PI3K Inhibitors in Advanced Breast Cancer: The Past, The Present, New Challenges and Future Perspectives
Abstract
:Simple Summary
Abstract
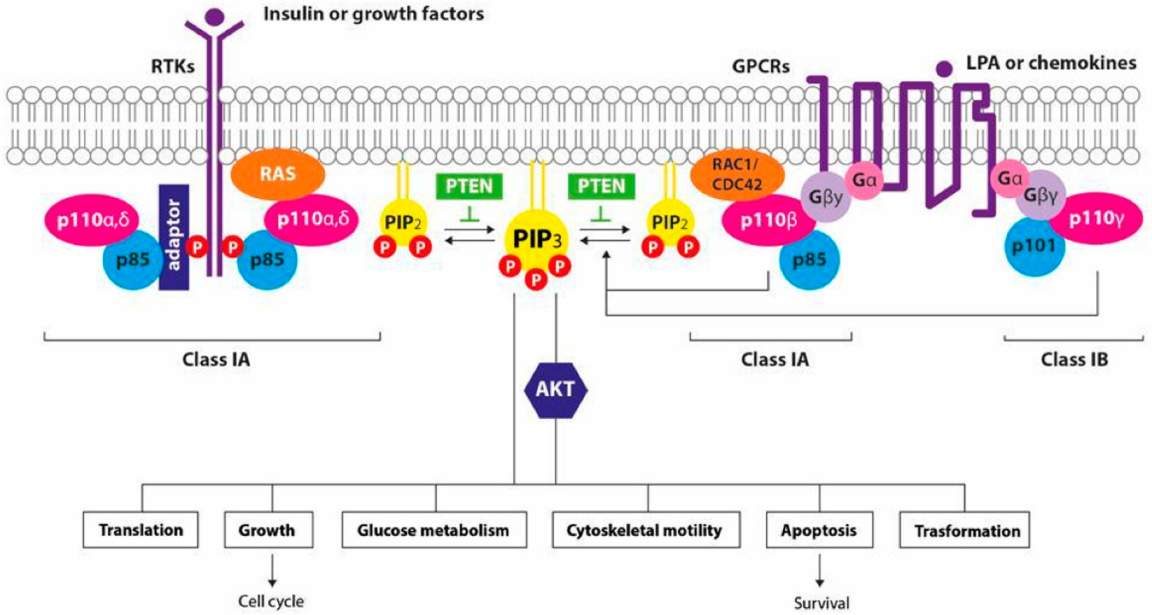
1. Introduction
2. Pan-PI3K Inhibitors
2.1. Pan-PI3K Inhibitors in HR+/HER2− Breast Cancer Subtypes
2.1.1. Buparlisib
2.1.2. Pictilisib
2.2. Pan-PI3K Inhibitors in HER2+ Breast Cancer Subtypes
2.3. Pan-PI3K Inhibitors in Triple Negative Breast Cancer Subtypes
3. PI3K Isoform-Specific Inhibitors
3.1. PI3K Isoform-Specific Inhibitors in HR+/HER2− Breast Cancer Subtypes
3.1.1. Alpelisib
3.1.2. Taselisib
3.2. PI3K Isoform-Specific Inhibitors in HER2+ Breast Cancer Subtypes
3.3. PI3K Isoform-Specific Inhibitors in Triple Negative Breast Cancer
4. PIK3 Inhibitors: New Perspectives
4.1. PI3K Inhibitors in HR+ HER2− Breast Cancer Subtypes
4.2. PI3K Inhibitors in HER2 + Breast Cancer Subtypes
4.3. PI3K Inhibitors in Triple Negative Breast Cancer Subtypes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heer, E.; Harper, A. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørli, T. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumors. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Kawaji, H.; Kubo, M. Comprehensive molecular profiling broadens treatment options for breast cancer patients. Cancer Med. 2021, 10, 529–539. [Google Scholar] [CrossRef]
- Guerrero-Zotano, A.; Mayer, I.A. PI3K/AKT/mTOR: Role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev. 2016, 35, 515–524. [Google Scholar] [CrossRef]
- Fruman, D.; Chiu, H. The PI3K pathway in human disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef] [Green Version]
- Engelman, J.; Luo, J. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006, 7, 606–619. [Google Scholar] [CrossRef]
- Thorpe, L.; Yuzugullu, H. PI3K in cancer: Divergent roles of isoforms, modes of activation, and therapeutic targeting. Nat. Rev. Cancer 2015, 15, 7–24. [Google Scholar] [CrossRef]
- Ellis, H.; Ma, C.X. PI3K Inhibitors in Breast Cancer Therapy. Curr. Oncol. Rep. 2019, 21, 110. [Google Scholar] [CrossRef]
- Saal, L.; Holm, K. PIK3CA Mutations Correlate with Hormone Receptors, Node Metastasis, and ERBB2, and Are Mutually Exclusive with PTEN Loss in Human Breast Carcinoma. Cancer Res. 2005, 65, 2554–2559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loi, S.; Michiels, S.; Lambrechts, D.; Fumagalli, D.; Claes, B.; Kellokumpu-Lehtinen, P.L.; Sotiriou, C. Somatic Mutation Profiling and Associations With Prognosis and Trastuzumab Benefit in Early Breast Cancer. J. Natl. Cancer Inst. 2013, 105, 960–967. [Google Scholar] [CrossRef] [PubMed]
- López-Knowles, E.; O’Toole, S.A.; McNeil, C.M.; Millar, E.K.; Qiu, M.R.; Crea, P.; Sutherland, R.L. PI3K pathway activation in breast cancer is associated with the basal-like phenotype and cancer-specific mortality. Int. J. Cancer 2009, 126, 1121–1131. [Google Scholar] [CrossRef]
- Fabi, A.; Metro, G.; Di Benedetto, A.; Nisticò, C.; Vici, P.; Melucci, E.; Antoniani, B. Clinical significance of PTEN and p-Akt co-expression in HER2-positive metastatic breast cancer patients treated with trastuzumab-based therapies. Oncology 2010, 78, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.G.; Kang, S. Oncogenic PI3K deregulates transcription and translation. Nat. Rev. Cancer 2005, 5, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Vivanco, I.; Sawyers, C.L. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat. Rev. Cancer 2002, 2, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Santiskulvong, C. Effect of PI3K/Akt pathway inhibition-mediated G1 arrest on chemosensitization in ovarian cancer cells. Anticancer Res. 2012, 32, 445–452. [Google Scholar]
- Carden, C.P.; Stewart, A. The association of PI3 kinase signaling and chemoresistance in advanced ovarian cancer. Mol. Cancer Ther. 2012, 11, 1609–1617. [Google Scholar] [CrossRef] [Green Version]
- Garces, A.E.; Stocks, M.J. PI3K Clinical Candidates and Recent Inhibitor Design Strategies: A Medicinal Chemistry Perspective. Med. Chem. 2019, 62, 4815–4850. [Google Scholar] [CrossRef]
- Yang, J.; Nie, J. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef] [Green Version]
- Verret, B.; Cortes, J. Efficacy of PI3K inhibitors in advanced breast cancer. Ann. Oncol. 2019, 30, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Janku, F.; Yap, T.A. Targeting the PI3K pathway in cancer: Are we making headway? Nat. Rev. Clin. Oncol. 2018, 15, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Im, S.A.; Iwata, H.; Cortés, J.; De Laurentiis, M.; Jiang, Z.; Campone, M. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 904–916. [Google Scholar] [CrossRef]
- Miller, T.; Hennessy, W. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J. Clin. Investig. 2010, 120, 2406–2413. [Google Scholar] [CrossRef] [Green Version]
- Miller, T.; Rexer, W. Mutations in the phosphatidylinositol 3-kinase pathway: Role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 2011, 13, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maira, S.M.; Pecchi, S. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol. Cancer Ther. 2012, 11, 317–328. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, C.G.; Ma, C.X. Preclinical modeling of combined phosphatidylinositol-3-kinase inhibition with endocrine therapy for estrogen receptor-positive breast cancer. Breast Cancer Res. 2011, 13, R21. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.X.; Luo, J. A phase I trial of BKM120 (Buparlisib) in combination with fulvestrant in postmenopausal women with estrogen receptor-positive metastatic breast cancer. Clin. Cancer Res. 2016, 22, 1583–1591. [Google Scholar] [CrossRef] [Green Version]
- Bendell, J.C.; Rodon, J. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2012, 30, 282–290. [Google Scholar] [CrossRef]
- Mayer, I.A.; Abramson, V.G. Stand up to cancer phase Ib study of pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. 2014, 32, 1202–1209. [Google Scholar] [CrossRef]
- Di Leo, A.; Johnston, S. Buparlisib plus fulvestrant in postme nopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018, 19, 87–100. [Google Scholar] [CrossRef]
- Baselga, J.; Sellami, D. Abstract A050: PIK3CA mutation status in tumor tissue and ctDNA as a biomarker for PFS in patients with HRþ, HER2- ABC treated with buparlisib or placebo plus fulvestrant: Results from the BELLE-2 and BELLE-3 randomized studies. Mol. Cancer Ther. 2018, 17 (Suppl. 1), A050. [Google Scholar]
- Chang, D.Y.; Ma, W.L. Role of Alpelisib in the Treatment of PIK3CA-Mutated Breast Cancer: Patient Selection and Clinical Perspectives (Review). Ther. Clin. Risk Manag. 2021, 17, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Nanni, P.; Nicoletti, G. Multiorgan metastasis of human HER-2+ breast cancer in Rag2−/−;Il2rg−/− mice and treatment with PI3K inhibitor. PLoS ONE 2012, 7, e39626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, M.; Chan, A.; Dirix, L.; O’Shaughnessy, J.; Hegg, R.; Manikhas, A.; Delaloge, S. A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2- advanced breast cancer (BELLE-4). Ann. Oncol. 2017, 28, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Folkes, A.; Ahmadi., K. The identification of 2-(1Hindazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3, 2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J. Med. Chem. 2008, 51, 5522–5532. [Google Scholar] [CrossRef] [PubMed]
- Sarker, D.; Ang, J.E. First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clin. Cancer Res. 2015, 21, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Schmid, P.; Pinder, S.E. Phase II randomized preoperative window-of-opportunity study of the PI3K inhibitor pictilisib plus anastrozole compared with anastrozole alone in patients with estrogen receptor-positive breast cancer. J. Clin. Oncol. 2016, 34, 1987–1994. [Google Scholar] [CrossRef] [Green Version]
- Krop, I.E.; Mayer, I.A. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016, 17, 811–821. [Google Scholar] [CrossRef] [Green Version]
- Vuylsteke, P.; Huizing, M. Pictilisib PI3Kinase inhibitor (a phosphatidylinositol 3-kinase [PI3K] inhibitor) plus paclitaxel for the treatment of hormone receptor-positive, HER2-negative, locally recurrent, or metastatic breast cancer: Interim analysis of the multicentre, placebo-controlled, phase II randomised PEGGY study. Ann. Oncol. 2016, 27, 2059–2066. [Google Scholar]
- Bahrami, A.; Khazaei, M. The therapeutic potential of PI3K/AKT/ mTOR inhibitors in breast cancer: Rationale and progress. J. Cell Biochem. 2018, 119, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Berns, K.; Horlings, H.M. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 2007, 12, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pernas, S.; Tolaney, S.M. HER2-positive breast cancer: New therapeutic frontiers and overcoming resistance. Ther. Adv. Med. Oncol. 2019, 11, 1758835919833519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baselga, J.; Cortes, J. Biomarker analyses in CLEOPATRA: A phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, firstline metastatic breast cancer. J. Clin. Oncol. 2014, 32, 3753–3761. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Lewis, G. Relationship between tumor biomarkers and efficacy in EMILIA, a phase III study of trastuzumab emtansine in HER2-positive metastatic breast cancer. Clin. Cancer Res. 2016, 22, 3755–3763. [Google Scholar] [CrossRef] [Green Version]
- Chandarlapaty, S.; Sakr, R.A. Frequent mutational activation of the PI3K-AKT pathway in trastuzumab-resistant breast cancer. Clin. Cancer Res. 2012, 18, 6784–6791. [Google Scholar] [CrossRef] [Green Version]
- Wilks, S. Potential of overcoming resistance to HER2-targeted therapies through the PI3K/Akt/mTOR pathway. Breast 2015, 24, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Prever, L. Targeting PI3K/AKT/mTOR Signaling Pathway in Breast Cancer (Review). Cancers 2021, 13, 3517. [Google Scholar] [CrossRef]
- Hanker, A.B.; Pfefferl, A.D. Mutant PIK3CA accelerates HER2-driven transgenic mammary tumors and induces resistance to combinations of anti-HER2 therapies. Proc. Natl. Acad. Sci. USA 2013, 110, 14372–14377. [Google Scholar] [CrossRef] [Green Version]
- Saura, C.; Bendell, J. Phase Ib study of buparlisib plus trastuzumab in patients with HER2-positive advanced or metastatic breast cancer that has progressed on Trastuzumab-based therapy. Clin. Cancer Res. 2014, 20, 1935–1945. [Google Scholar] [CrossRef] [Green Version]
- Pistilli, B.; Pluard, T. Phase II study of buparlisib (BKM120) and trastuzumab in patients with HER2+ locally advanced or metastatic breast cancer resistant to trastuzumab-based therapy. Breast Cancer Res. Treat. 2018, 168, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.; Rezai, K. PIKHER2: A phase IB study evaluating buparlisib in combination with lapatinib in trastuzumabresistant HER2-positive advanced breast cancer. Eur. J. Cancer 2017, 86, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Richmond, A. The Role of PI3K Inhibition in the Treatment of Breast Cancer, Alone or Combined with Immune Checkpoint Inhibitors. Front. Mol. Biosci. 2021, 8, 648663. [Google Scholar] [CrossRef] [PubMed]
- Poggio, F.; Bruzzone, M. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: A systematic review and meta-analysis. Ann. Oncol. 2018, 29, 1497–1508. [Google Scholar] [CrossRef]
- Byrski, T.; Dent, R. Results of a phase II open-label, non-randomized trial of cisplatin chemotherapy in patients with BRCA1-positive metastatic breast cancer. Breast Cancer Res. 2012, 14, R110. [Google Scholar] [CrossRef] [Green Version]
- Tutt, A.; Tovey, H. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: The TNT Trial. Nat. Med. 2018, 24, 628–637. [Google Scholar] [CrossRef] [Green Version]
- Robson, M.; Im, S.A. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Litton, J.K.; Rugo, H.S. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef]
- Turner, N.C.; Telli, M.L. Final results of a phase 2 study of talazoparib (TALA) following platinum or multiple cytotoxic regimens in advanced breast cancer patients (pts) with germline BRCA1/2 mutations (ABRAZO). J. Clin. Oncol. 2017, 35 (Suppl. 15), 1007. [Google Scholar] [CrossRef]
- Andrè, F.; Zielinski, C.C. Optimal strategies for the treatment of metastatic triple-negative breast cancer with currently approved agents. Ann. Oncol. 2012, 23, vi46–vi51. [Google Scholar] [CrossRef]
- Shah, S.P.; Roth, A. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 2012, 486, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.; Turner, N.C. Targeting the PI3-kinase pathway in triple negative breast cancer. Ann. Oncol. 2019, 30, 1051–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drullinsky, P.R.; Hurvitz, S.A. Mechanistic basis for PI3K inhibitor antitumor activity and adverse reactions in advanced breast cancer. Breast Cancer Res. Treat. 2020, 181, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Chen, J.Q. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 2016, 6, 202–216. [Google Scholar] [CrossRef] [Green Version]
- LoRusso, P. Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J. Clin. Oncol. 2016, 34, 3803–3815. [Google Scholar] [CrossRef]
- Ibrahim, Y.H.; Garcia, G. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov. 2012, 2, 1036–1047. [Google Scholar] [CrossRef] [Green Version]
- Juvekar, A.; Burga, L.N. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov. 2012, 2, 1048–1063. [Google Scholar] [CrossRef] [Green Version]
- Matulonis, U.A.; Wulf, G.M. Phase I dose escalation study of the PI3kinase pathway inhibitor BKM120 and the oral poly (ADP ribose) polymerase (PARP) inhibitor olaparib for the treatment of highgrade serous ovarian and breast cancer. Ann. Oncol. 2017, 28, 512–518. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.M. Overall survival (os) results from SOLAR-1, a phase III study of alpelisib (ALP) + fulvestrant (FUL) for hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) advanced breast cancer (ABC). Ann. Oncol. 2020, 31 (Suppl. 4), S1150–S1151.76. [Google Scholar] [CrossRef]
- Baselga, J.; Dent, S.F. Phase III study of taselisib (GDC-0032) + fulvestrant (FULV) v FULV in patients (pts) with estrogen receptor (ER)-positive, PIK3CA-mutant (MUT), locally advanced or metastatic breast cancer (MBC): Primary analysis from SANDPIPER. J. Clin. Oncol. 2018, 36 (Suppl. 18), LBA1006. [Google Scholar] [CrossRef]
- Rugo, H.S.; Lerebours, F. Alpelisib (ALP) + fulvestrant (FUL) in patients (pts) with PIK3CA-mutated (mut) hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) advanced breast cancer (ABC) previously treated with cyclin-dependent kinase 4/6 inhibitor (CDKi) + aromatase inhibitor (AI): BYLieve study results. J. Clin. Oncol. 2020, 38, 1006. [Google Scholar]
- Mavratzas, A.; Marmé, F. Alpelisib in the treatment of metastatic HR+ breast cancer with PIK3CA mutations. Future Oncol. 2021, 17, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Juric, D.; Rodon, J. Phosphatidylinositol 3-kinase α-selective inhibition with alpelisib (BYL719) in PIK3CA-altered solid tumors: Results from the first-in-human study. J. Clin. Oncol. 2018, 36, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Juric, D.; Janku, F. Alpelisib Plus Fulvestrant in PIK3CA-altered and PIK3CA-wild-Type Estrogen Receptor-Positive Advanced Breast Cancer. JAMA Oncol. 2019, 5, e184475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertucci, F.; Finetti, P. Comparative genomic analysis of primary tumors and metastases in breast cancer. Oncotarget 2016, 7, 27208–27219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeselsohn, R.; Yelensky, R. Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin. Cancer Res. 2014, 20, 1757–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, M.J.; Jelovac, D. Detection of tumor PIK3CA status in metastatic breast cancer using peripheral blood. Clin. Cancer Res. 2012, 18, 3462–3469. [Google Scholar] [CrossRef] [Green Version]
- Juric, D.; Andre, F. Abstract P4-10-04: Clinical outcomes of alpelisib in hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer by next-generation sequencing-detected PIK3CA alteration status and phosphatase and tensin homolog loss: Biomarker analysis from the SOLAR-1 study. Cancer Res. 2020, 80 (Suppl. 4), P4-10-04. [Google Scholar]
- Lu, Y.S.; Lee, K.S. A phase Ib study of alpelisib or buparlisib combined with tamoxifen plus goserelin in premenopausal women with HR-positive HER2-negative advanced breast cancer. Clin. Cancer Res. 2020, 27, 408–417. [Google Scholar] [CrossRef]
- Juric, D.; Krop, I.K. Abstract LB-64: GDC-0032, a beta isoform-sparing PI3K inhibitor: Results of a first-in-human phase Ia dose escalation study. Cancer Res. 2013, 73, LB-64. [Google Scholar]
- Olivero, A.; Heffron, T. Discovery of GDC-0032: A beta-sparing PI3K inhibitor active against PIK3CA mutant tumors. Cancer Res. 2013, 73, DDT02-01. [Google Scholar]
- Juric, D.; Krop, I. Phase I dose-escalation study of taselisib, an oral PI3K inhibitor, in patients with advanced solid tumors. Cancer Discov. 2017, 7, 704–715. [Google Scholar] [CrossRef] [Green Version]
- Ndubaku, C.O.; Heffron, T.P. Discovery of 2-{3-[2-(1-isopropyl-3-methyl-1H-1,2-4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl]-1H-pyrazol-1-yl}-2-methylpropanamide (GDC-032): A b-sparing phosphoinositide 3-kinase inhibitor with high unbound exposure and robust in vivo antitumor activity. J. Med. Chem. 2013, 56, 4597–4610. [Google Scholar]
- Pohlmann, P.R.; Mayer, I.A. Resistance to Trastuzumab in Breast Cancer. Clin. Cancer Res. 2009, 15, 7479–7491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junttila, T.T.; Akita, R.W. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell 2009, 15, 429–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, S.; Shah, A.N. Phase I study of alpelisib (BYL-719) and trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) after trastuzumab and taxane therapy. Breast Cancer Res. Treat. 2018, 171, 371–381. [Google Scholar] [CrossRef]
- Barok, M.; Tanner, M. Trastuzumab-DM1 causes tumour growth inhibition by mitotic catastrophe in trastuzumab-resistant breast cancer cells in vivo. Breast Cancer Res. 2011, 13, R46. [Google Scholar] [CrossRef] [Green Version]
- Merlino, G.; Fiascarelli, A. Abstract 2160: MEN1611, a novel α-selective PI3K inhibitor in solid tumors. Cancer Res. 2018, 78 (Suppl. 13), 2160. [Google Scholar]
- Fiascarelli, A.; Merlino, G. Characterization of the mechanism of action and efficacy of MEN1611 (PA799), a novel PI3K inhibitor, in breast cancer preclinical models. Ann Oncol. 2019, 30 (Suppl. 5), v781–v782. [Google Scholar] [CrossRef]
- Hansen, A.R.; Shapiro, G. A first in human phase I study of AZD8186, a potent and selective inhibitor of PI3K in patients with advanced solid tumours as monotherapy and in combination with the dual mTORC1/2 inhibitor vistusertib (AZD2014) or abiraterone acetate. J. Clin. Oncol. 2017, 35, 2570. [Google Scholar] [CrossRef]
- Owusu-Brackett, N.; Zhao, M. Targeting PI3K alone and in combination with chemotherapy or immunotherapy in tumors with PTEN loss. Oncotarget 2020, 11, 969–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Zhu, G. The upregulation of PI3K/Akt and MAP kinase pathways is associated with resistance of microtubule-targeting drugs in prostate cancer. J. Cell. Biochem. 2015, 116, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Rodon, J.; Curigliano, G. A Phase Ib, open-label, dose-finding study of alpelisib in combination with paclitaxel in patients with advanced solid tumors. Oncotarget 2018, 9, 31709–31718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Abramson, V.G. Safety and efficacy results from Phase I study of BYL719 plus nab-paclitaxel in HER-2 negative metastatic breast cancer. Cancer Res. 2017, 77 (Suppl. 4), P6-11-08. [Google Scholar]
- Sharma, P.; Abramson, V.G. Clinical and biomarker results from phase I/II study of PI3K inhibitor BYL 719 (alpelisib) plus nab-paclitaxel in HER2-negative metastatic breast cancer. J. Clin. Oncol. 2018, 36, 1018. [Google Scholar] [CrossRef]
- Asghar, U.S.; Barr, A.R. Single-cell dynamics determines response to CDK4/6 inhibition in triple-negative breast cancer. Clin. Cancer Res. 2017, 23, 5561–5572. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, B.D.; Bauer, J.A. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res. 2014, 16, 406. [Google Scholar] [CrossRef] [Green Version]
- Lopez, J.S.; Miralles, M.S. PIPA: A phase Ib study of selective ß-isoform sparing phosphatidylinositol 3-kinase (PI3K) inhibitor taselisib (T) plus palbociclib (P) in patients (pts) with advanced solid cancers—Safety, tolerability, pharmacokinetic (PK), and pharmacodynamic (PD) analysis of the doublet combination. J. Clin. Oncol. 2019, 37 (Suppl. 15), 3087–3308. [Google Scholar]
- Teo, Z.L.; Versaci, S. Combined CDK4/6 and PI3Kα Inhibition Is Synergistic and Immunogenic in Triple-Negative Breast Cancer. Cancer Res. 2017, 77, 6340–6352. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://clinicaltrials.gov/ct2/show/study/NCT04191499 (accessed on 8 February 2022).
- Available online: https://clinicaltrials.gov/ct2/show/NCT04355520 (accessed on 15 February 2022).
- Available online: https://clinicaltrials.gov/ct2/show/NCT05025735 (accessed on 15 February 2022).
- Available online: https://clinicaltrials.gov/ct2/show/NCT03006172 (accessed on 8 February 2022).
- Available online: https://clinicaltrials.gov/ct2/show/study/NCT04108858 (accessed on 15 February 2022).
- Available online: https://clinicaltrials.gov/ct2/show/study/NCT04208178 (accessed on 15 February 2022).
- Available online: https://clinicaltrials.gov/ct2/show/NCT03767335 (accessed on 15 February 2022).
- Available online: https://www.clinicaltrials.gov/ct2/show/NCT04586335 (accessed on 16 February 2022).
- Available online: https://clinicaltrials.gov/ct2/show/study/NCT03218826 (accessed on 16 February 2022).
- Available online: https://www.clinicaltrials.gov/ct2/show/NCT04251533 (accessed on 15 February 2022).
- Available online: https://clinicaltrials.gov/ct2/show/NCT03961698 (accessed on 16 February 2022).
- Available online: https://clinicaltrials.gov/ct2/show/NCT02637531 (accessed on 16 February 2022).
- Available online: https://clinicaltrials.gov/ct2/show/NCT02646748 (accessed on 16 February 2022).
- Available online: https://clinicaltrials.gov/ct2/show/study/NCT04345913 (accessed on 16 February 2022).
- Available online: https://clinicaltrials.gov/ct2/show/record/NCT02457910 (accessed on 16 February 2022).
- Available online: https://clinicaltrials.gov/ct2/show/NCT03207529 (accessed on 16 February 2022).
- Eltantawy, A.; Vallejos, X. Copanlisib: An Intravenous Phosphatidylinositol 3-Kinase (PI3K) Inhibitor for the Treatment of Relapsed Follicular Lymphoma. Ann. Pharmacother. 2019, 53, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov/ct2/show/NCT05063786 (accessed on 15 February 2022).
- Kaneda, M.; Messer, K. PI3Kγ is a molecular switch that controls immune suppression. Nature 2016, 539, 437–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Henau, O.; Rausch, M. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature 2016, 539, 443–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Trials | Phase | Patient Population | Targeted Therapy | Treatment | mPFS (Months) | mOS (Months) | ORr (%) | |
|---|---|---|---|---|---|---|---|---|
| ITT | Mutated PIK3CA | ITT | ITT | |||||
| BELLE-2 (Baselga)[23] NCT01610284 | III | HR+/HER2− LABC or MBC, resistant to AI | pan-PI3K inhibitor | Buparlisib + Fulvestrant | 6.9 (HR 0.78) | 7.0 (HR 0.58) | NA | 11.8 |
| Placebo + Fulvestrant | 5.0 | 3.2 | NA | 7.7 | ||||
| BELLE-3 (Di Leo)[18] NCT01633060 | III | HR+/HER2− LABC or MBC, resistant to mTOR | Buparlisib + Fulvestrant | 3.9 (HR 0.67) | 4.2 (HR 0.46) | NA | 8 | |
| Placebo + Fulvestrant | 1.8 | 1.6 | NA | 2 | ||||
| BELLE-4 (Martin)[21] NCT01572727 | II-III | HER2− LABC or MBC | Buparlisib + Paclitaxel | 8.0 (HR 1.18) | 9.1 (HR 1.17) | NA | 22.6 | |
| Placebo + Paclitaxel | 9.2 | 9.2 | NA | 27.1 | ||||
| FERGI (Krop)[24] NCT01437566 | II | HR+/HER2− LABC or MBC, resistant to AI (only PIK3CA | Pictilisib + Fulvestrant | 6.6 (HR 0.74) | 6.5 (HR 0.73) | NA | 7.9 | |
| Placebo + Fulvestrant | 5.1 | 5.1 | NA | 6.3 | ||||
| PEGGY (Vuylsteke) [25] NCT01740336 | II | HR+/HER2− LABC or MBC first/second line CT | Pictilisib + Paclitaxel | 8.2 (HR 0.83) | 7.3 (HR 1.06) | NA | 22 | |
| Placebo + Paclitaxel | 7.8 | 5.8 | NA | 19.6 | ||||
| Trial | Phase | Patient Population | Targeted Therapy | Treatment | mPFS (Months) | mOS (Months) | Orr (%) | |
|---|---|---|---|---|---|---|---|---|
| Wild Type PI3KCA | Mutated PIK3CA | Mutated PIK3CA | Mutated PIK3CA | |||||
| SOLAR-1 (Andrè) [69,70] NCT02437318 | III | HR+/HER2− MBC, resistant to AI | PI3Kα inhibitor | Alpelisib + Fulvestrant | 7.4 (HR 0.85) | 11.0 (HR 0.65) | 39.3 (HR 0.86) | 26.6 |
| Placebo + Fulvestrant | 5.6 | 5.7 | 31.4 | 12.8 | ||||
| SANDPIPER (Baselga)[71] NCT02340221 | III | HR+/HER2− LABC or MBC, resistant to AI | Taselisib + Fulvestrant | 5.6 (HR 0.69) | 7.4 (HR 0.70) | NA | 28 | |
| Placebo + Fulvestrant | 4.0 | 5.4 | NA | 11.9 | ||||
| BYLieve (Rugo)[72] NCT03056755 | II | HR+/HER2− PIK3CA-mutated MBC, after CDKi + ET or CT or ET | Alpelisib + AI +/− LHRHa | - | NA | NA | NA | |
| Alpelisib + Fulvestrant +/− LHRHa | - | 7.5 | NA | 21 | ||||
| BC Subtypes | Trials | Phase | Patient Population | Targeted Therapy | Treatment | Primary Endpoint | Secondary Endpoint |
|---|---|---|---|---|---|---|---|
| HR+/ HER2− | NCT04191499 [101] (INAVO 120) | II/III | 400 | PI3Kα-inhibitor | Inavolisib (GDC-0077) Palbociclib Fulvestrant | PFS | ORR BOR DOR CBR OS TTD AE |
| NCT04355520 [102] | I/II | 42 | PI3K α/δ-inhibitor | TQ-B3525 Fulvestrant | DLT | ORR DCR DOR PFS OS | |
| NCT05025735 [103] | II | 25 | PI3Kα-inhibitor | Alpelisib Dapagliflozin Fulvestrant | Incidence of all grade hyperglycemia | Incidence of grade 3/4 hyperglycemia ORR PFS | |
| HER2+ | NCT03006172 [104] | I | 256 | PI3Kα-inhibitor | Inavolisib (GDC-0077) Fulvestrant Letrozole Palbociclib Metformin Trastuzumab Pertuzumab | DLTs | AUC Half-Life Cmax Cmin Time to Cmax of Inavolisib Apparent Clearance of Inavolisib ORR CBR DOR PFS |
| NCT04108858 [105] | Ib/II | 102 | pan-PI3K—PI3K α/δ-inhibitor | Copanlisib Petruzumab Trastuzumab | AEs and SAEs DLTs PFS | PFS OS AEs and SAEs | |
| NCT04208178 [106] | III | 588 | PI3Kα-inhibitor | Alpelisib Pertuzumab Trastuzumab | DLTs PFS | OS ORR CBR TTR DOR | |
| NCT03767335 (B-PRECISE 01)[107] | I | 48 | PI3K α/β/γ-inhibitor | MEN1611 Trastuzumab Fulvestrant | MTD | TEAEs OS PFS | |
| NCT05063786 (ALPHABET)1 [9] | III | 300 | PI3Kα-inhibitor | Alpelisib Trastuzumab Fulvestrant Vinorelbine/ Capecitabine or Eribulin | PFS | OS OR Safety profile | |
| HR+/ HER2 and TNBC | NCT04586335 [108] | Ib | 350 | PI3Kα-inhibitor | CYH33 Olaparib | DLT ORR | AEs DCR Pharmacokinetic measures |
| NCT03218826 [109] | I | 58 | PI3Kβ-inhibitor | AZD8186 Docetaxel | MTD AEs | ORR CBR Drug—Drug interaction | |
| TNBC | NCT04251533 (EPIK B3)[110] | III | 566 | PI3Kα-inhibitor | Alpelisib Nab-paclitaxel | PFS ORR | OS CBR ORr TTR DOR |
| NCT03961698 (MARIO-3)[111] | II | 90 | PI3Kγ-inhibitor | IPI-549 (eganelisib) Nab-paclitaxel Atezolizumab | CR | TEAEs SAEs AEs ORr TTR DOR PFS | |
| NCT02637531 [112] | I | 219 | PI3Kγ-inhibitor | IPI-549 (eganelisib) Nivolumab | DLT AEs | AEs and safety laboratory Values Plasma concentrations of IPI-549 ORr CR PR DOR PFS OS | |
| NCT02646748 [113] | Ib | 159 | PI3Kδ-inhibitor | INCB050465 Pembrolizumab | Safety and tolerability profile | ORr Change in the number of TILs and the ratio of CD8+ lymphocytes to FOXP3+ cells infiltrating tumor post-treatment versus pretreatment by IHC | |
| NCT04345913 [114] | I/II | 18 | pan-PI3K-inhibitor / PI3K α/δ-inhibitor | Copanlisib Eribulina | MTD PFS | ORR CBR PFS | |
| TNBC AR+ | NCT02457910 [115] | I/II | 30 | PI3K α/δ/γ- inhibitor | Taselisib Enzalutamide | CBR MTD | PFS Pharmacokinetic profile |
| NCT03207529 [116] | I | 28 | PI3Kα-inhibitor | Alpelisib Enzalutamide | MDT RP2D | DLT Safety profile |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuso, P.; Muratore, M.; D’Angelo, T.; Paris, I.; Carbognin, L.; Tiberi, G.; Pavese, F.; Duranti, S.; Orlandi, A.; Tortora, G.; et al. PI3K Inhibitors in Advanced Breast Cancer: The Past, The Present, New Challenges and Future Perspectives. Cancers 2022, 14, 2161. https://doi.org/10.3390/cancers14092161
Fuso P, Muratore M, D’Angelo T, Paris I, Carbognin L, Tiberi G, Pavese F, Duranti S, Orlandi A, Tortora G, et al. PI3K Inhibitors in Advanced Breast Cancer: The Past, The Present, New Challenges and Future Perspectives. Cancers. 2022; 14(9):2161. https://doi.org/10.3390/cancers14092161
Chicago/Turabian StyleFuso, Paola, Margherita Muratore, Tatiana D’Angelo, Ida Paris, Luisa Carbognin, Giordana Tiberi, Francesco Pavese, Simona Duranti, Armando Orlandi, Giampaolo Tortora, and et al. 2022. "PI3K Inhibitors in Advanced Breast Cancer: The Past, The Present, New Challenges and Future Perspectives" Cancers 14, no. 9: 2161. https://doi.org/10.3390/cancers14092161
APA StyleFuso, P., Muratore, M., D’Angelo, T., Paris, I., Carbognin, L., Tiberi, G., Pavese, F., Duranti, S., Orlandi, A., Tortora, G., Scambia, G., & Fabi, A. (2022). PI3K Inhibitors in Advanced Breast Cancer: The Past, The Present, New Challenges and Future Perspectives. Cancers, 14(9), 2161. https://doi.org/10.3390/cancers14092161









