Recent Trends and In-Hospital Mortality of Transarterial Chemoembolization (TACE) in Germany: A Systematic Analysis of Hospital Discharge Data between 2010 and 2019
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection Criteria and Variables
2.3. Statistical Analysis
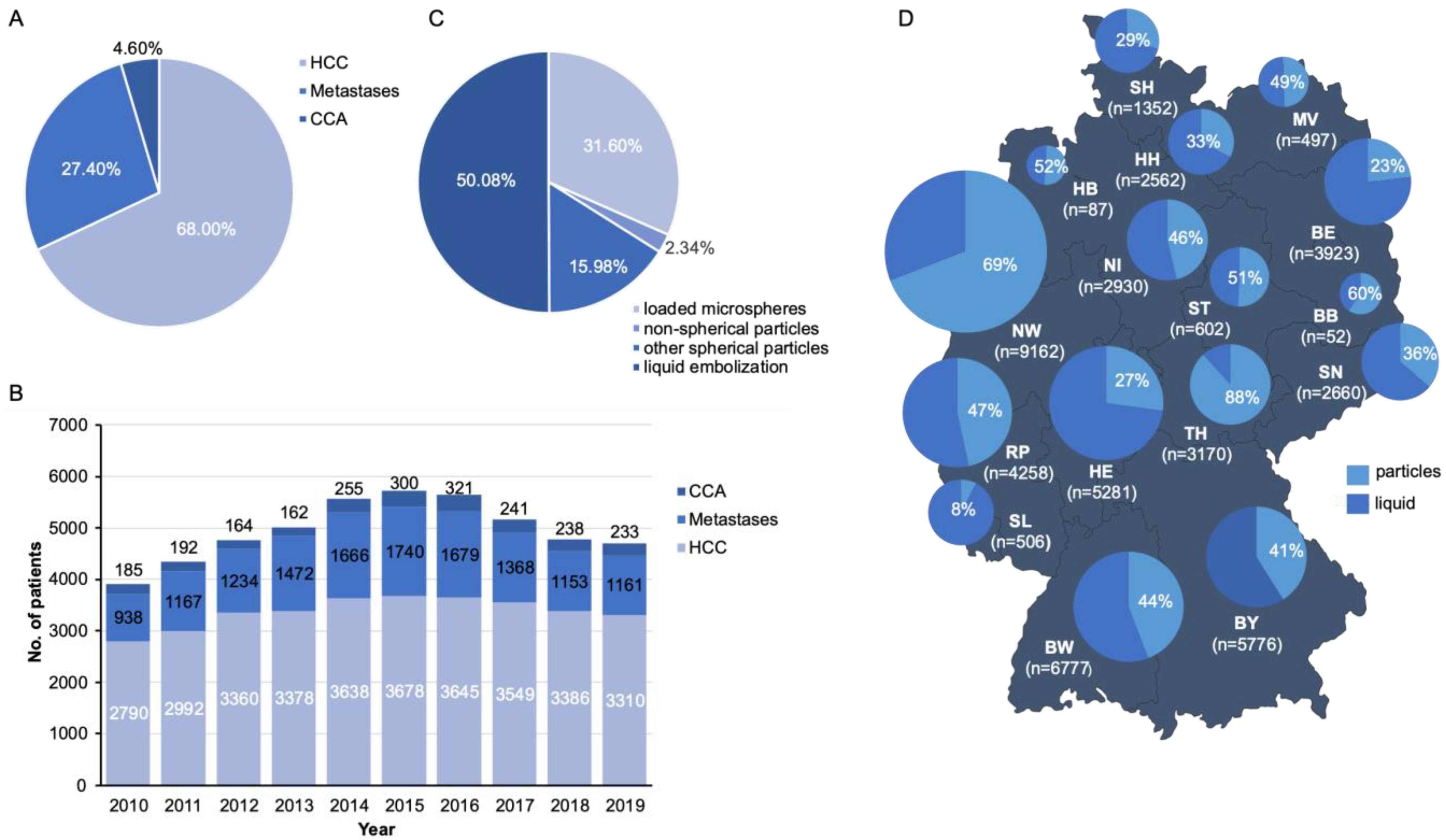
3. Results
3.1. Current Trends of TACE in Germany between 2010 and 2019
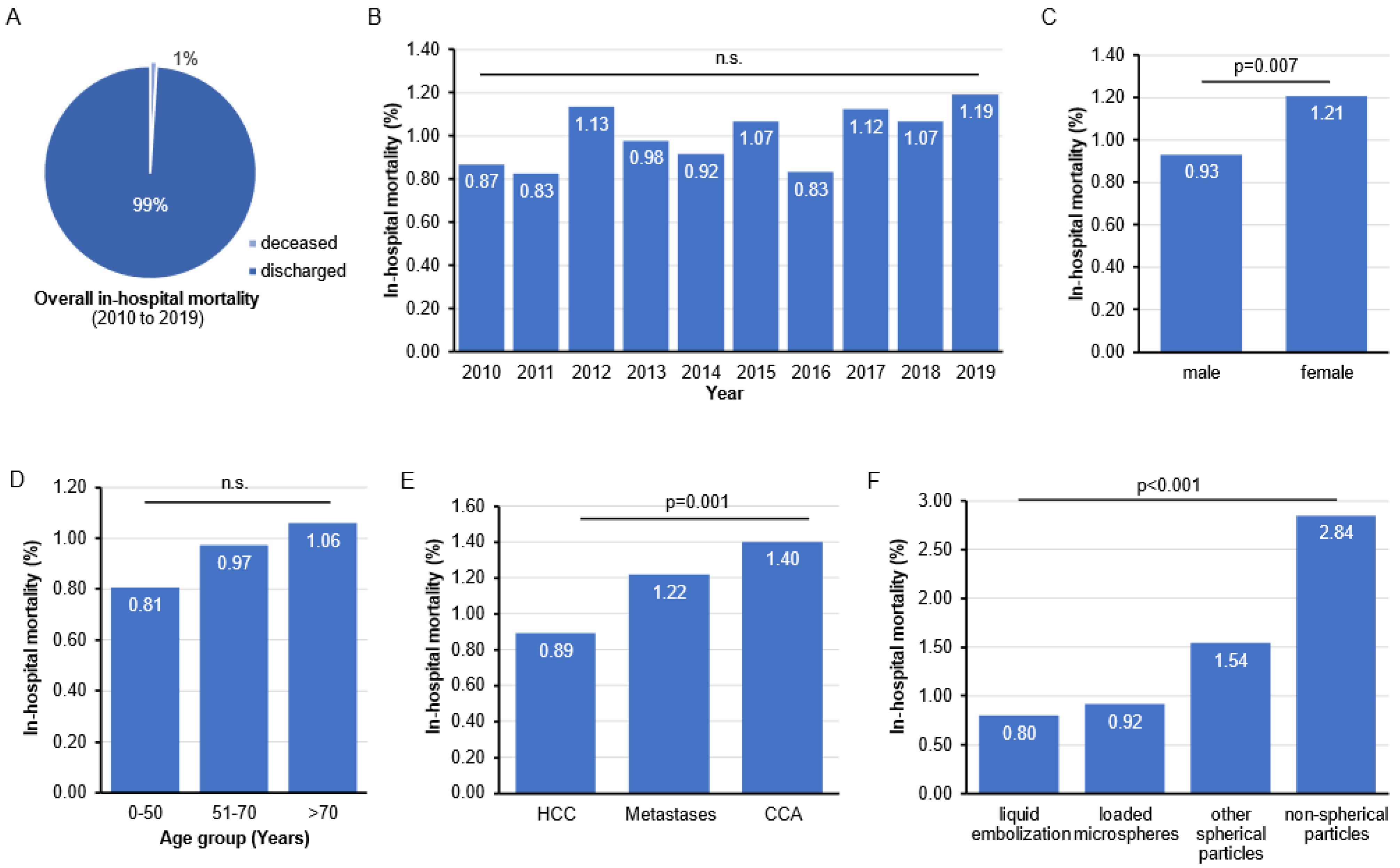
3.2. In-Hospital Mortality Rates following TACE in Germany
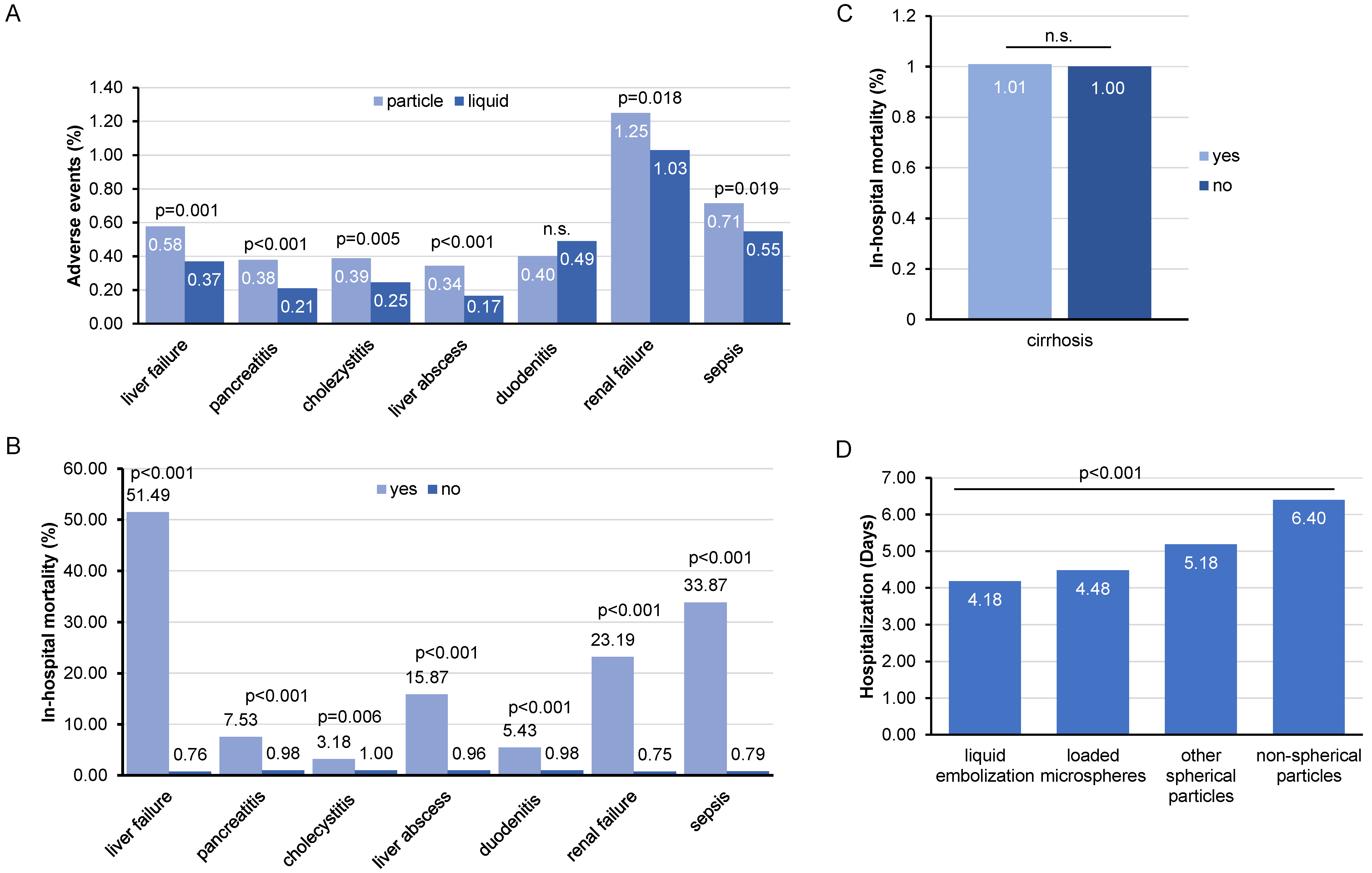
3.3. Post-Interventional Complications following TACE and Their Impact on In-Hospital Mortality
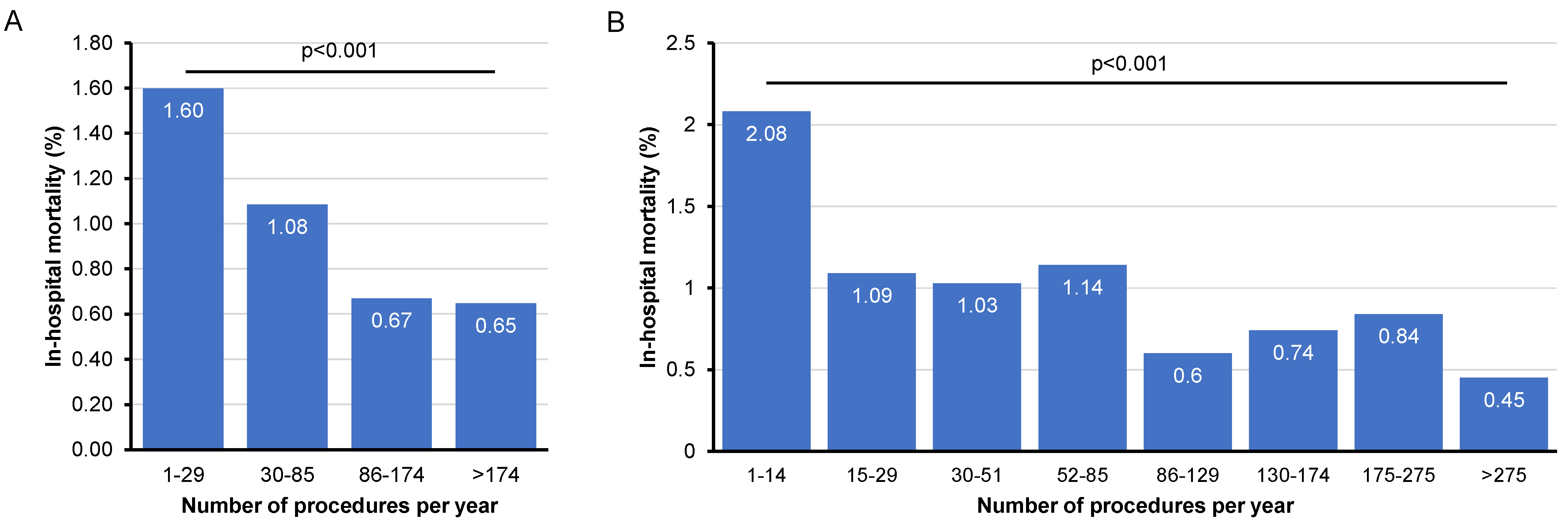
3.4. Influence of Hospital Case Volume on In-Hospital Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamada, R.; Sato, M.; Kawabata, M.; Nakatsuka, H.; Nakamura, K.; Takashima, S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology 1983, 148, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Zangos, S.; Balzer, J.O.; Nabil, M.; Rao, P.; Eichler, K.; Bechstein, W.O.; Zeuzem, S.; Abdelkader, A. Transarterial chemoembolization (TACE) in hepatocellular carcinoma: Technique, indication and results. RoFo Fortschr. Auf Dem Geb. Rontgenstrahlen Nukl. 2007, 179, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Mahnken, A.H. Transarterial ablative therapy of hepatocellular carcinoma. Radiologe 2014, 54, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Song, J.E.; Kim, D.Y. Conventional vs drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma. World J. Hepatol. 2017, 9, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R. Loco-regional treatment of hepatocellular carcinoma. Hepatology 2010, 52, 762–773. [Google Scholar] [CrossRef]
- Llovet, J.M.; Real, M.I.; Montaña, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Solà, R.; et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet 2002, 359, 1734–1739. [Google Scholar] [CrossRef]
- Lo, C.M.; Ngan, H.; Tso, W.K.; Liu, C.L.; Lam, C.M.; Poon, R.T.; Fan, S.T.; Wong, J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002, 35, 1164–1171. [Google Scholar] [CrossRef]
- Osuga, K.; Maeda, N.; Higashihara, H.; Hori, S.; Nakazawa, T.; Tanaka, K.; Nakamura, M.; Kishimoto, K.; Ono, Y.; Tomiyama, N. Current status of embolic agents for liver tumor embolization. Int. J. Clin. Oncol. 2012, 17, 306–315. [Google Scholar] [CrossRef]
- Javanbakht, S.; Namazi, H. Doxorubicin loaded carboxymethyl cellulose/graphene quantum dot nanocomposite hydrogel films as a potential anticancer drug delivery system. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 87, 50–59. [Google Scholar] [CrossRef]
- Luo, J.; Xu, L.; Li, L.; Zhang, J.; Zhang, M.; Xu, M. Comparison of treatments for hepatocellular carcinoma patients with portal vein thrombosis: A systematic review and network meta-analysis. Ann. Transl. Med. 2021, 9, 1450. [Google Scholar] [CrossRef]
- Sneiders, D.; Boteon, A.; Lerut, J.; Iesari, S.; Gilbo, N.; Blasi, F.; Larghi Laureiro, Z.; Orlacchio, A.; Tisone, G.; Lai, Q.; et al. Transarterial chemoembolization of hepatocellular carcinoma before liver transplantation and risk of post-transplant vascular complications: A multicentre observational cohort and propensity score-matched analysis. Br. J. Surg. 2021, 108, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Jia, Z.; Ying, X.; Zhang, D.; Li, S.; Tian, F.; Jiang, G. The incidence and outcome of major complication following conventional TAE/TACE for hepatocellular carcinoma. Medicine 2016, 95, e5606. [Google Scholar] [CrossRef] [PubMed]
- Marcacuzco Quinto, A.; Nutu, O.A.; San Román Manso, R.; Justo Alonso, I.; Calvo Pulido, J.; Manrique Municio, A.; García-Sesma, Á.; Loinaz Segurola, C.; Martínez Caballero, J.; Jiménez Romero, L.C. Complications of transarterial chemoembolization (TACE) in the treatment of liver tumors. Cir. Esp. 2018, 96, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Garwood, E.R.; Fidelman, N.; Hoch, S.E.; Kerlan, R.K., Jr.; Yao, F.Y. Morbidity and mortality following transarterial liver chemoembolization in patients with hepatocellular carcinoma and synthetic hepatic dysfunction. Liver Transplant. 2013, 19, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Ren, Z.; Ye, S.; Sharma, D.; Lin, Z.; Gan, Y.; Chen, Y.; Ge, N.; Ma, Z.; Wu, Z.; et al. Study of severe and rare complications of transarterial chemoembolization (TACE) for liver cancer. Eur. J. Radiol. 2006, 59, 407–412. [Google Scholar] [CrossRef]
- Cohen, M.J.; Bloom, A.I.; Barak, O.; Klimov, A.; Nesher, T.; Shouval, D.; Levi, I.; Shibolet, O. Trans-arterial chemo-embolization is safe and effective for very elderly patients with hepatocellular carcinoma. World J. Gastroenterol. 2013, 19, 2521–2528. [Google Scholar] [CrossRef]
- Nishikawa, H.; Arimoto, A.; Wakasa, T.; Kita, R.; Kimura, T.; Osaki, Y. Effect of transcatheter arterial chemoembolization prior to surgical resection for hepatocellular carcinoma. Int. J. Oncol. 2013, 42, 151–160. [Google Scholar] [CrossRef]
- Varela, M.; Real, M.I.; Burrel, M.; Forner, A.; Sala, M.; Brunet, M.; Ayuso, C.; Castells, L.; Montañá, X.; Llovet, J.M.; et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: Efficacy and doxorubicin pharmacokinetics. J. Hepatol. 2007, 46, 474–481. [Google Scholar] [CrossRef]
- Nouri, Y.M.; Kim, J.H.; Yoon, H.K.; Ko, H.K.; Shin, J.H.; Gwon, D.I. Update on Transarterial Chemoembolization with Drug-Eluting Microspheres for Hepatocellular Carcinoma. Korean J. Radiol. 2019, 20, 34–49. [Google Scholar] [CrossRef]
- Li, H.; Wu, F.; Duan, M.; Zhang, G. Drug-eluting bead transarterial chemoembolization (TACE) vs conventional TACE in treating hepatocellular carcinoma patients with multiple conventional TACE treatments history: A comparison of efficacy and safety. Medicine 2019, 98, e15314. [Google Scholar] [CrossRef]
- Lammer, J.; Malagari, K.; Vogl, T.; Pilleul, F.; Denys, A.; Watkinson, A.; Pitton, M.; Sergent, G.; Pfammatter, T.; Terraz, S.; et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: Results of the PRECISION V study. Cardiovasc. Interv. Radiol. 2010, 33, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Recchia, F.; Passalacqua, G.; Filauri, P.; Doddi, M.; Boscarato, P.; Candeloro, G.; Necozione, S.; Desideri, G.; Rea, S. Chemoembolization of unresectable hepatocellular carcinoma: Decreased toxicity with slow-release doxorubicin-eluting beads compared with lipiodol. Oncol. Rep. 2012, 27, 1377–1383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sacco, R.; Bargellini, I.; Bertini, M.; Bozzi, E.; Romano, A.; Petruzzi, P.; Tumino, E.; Ginanni, B.; Federici, G.; Cioni, R.; et al. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J. Vasc. Interv. Radiol. 2011, 22, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- van Malenstein, H.; Maleux, G.; Vandecaveye, V.; Heye, S.; Laleman, W.; van Pelt, J.; Vaninbroukx, J.; Nevens, F.; Verslype, C. A randomized phase II study of drug-eluting beads versus transarterial chemoembolization for unresectable hepatocellular carcinoma. Onkologie 2011, 34, 368–376. [Google Scholar] [CrossRef]
- Scartozzi, M.; Baroni, G.S.; Faloppi, L.; Paolo, M.D.; Pierantoni, C.; Candelari, R.; Berardi, R.; Antognoli, S.; Mincarelli, C.; Risaliti, A.; et al. Trans-arterial chemo-embolization (TACE), with either lipiodol (traditional TACE) or drug-eluting microspheres (precision TACE, pTACE) in the treatment of hepatocellular carcinoma: Efficacy and safety results from a large mono-institutional analysis. J. Exp. Clin. Cancer Res. 2010, 29, 164. [Google Scholar] [CrossRef]
- Bzeizi, K.I.; Arabi, M.; Jamshidi, N.; Albenmousa, A.; Sanai, F.M.; Al-Hamoudi, W.; Alghamdi, S.; Broering, D.; Alqahtani, S.A. Conventional Transarterial Chemoembolization Versus Drug-Eluting Beads in Patients with Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 6172. [Google Scholar] [CrossRef]
- Song, M.J.; Chun, H.J.; Song, D.S.; Kim, H.Y.; Yoo, S.H.; Park, C.H.; Bae, S.H.; Choi, J.Y.; Chang, U.I.; Yang, J.M.; et al. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J. Hepatol. 2012, 57, 1244–1250. [Google Scholar] [CrossRef]
- Dhanasekaran, R.; Kooby, D.A.; Staley, C.A.; Kauh, J.S.; Khanna, V.; Kim, H.S. Comparison of conventional transarterial chemoembolization (TACE) and chemoembolization with doxorubicin drug eluting beads (DEB) for unresectable hepatocelluar carcinoma (HCC). J. Surg. Oncol. 2010, 101, 476–480. [Google Scholar] [CrossRef]
- Meyer, T.; Kirkwood, A.; Roughton, M.; Beare, S.; Tsochatzis, E.; Yu, D.; Davies, N.; Williams, E.; Pereira, S.P.; Hochhauser, D.; et al. A randomised phase II/III trial of 3-weekly cisplatin-based sequential transarterial chemoembolisation vs embolisation alone for hepatocellular carcinoma. Br. J. Cancer 2013, 108, 1252–1259. [Google Scholar] [CrossRef]
- Malagari, K.; Pomoni, M.; Kelekis, A.; Pomoni, A.; Dourakis, S.; Spyridopoulos, T.; Moschouris, H.; Emmanouil, E.; Rizos, S.; Kelekis, D. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc. Interv. Radiol. 2010, 33, 541–551. [Google Scholar] [CrossRef]
- Facciorusso, A.; Di Maso, M.; Muscatiello, N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: A meta-analysis. Dig. Liver Dis. 2016, 48, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.B.; Wang, X.B.; Peng, Y.C.; Zhu, S.L.; Ma, L.; Xiang, B.D.; Gong, W.F.; Chen, J.; You, X.M.; Jiang, J.H.; et al. Systematic review comparing the safety and efficacy of conventional and drug-eluting bead transarterial chemoembolization for inoperable hepatocellular carcinoma. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2015, 45, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yuan, P.; Chen, B.; Sun, J.; Shen, H.; Qian, Y. Evaluation of drug-eluting beads versus conventional transcatheter arterial chemoembolization in patients with unresectable hepatocellular carcinoma: A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.H.; Zhang, L.; Ren, Z.G.; Ye, S.L. Efficacy and safety of cTACE versus DEB-TACE in patients with hepatocellular carcinoma: A meta-analysis. J. Dig. Dis. 2016, 17, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, C.; Wei, X.; Shen, K.; Shu, Y.; Wan, X.; Sun, J.; Ren, X.; Dong, Y.; Liu, Y.; et al. A comparison between drug-eluting bead-transarterial chemoembolization and conventional transarterial chemoembolization in patients with hepatocellular carcinoma: A meta-analysis of six randomized controlled trials. J. Cancer Res. Ther. 2020, 16, 243–249. [Google Scholar] [CrossRef]
- Poursaid, A.; Jensen, M.M.; Huo, E.; Ghandehari, H. Polymeric materials for embolic and chemoembolic applications. J. Control. Release 2016, 240, 414–433. [Google Scholar] [CrossRef]
| Study Population | |
|---|---|
| Total number of TACE procedures | 49,595 |
| In-hospital death (total) | 497 |
| In-hospital mortality rate (%) | 1.00 |
| Sex (total) | |
| Male | 36,567 |
| Female | 12,028 |
| Age (Mean and SD) | 67.71 (10.40) |
| Age group (total) | |
| 0–17 years | 11 |
| 18–30 years | 112 |
| 31–50 years | 2853 |
| 51–70 years | 24,639 |
| >70 years | 21,980 |
| Federal state (total) | |
| Baden-Württemberg | 6777 |
| Bavaria | 5776 |
| Berlin | 3923 |
| Brandenburg | 52 |
| Bremen | 87 |
| Hamburg | 2562 |
| Hesse | 5281 |
| Lower Saxony | 2930 |
| Mecklenburg-Western Pomerania | 497 |
| North Rhine-Westphalia | 9162 |
| Rhineland-Palatinate | 4258 |
| Saarland | 506 |
| Saxony | 2660 |
| Saxony-Anhalt | 602 |
| Schleswig-Holstein | 1352 |
| Thuringia | 3170 |
| Underlying diagnosis for TACE (total) | |
| Hepatocellular Carcinoma (HCC) | 33,726 |
| Cholangiocellular Carcinoma (CCA) | 13,578 |
| Liver Metastases | 2291 |
| Acute or subacute liver failure (total) | |
| Yes | 235 |
| No | 49,360 |
| Acute pancreatitis (total) | |
| Yes | 146 |
| No | 49,449 |
| Cholecystitis (total) | |
| Yes | 157 |
| No | 49,438 |
| Liver abscess (total) | |
| Yes | 126 |
| No | 49,469 |
| Duodenitis (total) | |
| Yes | 221 |
| No | 49,374 |
| Gastritis (total) | |
| Yes | 1857 |
| No | 47,738 |
| Acute kidney failure (total) | |
| Yes | 565 |
| No | 49,030 |
| Sepsis (total) | |
| Yes | 313 |
| No | 49,282 |
| Cirrhosis (total) | |
| Yes | 16,552 |
| No | 49,282 |
| TACE procedures (total) | |
| Selective embolization with drug-eluting particles | 15,670 |
| Selective embolization with non-spherical particles | 1160 |
| Selective embolization with spherical particles | 7926 |
| Selective embolization with embolizing liquids | 24,830 |
| Annual TACE case volume groups based on quartiles (total) | |
| LVC 1–29 (cases/year) | 12,507 |
| MLVC (30–85 cases/year) | 12,363 |
| MHVC (86–174 cases/year) | 12,369 |
| HVC (>174 cases/year) | 12,356 |
| Annual TACE case volume groups based on octiles (total) | |
| 1–14 cases/year | 6452 |
| 15–29 cases/year | 6055 |
| 30–51 cases/year | 6146 |
| 52–85 cases/year | 6217 |
| 86–129 cases/year | 6127 |
| 130–174 cases/year | 6242 |
| 175–275 cases/year | 6321 |
| >275 cases/year | 6035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krieg, S.; Essing, T.; Krieg, A.; Roderburg, C.; Luedde, T.; Loosen, S.H. Recent Trends and In-Hospital Mortality of Transarterial Chemoembolization (TACE) in Germany: A Systematic Analysis of Hospital Discharge Data between 2010 and 2019. Cancers 2022, 14, 2088. https://doi.org/10.3390/cancers14092088
Krieg S, Essing T, Krieg A, Roderburg C, Luedde T, Loosen SH. Recent Trends and In-Hospital Mortality of Transarterial Chemoembolization (TACE) in Germany: A Systematic Analysis of Hospital Discharge Data between 2010 and 2019. Cancers. 2022; 14(9):2088. https://doi.org/10.3390/cancers14092088
Chicago/Turabian StyleKrieg, Sarah, Tobias Essing, Andreas Krieg, Christoph Roderburg, Tom Luedde, and Sven H. Loosen. 2022. "Recent Trends and In-Hospital Mortality of Transarterial Chemoembolization (TACE) in Germany: A Systematic Analysis of Hospital Discharge Data between 2010 and 2019" Cancers 14, no. 9: 2088. https://doi.org/10.3390/cancers14092088
APA StyleKrieg, S., Essing, T., Krieg, A., Roderburg, C., Luedde, T., & Loosen, S. H. (2022). Recent Trends and In-Hospital Mortality of Transarterial Chemoembolization (TACE) in Germany: A Systematic Analysis of Hospital Discharge Data between 2010 and 2019. Cancers, 14(9), 2088. https://doi.org/10.3390/cancers14092088









