zhang, Y.; Zhang, Z.G.; Wang, X.F.; Shi, Y.G. Platelets Promote Breast Cancer Cell MCF-7 Metastasis by Direct Interaction: Surface Integrin A2β1-Contacting-Mediated Activation of Wnt-β-Catenin Pathway. Cell Commun. Signal. 2019, 17, 142. [Google Scholar] [CrossRef]
- Kim, Y.J.; Borsig, L.; Varki, N.M.; Varki, A. P-Selectin Deficiency Attenuates Tumor Growth and Metastasis. Proc. Natl. Acad. Sci. USA 1998, 95, 9325–9330. [Google Scholar] [CrossRef]
- Ugen, K.K.; Mahalingam, M.; Klein, P.A.; Kao, K.-J. Inhibition of Tumor Cell-Induced Platelet Aggregation and Experimental Tumor Metastasis by the Synthetic Gly-Arg-Gly-Asp-Ser Peptide. JNCI J. Natl. Cancer Inst. 1988, 80, 1461–1466. [Google Scholar] [CrossRef]
- Bastida, E.; Ordinas, A.; Escolar, G.; Jamieson, G. Tissue Factor in Microvesicles Shed from U87MG Human Glioblastoma Cells Induces Coagulation, Platelet Aggregation, and Thrombogenesis. Blood 1984, 64, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Warren, B.A.; Vales, O. The Adhesion of Thromboplastic Tumour Emboli to Vessel Walls in Vivo. Br. J. Exp. Pathol. 1972, 53, 301–313. [Google Scholar] [PubMed]
- Mezouar, S.; Frère, C.; Darbousset, R.; Mege, D.; Crescence, L.; Dignat-George, F.; Panicot-Dubois, L.; Dubois, C. Role of Platelets in Cancer and Cancer-Associated Thrombosis: Experimental and Clinical Evidences. Thromb. Res. 2016, 139, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Hisada, Y.; Mackman, N. Tissue Factor and Extracellular Vesicles: Activation of Coagulation and Impact on Survival in Cancer. Cancers 2021, 13, 3839. [Google Scholar] [CrossRef]
- Zucchella, M.; Dezza, L.; Pacchiarini, L.; Meloni, F.; Tacconi, F.; Bonomi, E.; Grignani, G.; Notario, A. Human Tumour Cells Cultured “in Vitro” Activate Platelet Function by Producing ADP or Thrombin. Haematologica 1989, 74, 541–545. [Google Scholar]
- Thomas, G.M.; Panicot-Dubois, L.; Lacroix, R.; Dignat-George, F.; Lombardo, D.; Dubois, C. Cancer Cell-Derived Microparticles Bearing P-Selectin Glycoprotein Ligand 1 Accelerate Thrombus Formation in Vivo. J. Exp. Med. 2009, 206, 1913–1927. [Google Scholar] [CrossRef]
- Longenecker, G.L.; Beyers, B.J.; Bowen, R.J.; King, T. Human Rhabdosarcoma Cell-Induced Aggregation of Blood Platelets. Cancer Res. 1989, 49, 16–19. [Google Scholar]
- Honn, K.V.; Cavanaugh, P.; Evens, C.; Taylor, J.D.; Sloane, B.F. Tumor Cell-Platelet Aggregation: Induced by Cathepsin B-like Proteinase and Inhibited by Prostacyclin. Science 1982, 217, 540–542. [Google Scholar] [CrossRef]
- Demers, M.; Krause, D.S.; Schatzberg, D.; Martinod, K.; Voorhees, J.R.; Fuchs, T.A.; Scadden, D.T.; Wagner, D.D. Cancers Predispose Neutrophils to Release Extracellular DNA Traps That Contribute to Cancer-Associated Thrombosis. Proc. Natl. Acad. Sci. USA 2012, 109, 13076–13081. [Google Scholar] [CrossRef]
- Cedervall, J.; Zhang, Y.; Huang, H.; Zhang, L.; Femel, J.; Dimberg, A.; Olsson, A.K. Neutrophil Extracellular Traps Accumulate in Peripheral Blood Vessels and Compromise Organ Function in Tumor-Bearing Animals. Cancer Res. 2015, 75, 2653–2662. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.L.; Nick, A.M.; McNeish, I.A.; Balkwill, F.; Han, H.D.; Bottsford-Miller, J.; Rupaimoole, R.; Armaiz-Pena, G.N.; Pecot, C.V.; Coward, J.; et al. Paraneoplastic Thrombocytosis in Ovarian Cancer. N. Engl. J. Med. 2012, 366, 610–618. [Google Scholar] [CrossRef]
- Kaser, A.; Brandacher, G.; Steurer, W.; Kaser, S.; Offner, F.A.; Zoller, H.; Theurl, I.; Widder, W.; Molnar, C.; Ludwiczek, O.; et al. Interleukin-6 Stimulates Thrombopoiesis through Thrombopoietin: Role in Inflammatory Thrombocytosis. Blood 2001, 98, 2720–2725. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Takahashi, T.; Nakamura, K.; Tsuyuoka, R.; Okuno, Y.; Enomoto, T.; Fukumoto, M.; Imura, H. Thrombocytosis in Patients with Tumors Producing Colony-Stimulating Factor. Blood 1992, 80, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Takahashi, T.; Miyazaki, H.; Matsumoto, A.; Kato, T.; Nakamura, K.; Iho, S.; Okuno, Y.; Nakao, K. Production of Thrombopoietin by Human Carcinomas and Its Novel Isoforms. Blood 1999, 94, 1952–1960. [Google Scholar] [CrossRef] [PubMed]
- Ryu, T.; Nishimura, S.; Miura, H.; Yamada, H.; Morita, H.; Miyazaki, H.; Kitamura, S.; Miura, Y.; Saito, T. Thrombopoietin-Producing Hepatocellular Carcinoma. Intern. Med. 2003, 42, 730–734. [Google Scholar] [CrossRef]
- Peterson, J.E.; Zurakowski, D.; Italiano, J.E.; Michel, L.V.; Connors, S.; Oenick, M.; D’Amato, R.J.; Klement, G.L.; Folkman, J. VEGF, PF4 and PDGF Are Elevated in Platelets of Colorectal Cancer Patients. Angiogenesis 2012, 15, 265–273. [Google Scholar] [CrossRef]
- Nilsson, R.J.A.; Balaj, L.; Hulleman, E.; Van Rijn, S.; Pegtel, D.M.; Walraven, M.; Widmark, A.; Gerritsen, W.R.; Verheul, H.M.; Vandertop, W.P.; et al. Blood Platelets Contain Tumor-Derived RNA Biomarkers. Blood 2011, 118, 3680–3683. [Google Scholar] [CrossRef]
- Plantureux, L.; Mège, D.; Crescence, L.; Dignat-George, F.; Dubois, C.; Panicot-Dubois, L. Impacts of Cancer on Platelet Production, Activation and Education and Mechanisms of Cancer-Associated Thrombosis. Cancers 2018, 10, 441. [Google Scholar] [CrossRef]
- Calverley, D.C.; Phang, T.L.; Choudhury, Q.G.; Gao, B.; Oton, A.B.; Weyant, M.J.; Geraci, M.W. Significant Downregulation of Platelet Gene Expression in Metastatic Lung Cancer. Clin. Transl. Sci. 2010, 3, 227–232. [Google Scholar] [CrossRef]
- Liefaard, M.C.; Lips, E.; Best, M.; Sol, N.; In ’T Veld, S.; Rookus, M.; Sonke, G.S.; Tannous, B.A.; Wesseling, J.; Würdinger, T. RNA Signatures from Tumor-Educated Platelets (TEP) Enable Detection of Early-Stage Breast Cancer. Ann. Oncol. 2019, 30, iii13. [Google Scholar] [CrossRef]
- Zaslavsky, A.; Baek, K.H.; Lynch, R.C.; Short, S.; Grillo, J.; Folkman, J.; Italiano, J.E.; Ryeom, S. Platelet-Derived Thrombospondin-1 Is a Critical Negative Regulator and Potential Biomarker of Angiogenesis. Blood 2010, 115, 4605–4613. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Bronson, S.M.; Pal-Nath, D.; Miller, T.W.; Soto-Pantoja, D.R.; Roberts, D.D. Functions of Thrombospondin-1 in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 4570. [Google Scholar] [CrossRef] [PubMed]
- Haas, S.; Hansson, J.; Klimmeck, D.; Loeffler, D.; Velten, L.; Uckelmann, H.; Wurzer, S.; Prendergast, Á.M.; Schnell, A.; Hexel, K.; et al. Inflammation-Induced Emergency Megakaryopoiesis Driven by Hematopoietic Stem Cell-like Megakaryocyte Progenitors. Cell Stem Cell 2015, 17, 422–434. [Google Scholar] [CrossRef]
- Jain, S.; Russell, S.; Ware, J. Platelet Glycoprotein VI Facilitates Experimental Lung Metastasis in Syngenic Mouse Models. J. Thromb. Haemost. 2009, 7, 1713–1717. [Google Scholar] [CrossRef]
- Zhang, Y.; Cedervall, J.; Hamidi, A.; Herre, M.; Viitaniemi, K.; D’Amico, G.; Miao, Z.; Unnithan, R.V.M.; Vaccaro, A.; van Hooren, L.; et al. Platelet-Specific PDGFB Ablation Impairs Tumor Vessel Integrity and Promotes Metastasis. Cancer Res. 2020, 80, 3345–3358. [Google Scholar] [CrossRef]
- Coupland, L.A.; Chong, B.H.; Parish, C.R. Platelets and P-Selectin Control Tumor Cell Metastasis in an Organ-Specific Manner and Independently of NK Cells. Cancer Res. 2012, 72, 4662–4671. [Google Scholar] [CrossRef]
- Cedervall, J.; Zhang, Y.; Ringvall, M.; Thulin, Å.; Moustakas, A.; Jahnen-Dechent, W.; Siegbahn, A.; Olsson, A.K. HRG Regulates Tumor Progression, Epithelial to Mesenchymal Transition and Metastasis via Platelet-Induced Signaling in the Pre-Tumorigenic Microenvironment. Angiogenesis 2013, 16, 889–902. [Google Scholar] [CrossRef]
- Labelle, M.; Begum, S.; Hynes, R.O. Direct Signaling between Platelets and Cancer Cells Induces an Epithelial-Mesenchymal-Like Transition and Promotes Metastasis. Cancer Cell 2011, 20, 576–590. [Google Scholar] [CrossRef]
- Gugnoni, M.; Sancisi, V.; Gandolfi, G.; Manzotti, G.; Ragazzi, M.; Giordano, D.; Tamagnini, I.; Tigano, M.; Frasoldati, A.; Piana, S.; et al. Cadherin-6 Promotes EMT and Cancer Metastasis by Restraining Autophagy. Oncogene 2017, 36, 667–677. [Google Scholar] [CrossRef]
- Nieswandt, B.; Hafner, M.; Echtenacher, B.; Männel, D.N. Lysis of Tumor Cells by Natural Killer Cells in Mice Is Impeded by Platelets. Cancer Res. 1999, 59, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P. Potential Role of Platelets in the Pathogenesis of Tumor Metastasis. Blood 1984, 63, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.R.; Zuka, M.; Lorger, M.; Tschan, M.; Torbett, B.E.; Zijlstra, A.; Quigley, J.P.; Staflin, K.; Eliceiri, B.P.; Krueger, J.S.; et al. Activated Tumor Cell Integrin Avβ3 Cooperates with Platelets to Promote Extravasation and Metastasis from the Blood Stream. Thromb. Res. 2016, 140, S27–S36. [Google Scholar] [CrossRef]
- Cho, M.S.; Bottsford-Miller, J.; Vasquez, H.G.; Stone, R.; Zand, B.; Kroll, M.H.; Sood, A.K.; Afshar-Kharghan, V. Platelets Increase the Proliferation of Ovarian Cancer Cells. Blood 2012, 120, 4869–4872. [Google Scholar] [CrossRef] [PubMed]
- Mikami, J.; Kurokawa, Y.; Takahashi, T.; Miyazaki, Y.; Yamasaki, M.; Miyata, H.; Nakajima, K.; Takiguchi, S.; Mori, M.; Doki, Y. Antitumor Effect of Antiplatelet Agents in Gastric Cancer Cells: An in Vivo and in Vitro Study. Gastric Cancer 2016, 19, 817–826. [Google Scholar] [CrossRef][Green Version]
- Egan, K.; Crowley, D.; Smyth, P.; O’Toole, S.; Spillane, C.; Martin, C.; Gallagher, M.; Canney, A.; Norris, L.; Conlon, N.; et al. Platelet Adhesion and Degranulation Induce Pro-Survival and pro-Angiogenic Signalling in Ovarian Cancer Cells. PLoS ONE 2011, 6, e26125. [Google Scholar] [CrossRef]
- Brockmann, M.A.; Bender, B.; Plaxina, E.; Nolte, I.; Erber, R.; Lamszus, K.; Groden, C.; Schilling, L. Differential Effects of Tumor-Platelet Interaction in Vitro and in Vivo in Glioblastoma. J. Neurooncol. 2011, 105, 45–56. [Google Scholar] [CrossRef]
- Sagawa, T.; Tominaga, A.; Kodama, T.; Okada, M. Cytotoxicity of Unstimulated and Thrombin-Activated Platelets to Human Tumour Cells. Immunology 1993, 78, 650. [Google Scholar]
- He, A.D.; Xie, W.; Song, W.; Ma, Y.Y.; Liu, G.; Liang, M.L.; Da, X.W.; Yao, G.Q.; Zhang, B.X.; Gao, C.J.; et al. Platelet Releasates Promote the Proliferation of Hepatocellular Carcinoma Cells by Suppressing the Expression of KLF6. Sci. Rep. 2017, 7, 3989. [Google Scholar] [CrossRef]
- Ibele, G.M.; Kay, N.E.; Johnson, G.J.; Jacob, H.S. Human Platelets Exert Cytotoxic Effects on Tumor Cells. Blood 1985, 65, 1252–1255. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H. Platelet-Induced Inhibition of Tumor Cell Growth. Thromb. Res. 2008, 123, 324–330. [Google Scholar] [CrossRef]
- Okada, M.; Sagawa, T.; Tominaga, A.; Kodama, T.; Hitsumoto, Y. Two Mechanisms for Platelet-Mediated Killing of Tumour Cells: One Cyclo-Oxygenase Dependent and the Other Nitric Oxide Dependent. Immunology 1996, 89, 158–164. [Google Scholar] [CrossRef]
- Michael, J.V.; Wurtzel, J.G.T.; Mao, G.F.; Rao, A.K.; Kolpakov, M.A.; Sabri, A.; Hoffman, N.E.; Rajan, S.; Tomar, D.; Madesh, M.; et al. Platelet Microparticles Infiltrating Solid Tumors Transfer MiRNAs That Suppress Tumor Growth. Blood 2017, 130, 567–580. [Google Scholar] [CrossRef]
- Demers, M.; Ho-Tin-Noé, B.; Schatzberg, D.; Yang, J.J.; Wagner, D.D. Increased Efficacy of Breast Cancer Chemotherapy in Thrombocytopenic Mice. Cancer Res. 2011, 71, 1540–1549. [Google Scholar] [CrossRef]
- Li, R.; Ren, M.; Chen, N.; Luo, M.; Deng, X.; Xia, J.; Yu, G.; Liu, J.; He, B.; Zhang, X.; et al. Presence of Intratumoral Platelets Is Associated with Tumor Vessel Structure and Metastasis. BMC Cancer 2014, 14, 167. [Google Scholar] [CrossRef]
- Volz, J.; Mammadova-Bach, E.; Gil-Pulido, J.; Nandigama, R.; Remer, K.; Sorokin, L.; Zernecke, A.; Abrams, S.I.; Ergün, S.; Henke, E.; et al. Inhibition of Platelet GPVI Induces Intratumor Hemorrhage and Increases Efficacy of Chemotherapy in Mice. Blood 2019, 133, 2696–2706. [Google Scholar] [CrossRef]
- Haemmerle, M.; Bottsford-Miller, J.; Pradeep, S.; Taylor, M.L.; Choi, H.J.; Hansen, J.M.; Dalton, H.J.; Stone, R.L.; Cho, M.S.; Nick, A.M.; et al. FAK Regulates Platelet Extravasation and Tumor Growth after Antiangiogenic Therapy Withdrawal. J. Clin. Investig. 2016, 126, 1885–1896. [Google Scholar] [CrossRef]
- Hu, Q.; Hisamatsu, T.; Haemmerle, M.; Cho, M.S.; Pradeep, S.; Rupaimoole, R.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Wong, S.T.C.; Sood, A.K.; et al. Role of Platelet-Derived Tgfβ1 in the Progression of Ovarian Cancer. Clin. Cancer Res. 2017, 23, 5611–5621. [Google Scholar] [CrossRef]
- Zaslavsky, A.B.; Adams, M.P.; Cao, X.; Maj, T.; Choi, J.E.; Stangl-Kremser, J.; Patel, S.; Putelo, A.; Lee, S.K.; Nallandhighal, S.; et al. Platelet PD-L1 Suppresses Anti-Cancer Immune Cell Activity in PD-L1 Negative Tumors. Sci. Rep. 2020, 10, 19296. [Google Scholar] [CrossRef]
- Wurtzel, J.G.T.; Lazar, S.; Sikder, S.; Cai, K.Q.; Astsaturov, I.; Weyrich, A.S.; Rowley, J.W.; Goldfinger, L.E. Platelet MicroRNAs Inhibit Primary Tumor Growth via Broad Modulation of Tumor Cell MRNA Expression in Ectopic Pancreatic Cancer in Mice. PLoS ONE 2021, 16, e0261633. [Google Scholar] [CrossRef]
- Camez, A.; Dupuy, E.; Bellucci, S.; Calvo, F.; Bryckaert, M.C.; Tobelem, G. Human Platelet-Tumor Cell Interactions Vary with the Tumor Cell Lines. Invasion Metastasis 1986, 6, 321–334. [Google Scholar]
- Fabricius, H.Å.; Starzonek, S.; Lange, T. The Role of Platelet Cell Surface P-Selectin for the Direct Platelet-Tumor Cell Contact During Metastasis Formation in Human Tumors. Front. Oncol. 2021, 11, 716. [Google Scholar] [CrossRef]
- Kassassir, H.; Karolczak, K.; Siewiera, K.M.; Wojkowska, D.W.; Braun, M.; Watala, C.W. Time-Dependent Interactions of Blood Platelets and Cancer Cells, Accompanied by Extramedullary Hematopoiesis, Lead to Increased Platelet Activation and Reactivity in a Mouse Orthotopic Model of Breast Cancer—Implications for Pulmonary and Liver Metastas. Aging (Albany NY) 2020, 12, 5091–5120. [Google Scholar] [CrossRef]
- Schlesinger, M. Role of Platelets and Platelet Receptors in Cancer Metastasis. J. Hematol. Oncol. 2018, 11, 125. [Google Scholar] [CrossRef]
- Katz, R.L.; Zaidi, T.M.; Ni, X. Liquid Biopsy: Recent Advances in the Detection of Circulating Tumor Cells and Their Clinical Applications. In Monographs in Clinical Cytology; Karger: Basel, Switzerland, 2020; Volume Voume 25, pp. 43–66. [Google Scholar]
- Miyashita, T.; Tajima, H.; Makino, I.; Nakagawara, H.; Kitagawa, H.; Fushida, S.; Harmon, J.W.; Ohta, T. Metastasis-Promoting Role of Extravasated Platelet Activation in Tumor. J. Surg. Res. 2015, 193, 289–294. [Google Scholar] [CrossRef]
- Zhang, S.R.; Yao, L.; Wang, W.Q.; Xu, J.Z.; Xu, H.X.; Jin, W.; Gao, H.L.; Wu, C.T.; Qi, Z.H.; Li, H.; et al. Tumor-Infiltrating Platelets Predict Postsurgical Survival in Patients with Pancreatic Ductal Adenocarcinoma. Ann. Surg. Oncol. 2018, 25, 3984–3993. [Google Scholar] [CrossRef]
- Xu, S.S.; Xu, H.X.; Wang, W.Q.; Li, S.; Li, H.; Li, T.J.; Zhang, W.H.; Liu, L.; Yu, X.J. Tumor-Infiltrating Platelets Predict Postoperative Recurrence and Survival in Resectable Pancreatic Neuroendocrine Tumor. World J. Gastroenterol. 2019, 25, 6248–6257. [Google Scholar] [CrossRef]
- Miyashita, T.; Tajima, H.; Gabata, R.; Okazaki, M.; Shimbashi, H.; Ohbatake, Y.; Okamoto, K.; Nakanuma, S.; Sakai, S.; Makino, I.; et al. Impact of Extravasated Platelet Activation and Podoplanin-Positive Cancer-Associated Fibroblasts in Pancreatic Cancer Stroma. Anticancer Res. 2019, 39, 5565–5572. [Google Scholar] [CrossRef]
- Saito, H.; Fushida, S.; Miyashita, T.; Oyama, K.; Yamaguchi, T.; Tsukada, T.; Kinoshita, J.; Tajima, H.; Ninomiya, I.; Ohta, T. Potential of Extravasated Platelet Aggregation as a Surrogate Marker for Overall Survival in Patients with Advanced Gastric Cancer Treated with Preoperative Docetaxel, Cisplatin and S-1: A Retrospective Observational Study. BMC Cancer 2017, 17, 294. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Fushida, S.; Kinoshita, J.; Okazaki, M.; Ishikawa, S.; Ohbatake, Y.; Terai, S.; Okamoto, K.; Nakanuma, S.; Makino, I.; et al. Extravasated Platelet Aggregation Contributes to Tumor Progression via the Accumulation of Myeloid-Derived Suppressor Cells in Gastric Cancer with Peritoneal Metastasis. Oncol. Lett. 2020, 20, 1879–1887. [Google Scholar] [CrossRef]
- Qi, C.; Wei, B.; Zhou, W.; Yang, Y.; Li, B.; Guo, S.; Li, J.; Ye, J.; Li, J.; Zhang, Q.; et al. P-Selectin-Mediated Platelet Adhesion Promotes Tumor Growth. Oncotarget 2015, 6, 6584–6596. [Google Scholar] [CrossRef] [PubMed]
- Schoppmann, S.F.; Alidzanovic, L.; Schultheis, A.; Perkmann, T.; Brostjan, C.; Birner, P. Thrombocytes Correlate with Lymphangiogenesis in Human Esophageal Cancer and Mediate Growth of Lymphatic Endothelial Cells In Vitro. PLoS ONE 2013, 8, e66941. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Xu, Z.; Feng, W.; Zheng, M.; Xu, Z.; Gao, H.; Li, W.; Zhang, Y.; Zong, Y.; Lu, A.; et al. Platelet Infiltration Predicts Survival in Postsurgical Colorectal Cancer Patients. Int. J. Cancer 2022, 150, 509–520. [Google Scholar] [CrossRef]
- Qi, C.; Li, B.; Guo, S.; Wei, B.; Shao, C.; Li, J.; Yang, Y.; Zhang, Q.; Li, J.; He, X.; et al. P-Selectin-Mediated Adhesion between Platelets and Tumor Cells Promotes Intestinal Tumorigenesis in Apcmin/+ Mice. Int. J. Biol. Sci. 2015, 11, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Miyashita, T.; Inokuchi, M.; Hayashi, H.; Oyama, K.; Tajima, H.; Takamura, H.; Ninomiya, I.; Ahmed, A.K.; Harman, J.W.; et al. Platelets Surrounding Primary Tumor Cells Are Related to Chemoresistance. Oncol. Rep. 2016, 36, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Yap, M.L.; McFadyen, J.D.; Wang, X.; Zia, N.A.; Hohmann, J.D.; Ziegler, M.; Yao, Y.; Pham, A.; Harris, M.; Donnelly, P.S.; et al. Targeting Activated Platelets: A Unique and Potentially Universal Approach for Cancer Imaging. Theranostics 2017, 7, 2565–2574. [Google Scholar] [CrossRef]
- Costa, B.; Eisemann, T.; Strelau, J.; Spaan, I.; Korshunov, A.; Liu, H.K.; Bugert, P.; Angel, P.; Peterziel, H. Intratumoral Platelet Aggregate Formation in a Murine Preclinical Glioma Model Depends on Podoplanin Expression on Tumor Cells. Blood Adv. 2019, 3, 1092–1102. [Google Scholar] [CrossRef]
- Williamson, S.R.; Mast, K.J.; Cheng, L.; Idrees, M.T. Clear Cell Renal Cell Carcinoma with Intratumoral and Nodal Extramedullary Megakaryopoiesis: A Potential Diagnostic Pitfall. Hum. Pathol. 2014, 45, 1306–1309. [Google Scholar] [CrossRef]
- Cruz, R.J.; Vincenzi, R.; Ketzer, B.M.; Cecilio, A.L.; Cepeda, L.A. Spontaneous Intratumoral Bleeding and Rupture of Giant Gastric Stromal Tumor (>30 Cm) in a Young Patient. World J. Surg. Oncol. 2008, 6, 76. [Google Scholar] [CrossRef]
- Wakai, S.; Yamakawa, K.; Manaka, S.; Takakura, K. Spontaneous Intracranial Hemorrhage Caused by Brain Tumor: Its Incidence and Clinical Significance. Neurosurgery 1982, 10, 437–444. [Google Scholar] [CrossRef]
- De Arnaldo Silva Vellutini, E.; De Oliveira, M.F. Intradural Chordoma Presenting with Intratumoral Bleeding. J. Clin. Neurosci. 2016, 25, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Razazi, K.; Parrot, A.; Khalil, A.; Djibre, M.; Gounant, V.; Assouad, J.; Carette, M.F.; Fartoukh, M.; Cadranel, J. Severe Haemoptysis in Patients with Nonsmall Cell Lung Carcinoma. Eur. Respir. J. 2015, 45, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, M.; Nishida, N.; Furukawa, A.; Kanasaki, S.; Ohta, S.; Miki, Y. Imaging Findings of Primary Hepatic Carcinoid Tumor with an Emphasis on MR Imaging: Case Study. Springerplus 2014, 3, 607. [Google Scholar] [CrossRef][Green Version]
- Petito, E.; Momi, S.; Gresele, P. The Migration of Platelets and Their Interaction with Other Misgrating Cells. In Platelets in Thrombotic and Non-Thrombotic Disorders; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 337–351. ISBN 9783319474625. [Google Scholar]
- Nicolai, L.; Schiefelbein, K.; Lipsky, S.; Leunig, A.; Hoffknecht, M.; Pekayvaz, K.; Raude, B.; Marx, C.; Ehrlich, A.; Pircher, J.; et al. Vascular Surveillance by Haptotactic Blood Platelets in Inflammation and Infection. Nat. Commun. 2020, 11, 5778. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, F.; Ahmad, Z.; Rosenberger, G.; Fan, S.; Nicolai, L.; Busch, B.; Yavuz, G.; Luckner, M.; Ishikawa-Ankerhold, H.; Hennel, R.; et al. Migrating Platelets Are Mechano-Scavengers That Collect and Bundle Bacteria. Cell 2017, 171, 1368–1382.e23. [Google Scholar] [CrossRef]
- Noetzli, L.J.; French, S.L.; Machlus, K.R. New Insights into the Differentiation of Megakaryocytes from Hematopoietic Progenitors. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1288–1300. [Google Scholar] [CrossRef]
- Cho, W.C.; Mandavilli, S. Intratumoral Extramedullary Hematopoiesis in Solitary Fibrous Tumor of the Breast. Breast J. 2020, 26, 755–758. [Google Scholar] [CrossRef]
- Von Schweinitz, D.; Schmidt, D.; Fuchs, J.; Welte, K.; Pietsch, T. Extramedullar Hematopoiesis and Intratumoral Production of Cytokines in Childhood Hepatoblastoma. Pediatr. Res. 1995, 38, 555–563. [Google Scholar] [CrossRef]
- Beckner, M.E.; Lee, J.Y.K.; Schochet, S.S.; Chu, C.T. Intracranial Extramedullary Hematopoiesis Associated with Pilocytic Astrocytoma: A Case Report. Acta Neuropathol. 2003, 106, 584–587. [Google Scholar] [CrossRef]
- Setsu, Y.; Oka, K.; Naoi, Y.; Nagayama, R.; Moriya, T.; Matsumoto, T.; Yatabe, Y.; Mori, N. Breast Carcinoma with Myeloid Metaplasia - A Case Report. Pathol. Res. Pract. 1997, 193, 219–224. [Google Scholar] [CrossRef]
- Gros, A.; Syvannarath, V.; Lamrani, L.; Ollivier, V.; Loyau, S.; Goerge, T.; Nieswandt, B.; Jandrot-Perrus, M.; Ho-Tin-Noé, B. Single Platelets Seal Neutrophil-Induced Vascular Breaches via GPVI during Immune-Complex-Mediated Inflammation in Mice. Blood 2015, 126, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Magnus, N.; D’Asti, E.; Garnier, D.; Meehan, B.; Rak, J. Brain Neoplasms and Coagulation. Semin. Thromb. Hemost. 2013, 39, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, M.; Friedman, T.M.; Olson, J.J.; Brat, D.J. Intravascular Thrombosis in Central Nervous System Malignancies: A Potential Role in Astrocytoma Progression to Glioblastoma. Brain Pathol. 2008, 18, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Wechman, S.L.; Emdad, L.; Sarkar, D.; Das, S.K.; Fisher, P.B. Vascular Mimicry: Triggers, Molecular Interactions and in Vivo Models. Adv. Cancer Res. 2020, 148, 27–67. [Google Scholar] [CrossRef]
- Feng, W.; Madajka, M.; Kerr, B.A.; Mahabeleshwar, G.H.; Whiteheart, S.W.; Byzova, T.V. Anovel Role for Platelet Secretion in Angiogenesis: Mediating Bone Marrow-Derived Cell Mobilization and Homing. Blood 2011, 117, 3893–3902. [Google Scholar] [CrossRef]
- Jiang, L.; Luan, Y.; Miao, X.; Sun, C.; Li, K.; Huang, Z.; Xu, D.; Zhang, M.; Kong, F.; Li, N. Platelet Releasate Promotes Breast Cancer Growth and Angiogenesis via VEGF-Integrin Cooperative Signalling. Br. J. Cancer 2017, 117, 695–703. [Google Scholar] [CrossRef]
- Ho-Tin-Noé, B.; Goerge, T.; Cifuni, S.M.; Duerschmied, D.; Wagner, D.D. Platelet Granule Secretion Continuously Prevents Intratumor Hemorrhage. Cancer Res. 2008, 68, 6851–6858. [Google Scholar] [CrossRef]
- Ho-Tin-Noé, B.; Carbo, C.; Demers, M.; Cifuni, S.M.; Goerge, T.; Wagner, D.D. Innate Immune Cells Induce Hemorrhage in Tumors during Thrombocytopenia. Am. J. Pathol. 2009, 175, 1699–1708. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, J.; Liu, Z.; Chen, G.; Li, X.; Ren, H. Damaging Tumor Vessels with an Ultrasound-Triggered NO Release Nanosystem to Enhance Drug Accumulation and T Cells Infiltration. Int. J. Nanomedicine 2021, 16, 2597. [Google Scholar] [CrossRef]
- Bénézech, C.; Nayar, S.; Finney, B.A.; Withers, D.R.; Lowe, K.; Desanti, G.E.; Marriott, C.L.; Watson, S.P.; Caamaño, J.H.; Buckley, C.D.; et al. CLEC-2 Is Required for Development and Maintenance of Lymph Nodes. Blood 2014, 123, 3200–3207. [Google Scholar] [CrossRef]
- Haining, E.J.; Lowe, K.L.; Wichaiyo, S.; Kataru, R.P.; Nagy, Z.; Kavanagh, D.P.; Lax, S.; Di, Y.; Nieswandt, B.; Ho-Tin-Noé, B.; et al. Lymphatic Blood Filling in CLEC-2-Deficient Mouse Models. Platelets 2021, 32, 352–367. [Google Scholar] [CrossRef] [PubMed]
- Rayes, J.; Lax, S.; Zuidscherwoude, M.; Wichaiyo, S.; Grygielska, B.; Watson, S.; Watson, S.P. Platelets Modulate the Proinflammatory Phenotype of Macrophages via the Interaction of CLEC- 2 and Podoplanin. Res. Pract. Thromb. Haemost. 2017, 1 (Suppl. 1), 12(ASY 32.1). [Google Scholar]
- Labelle, M.; Begum, S.; Hynes, R.O. Platelets Guide the Formation of Early Metastatic Niches. Proc. Natl. Acad. Sci. USA 2014, 111, E3053–E3061. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).