KRAS Mutations Impact Clinical Outcome in Metastatic Non-Small Cell Lung Cancer
Abstract
:Simple Summary
Abstract
1. Background
2. Materials and Methods
2.1. Patient Population
2.2. Mutational Status
2.3. PD-L1 Expression
2.4. ICB Treatment
2.5. PT Treatment
2.6. Study Objectives
2.7. Statistical Analysis
3. Results
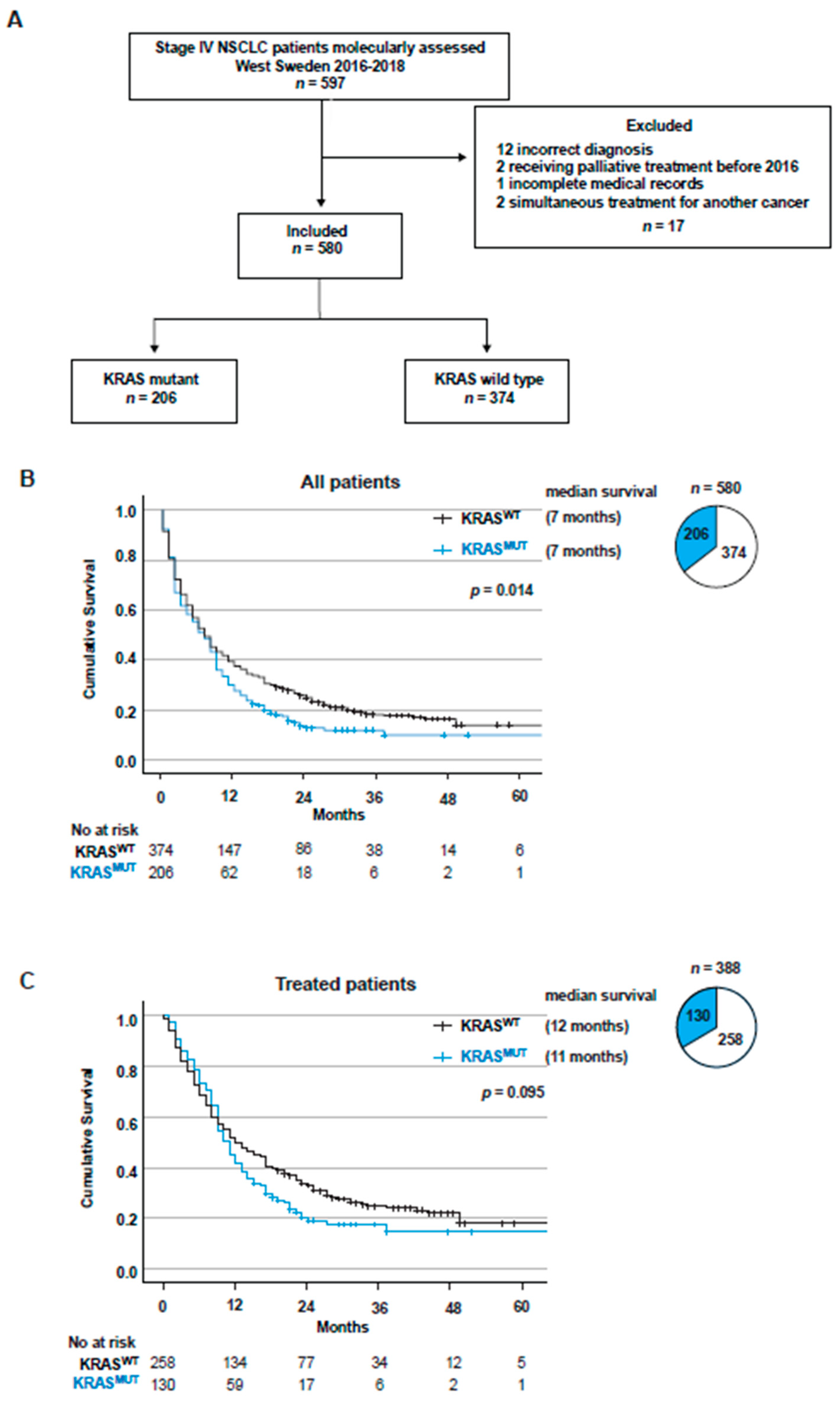
3.1. Patients and Tumor Characteristics
3.2. KRAS Mutations Are a Negative Factor for Overall Survival in Stage IV NSCLC
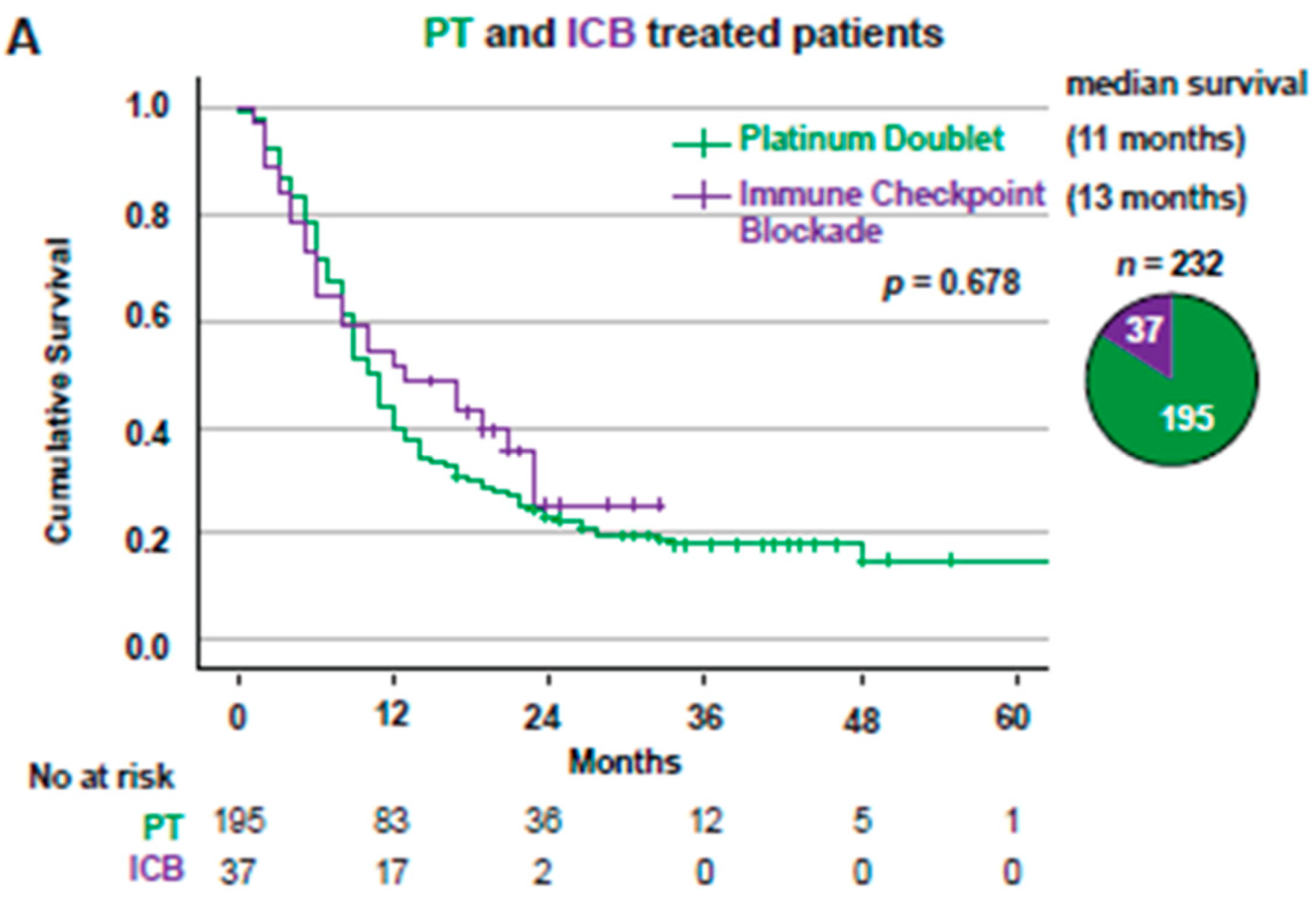
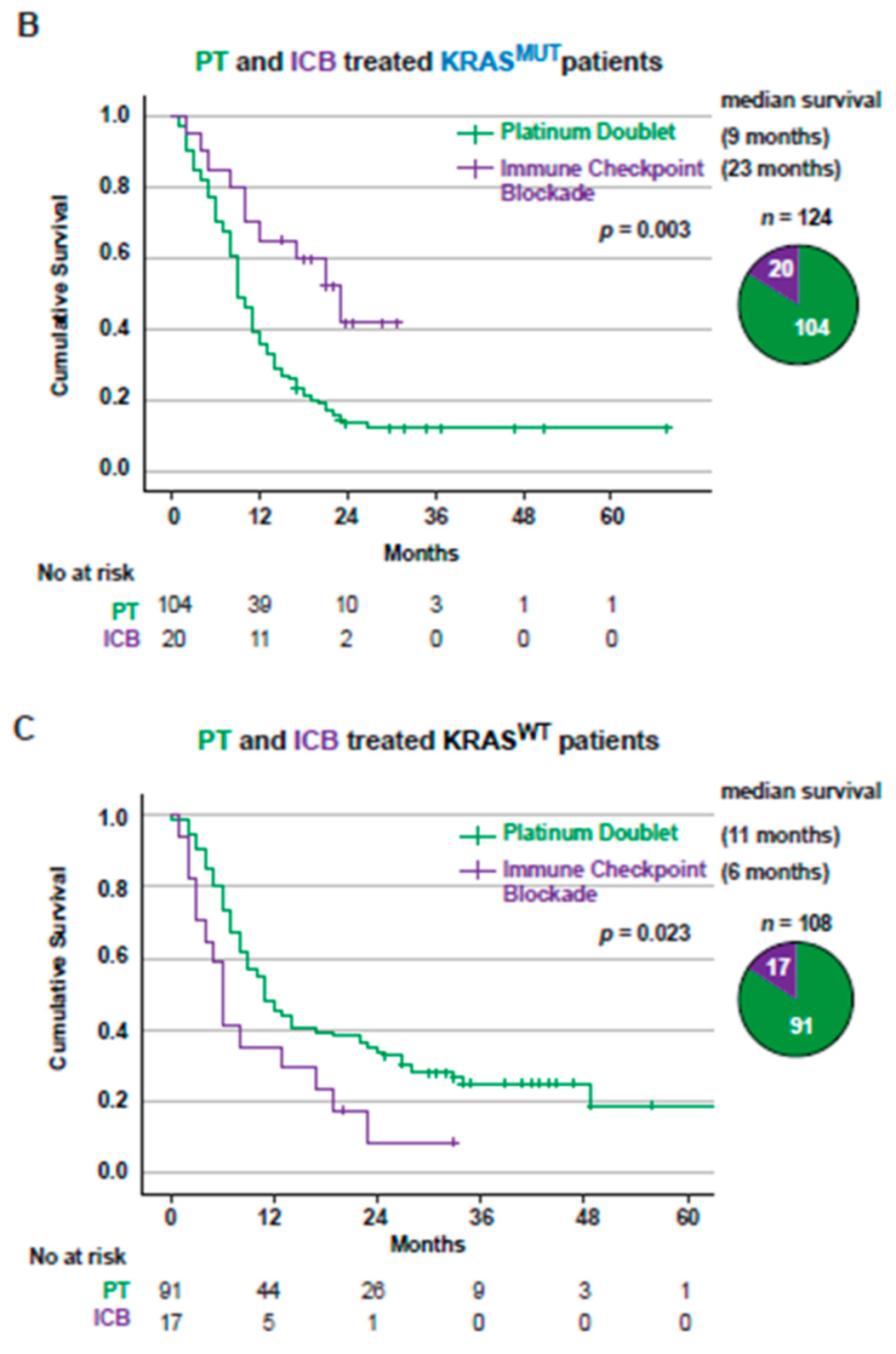
3.3. Overall Survival for Stage IV NSCLC Patients Treated with First-Line PT or ICB Stratified Based on KRAS Mutational Status
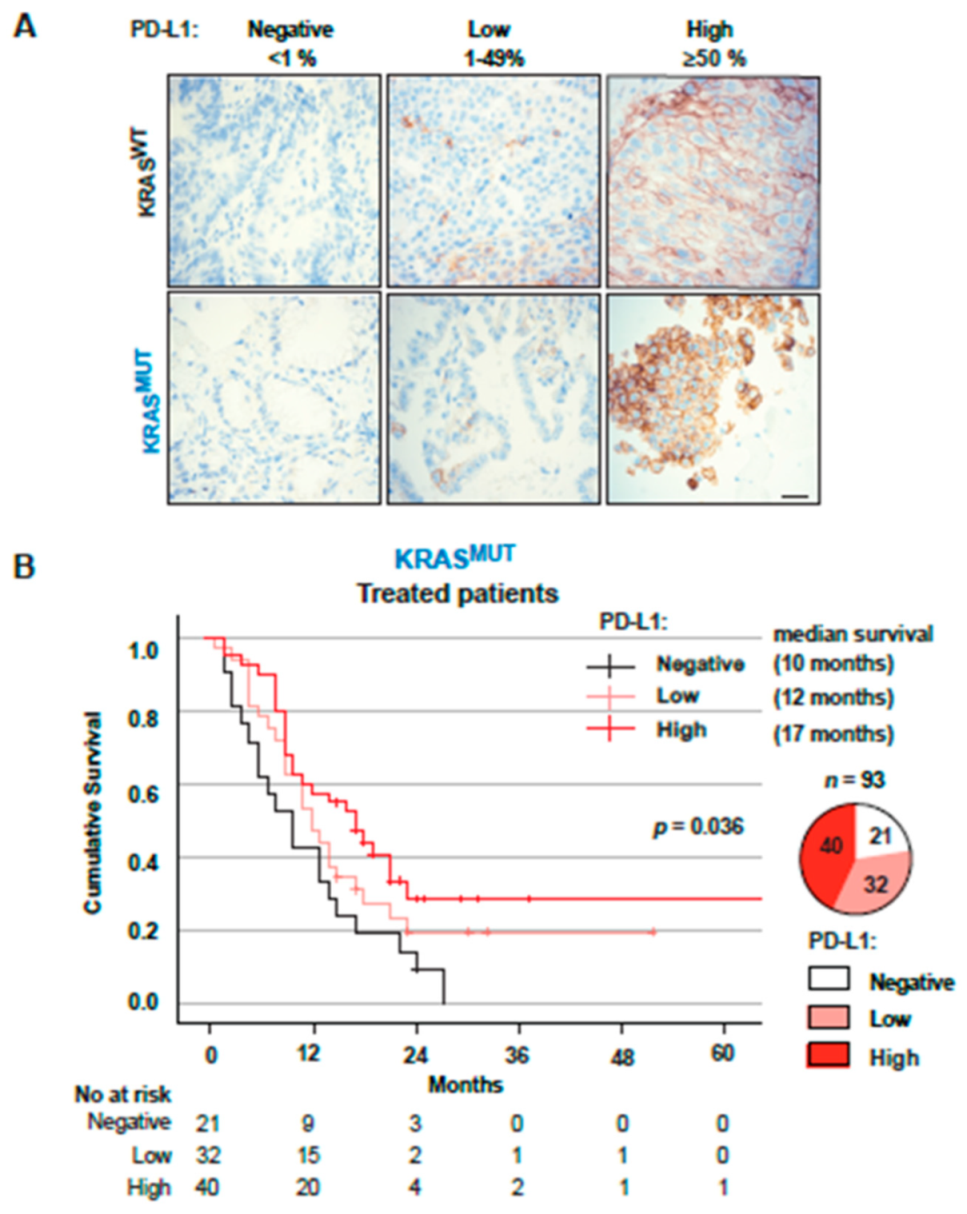
3.4. PD-L1 Expression in Tumors from Stage IV Patients Is a Positive Factor for Overall Survival in KRASMUT but Not KRASWT Patients
3.5. KRAS Mutations Are a Positive Factor for Overall Survival in Patients with PD-L1high Tumors Receiving Immunotherapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization, International Agency for Research on Cancer. Globocan 2020: Lung Cancer. International Agency for Research on Cancer. Available online: http://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf (accessed on 2 March 2021).
- Swedish Lung Cancer Registry. Available online: https://cancercentrum.se/samverkan/cancerdiagnoser/lunga-och-lungsack/vardprogram (accessed on 1 February 2022).
- El Osta, B.; Behera, M.; Kim, S.; Berry, L.D.; Sica, G.; Pillai, R.N.; Owonikoko, T.K.; Kris, M.G.; Johnson, B.E.; Kwiatkowski, D.J.; et al. Characteristics and Outcomes of Patients with Metastatic KRAS-Mutant Lung Adenocarcinomas: The Lung Cancer Mutation Consortium Experience. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2019, 14, 876–889. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- König, D.; Savic Prince, S.; Rothschild, S.I. Targeted Therapy in Advanced and Metastatic Non-Small Cell Lung Cancer. An Update on Treatment of the Most Important Actionable Oncogenic Driver Alterations. Cancers 2021, 13, 804. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes with Pembrolizumab versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score ≥ 50. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef]
- Borghaei, H.; Langer, C.J.; Paz-Ares, L.; Rodríguez-Abreu, D.; Halmos, B.; Garassino, M.C.; Houghton, B.; Kurata, T.; Cheng, Y.; Lin, J.; et al. Pembrolizumab plus chemotherapy versus chemotherapy alone in patients with advanced non-small cell lung cancer without tumor PD-L1 expression: A pooled analysis of 3 randomized controlled trials. Cancer 2020, 126, 4867–4877. [Google Scholar] [CrossRef]
- Ferrara, R.; Imbimbo, M.; Malouf, R.; Paget-Bailly, S.; Calais, F.; Marchal, C.; Westeel, V. Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer. Cochrane Database Syst. Rev. 2021, 4, Cd013257. [Google Scholar] [CrossRef]
- Frederickson, A.M.; Arndorfer, S.; Zhang, I.; Lorenzi, M.; Insinga, R.; Arunachalam, A.; Burke, T.A.; Simon, G.R. Pembrolizumab plus chemotherapy for first-line treatment of metastatic nonsquamous non-small-cell lung cancer: A network meta-analysis . Immunotherapy 2019, 11, 407–428. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Zhou, S.; Jiang, H.; Zhu, K.; Wang, R. Predictive effect of PD-L1 expression for immune checkpoint inhibitor (PD-1/PD-L1 inhibitors) treatment for non-small cell lung cancer: A meta-analysis. Int. Immunopharmacol. 2020, 80, 106214. [Google Scholar] [CrossRef] [PubMed]
- Adderley, H.; Blackhall, F.H.; Lindsay, C.R. KRAS-mutant non-small cell lung cancer: Converging small molecules and immune checkpoint inhibition. EBioMedicine 2019, 41, 711–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrer, I.; Zugazagoitia, J.; Herbertz, S.; John, W.; Paz-Ares, L.; Schmid-Bindert, G. KRAS-Mutant non-small cell lung cancer: From biology to therapy. Lung Cancer 2018, 124, 53–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brady, A.K.; McNeill, J.D.; Judy, B.; Bauml, J.; Evans, T.L.; Cohen, R.B.; Langer, C.; Vachani, A.; Aggarwal, C. Survival outcome according to KRAS mutation status in newly diagnosed patients with stage IV non-small cell lung cancer treated with platinum doublet chemotherapy. Oncotarget 2015, 6, 30287–30294. [Google Scholar] [CrossRef] [PubMed]
- Hames, M.L.; Chen, H.; Iams, W.; Aston, J.; Lovly, C.M.; Horn, L. Correlation between KRAS mutation status and response to chemotherapy in patients with advanced non-small cell lung cancer. Lung Cancer 2016, 92, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Marabese, M.; Ganzinelli, M.; Garassino, M.C.; Shepherd, F.A.; Piva, S.; Caiola, E.; Macerelli, M.; Bettini, A.; Lauricella, C.; Floriani, I.; et al. KRAS mutations affect prognosis of non-small-cell lung cancer patients treated with first-line platinum containing chemotherapy. Oncotarget 2015, 6, 34014–34022. [Google Scholar] [CrossRef] [Green Version]
- Mellema, W.W.; Dingemans, A.M.; Thunnissen, E.; Snijders, P.J.; Derks, J.; Heideman, D.A.; Van Suylen, R.; Smit, E.F. KRAS mutations in advanced nonsquamous non-small-cell lung cancer patients treated with first-line platinum-based chemotherapy have no predictive value. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2013, 8, 1190–1195. [Google Scholar] [CrossRef] [Green Version]
- Rodenhuis, S.; Boerrigter, L.; Top, B.; Slebos, R.J.; Mooi, W.J.; van’t Veer, L.; van Zandwijk, N. Mutational activation of the K-ras oncogene and the effect of chemotherapy in advanced adenocarcinoma of the lung: A prospective study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997, 15, 285–291. [Google Scholar] [CrossRef]
- Meng, D.; Yuan, M.; Li, X.; Chen, L.; Yang, J.; Zhao, X.; Ma, W.; Xin, J. Prognostic value of K-RAS mutations in patients with non-small cell lung cancer: A systematic review with meta-analysis. Lung Cancer 2013, 81, 1–10. [Google Scholar] [CrossRef]
- Addeo, A.; Banna, G.L.; Friedlaender, A. KRAS G12C Mutations in NSCLC: From Target to Resistance. Cancers 2021, 13, 2541. [Google Scholar] [CrossRef] [PubMed]
- Burns, T.F.; Borghaei, H.; Ramalingam, S.S.; Mok, T.S.; Peters, S. Targeting KRAS-Mutant Non-Small-Cell Lung Cancer: One Mutation at a Time, with a Focus on KRAS G12C Mutations. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 4208–4218. [Google Scholar] [CrossRef] [PubMed]
- Indini, A.; Rijavec, E.; Ghidini, M.; Cortellini, A.; Grossi, F. Targeting KRAS in Solid Tumors: Current Challenges and Future Opportunities of Novel KRAS Inhibitors. Pharmaceutics 2021, 13, 653. [Google Scholar] [CrossRef]
- Mathieu, M.; Steier, V.; Fassy, F.; Delorme, C.; Papin, D.; Genet, B.; Duffieux, F.; Bertrand, T.; Delarbre, L.; Le-Borgne, H.; et al. KRAS G12C fragment screening renders new binding pockets. Small GTPases 2021, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Liu, Q.; Fan, X.X.; Leung, E.L.; Yao, X.J.; Liu, L. Resistance looms for KRAS G12C inhibitors and rational tackling strategies. Pharm. Ther. 2022, 229, 108050. [Google Scholar] [CrossRef]
- Zhao, Y.; Murciano-Goroff, Y.R.; Xue, J.Y.; Ang, A.; Lucas, J.; Mai, T.T.; Da Cruz Paula, A.F.; Saiki, A.Y.; Mohn, D.; Achanta, P.; et al. Diverse alterations associated with resistance to KRAS(G12C) inhibition. Nature 2021, 599, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zheng, S.; Jin, R.; Wang, X.; Wang, F.; Zang, R.; Xu, H.; Lu, Z.; Huang, J.; Lei, Y.; et al. The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett. 2020, 470, 95–105. [Google Scholar] [CrossRef]
- Cinausero, M.; Laprovitera, N.; De Maglio, G.; Gerratana, L.; Riefolo, M.; Macerelli, M.; Fiorentino, M.; Porcellini, E.; Buoro, V.; Gelsomino, F.; et al. KRAS and ERBB-family genetic alterations affect response to PD-1 inhibitors in metastatic nonsquamous NSCLC. Ther. Adv. Med. Oncol. 2019, 11, 1758835919885540. [Google Scholar] [CrossRef] [Green Version]
- Gianoncelli, L.; Spitaleri, G.; Passaro, A.; Radice, D.; Fumagalli, C.; Del Signore, E.; Stati, V.; Catania, C.M.; Guerini-Rocco, E.; Barberis, M.; et al. Efficacy of Anti-PD1/PD-L1 Therapy (IO) in KRAS Mutant Non-small Cell Lung Cancer Patients: A Retrospective Analysis. Anticancer Res. 2020, 40, 427–433. [Google Scholar] [CrossRef]
- Karatrasoglou, E.A.; Chatziandreou, I.; Sakellariou, S.; Stamopoulos, K.; Kavantzas, N.; Lazaris, A.C.; Korkolopoulou, P.; Saetta, A.A. Association between PD-L1 expression and driver gene mutations in non-small cell lung cancer patients: Correlation with clinical data. Virchows Arch. Int. J. Pathol. 2020, 477, 207–217. [Google Scholar] [CrossRef]
- Torralvo, J.; Friedlaender, A.; Achard, V.; Addeo, A. The Activity of Immune Checkpoint Inhibition in KRAS Mutated Non-small Cell Lung Cancer: A Single Centre Experience. Cancer Genom. Proteom. 2019, 16, 577–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeanson, A.; Tomasini, P.; Souquet-Bressand, M.; Brandone, N.; Boucekine, M.; Grangeon, M.; Chaleat, S.; Khobta, N.; Milia, J.; Mhanna, L.; et al. Efficacy of Immune Checkpoint Inhibitors in KRAS-Mutant Non-Small Cell Lung Cancer (NSCLC). J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2019, 14, 1095–1101. [Google Scholar] [CrossRef]
- Herbst, R.; Jassem, J.; Abogunrin, S.; James, D.; McCool, R.; Belleli, R.; Giaccone, G.; De Marinis, F. A Network Meta-Analysis of Cancer Immunotherapies versus Chemotherapy for First-Line Treatment of Patients with Non-Small Cell Lung Cancer and High Programmed Death-Ligand 1 Expression. Front. Oncol. 2021, 11, 676732. [Google Scholar] [CrossRef] [PubMed]
- Arak, H.; Aytekin, A.; Canoz, O.; Ozkan, M. Prognostic and Predictive Significance of PD-L1 Expression in Non-Small Cell Lung Cancer Patients: A Single-Center Experience. Turk. Patoloji Derg. 2021, 37, 239–248. [Google Scholar] [CrossRef]
- Wang, A.; Wang, H.Y.; Liu, Y.; Zhao, M.C.; Zhang, H.J.; Lu, Z.Y.; Fang, Y.C.; Chen, X.F.; Liu, G.T. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: A meta-analysis. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2015, 41, 450–456. [Google Scholar] [CrossRef]
- Noordhof, A.L.; Damhuis, R.A.M.; Hendriks, L.E.L.; de Langen, A.J.; Timens, W.; Venmans, B.J.W.; van Geffen, W.H. Prognostic impact of KRAS mutation status for patients with stage IV adenocarcinoma of the lung treated with first-line pembrolizumab monotherapy. Lung Cancer 2021, 155, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.; Shen, R.; Ang, D.C.; Johnson, M.L.; D’Angelo, S.P.; Paik, P.K.; Brzostowski, E.B.; Riely, G.J.; Kris, M.G.; Zakowski, M.F.; et al. Molecular Epidemiology of EGFR and KRAS Mutations in 3,026 Lung Adenocarcinomas: Higher Susceptibility of Women to Smoking-Related KRAS Mutant. Cancers 2012, 18, 6169–6177. [Google Scholar] [CrossRef] [Green Version]
- Darabi, S.; Braxton, D.R.; Eisenberg, B.L.; Demeure, M.J. Predictive Biomarkers for Immunotherapy Response Beyond PD-1/PD-L1. Oncology 2020, 34, 321–327. [Google Scholar] [CrossRef]
- Niu, M.; Yi, M.; Li, N.; Luo, S.; Wu, K. Predictive biomarkers of anti-PD-1/PD-L1 therapy in NSCLC. Exp. Hematol. Oncol. 2021, 10, 18. [Google Scholar] [CrossRef]
- Hallqvist, A.; Enlund, F.; Andersson, C.; Sjögren, H.; Hussein, A.; Holmberg, E.; Nyman, J. Mutated KRAS Is an Independent Negative Prognostic Factor for Survival in NSCLC Stage III Disease Treated with High-Dose Radiotherapy. Lung Cancer Int. 2012, 2012, 587424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebastian, M.; Eberhardt, W.E.E.; Hoffknecht, P.; Metzenmacher, M.; Wehler, T.; Kokowski, K.; Alt, J.; Schütte, W.; Büttner, R.; Heukamp, L.C.; et al. KRAS G12C-mutated advanced non-small cell lung cancer: A real-world cohort from the German prospective, observational, nation-wide CRISP Registry (AIO-TRK-0315). Lung Cancer 2021, 154, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Lopes, G.; Kowalski, D.M.; Kasahara, K.; Wu, Y.L.; De Castro, G.; Cho, B.C.; Turna, H.Z.; Cristescu, R.; Aurora-Garg, D.; et al. LBA4 Association of KRAS mutational status with response to pembrolizumab monotherapy given as first-line therapy for PD-L1-positive advanced non-squamous NSCLC in Keynote-042. Ann. Oncol. 2019, 30, xi63–xi64. [Google Scholar] [CrossRef]
- Landre, T.; Justeau, G.; Assié, J.B.; Chouahnia, K.; Davoine, C.; Taleb, C.; Chouaïd, C.; Duchemann, B. Anti-PD-(L)1 for KRAS-mutant advanced non-small-cell lung cancers: A meta-analysis of randomized-controlled trials. Cancer Immunol. Immunother. 2022, 71, 719–726. [Google Scholar] [CrossRef]




| Characteristics of All Patients Stage IV NSCLC | Total | KRAS WT | KRAS MUT |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Total | 580 (100) | 374 (64, 5) | 206 (35, 5) |
| Age in years, median (range) | 71 (24–94) | 70,5 (24–94) | 71 (46–90) |
| Sex | |||
| Male | 254 (43, 8) | 173 (46, 3) | 81 (39, 3) |
| Female | 326 (56, 2) | 201 (53, 7) | 125 (60, 7) |
| Smoking history | |||
| Current smoker | 192 (33, 1) | 117 (31, 4) | 75 (36, 4) |
| Former smoker | 270 (46, 6) | 152 (40, 6) | 118 (57, 3) |
| Never smoker | 116 (20) | 103 (27, 5) | 13 (6, 3) |
| Missing | 2 (0, 3) | 2 (0, 5) | 0 |
| Performance status | |||
| ECOG 0 | 79 (13, 6) | 48 (12, 8) | 31 (15, 0) |
| ECOG 1 | 243 (41, 9) | 142 (38, 0) | 101 (49, 0) |
| ECOG 2 | 154 (26, 6) | 99 (26, 5) | 55 (26, 7) |
| ECOG 3 | 74 (12, 8) | 57 (15, 2) | 17 (8, 3) |
| ECOG 4 | 19 (3, 3) | 17 (4, 5) | 2 (1, 0) |
| Missing | 11 (1, 9) | 11 (2, 9) | 0 |
| Histology | |||
| Adenocarcinoma | 498 (85, 9) | 316 (84, 5) | 128 (85, 9) |
| NSCLC NOS | 50 (8, 6) | 28 (7, 5) | 19 (12, 8) |
| Squamous cell carcinoma | 32 (5, 5) | 30 (8, 0) | 2 (1, 3) |
| Mutation status | |||
| None known | 231 (39, 8) | 231 (71, 8) | |
| ALK | 19 (3, 3) | 19 (5, 1) | |
| EGFR | 85 (14, 7) | 85 (22, 7) | |
| BRAF | 20 (3, 4) | 20 (5, 3) | |
| Other | 15 (2, 6) | 15 (4) | |
| ROS1 | 2 (0, 3) | 2 (0, 5) | |
| RET | 2 (0, 3) | 2 (0, 5) | |
| KRAS | 206 (35, 5) | ||
| KRAS submutation | |||
| G12A | 14 (6, 8) | ||
| G12C | 83 (40, 3) | ||
| G12D | 23 (11, 2) | ||
| G12V | 43 (20, 9) | ||
| Q61H | 10 (4, 9) | ||
| Others | 33 (16) | ||
| At last follow-up | |||
| Alive | 97 (16, 7) | 69 (18, 4) | 28 (13, 6) |
| Deceased | 483 (83, 3) | 305 (81, 6) | 178 (86, 4) |
| Survival | |||
| Median survival (months) | 7 | 7 | 7 |
| No. of metastatic locations at diagnosis | |||
| 1 | 361 (62, 2) | 234 (62, 6) | 127 (61, 7) |
| 2 | 153 (26, 4) | 101 (27, 0) | 52 (25, 2) |
| 3 | 47 (8, 1) | 30 (8, 0 | 17 (8, 3) |
| >3 | 11 (1, 9) | 3 (0, 8) | 8 (3, 9) |
| Missing | 8 (1, 4) | 6 (1, 6) | 2 (1, 0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eklund, E.A.; Wiel, C.; Fagman, H.; Akyürek, L.M.; Raghavan, S.; Nyman, J.; Hallqvist, A.; Sayin, V.I. KRAS Mutations Impact Clinical Outcome in Metastatic Non-Small Cell Lung Cancer. Cancers 2022, 14, 2063. https://doi.org/10.3390/cancers14092063
Eklund EA, Wiel C, Fagman H, Akyürek LM, Raghavan S, Nyman J, Hallqvist A, Sayin VI. KRAS Mutations Impact Clinical Outcome in Metastatic Non-Small Cell Lung Cancer. Cancers. 2022; 14(9):2063. https://doi.org/10.3390/cancers14092063
Chicago/Turabian StyleEklund, Ella A., Clotilde Wiel, Henrik Fagman, Levent M. Akyürek, Sukanya Raghavan, Jan Nyman, Andreas Hallqvist, and Volkan I. Sayin. 2022. "KRAS Mutations Impact Clinical Outcome in Metastatic Non-Small Cell Lung Cancer" Cancers 14, no. 9: 2063. https://doi.org/10.3390/cancers14092063
APA StyleEklund, E. A., Wiel, C., Fagman, H., Akyürek, L. M., Raghavan, S., Nyman, J., Hallqvist, A., & Sayin, V. I. (2022). KRAS Mutations Impact Clinical Outcome in Metastatic Non-Small Cell Lung Cancer. Cancers, 14(9), 2063. https://doi.org/10.3390/cancers14092063








