How Do the Outcomes of Radiation-Associated Pelvic and Sacral Bone Sarcomas Compare to Primary Osteosarcomas following Surgical Resection?
Abstract
:Simple Summary
Abstract
1. Background
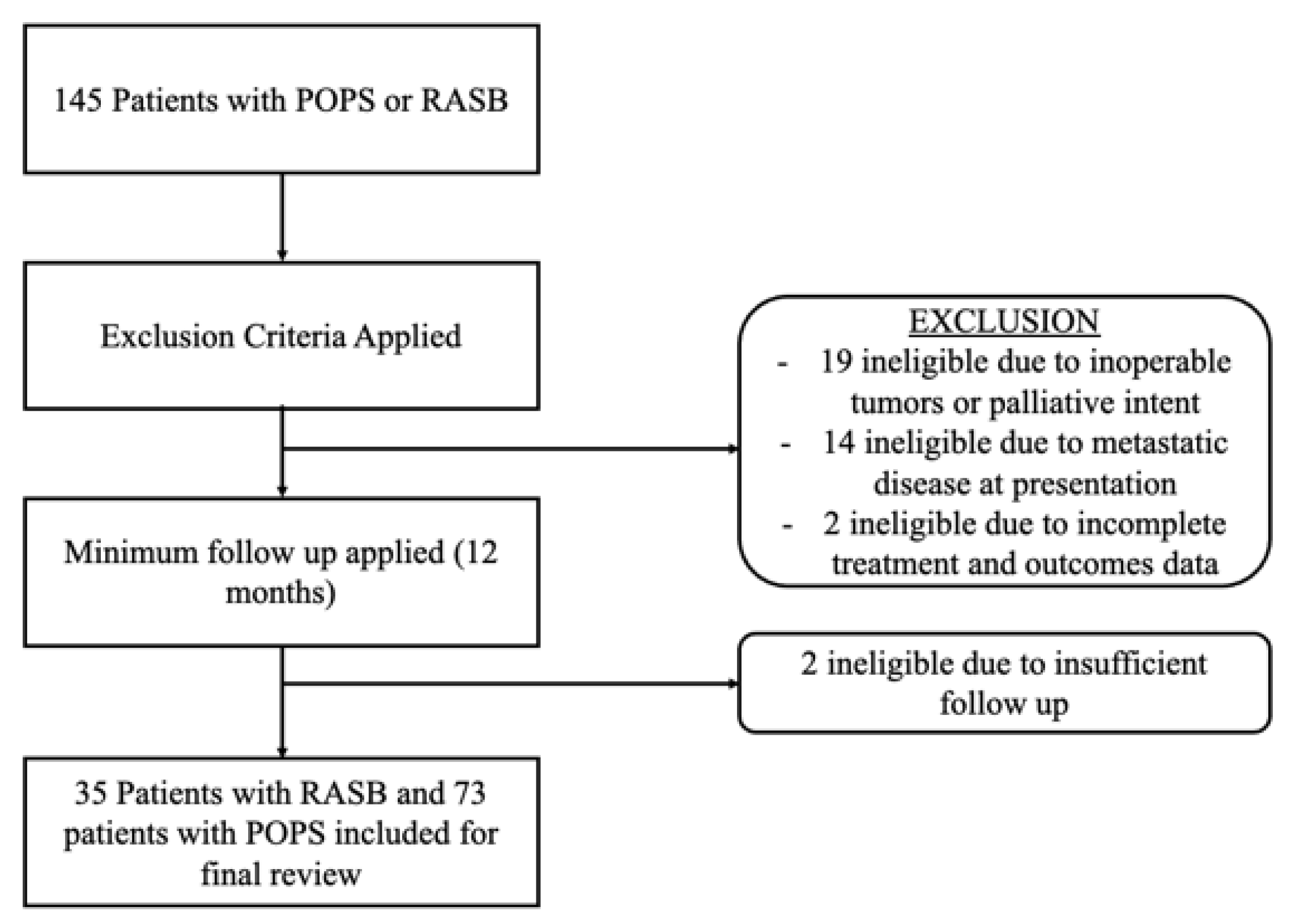
2. Methods
Statistical Analysis
3. Results
3.1. Patient Demographics and Treatment Details
3.2. Comparison of Patient and Treatment between RASB and POPS
3.3. Surgical Outcomes
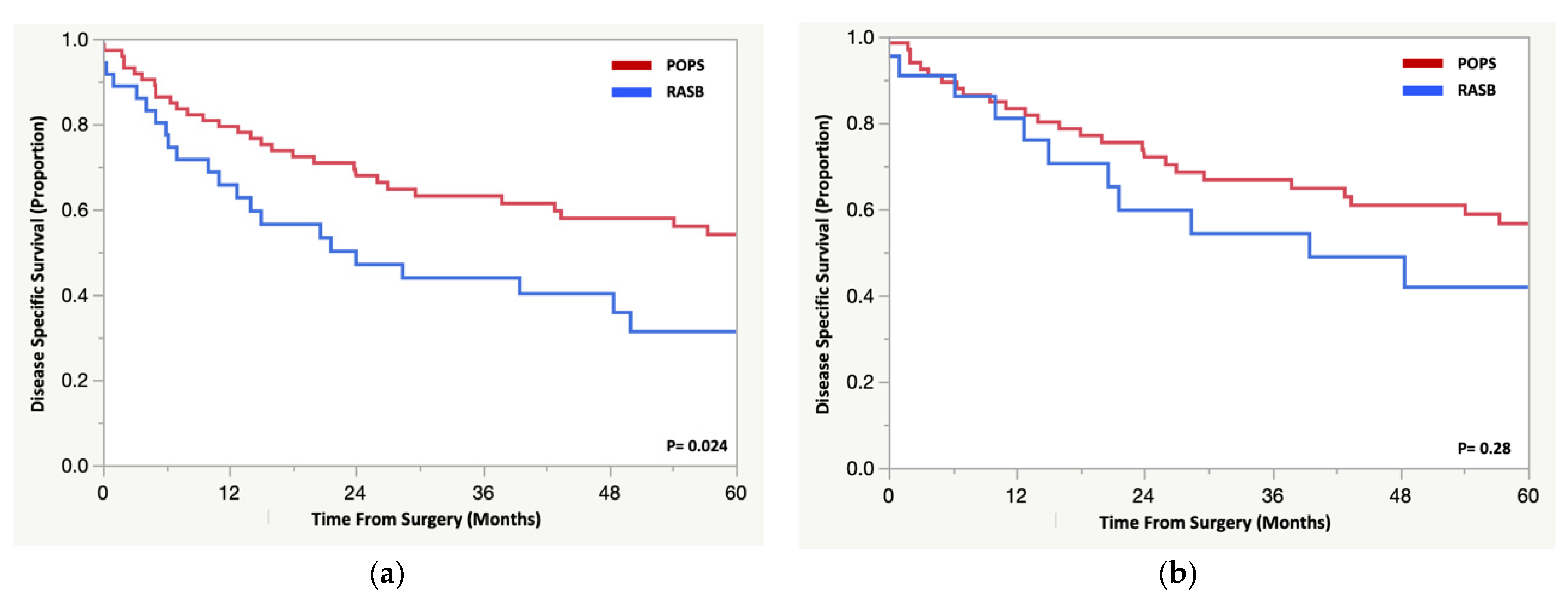
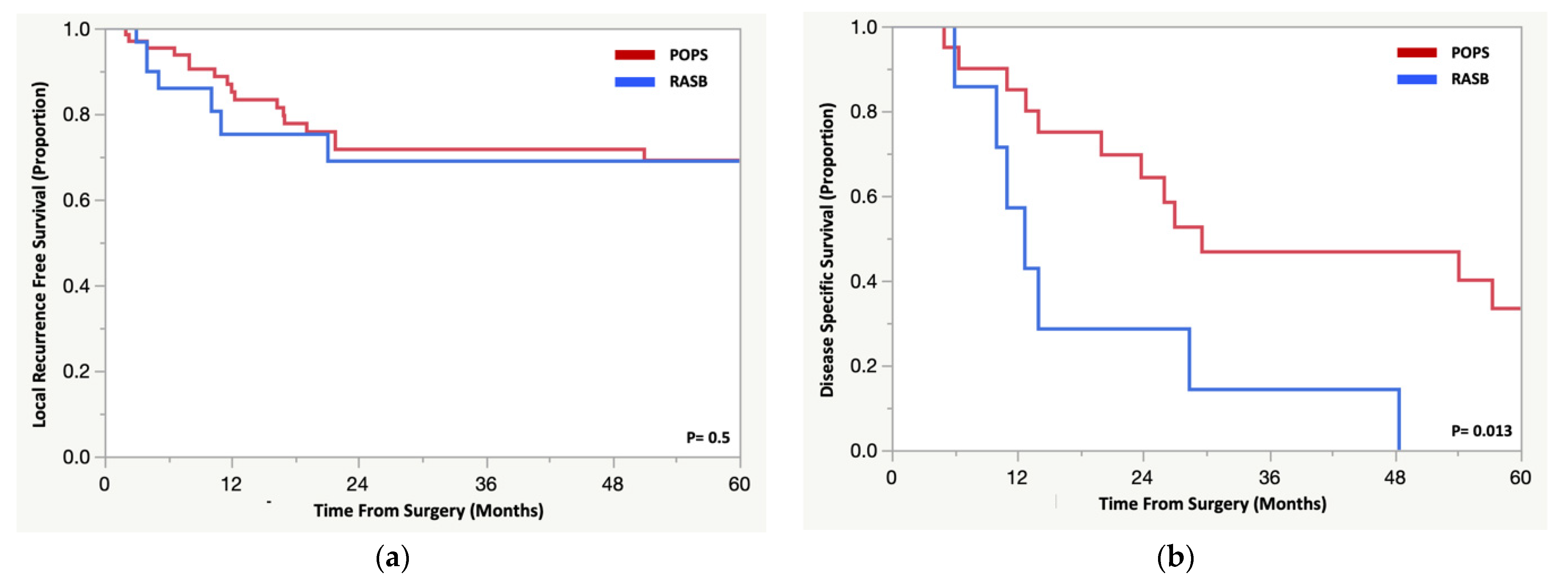
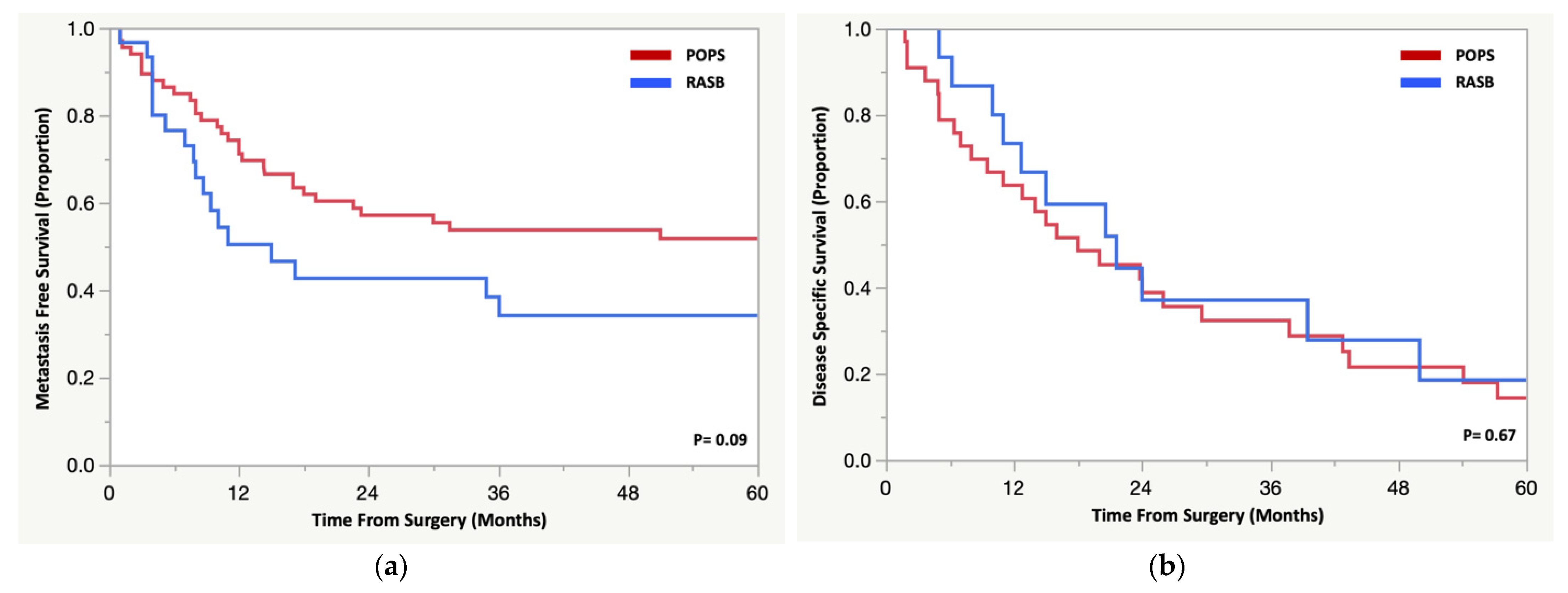
3.4. Oncologic Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gladdy, R.A.; Qin, L.X.; Moraco, N.; Edgar, M.A.; Antonescu, C.R.; Alektiar, K.M.; Brennan, M.F.; Singer, S. Do radiation-associated soft tissue sarcomas have the same prognosis as sporadic soft tissue sarcomas? J. Clin. Oncol. 2010, 28, 2064–2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, A.M.; Dickie, C.I.; Catton, C.N.; Chung, P.W.; Ferguson, P.C.; Wunder, J.S.; O’Sullivan, B. The influence of time interval between preoperative radiation and surgical resection on the development of wound healing complications in extremity soft tissue sarcoma. Ann. Surg. Oncol. 2015, 22, 2824–2830. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Davis, A.M.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Kandel, R.; Goddard, K.; Sadura, A.; et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet 2002, 359, 2235–2241. [Google Scholar] [CrossRef]
- Davis, A.M.; O’Sullivan, B.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Hammond, A.; Benk, V.; Kandel, R.; et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother. Oncol. 2005, 75, 48–53. [Google Scholar] [CrossRef]
- Bjerkehagen, B.; Småstuen, M.C.; Hall, K.S.; Skjeldal, S.; Smeland, S.; Fosså, S.D. Why do patients with radiation-induced sarcomas have a poor sarcoma-related survival? Br. J. Cancer 2012, 106, 297–306. [Google Scholar] [CrossRef]
- Huang, J.; Mackillop, W.J. Increased risk of soft tissue sarcoma after radiotherapy in women with breast carcinoma. Cancer 2001, 92, 172–180. [Google Scholar] [CrossRef]
- Inoue, Y.Z.; Frassica, F.J.; Sim, F.H.; Unni, K.K.; Petersen, I.A.; McLeod, R.A. Clinicopathologic features and treatment of postirradiation sarcoma of bone and soft tissue. J. Surg. Oncol. 2000, 75, 42–50. [Google Scholar] [CrossRef]
- Kalra, S.; Grimer, R.J.; Spooner, D.; Carter, S.R.; Tillman, R.M.; Abudu, A. Radiation-induced sarcomas of bone: Factors that affect outcome. J. Bone Jt. Surg. Br. 2007, 89, 808–813. [Google Scholar] [CrossRef] [Green Version]
- Kirova, Y.M.; Gambotti, L.; De Rycke, Y.; Vilcoq, J.R.; Asselain, B.; Fourquet, A. Risk of second malignancies after adjuvant radiotherapy for breast cancer: A large-scale, single-institution review. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 359–363. [Google Scholar] [CrossRef]
- Mark, R.J.; Poen, J.; Tran, L.M.; Fu, Y.S.; Selch, M.T.; Parker, R.G. Postirradiation sarcomas. A single-institution study and review of the literature. Cancer 1994, 73, 2653–2662. [Google Scholar] [CrossRef]
- Shaheen, M.; Deheshi, B.M.; Riad, S.; Werier, J.; Holt, G.E.; Ferguson, P.C.; Wunder, J.S. Prognosis of radiation-induced bone sarcoma is similar to primary osteosarcoma. Clin. Orthop. Relat. Res. 2006, 450, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Suit, H.; Goldberg, S.; Niemierko, A.; Ancukiewicz, M.; Hall, E.; Goitein, M.; Wong, W.; Paganetti, H. Secondary carcinogenesis in patients treated with radiation: A review of data on radiation-induced cancers in human, non-human primate, canine and rodent subjects. Radiat. Res. 2007, 167, 12–42. [Google Scholar] [CrossRef] [PubMed]
- Riad, S.; Biau, D.; Holt, G.E.; Werier, J.; Turcotte, R.E.; Ferguson, P.C.; Griffin, A.M.; Dickie, C.I.; Chung, P.W.; Catton, C.N.; et al. The clinical and functional outcome for patients with radiation-induced soft tissue sarcoma. Cancer 2012, 118, 2682–2692. [Google Scholar] [CrossRef]
- Senchenkov, A.; Moran, S.L.; Petty, P.M.; Knoetgen, J., 3rd; Clay, R.P.; Bite, U.; Barnes, S.A.; Sim, F.H. Predictors of complications and outcomes of external hemipelvectomy wounds: Account of 160 consecutive cases. Ann. Surg. Oncol. 2008, 15, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Ogura, K.; Boland, P.J.; Fabbri, N.; Healey, J.H. Rate and risk factors for wound complications after internal hemipelvectomy. Bone Jt. J. 2020, 102-B, 280–284. [Google Scholar] [CrossRef]
- Hulen, C.A.; Temple, H.T.; Fox, W.P.; Sama, A.A.; Green, B.A.; Eismont, F.J. Oncologic and functional outcome following sacrectomy for sacral chordoma. J. Bone Jt. Surg. Am. 2006, 88, 1532–1539. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, H.S.; Yun, J.Y.; Han, I. Neuropathic pain after sarcoma surgery: Prevalence and predisposing factors. Medicine 2018, 97, e10852. [Google Scholar] [CrossRef]
- Ranft, A.; Seidel, C.; Hoffmann, C.; Paulussen, M.; Warby, A.C.; van den Berg, H.; Ladenstein, R.; Rossig, C.; Dirksen, U.; Rosenbaum, D.; et al. Quality of Survivorship in a Rare Disease: Clinicofunctional Outcome and Physical Activity in an Observational Cohort Study of 618 Long-Term Survivors of Ewing Sarcoma. J. Clin. Oncol. 2017, 35, 1704–1712. [Google Scholar] [CrossRef]
- Arlen, M.; Higinbotham, N.L.; Huvos, A.G.; Marcove, R.C.; Miller, T.; Shah, I.C. Radiation-induced sarcoma of bone. Cancer 1971, 28, 1087–1099. [Google Scholar] [CrossRef]
- Wiklund, T.A.; Blomqvist, C.P.; Raty, J.; Elomaa, I.; Rissanen, P.; Miettinen, M. Postirradiation sarcoma. Analysis of a nationwide cancer registry material. Cancer 1991, 68, 524–531. [Google Scholar] [CrossRef]
- Gundle, K.R.; Kafchinski, L.; Gupta, S.; Griffin, A.M.; Dickson, B.C.; Chung, P.W.; Catton, C.N.; O’Sullivan, B.; Wunder, J.S.; Ferguson, P.C. Analysis of Margin Classification Systems for Assessing the Risk of Local Recurrence After Soft Tissue Sarcoma Resection. J. Clin. Oncol. 2018, 36, 704–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielack, S.S.; Kempf-Bielack, B.; Delling, G.; Exner, G.U.; Flege, S.; Helmke, K.; Kotz, R.; Salzer-Kuntschik, M.; Werner, M.; Winkelmann, W.; et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002, 20, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Thijssens, K.M.; van Ginkel, R.J.; Suurmeijer, A.J.; Pras, E.; van der Graaf, W.T.; Hollander, M.; Hoekstra, H.J. Radiation-induced sarcoma: A challenge for the surgeon. Ann. Surg. Oncol. 2005, 12, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, A.; Lewis, V.O.; Satcher, R.L.; Moon, B.S.; Lin, P.P. What are the factors that affect survival and relapse after local recurrence of osteosarcoma? Clin. Orthop. Relat. Res. 2014, 472, 3188–3195. [Google Scholar] [CrossRef] [Green Version]
- Pakos, E.E.; Nearchou, A.D.; Grimer, R.J.; Koumoullis, H.D.; Abudu, A.; Bramer, J.A.; Jeys, L.M.; Franchi, A.; Scoccianti, G.; Campanacci, D.; et al. Prognostic factors and outcomes for osteosarcoma: An international collaboration. Eur. J. Cancer 2009, 45, 2367–2375. [Google Scholar] [CrossRef]
- Bacci, G.; Forni, C.; Longhi, A.; Ferrari, S.; Mercuri, M.; Bertoni, F.; Serra, M.; Briccoli, A.; Balladelli, A.; Picci, P. Local recurrence and local control of non-metastatic osteosarcoma of the extremities: A 27-year experience in a single institution. J. Surg. Oncol. 2007, 96, 118–123. [Google Scholar] [CrossRef]
- Bacci, G.; Longhi, A.; Forni, C.; Fabbri, N.; Briccoli, A.; Barbieri, E.; Mercuri, M.; Balladelli, A.; Ferrari, S.; Picci, P. Neoadjuvant chemotherapy for radioinduced osteosarcoma of the extremity: The Rizzoli experience in 20 cases. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 505–511. [Google Scholar] [CrossRef]
- Whelan, J.S.; Jinks, R.C.; McTiernan, A.; Sydes, M.R.; Hook, J.M.; Trani, L.; Uscinska, B.; Bramwell, V.; Lewis, I.J.; Nooij, M.A.; et al. Survival from high-grade localised extremity osteosarcoma: Combined results and prognostic factors from three European Osteosarcoma Intergroup randomised controlled trials. Ann. Oncol. 2012, 23, 1607–1616. [Google Scholar] [CrossRef]
- Souhami, R.L.; Craft, A.W.; Van der Eijken, J.W.; Nooij, M.; Spooner, D.; Bramwell, V.H.; Wierzbicki, R.; Malcolm, A.J.; Kirkpatrick, A.; Uscinska, B.M.; et al. Randomised trial of two regimens of chemotherapy in operable osteosarcoma: A study of the European Osteosarcoma Intergroup. Lancet 1997, 350, 911–917. [Google Scholar] [CrossRef]
- Bramwell, V.H.; Burgers, M.; Sneath, R.; Souhami, R.; van Oosterom, A.T.; Voute, P.A.; Rouesse, J.; Spooner, D.; Craft, A.W.; Somers, R.; et al. A comparison of two short intensive adjuvant chemotherapy regimens in operable osteosarcoma of limbs in children and young adults: The first study of the European Osteosarcoma Intergroup. J. Clin. Oncol. 1992, 10, 1579–1591. [Google Scholar] [CrossRef]
| n | % | |
|---|---|---|
| Histology (n = 33) | ||
| Gastrointestinal | 6 | 18% |
| Genitourinary | 9 | 28% |
| Hematologic (Acute lymphocytic leukemia and unspecified lymphoma) | 2 | 6% |
| Musculoskeletal/Sarcoma | 8 | 24% |
| Reproductive | 7 | 21% |
| Skin | 1 | 3% |
| Treatment (n = 32) | ||
| Radiotherapy Alone | 6 | 17% |
| Surgery with Adjuvant or Neoadjuvant Radiotherapy | 29 | 83% |
| Median Dosage (Gy) | 50 (25–66) | |
| Median Latency (yrs) | 12.7 (3–27) | |
| Secondary Malignancy Histology (n = 35) | ||
| Osteosarcoma | 23 | 66% |
| Spindle Cell Sarcoma | 11 | 31% |
| Chondrosarcoma | 1 | 3% |
| Radiation-Associated Sarcoma of Pelvis (n = 35) | Primary Osteosarcoma/Spindle Cell Sarcoma of Pelvis (n = 73) | ||
|---|---|---|---|
| n (%) | n (%) | p-Value # | |
| Median Age (years) | 57 (14–84) | 38 (12–81) | <0.001 |
| Gender | |||
| Male | 20 (58) | 42 (58) | 0.94 |
| Female | 15 (42) | 31 (42) | |
| Location | |||
| Pelvis | 19 (54) | 50 (68) | <0.001 |
| Sacrum | 15 (43) | 10 (14) | |
| Combined | 1 (3) | 13 (18) | |
| Median Tumor Size | 9 (3–20) | 11 (3–28) | 0.1 |
| Grade | 0.27 | ||
| Intermediate | 4 (11) | 4 (6) | |
| High/undifferentiated | 31 (89) | 68 (93) | |
| Unavailable | 0 | 1 (1) | |
| Chemotherapy | 0.011 | ||
| Yes | 25 (71) | 66 (90) | |
| No | 10 (29) | 7 (10) | |
| Radiotherapy | 0.51 | ||
| Yes | 6 (17) | 9 (12) | |
| No | 29 (83) | 64 (88) | |
| Type of Surgery | 0.16 | ||
| Limb Salvage | 28 (80) | 49 (67) | |
| Amputation | 7 (20) | 24 (33) | |
| Type of Closure | 0.18 | ||
| Primary | 34 (97) | 66 (90) | |
| Flap | 1 (3) | 7 (10) | |
| Margin Status | 0.35 | ||
| Positive | 6 (17) | 8 (11) | |
| Negative | 28 (80) | 65 (89) | |
| Unavailable | 1 (3) | 0 | |
| Complications | 0.28 | ||
| Yes | 29 (78) | 55 (65) | |
| No | 8 (22) | 39 (35) | |
| Perioperative Death within 90 days of Surgery | 0.03 | ||
| Yes | 6 (17) | 3 (4) | |
| No | 29 (83) | 70 (96) | |
| Median time to local recurrence (months) | 5 (3–21.1) | 14 (2–200) | 0.07 |
| Median time to metastasis (months) | 8 (3.5–52.1) | 11 (3–141) | 0.69 |
| Median time to death (months) | 11 (0–50) | 13 (0–57.3) | 0.53 |
| Radiation-Associated Sarcoma | Primary Osteosarcoma/Spindle Cell Sarcoma | ||
|---|---|---|---|
| n (%) | n (%) | p-Value # | |
| Number of Agents Received | 34 (97) | 70 (96) | <0.0001 |
| 0 (Family refused or patient not deemed a medical candidate) | 14 (41) | 7 (10) | |
| 2 (Adriamycin + Cisplatin or Ifosfamide + Adriamycin or Etoposide) | 14 (41) | 25 (36) | |
| 3 (Methotrexate + Adriamycin + Cisplatin) | 6 (18) | 38 (54) | |
| Chemotherapy Agent Information Unavailable | 1 (3) | 3 (4) | |
| Number of Chemotherapy Cycles Received | 0.009 | ||
| 4 or Fewer Cycles | 11 (69) | 14 (31) | |
| 5 or More Cycles | 5 (31) | 31 (69) | |
| Chemotherapy Cycle Information Unavailable | 4 (20%) | 18 (29%) | |
| Percent Necrosis for Patients Receiving Neoadjuvant Chemotherapy | 0.38 | ||
| >90% | 7 (27) | 14 (22) | |
| ≤90% | 19 (73) | 50 (78) | |
| Median Radiotherapy Dosing (range, Gy) | 45 (30–50) | 52 (15–56.25) | |
| Timing of Radiotherapy | |||
| Neoadjuvant | 6 (100) | 8 (89) | |
| Adjuvant | 0 (0) | 1 (11) |
| Radiation-Associated Sarcoma (n = 35) | Primary Osteosarcoma/Spindle Cell Sarcoma (n = 73) | |||||
|---|---|---|---|---|---|---|
| HR | Confidence Interval | p-Value | HR | Confidence Interval | p-Value # | |
| Age (years) | ||||||
| <40 | ref | ref | ||||
| >40 | 2.6 | 0.75–8.75 | 0.13 | 1.9 | 0.94–3.84 | 0.08 |
| Gender | ||||||
| Male | ref | ref | ||||
| Female | 0.6 | 0.26–1.53 | 0.31 | 0.7 | 0.32–1.37 | 0.26 |
| Location | ||||||
| Pelvis | ref | |||||
| Sacrum | 1.1 | 0.48–2.59 | 0.8 | 1.2 | 0.41–3.44 | 0.76 |
| Combined | n/a | n/a | n/a | 0.8 | 0.3–2.08 | 0.63 |
| Tumor Size * | ||||||
| <11 cm | ref | ref | ||||
| >11 cm | 2.2 | 0.88–5.55 | 0.09 | 2.1 | 1–4.49 | 0.05 |
| Histologic Grade | ||||||
| Intermediate | ref | ref | ||||
| High/undifferentiated | 0.7 | 0.21–2.48 | 0.61 | 0.9 | 0.21–3.65 | 0.64 |
| Chemotherapy | ||||||
| No | ref | ref | ||||
| Yes | 0.4 | 0.19–1.02 | 0.06 | 0.4 | 0.13–0.92 | 0.03 |
| >90% Necrosis following Chemotherapy | ||||||
| Yes | - | - | - | ref | ||
| No | - | - | - | 3.1 | 0.72–13.1 | 0.12 |
| Radiotherapy | ||||||
| No | ref | ref | ||||
| Yes | 1.7 | 0.62–4.63 | 0.3 | 0.4 | 0.09–1.58 | 0.18 |
| Type of Surgery | ||||||
| Amputation | ref | ref | ||||
| Limb Salvage | 0.7 | 0.25–1.88 | 0.46 | 0.8 | 0.41–1.73 | 0.64 |
| Type of Wound Closure | ||||||
| Primary | ref | ref | ||||
| Flap | 4.4 | 0.54–35.9 | 0.17 | 0.5 | 0.12–2.13 | 0.35 |
| Margin Status | ||||||
| Negative | ref | ref | ||||
| Positive | 1.6 | 0.6–4.04 | 0.37 | 1.6 | 0.52–4.82 | 0.41 |
| Complications | ||||||
| No | ref | ref | ||||
| Yes | 1.9 | 0.57–6.47 | 0.3 | 0.7 | 0.34–1.43 | 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarides, A.L.; Burke, Z.D.C.; Gundavda, M.K.; Novak, R.; Ghert, M.; Wilson, D.A.; Rose, P.S.; Wong, P.; Griffin, A.M.; Ferguson, P.C.; et al. How Do the Outcomes of Radiation-Associated Pelvic and Sacral Bone Sarcomas Compare to Primary Osteosarcomas following Surgical Resection? Cancers 2022, 14, 2179. https://doi.org/10.3390/cancers14092179
Lazarides AL, Burke ZDC, Gundavda MK, Novak R, Ghert M, Wilson DA, Rose PS, Wong P, Griffin AM, Ferguson PC, et al. How Do the Outcomes of Radiation-Associated Pelvic and Sacral Bone Sarcomas Compare to Primary Osteosarcomas following Surgical Resection? Cancers. 2022; 14(9):2179. https://doi.org/10.3390/cancers14092179
Chicago/Turabian StyleLazarides, Alexander L., Zachary D. C. Burke, Manit K. Gundavda, Rostislav Novak, Michelle Ghert, David A. Wilson, Peter S. Rose, Philip Wong, Anthony M. Griffin, Peter C. Ferguson, and et al. 2022. "How Do the Outcomes of Radiation-Associated Pelvic and Sacral Bone Sarcomas Compare to Primary Osteosarcomas following Surgical Resection?" Cancers 14, no. 9: 2179. https://doi.org/10.3390/cancers14092179
APA StyleLazarides, A. L., Burke, Z. D. C., Gundavda, M. K., Novak, R., Ghert, M., Wilson, D. A., Rose, P. S., Wong, P., Griffin, A. M., Ferguson, P. C., Wunder, J. S., Houdek, M. T., & Tsoi, K. M. (2022). How Do the Outcomes of Radiation-Associated Pelvic and Sacral Bone Sarcomas Compare to Primary Osteosarcomas following Surgical Resection? Cancers, 14(9), 2179. https://doi.org/10.3390/cancers14092179







