Bioelectrical Phase Angle in Patients with Breast Cancer: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Eligibility Criteria and Study Selection
2.3. Data Extraction
2.4. Risk of Bias Assessment
3. Results
3.1. Selected Studies
3.2. BIA Methods, Instrument, and Measurement Conditions
3.3. Risk of Bias
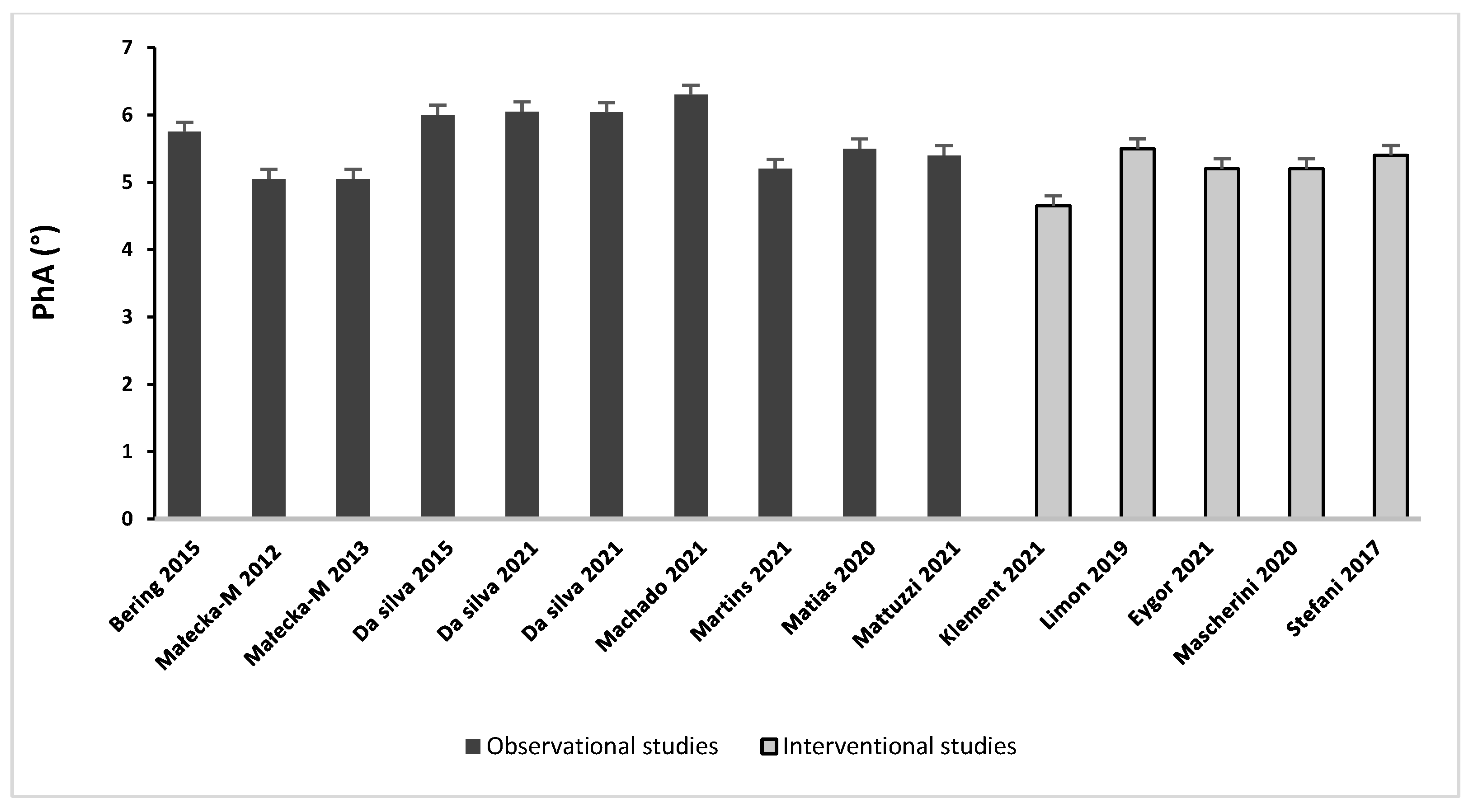
3.4. BIA-Derived PhA in BC Patients
3.4.1. Observational Studies
Changes between Categories of BC Patients
Changes after Cancer Treatments in the Short (<10 Months) and Long Term (>2 Years)
Association between PhA and Survival
Associations between PhA, Nutritional Status, and Muscle Strength
3.4.2. Interventional Studies
Changes after Nutritional Program
Changes after Hatha Yoga and Exercise Programs
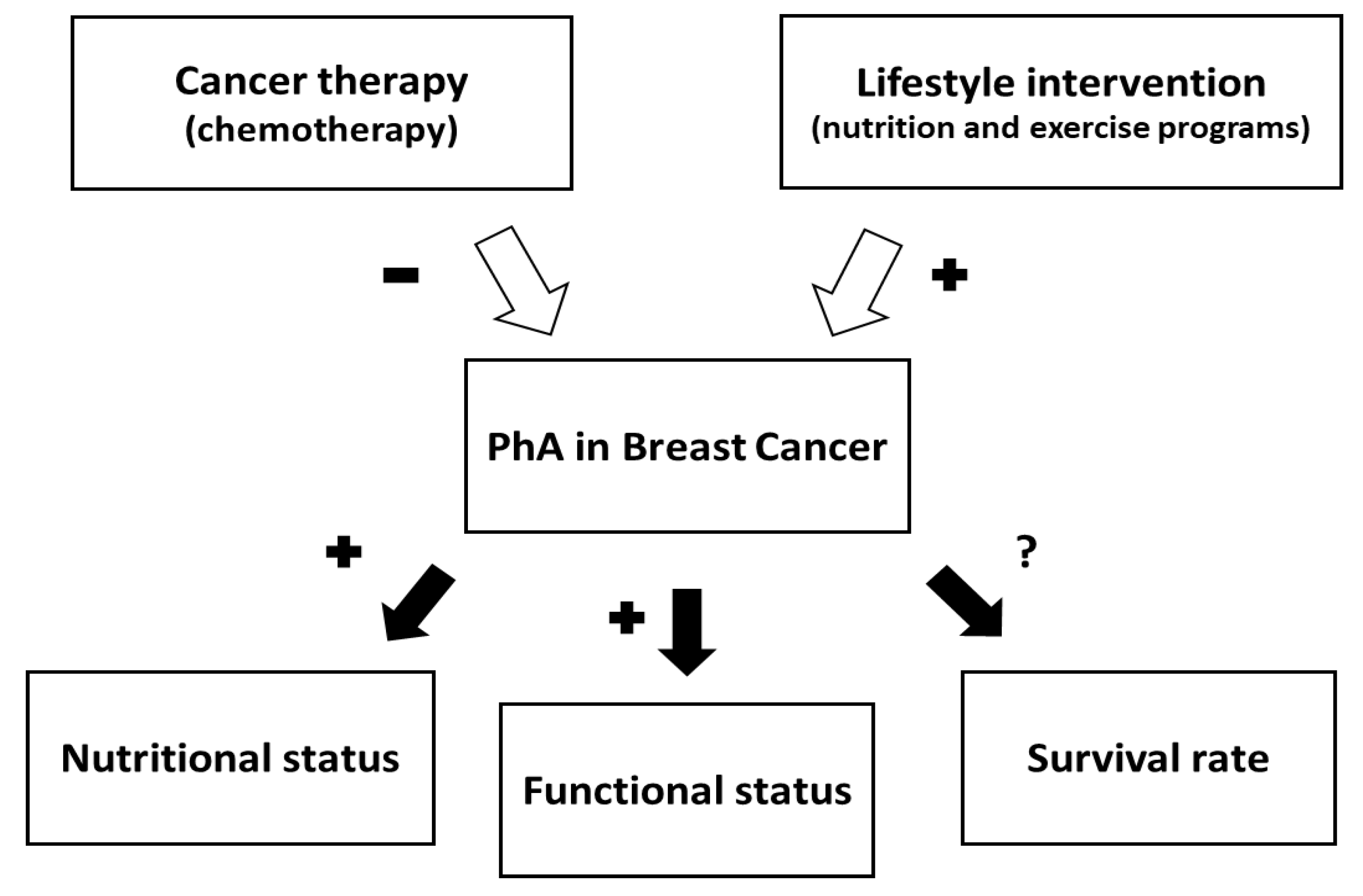
4. Discussion
Strength and Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- De Santis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Dafni, U.; Tsourti, Z.; Alatsathianos, I. Breast Cancer Statistics in the European Union: Incidence and Survival across European Countries. Breast Care 2019, 14, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Demark-Wahnefried, W.; Peterson, B.L.; Winer, E.P.; Marks, L.; Aziz, N.; Marcom, P.K.; Blackwell, K.; Rimer, B.K. Changes in Weight, Body Composition, and Factors Influencing Energy Balance among Premenopausal Breast Cancer Patients Receiving Adjuvant Chemotherapy. J. Clin. Oncol. 2001, 19, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Thomson, Z.O.; Reeves, M.M. Can Weight Gain Be Prevented in Women Receiving Treatment for Breast Cancer? A Systematic Review of Intervention Studies: Weight Gain Prevention Trials in Breast Cancer. Obes. Rev. 2017, 18, 1364–1373. [Google Scholar] [CrossRef]
- Pedersen, B.; Delmar, C.; Lörincz, T.; Falkmer, U.; Grønkjær, M. Investigating Changes in Weight and Body Composition among Women in Adjuvant Treatment for Breast Cancer: A Scoping Review. Cancer Nurs. 2019, 42, 91–105. [Google Scholar] [CrossRef]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and Adverse Breast Cancer Risk and Outcome: Mechanistic Insights and Strategies for Intervention: Breast Cancer, Inflammation, and Obesity. CA A Cancer J. Clin. 2017, 67, 378–397. [Google Scholar] [CrossRef]
- Choi, Y.; Oh, D.-Y.; Kim, T.-Y.; Lee, K.-H.; Han, S.-W.; Im, S.-A.; Kim, T.-Y.; Bang, Y.-J. Skeletal Muscle Depletion Predicts the Prognosis of Patients with Advanced Pancreatic Cancer Undergoing Palliative Chemotherapy, Independent of Body Mass Index. PLoS ONE 2015, 10, e0139749. [Google Scholar] [CrossRef] [Green Version]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical Phase Angle and Impedance Vector Analysis–Clinical Relevance and Applicability of Impedance Parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef]
- Chan, D.S.M.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Navarro Rosenblatt, D.; Thune, I.; Vieira, R.; Norat, T. Body Mass Index and Survival in Women with Breast Cancer-Systematic Literature Review and Meta-Analysis of 82 Follow-up Studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef]
- Nyrop, K.A.; Deal, A.M.; Shachar, S.S.; Park, J.; Choi, S.K.; Lee, J.T.; O’Hare, E.A.; Wheless, A.; Carey, L.A.; Muss, H.B. Weight Trajectories in Women Receiving Systemic Adjuvant Therapy for Breast Cancer. Breast Cancer Res. Treat. 2020, 179, 709–720. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Kyle, U.G.; Kondrup, J. Assessment of Adult Malnutrition and Prognosis with Bioelectrical Impedance Analysis: Phase Angle and Impedance Ratio. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.; Karimi, E.; Vingrys, K.; Shirani, F. Is Phase Angle a Valuable Prognostic Tool in Cancer Patients’ Survival? A Systematic Review and Meta-Analysis of Available Literature. Clin. Nutr. 2021, 40, 3182–3190. [Google Scholar] [CrossRef] [PubMed]
- Matthews, L.; Bates, A.; Wootton, S.A.; Levett, D. The Use of Bioelectrical Impedance Analysis to Predict Post-Operative Complications in Adult Patients Having Surgery for Cancer: A Systematic Review. Clin. Nutr. 2021, 40, 2914–2922. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Lammersfeld, C.A.; Vashi, P.G.; King, J.; Dahlk, S.L.; Grutsch, J.F.; Lis, C.G. Bioelectrical Impedance Phase Angle as a Prognostic Indicator in Breast Cancer. BMC Cancer 2008, 8, 249. [Google Scholar] [CrossRef] [Green Version]
- Bell, K.E.; Schmidt, S.; Pfeiffer, A.; Bos, L.; Earthman, C.; Russell, C.; Mourtzakis, M. Bioelectrical Impedance Analysis Overestimates Fat-Free Mass in Breast Cancer Patients Undergoing Treatment. Nutr. Clin. Pr. 2020, 35, 1029–1040. [Google Scholar] [CrossRef]
- Gonzalez, M.C.; Barbosa-Silva, T.G.; Bielemann, R.M.; Gallagher, D.; Heymsfield, S.B. Phase Angle and Its Determinants in Healthy Subjects: Influence of Body Composition. Am. J. Clin. Nutr. 2016, 103, 712–716. [Google Scholar] [CrossRef] [Green Version]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical Impedance Analysis—Part I: Review of Principles and Methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Martins, A.D.; Oliveira, R.; Brito, J.P.; Costa, T.; Ramalho, F.; Pimenta, N.; Santos-Rocha, R. Phase Angle Cutoff Value as a Marker of the Health Status and Functional Capacity in Breast Cancer Survivors. Physiol. Behav. 2021, 235, 113400. [Google Scholar] [CrossRef]
- Lee, K.; Sami, N.; Sweeney, F.C.; Dieli-Conwright, C.M. Body Composition with Dual-Energy X-ray Absorptiometry and Bioelectrical Impedance Analysis in Breast Cancer Survivors. Nutr. Clin. Pract. 2019, 34, 421–427. [Google Scholar] [CrossRef]
- Cioffi, I.; Marra, M.; Imperatore, N.; Pagano, M.C.; Santarpia, L.; Alfonsi, L.; Testa, A.; Sammarco, R.; Contaldo, F.; Castiglione, F.; et al. Assessment of Bioelectrical Phase Angle as a Predictor of Nutritional Status in Patients with Crohn’s Disease: A Cross Sectional Study. Clin. Nutr. 2020, 39, 1564–1571. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.M.E.; Queiroz, M.; de Albuquerque, N.M.C.; Rodrigues, J.; Wiegert, E.V.M.; Calixto-Lima, L.; de Oliveira, L.C. The Prognostic Role of Phase Angle in Advanced Cancer Patients: A Systematic Review. Nutr. Clin. Pract. 2018, 33, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Toso, S.; Piccoli, A.; Gusella, M.; Menon, D.; Bononi, A.; Crepaldi, G.; Ferrazzi, E. Altered Tissue Electric Properties in Lung Cancer Patients as Detected by Bioelectric Impedance Vector Analysis. Nutrition 2000, 16, 120–124. [Google Scholar] [CrossRef]
- Santarpia, L.; Marra, M.; Montagnese, C.; Alfonsi, L.; Pasanisi, F.; Contaldo, F. Prognostic Significance of Bioelectrical Impedance Phase Angle in Advanced Cancer: Preliminary Observations. Nutrition 2009, 25, 930–931. [Google Scholar] [CrossRef] [PubMed]
- Morlino, D.; Marra, M.; Cioffi, I.; Sammarco, R.; Speranza, E.; Di Vincenzo, O.; De Caprio, C.; De Filippo, E.; Pasanisi, F. A Proposal for Reference Values of Hand Grip Strength in Women with Different Body Mass Indexes. Nutrition 2021, 87–88, 111199. [Google Scholar] [CrossRef]
- Di Vincenzo, O.; Marra, M.; Sacco, A.M.; Pasanisi, F.; Scalfi, L. Bioelectrical Impedance (BIA)-Derived Phase Angle in Adults with Obesity: A Systematic Review. Clin. Nutr. 2021, 40, 5238–5248. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Zocher, D.; Bosy-Westphal, A.; Szramek, A.; Scheufele, R.; Smoliner, C.; Pirlich, M. Cutoff Percentiles of Bioelectrical Phase Angle Predict Functionality, Quality of Life, and Mortality in Patients with Cancer. Am. J. Clin. Nutr. 2010, 92, 612–619. [Google Scholar] [CrossRef] [Green Version]
- Hui, D.; Dev, R.; Pimental, L.; Park, M.; Cerana, M.A.; Liu, D.; Bruera, E. Association Between Multi-Frequency Phase Angle and Survival in Patients With Advanced Cancer. J. Pain Symptom. Manag. 2017, 53, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- National Institution of Health: U. S Department of Health and Human Services. Study Quality Assessment Tool 2013, Last Updated July 2021. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 7 August 2021).
- Bering, T.; Maurício, S.F.; Silva, J.B.; Correia, M.I.T.D. Nutritional and Metabolic Status of Breast Cancer Women. Nutr. Hosp. 2014, 31, 751–758. [Google Scholar] [CrossRef]
- Ramos Silva, B.; Mialich, M.S.; Cruz, L.P.; Rufato, S.; Gozzo, T.; Jordao, A.A. Performance of Functionality Measures and Phase Angle in Women Exposed to Chemotherapy for Early Breast Cancer. Clin. Nutr. ESPEN 2021, 42, 105–116. [Google Scholar] [CrossRef]
- Ramos Silva, B.; Rufato, S.; Mialich, M.S.; Cruz, L.P.; Gozzo, T.; Jordao, A.A. Metabolic Syndrome and Unfavorable Outcomes on Body Composition and in Visceral Adiposities Indexes among Early Breast Cancer Women Post-Chemotherapy. Clin. Nutr. ESPEN 2021, 44, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.M.Q.; Justa, R.M.D.E.; Lopes da Costa, S.; Barbosa, M.C.; Damasceno, N.R.T.; Verde, S.M.M.L. Bioelectrical Impedance Vector Applied to Body Composition Evaluation of Women Survivors of Breast Cancer: A Longitudinal Study. Clin. Nutr. ESPEN 2021, 44, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Koyama Silva, E.Y.; Ferreira Carioca, A.A.; Moreira Lima Verde, S.M.; da Conceição Quintaneiro, E.; Raquel Teixeira Damasceno, N. Effect of Chemotherapy on Dietary Glycemic Index and Load in Patients with Breast Cancer and Their Relationships to Body Fat and Phase Angle. Nutr. Cancer 2015, 67, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Mazzutti, F.S.; Custódio, I.D.D.; Lima, M.T.M.; Carvalho, K.P.; Pereira, T.S.S.; del Molina, M.C.B.; Canto, P.P.L.; Paiva, C.E.; Maia, Y.C. Breast Cancer Survivors Undergoing Endocrine Therapy Have a Worrying Risk Factor Profile for Cardiovascular Diseases. Nutrients 2021, 13, 1114. [Google Scholar] [CrossRef]
- Stefani, L.; Palmerini, D.; Corezzi, M.; Mascherini, G.; Petri, C.; Klika, R.; Galanti, G. Total Body Water Distribution in Breast Cancer Survivors Following Cancer Rehabilitation. JFMK 2017, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Mascherini, G.; Tosi, B.; Giannelli, C.; Ermini, E.; Osti, L.; Galanti, G. Adjuvant Therapy Reduces Fat Mass Loss during Exercise Prescription in Breast Cancer Survivors. JFMK 2020, 5, 49. [Google Scholar] [CrossRef]
- Małecka-Massalska, T.; Chara, K.; Smolen, A.; Kurylcio, A.; Polkowski, W.; Lupa-Zatwarnicka, K. Bioimpedance Vector Pattern in Women with Breast Cancer Detected by Bioelectric Impedance Vector Analysis. Preliminary Observations. Ann. Agric. Environ. Med. 2012, 19, 697–700. [Google Scholar]
- Małecka-Massalska, T.; Chara, K.; Gołębiowski, P.; Władysiuk, M.; Smoleń, A.; Kurylcio, A.; Zuchora, B.; Zubrzycki, J.; Orłowska-Kowalik, G.; Lupa-Zatwarnicka, K.; et al. Altered Tissue Electrical Properties in Women with Breast Cancer--Preliminary Observations. Ann. Agric. Environ. Med. 2013, 20, 523–527. [Google Scholar]
- Matias, C.N.; Cavaco-Silva, J.; Reis, M.; Campa, F.; Toselli, S.; Sardinha, L.; Silva, A.M. Phase Angle as a Marker of Muscular Strength in Breast Cancer Survivors. IJERPH 2020, 17, 4452. [Google Scholar] [CrossRef]
- Klement, R.J.; Koebrunner, P.S.; Krage, K.; Weigel, M.M.; Sweeney, R.A. Short-Term Effects of a Paleolithic Lifestyle Intervention in Breast Cancer Patients Undergoing Radiotherapy: A Pilot and Feasibility Study. Med. Oncol. 2021, 38, 1. [Google Scholar] [CrossRef]
- Limon-Miro, A.T.; Valencia, M.E.; Lopez-Teros, V.; Guzman-Leon, A.E.; Mendivil-Alvarado, H.; Astiazaran-Garcia, H. Bioelectric Impedance Vector Analysis (BIVA) in Breast Cancer Patients: A Tool for Research and Clinical Practice. Medicina 2019, 55, 663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyigör, S.; Apaydin, S.; Yesil, H.; Tanıgor, G.; Hopanci Bicakli, D. Effects of Yoga on Phase Angle and Quality of Life in Patients with Breast Cancer: A Randomized, Single-Blind, Controlled Trial. Complement. Med. Res. 2021, 28, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Guidelines on Nutrition in Cancer Patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, R.J.; Aziz, N.; Albanes, D.; Hartman, T.; Danforth, D.; Hill, S.; Sebring, N.; Reynolds, J.C.; Yanovski, J.A. Weight and Body Composition Changes during and after Adjuvant Chemotherapy in Women with Breast Cancer. J. Clin. Endocrinol. Metab. 2004, 89, 2248–2253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Sebastiano, K.M.; Mourtzakis, M. A Critical Evaluation of Body Composition Modalities Used to Assess Adipose and Skeletal Muscle Tissue in Cancer. Appl. Physiol. Nutr. Metab. 2012, 37, 811–821. [Google Scholar] [CrossRef]
- Vance, V.; Mourtzakis, M.; McCargar, L.; Hanning, R. Weight Gain in Breast Cancer Survivors: Prevalence, Pattern and Health Consequences. Obes Rev. 2011, 12, 282–294. [Google Scholar] [CrossRef]
- Wang, C.; Hou, X.-H.; Zhang, M.-L.; Bao, Y.-Q.; Zou, Y.-H.; Zhong, W.-H.; Xiang, K.-S.; Jia, W.-P. Comparison of Body Mass Index with Body Fat Percentage in the Evaluation of Obesity in Chinese. Biomed. Environ. Sci. 2010, 23, 173–179. [Google Scholar] [CrossRef]
- Trestini, I.; Carbognin, L.; Monteverdi, S.; Zanelli, S.; De Toma, A.; Bonaiuto, C.; Nortilli, R.; Fiorio, E.; Pilotto, S.; Di Maio, M.; et al. Clinical Implication of Changes in Body Composition and Weight in Patients with Early-Stage and Metastatic Breast Cancer. Crit. Rev. Oncol. Hematol. 2018, 129, 54–66. [Google Scholar] [CrossRef]
- Iwase, T.; Wang, X.; Shrimanker, T.V.; Kolonin, M.G.; Ueno, N.T. Body Composition and Breast Cancer Risk and Treatment: Mechanisms and Impact. Breast Cancer Res. Treat. 2021, 186, 273–283. [Google Scholar] [CrossRef]
- McTiernan, A. Weight, Physical Activity and Breast Cancer Survival. Proc. Nutr. Soc. 2018, 77, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Engin, A. Obesity-Associated Breast Cancer: Analysis of Risk Factors. Adv. Exp. Med. Biol. 2017, 960, 571–606. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F.; on behalf of the SCRINIO Working Group. Screening the Nutritional Status in Oncology: A Preliminary Report on 1000 Outpatients. Support. Care Cancer 2009, 17, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Hébuterne, X.; Lemarié, E.; Michallet, M.; de Montreuil, C.B.; Schneider, S.M.; Goldwasser, F. Prevalence of Malnutrition and Current Use of Nutrition Support in Patients With Cancer. JPEN J. Parenter Enter. Nutr. 2014, 38, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Ferrão, B.; Neves, P.M.; Santos, T.; Capelas, M.L.; Mäkitie, A.; Ravasco, P. Body Composition Changes in Patients with Head and Neck Cancer under Active Treatment: A Scoping Review. Support. Care Cancer 2020, 28, 4613–4625. [Google Scholar] [CrossRef]
- Małecka-Massalska, T.; Smolen, A.; Morshed, K. Altered Tissue Electrical Properties in Squamous Cell Carcinoma in Head and Neck Tumors: Preliminary Observations. Head Neck. 2013, 35, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, M.; Dickinson, A.; Nikander, P.; Orell, H.; Mäkitie, A. Low-Phase Angle in Body Composition Measurements Correlates with Prolonged Hospital Stay in Head and Neck Cancer Patients. Acta Otolaryngol. 2019, 139, 383–387. [Google Scholar] [CrossRef]
- Paixão, E.M.S.; Gonzalez, M.C.; Ito, M.K. A Prospective Study on the Radiation Therapy Associated Changes in Body Weight and Bioelectrical Standardized Phase Angle. Clin. Nutr. 2015, 34, 496–500. [Google Scholar] [CrossRef]
- Limon-Miro, A.T.; Lopez-Teros, V.; Astiazaran-Garcia, H. Dietary Guidelines for Breast Cancer Patients: A Critical Review. Adv. Nutr. 2017, 8, 613–623. [Google Scholar] [CrossRef]
- Paiva, S.I.; Borges, L.R.; Halpern-Silveira, D.; Assunção, M.C.F.; Barros, A.J.D.; Gonzalez, M.C. Standardized Phase Angle from Bioelectrical Impedance Analysis as Prognostic Factor for Survival in Patients with Cancer. Support. Care Cancer 2011, 19, 187–192. [Google Scholar] [CrossRef]
- Stegel, P.; Kozjek, N.R.; Brumen, B.A.; Strojan, P. Bioelectrical Impedance Phase Angle as Indicator and Predictor of Cachexia in Head and Neck Cancer Patients Treated with (Chemo)Radiotherapy. Eur. J. Clin. Nutr. 2016, 70, 602–606. [Google Scholar] [CrossRef]
- Kowshik, V.; Velkumary, S.; Sethi, P.; Feula, J.M.; Subhashri, S.; Abirami, M. Association of Handgrip Strength and Endurance with Body Composition in Head and Neck Cancer Patients. J. Fam. Med. Prim. Care 2021, 10, 910–916. [Google Scholar] [CrossRef]
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and Breast Cancer: A Literature Review on Prevention, Treatment and Recurrence. Nutrients 2019, 11, E1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cereda, E.; Turri, A.; Klersy, C.; Cappello, S.; Ferrari, A.; Filippi, A.R.; Brugnatelli, S.; Caraccia, M.; Chiellino, S.; Borioli, V.; et al. Whey Protein Isolate Supplementation Improves Body Composition, Muscle Strength, and Treatment Tolerance in Malnourished Advanced Cancer Patients Undergoing Chemotherapy. Cancer Med. 2019, 8, 6923–6932. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.D.; Oliveira, R.; Brito, J.P.; Costa, T.; Silva, J.; Ramalho, F.; Santos-Rocha, R.; Pimenta, N. Effect of Exercise on Phase Angle in Cancer Patients: A Systematic Review. J. Sports Med. Phys. Fit. 2021. [Google Scholar] [CrossRef]
- Buckinx, F.; Reginster, J.-Y.; Dardenne, N.; Croisiser, J.-L.; Kaux, J.-F.; Beaudart, C.; Slomian, J.; Bruyère, O. Concordance between Muscle Mass Assessed by Bioelectrical Impedance Analysis and by Dual Energy X-ray Absorptiometry: A Cross-Sectional Study. BMC Musculoskelet Disord. 2015, 16, 60. [Google Scholar] [CrossRef] [Green Version]
- Winters-Stone, K.M.; Dobek, J.; Bennett, J.A.; Nail, L.M.; Leo, M.C.; Schwartz, A. The Effect of Resistance Training on Muscle Strength and Physical Function in Older, Postmenopa.ausal Breast Cancer Survivors: A Randomized Controlled Trial. J. Cancer Surviv. 2012, 6, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Christensen, J.F.; Jones, L.W.; Andersen, J.L.; Daugaard, G.; Rorth, M.; Hojman, P. Muscle Dysfunction in Cancer Patients. Ann. Oncol. 2014, 25, 947–958. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G.; Dahlk, S.L.; Vashi, P.G.; Grutsch, J.F.; Lammersfeld, C.A. Bioelectrical Impedance Phase Angle as a Prognostic Indicator in Advanced Pancreatic Cancer. Br. J. Nutr. 2004, 92, 957–962. [Google Scholar] [CrossRef] [Green Version]
- Norman, K.; Wirth, R.; Neubauer, M.; Eckardt, R.; Stobäus, N. The Bioimpedance Phase Angle Predicts Low Muscle Strength, Impaired Quality of Life, and Increased Mortality in Old Patients with Cancer. J. Am. Med. Dir. Assoc. 2015, 16, 173.e17–173.e22. [Google Scholar] [CrossRef]
- Custódio Martins, P.; de Lima, T.R.; Silva, A.M.; Santos Silva, D.A. Association of Phase Angle with Muscle Strength and Aerobic Fitness in Different Populations: A Systematic Review. Nutrition 2022, 93, 111489. [Google Scholar] [CrossRef]
- Navigante, A.; Morgado, P.C.; Casbarien, O.; Delgado, N.L.; Giglio, R.; Perman, M. Relationship between Weakness and Phase Angle in Advanced Cancer Patients with Fatigue. Support. Care Cancer 2013, 21, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
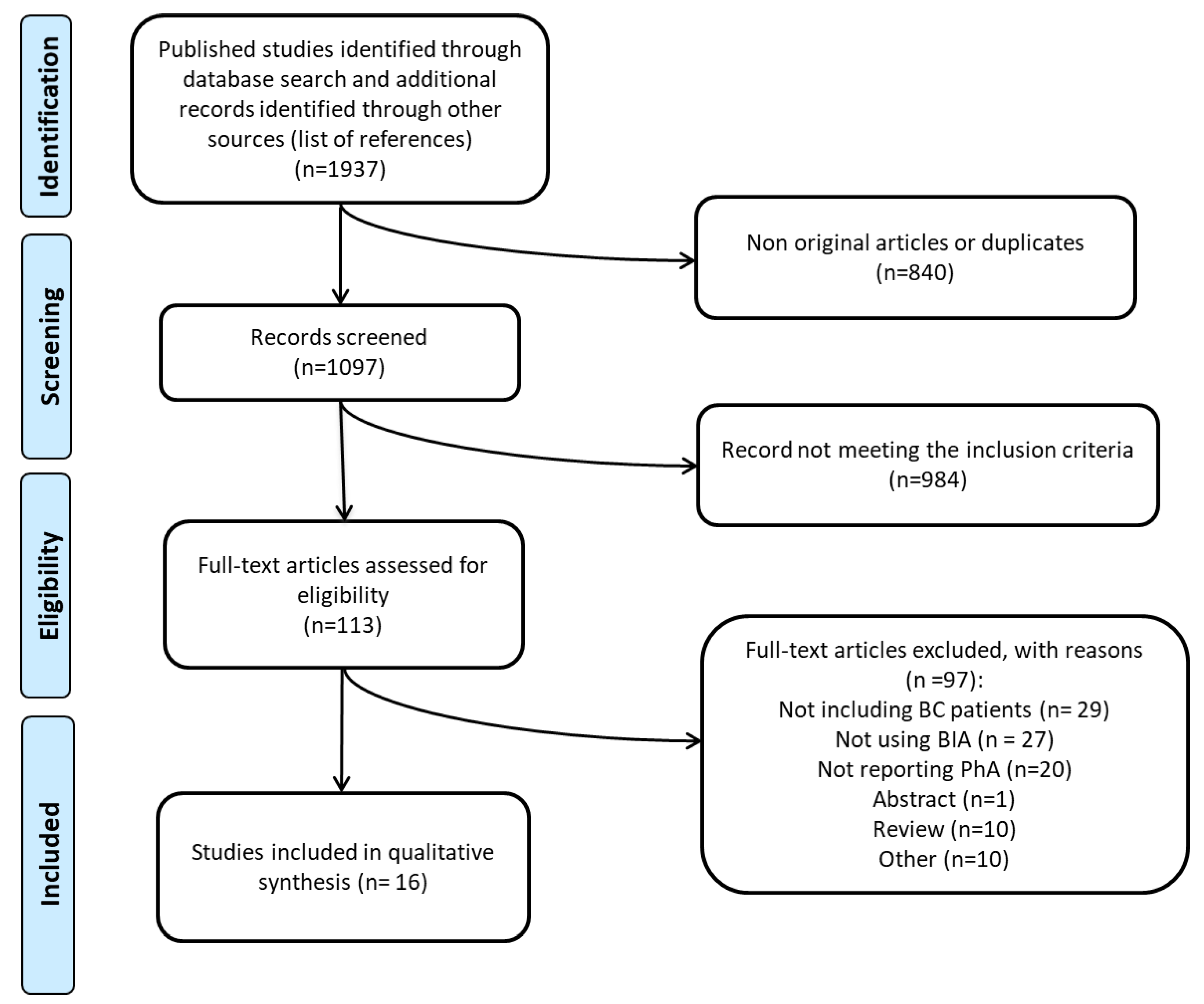
| Author, Year [Ref.] | Design | Study Population | N | Study Groups | Age (Years) | BMI (kg/m2) | BIA Methods/ Instrument | Measurements/Position |
|---|---|---|---|---|---|---|---|---|
| Observational Studies | ||||||||
| Bering et al., 2015 [30] | Cross-sectional | Pre/postmenopausal BC women; stage I–IV; age range: 31–79 y | 64 | MetS (n = 28) No-MetS (n = 36) | 53.2 ± 11.6 ‡ | 30.9 ± 5.5 * 25.3 ± 4.3 | NR | Fast and rest not specified |
| Małecka-Massalska et al., 2012 [38] | Cross-sectional | Pre/postmenopausal BC women; age range: 31–82 y; pre-surgery | 68 | BC (n = 34) C (n = 34) | 53.88 ± 10.84 53.79 ± 10.18 | 26.97 ± 3.99 27.27 ± 7.66 | Multifrequency ImpediMed SFB7 BioImp v1.5 | 10 min rest supine position |
| Małecka-Massalska et al., 2013 [39] | Cross-sectional | Pre/postmenopausal BC women; age range: 31–82 y; pre-surgery | 68 | BC (n = 34) C (n = 34) | 53.88 ± 10.84 53.79 ± 10.18 | 26.0 ± 3.99 * 29.61 ± 7.66 | Multifrequency ImpediMed SFB7 BioImp v1.5 | 10 min rest supine position |
| Martins et al., 2021 [18] | Cross-sectional | Pre/postmenopausal BC survivors; stage I–IIIA; age range: 30–69 y; post-surgery | 25 | G 1 (n = 13) G 2 (n = 12) | 50.5 ± 8.6 51.1 ± 8.9 | 25.5 ± 3.9 27.3 ± 5.6 | Multifrequency Biospace Co InBody S10 | 8 h fast and 10 min rest; supine position |
| Matias et al., 2020 [40] | Cross-sectional | Post-surgery BC survivors | 41 | 54.6 ± 9.2 | 26.6 ± 4.6 | Multifrequency BIS 4200B | Overnight fast; 10 min rest; supine position | |
| Gupta et al., 2008 [14] | Retrospective cohort study | Pre/postmenopausal BC women; stage I–IV | 259 | 49 (25–74) ° | NR | Single frequency BIA-101Q: RJL Systems | NR Supine position | |
| da Silva et al., 2015 [34] | Prospective cohort study | Pre/postmenopausal BC women; stage I–-II | 25 | T0 T1 (7 mo) T2 (9 mo) | 46 (29–70) | 25.0 ± 4.0 26.0 ± 5.0 * 26.0 ± 4.0 * | Multifrequency Biodynamics 450 | Fast and rest not specified |
| da Silva et al., 2021 [31] | Prospective cohort study | Pre/postmenopausal BC women; stage I–III | 61 | T0 T1 (7 mo) | 46.4 (26–64) | 28.54 ± 5.46 28.95 ± 4.37 | Multifrequency BIS-BCM Fresenius Medical care | Fast and rest not specified; supine position |
| da Silva et al., 2021 [32] | Prospective cohort study | Pre/postmenopausal BC women; stage I–III | 61 | T0 T1 (7 mo) | 46.4 (26–64) | 28.53 ± 5.45 29.23 ± 5.47 | Multifrequency BIS-BCM Fresenius Medical care | Fast and rest not specified; supine position |
| Machado et al., 2021 [33] | Prospective cohort study | Pre/postmenopausal BC women, stage I–IV; aged ≥ 20 y | 35 | T0 T1 (5 y) | 50.6 ± 11.4 55.5 ± 9.7 | 27.8 ± 4.4 28.5 ± 4.2 | Multifrequency Biodynamics 450 | 12 h fast, 5 min rest; supine position |
| Mazzutti et al., 2021 [35] | Prospective cohort study | Postmenopausal BC survivors; stage I–III; post-surgery | 38 | T0 T1 (12 mo) T2 (24 mo) | 65 (58.5–69.5) ‡ ° | 28.5 ± 1.10 29.2 ± 0.93 29.4 ± 1.12 | Multifrequency Biodynamics 450 | Fast and rest not specified; supine position |
| Interventional studies | ||||||||
| Klement et al., 2020 [41] | Clinical Trial | Pre/postmenopausal BC women | 22 | PL (n = 11) T0 T1 SD (n = 11) T0 T1 | 58 (37–72) ° 58 (35–67) ° | 24.2 (19.9–30.0) ° NR 23.7 (18.8–28.0) ° NR | Multifrequency Seca514–515 BC analyzers | Fast and rest not specified; stand position |
| Limon-Miro et al., 2019 [42] | Clinical trial | BC women; stage I-IIB post- surgery | 9 | T0 T1 (6 mo) | 44 ± 12 | 30.7 (IQR 7–11) 29.5 (IQR 7–9) | Single frequency Impedimed Limited DF50 (BIVA) | 4 h fast; rest and position not specified |
| Eyigör et al., 2021 [43] | RCT | BC women; age range: 18–70 y; post-surgery | 31 | Y (n = 15) T0 T1 (10 weeks) C (n = 16) T0 T1 (10 weeks) | 51.40 ± 10.6 50.7 ± 7.6 | 26.0 ± 4.9 25.7 ± 4.5 25.6 ± 3.7 24.7 ± 3.5 | Tanita-305 body-fat analyzer | Fast and rest not specified; stand position |
| Mascherini et al., 2020 [37] | Clinical Trial | Postmenopausal BC women; stage < IIIC; age range: 21–65 y; post-surgery | 42 | T0 T1 (6 mo) | 52.0 ± 10.1 | 27.3 ± 4.2 26.1 ± 3.9 * | Single frequency BIA 101 Sport edition, Akern | Fast and rest not specified; supine position |
| Stefani et al., 2017 [36] | Clinical Trial | BC survivors; post-surgery and radiotherapy | 28 | T0 T1 (6 mo) T2 (12 mo) | 59 ± 9 | 26.7 ± 5.4 26.6 ± 5.6 26.8 ± 5.8 | Single frequency BIA 101, Akern | Fast and rest not specified; supine position |
| Author, Year, Ref. | Study Design | Stage of Care | N | Study Group | Weight (kg) | PhA (Degrees) | FFM (kg or %) | FM (kg or %) | Major Findings on PhA |
|---|---|---|---|---|---|---|---|---|---|
| Bering et al., 2015 [30] | Cross- sectional | Pre-chemotherapy and radiotherapy | 62 | MetS (n = 28) No-MetS (n = 36) | NR | 5.7 ± 0.8 5.8 ± 1.0 | (%): 57.7 ± 6.2 (%): 63.8 ± 7.9 * | (%): 42.2 ± 6.2 (%): 35.4 ± 6.5 * | PhA was similar between the two BC groups |
| Małecka-Massalska et al., 2012 [38] | Cross- sectional | Preoperative | 68 | BC (n = 34) C (n = 34) | 69.04 ± 12.56 70.07 ± 23.6 | 5.05 ± 0.66 5.22 ± 0.64 | NR | NR | PhA was similar between BC and C |
| Małecka-Massalska et al., 2013 [39] | Cross- sectional | Preoperative | 68 | BC (n = 34) C (n = 34) | 67.94 ± 12.56 79.07 ± 23.60 * | 5.05 ± 0.12 5.25 ± 0.11 | 43.3 ± 1.1 50.5 ± 1.3 * | 26 ± 3.9 29.6 ± 7.6 * | PhA was similar between BC and C |
| Martins et al., 2021 [18] | Cross- sectional | Completed therapy | 25 | G1 (n = 13) G2 (n = 12) | 66.8 ± 10.1 67.5 ± 14.8 | 5.2 ± 0.26 5.9 ± 0.3 * | 43.2 ± 4.9 43.2 ± 7.9 | 23.1 ± 8.9 25.5 ± 10.5 | Better health status in G2 compared to G1 |
| Matias et al., 2020 [40] | Cross- sectional | NR | 41 | 68.0 ± 11.7 | 5.5 ± 0.7 | NR | NR | PhA can predict muscular strength in BC survivors | |
| Gupta et al., 2008 [14] | Retrospective cohort study | NR | 259 | NR | 5.6 (1.5–8.9) ‡ | NR | NR | PhA seemed to be a strong predictor of survival in BC. | |
| da Silva et al., 2015 [34] | Prospective cohort study | Pre/post adjuvant chemotherapy | 25 | T0 T1 (7 mo) T2 (9 mo) | 64 ± 13 66 ± 13 * 67 ± 13 * | 6.0 ± 0.6 5.7 ± 0.6 * 5.9 ± 1.4 | NR | (%): 29.7 ± 6.1 (%): 29.5 ± 6.5 * (%): 30.7 ± 5.4 § | PhA significantly decreased (−5%) after treatments |
| da Silva et al., 2021 [31] | Prospective cohort study | Pre-/post (neo) adjuvant chemotherapy | 61 | T0 T1 (7 mo) | 71.7 ± 12.6 73.5 ± 12.6 | 6.05 ± 0.75 5.16 ± 0.77 * | 34 ± 7.1 32.5 ± 5.6 | 28.82 ± 9.09 28.78 ± 8.94 | PhA significantly decreased (−15%) after treatments |
| da Silva et al., 2021 [32] | Prospective cohort study | Pre-/post (neo) adjuvant chemotherapy | 61 | T0 T1(7 mo) | 71.7 ± 12.6 72.1 ± 12.4 | 6.04 ± 0.76 5.18 ± 0.76 * | 34 ± 7.1 33.8 ± 8.40 | 28.82 ± 9.09 28.86 ± 10.04 | PhA significantly decreased (−15%) after treatments |
| Machado et al., 2021 [33] | Prospective cohort study | Pre/post treatments | 35 | T0 T1 (5 y) | 67.4 ± 11.2 71.0 ± 12.0 | 6.3 ± 0.9 5.7 ± 0.6 * | 43.5 ± 3.3 44.7 ± 1.7 | (%): 35.4 ± 4.9 (%): 37.0 ± 2.5 * | PhA significantly decreased (−10%) after follow-up |
| Mazzutti et al., 2021 [35] | Prospective cohort study | During AIs treatments | 38 | T0 T1 (12 mo) T2 (24 mo) | NR | 5.4 ± 0.20 6.2 ± 0.11 * 6.1 ± 0.15 * | 42.3 ± 1.08 43.3 ± 0.87 43.0 ± 1.15 | (%): 40.5 ± 1.25 (%): 39.3 ± 1.02 (%): 39.7 ± 1.34 | PhA was lower at T0 compared to T1 and T2 |
| Author, Year, Ref. | Study Design | Stage of Care | N | Study Group | Weight (kg) | PhA (Degrees) | FFM (kg or %) | FM (kg or %) | Major Findings |
|---|---|---|---|---|---|---|---|---|---|
| Klement et al., 2020 [41] | Clinical trial | Undergoing radiotherapy | 22 | PL (n = 11) T0 T1 (39 days) SD (n = 11) T0 T1 (33 days) | 62.5 (54.1–88.4) − 0.4/week * 61.3 (48.1–75.2) NR | 4.81 (4.04–5.28) − 0.02/week 4.49 (3.87–5.37) 0.03/week * | 40.1 (34.2–51.5) 0.04/week 38.7 (31.5–48.7) −0.13/week * | 22.4 (16.5–37.7) − 0.34/week * 21.6 (15.9–31.7) 0.14/week | PhA values show an opposite trend between diets |
| Limon-Miro et al., 2019 [42] | Clinical trial | Pre-/post adjuvant chemo- and/or radiotherapy | 9 | T0 T1 (6 mo) | 79.2 (IQR 10–27) 73.4 (IQR 13–22) * | 5.5 (IQR 3–10) 7.6 (IQR 4–10) * | NR | NR | PhA significantly improved after nutrition intervention (+38%) |
| Mascherini et al., 2020 [37] | Clinical trial | Pre/post adjuvant hormone and/or chemotherapy | 42 | T0 T1 (6 mo) | 71.9 ± 10.8 68.7 ± 10.1 * | 5.2 ± 0.7 5.3 ± 0.7 | 46.7 ± 4.7 45.7 ± 4.4 * | 25 ± 8.1 22.6 ± 7.2 * | PhA remained stable before and after 6 mo from starting therapy (+2%) |
| Stefani et al., 2017 [36] | Clinical Trial | Completed therapy | 28 | T0 T1 (6 mo) T2 (12 mo) | 70.2 ± 9.9 69.9 ± 14.9 70.5 ± 15.8 | 5.4 ± 0.7 5.7 ± 0.8 * 5.9 ± 0.7 * | 45.9 ± 5.9 • 45.8 ± 5.9 • 46.7 ± 7.2 • | (%): 34.6 ± 8.3 (%): 34.4 ± 8.5 (%): 33.7 ± 10.3 | PhA significantly improved after exercise program (+9%) |
| Eyigör et al., 2021 [43] | RCT | Completed therapy | 31 | Y (n = 15) T0 T1 (10 weeks) C (n = 16) T0 T1 (10 weeks) | NR | 5.2 ± 0.7 5.2 ± 0.5 5.2 ± 0.4 5.2 ± 0.5 | NR | NR | PhA was not affected by Hatha yoga exercises. No difference was found between the two groups |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morlino, D.; Cioffi, I.; Marra, M.; Di Vincenzo, O.; Scalfi, L.; Pasanisi, F. Bioelectrical Phase Angle in Patients with Breast Cancer: A Systematic Review. Cancers 2022, 14, 2002. https://doi.org/10.3390/cancers14082002
Morlino D, Cioffi I, Marra M, Di Vincenzo O, Scalfi L, Pasanisi F. Bioelectrical Phase Angle in Patients with Breast Cancer: A Systematic Review. Cancers. 2022; 14(8):2002. https://doi.org/10.3390/cancers14082002
Chicago/Turabian StyleMorlino, Delia, Iolanda Cioffi, Maurizio Marra, Olivia Di Vincenzo, Luca Scalfi, and Fabrizio Pasanisi. 2022. "Bioelectrical Phase Angle in Patients with Breast Cancer: A Systematic Review" Cancers 14, no. 8: 2002. https://doi.org/10.3390/cancers14082002
APA StyleMorlino, D., Cioffi, I., Marra, M., Di Vincenzo, O., Scalfi, L., & Pasanisi, F. (2022). Bioelectrical Phase Angle in Patients with Breast Cancer: A Systematic Review. Cancers, 14(8), 2002. https://doi.org/10.3390/cancers14082002








