Suppression of the ABCA1 Cholesterol Transporter Impairs the Growth and Migration of Epithelial Ovarian Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohorts
2.2. Cell Lines and Maintenance
2.3. Quantitative PCR (qPCR)
2.4. Western Blots
2.5. siRNA Transfections
2.6. Growth Curves
2.7. Bromodeoxyuridine (BrdU) Incorporation Assays
2.8. Apoptosis Detection
2.9. Wound Healing Assay
2.10. Growth in 3D Spheroids
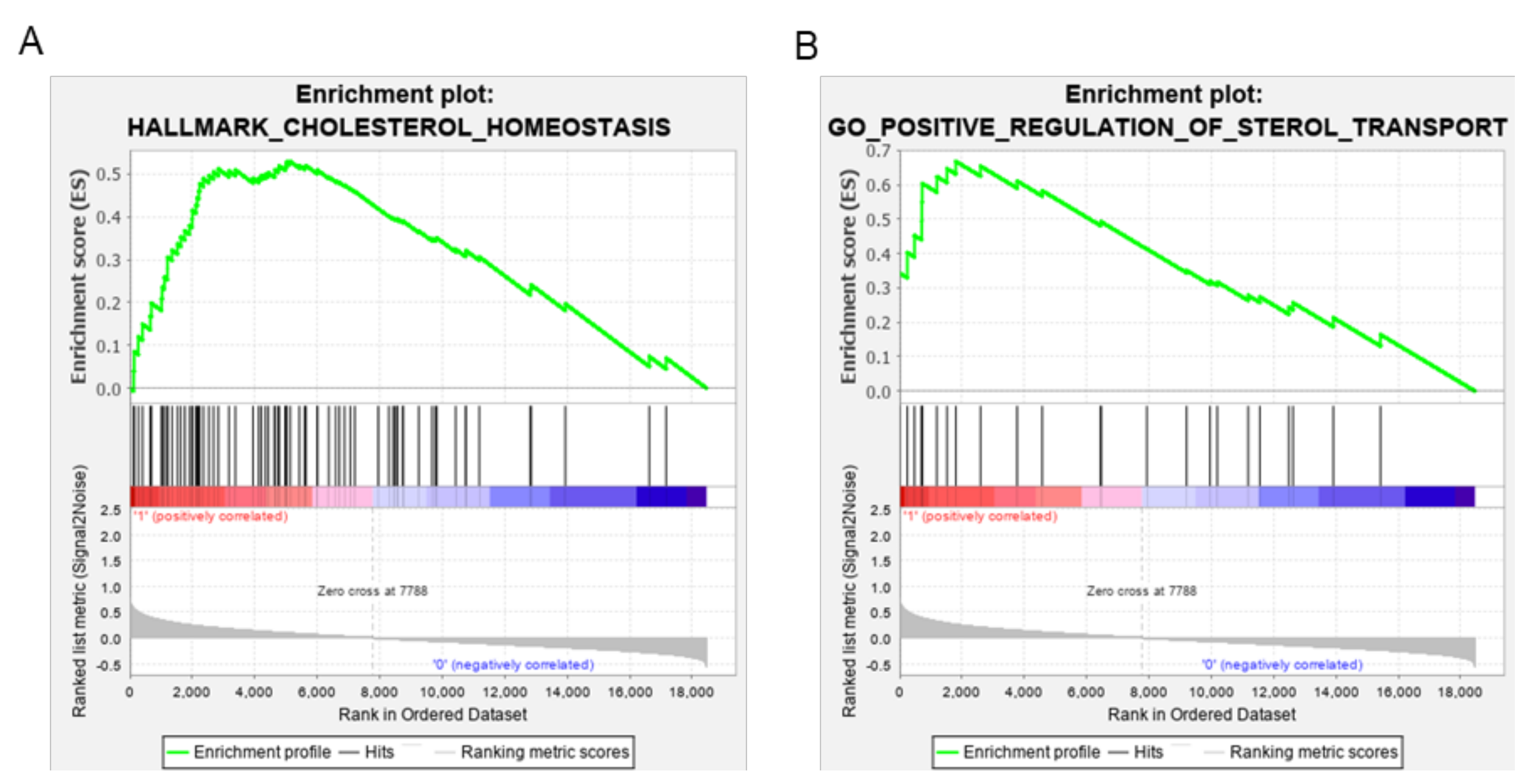
2.11. Gene Set Enrichment Analysis
2.12. Cholesterol Extraction and Quantification
2.13. Cell Viability Assay
2.14. General Statistical Analysis
3. Results
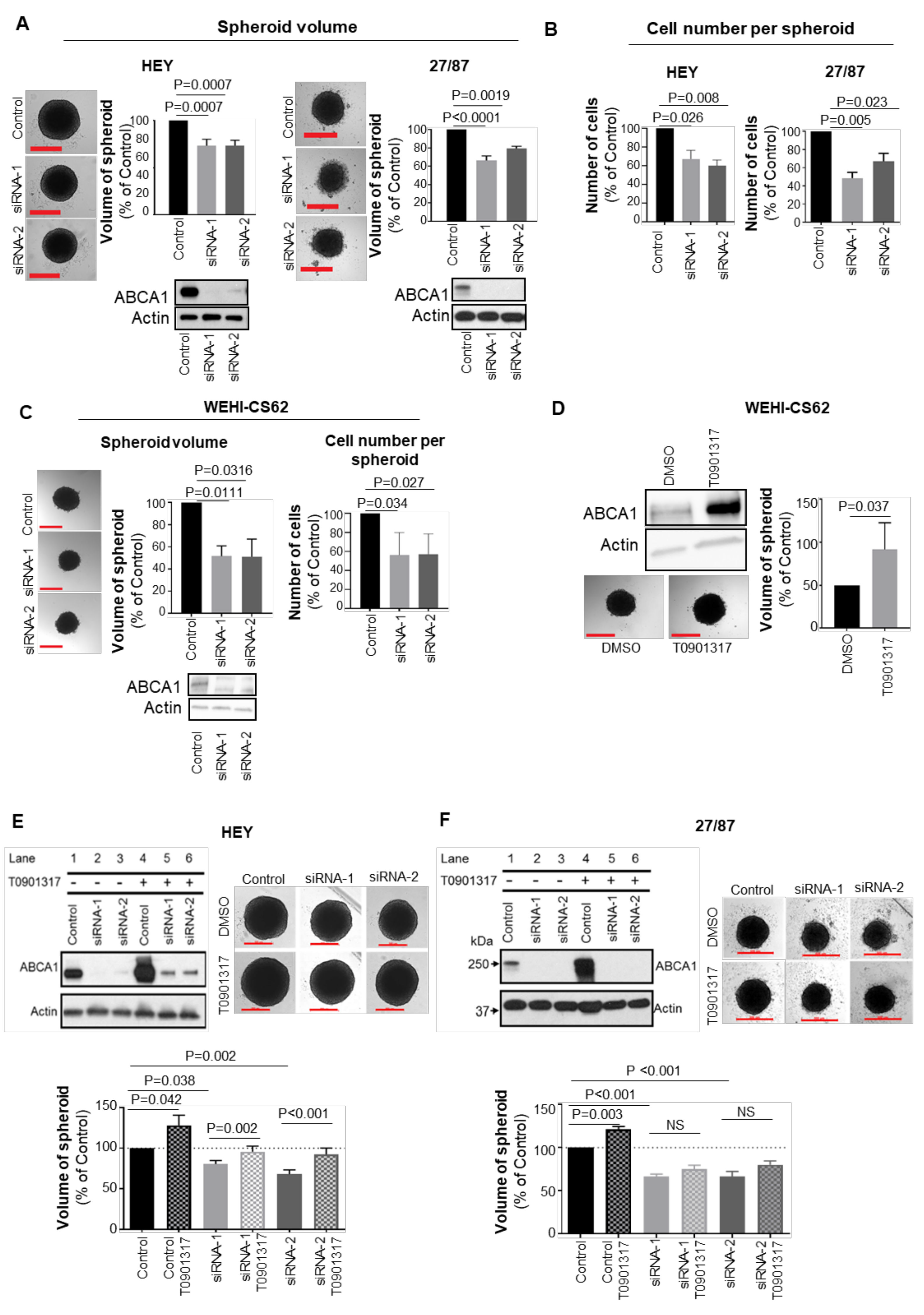
3.1. ABCA1 Suppression Impaired Malignant Phenotypes of Epithelial Ovarian Cancer Cells
3.2. ABCA1 Suppression Impaired the Development of Three-Dimensional Structures
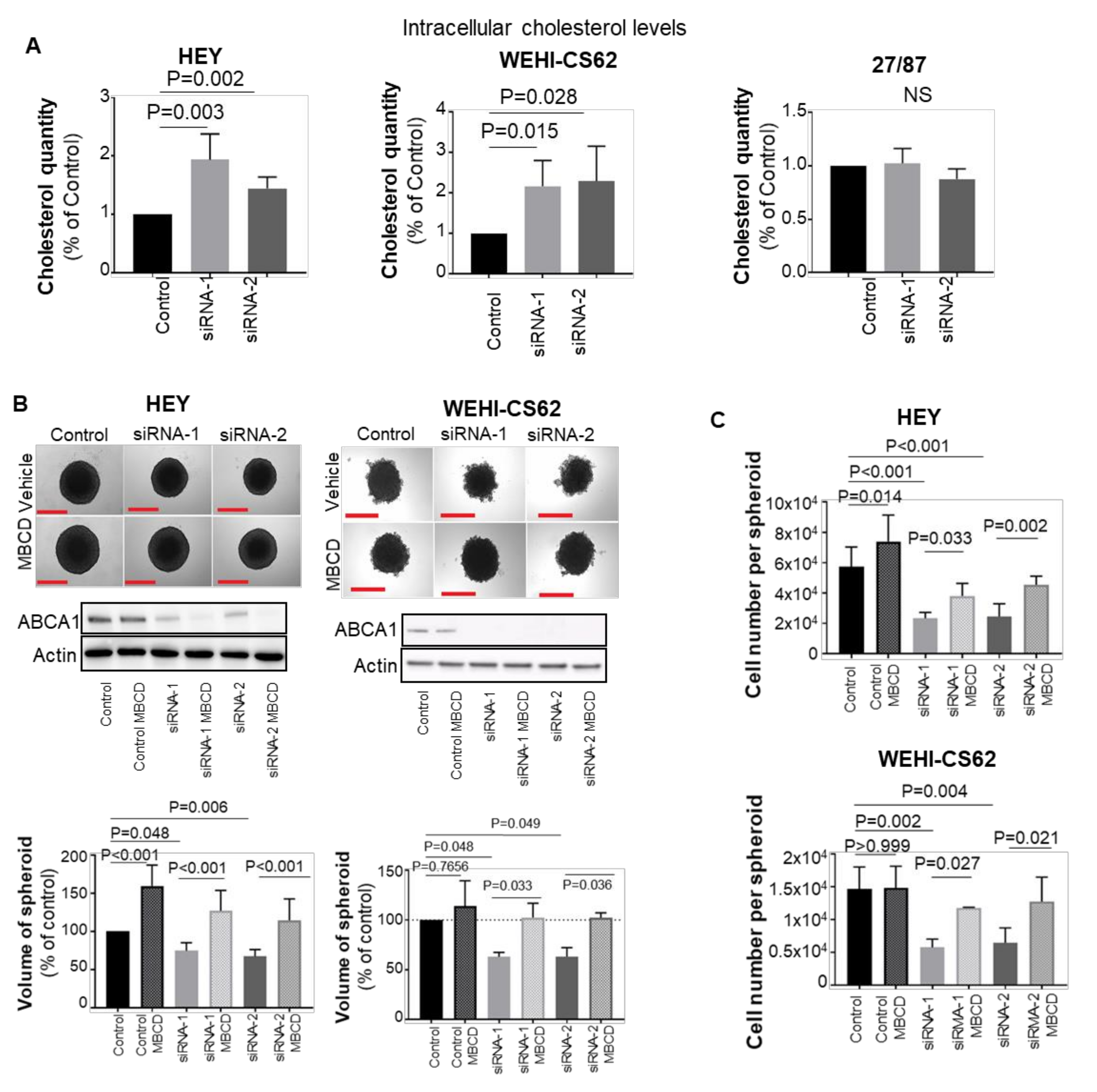
3.3. ABCA1 Suppression Induced Cholesterol Accumulation in EOC Cells
3.4. Blocking Cholesterol Efflux Using FDA-Approved Agents Impaired Malignant Phenotypes in EOC Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandra, A.; Pius, C.; Nabeel, M.; Nair, M.; Vishwanatha, J.; Ahmad, S.; Basha, R. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med. 2019, 8, 7018–7031. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.G. Morphological subtypes of ovarian carcinoma: A review with emphasis on new developments and pathogenesis. Pathology 2011, 43, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Primers 2016, 2, 16061. [Google Scholar] [CrossRef] [PubMed]
- Giusti, I.; Bianchi, S.; Nottola, S.A.; Macchiarelli, G.; Dolo, V. Clinical electron microscopy in the study of human ovarian tissues. EMBJ 2019, 14, 145–151. [Google Scholar]
- Coleman, R.L.; Monk, B.J.; Sood, A.K.; Herzog, T.J. Latest research and treatment of advanced-stage epithelial ovarian cancer. Nat. Rev. Clin. Oncol. 2013, 10, 211–224. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, P.Y. Recent perspectives of epithelial ovarian carcinoma. Oncol. Lett. 2016, 12, 3055–3058. [Google Scholar] [CrossRef]
- Narod, S. Can advanced-stage ovarian cancer be cured? Nat. Rev. Clin. Oncol. 2016, 13, 255–261. [Google Scholar] [CrossRef]
- Weidle, U.H.; Birzele, F.; Kollmorgen, G.; Rueger, R. Mechanisms and Targets Involved in Dissemination of Ovarian Cancer. CGP 2016, 13, 407–424. [Google Scholar] [CrossRef]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef]
- Worzfeld, T.; Von Strandmann, E.P.; Huber, M.; Adhikary, T.; Wagner, U.; Reinartz, S.; Müller, R. The Unique Molecular and Cellular Microenvironment of Ovarian Cancer. Front. Oncol. 2017, 7, 24. [Google Scholar] [CrossRef]
- Tang, H.; Chu, Y.; Huang, Z.; Cai, J.; Wang, Z. The metastatic phenotype shift toward myofibroblast of adipose-derived mesenchymal stem cells promotes ovarian cancer progression. Carcinogenesis 2020, 41, 182–193. [Google Scholar] [CrossRef]
- Ades, A.; Carvalho, J.P.; Graziani, S.R.; Amancio, R.F.; Souen, J.S.; Pinotti, J.A.; Maranhão, R. Uptake of a Cholesterol-Rich Emulsion by Neoplastic Ovarian Tissues. Gynecol. Oncol. 2001, 82, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Ladanyi, A.; Mukherjee, A.; Kenny, H.A.; Johnson, A.; Mitra, A.K.; Sundaresan, S.; Nieman, K.M.; Pascual, G.; Benitah, S.A.; Montag, A.; et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene 2018, 37, 2285–2301. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zheng, Y.; Pan, Q.; Chen, H.; Chen, F.; Wu, J.; Di, D. Expression of LXR β, ABCA1 and ABCG1 in human triple negative breast cancer tissues. Oncol. Rep. 2019, 42, 1869–1877. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, X.; Song, D.; Liu, X.; Gu, Y.; Xu, Z.; Wang, X.; Zhang, X.; Ye, Q.; Tong, Z.; et al. Cholesterol Induces Epithelial-to-Mesenchymal Transition of Prostate Cancer Cells by Suppressing Degradation of EGFR through APMAP. Cancer Res. 2019, 79, 3063–3075. [Google Scholar] [CrossRef] [PubMed]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef] [PubMed]
- Burns, V.E.; Kerppola, T.K. ATR-101 inhibits cholesterol efflux and cortisol secretion by ATP-binding cassette transporters, causing cytotoxic cholesterol accumulation in adrenocortical carcinoma cells. J. Cereb. Blood Flow Metab. 2017, 174, 3315–3332. [Google Scholar] [CrossRef] [PubMed]
- Kellner-Weibel, G.; Luke, S.J.; Rothblat, G.H. Cytotoxic cellular cholesterol is selectively removed by apoA-I via ABCA1. Atherosclerosis 2003, 171, 235–243. [Google Scholar] [CrossRef]
- Soumian, S.; Albrecht, C.; Davies, A.H.; Gibbs, R.G.J. ABCA1 and atherosclerosis. Vasc. Med. 2005, 10, 109–119. [Google Scholar] [CrossRef]
- Ma, L.; Dong, F.; Denis, M.; Feng, Y.; Wang, M.-D.; Zha, X. Ht31, a Protein Kinase A Anchoring Inhibitor, Induces Robust Cholesterol Efflux and Reverses Macrophage Foam Cell Formation through ATP-binding Cassette Transporter A1. J. Biol. Chem. 2011, 286, 3370–3378. [Google Scholar] [CrossRef]
- Torres-Adorno, A.M.; Vitrac, H.; Qi, Y.; Tan, L.; Levental, K.R.; Fan, Y.-Y.; Yang, P.; Chapkin, R.S.; Eckhardt, B.L.; Ueno, N.T. Eicosapentaenoic acid in combination with EPHA2 inhibition shows efficacy in preclinical models of triple-negative breast cancer by disrupting cellular cholesterol efflux. Oncogene 2019, 38, 2135–2150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Prijic, S.; Urban, B.C.; Tisza, M.J.; Zuo, Y.; Li, L.; Chang, J.T. Candidate Antimetastasis Drugs Suppress the Metastatic Capacity of Breast Cancer Cells by Reducing Membrane Fluidity. Cancer Res. 2016, 76, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Hedditch, E.L.; Gao, B.; Russell, A.J.; Lu, Y.; Emmanuel, C.; Beesley, J.; Johnatty, S.E.; Chen, X.; Harnett, P.; George, J.; et al. ABCA Transporter Gene Expression and Poor Outcome in Epithelial Ovarian Cancer. JNCI 2014, 106, dju149. [Google Scholar] [CrossRef]
- Topp, M.D.; Hartley, L.; Cook, M.; Heong, V.; Boehm, E.; McShane, L.; Pyman, J.; McNally, O.; Ananda, S.; Harrell, M.; et al. Molecular correlates of platinum response in human high-grade serous ovarian cancer patient-derived xenografts. Mol. Oncol. 2014, 8, 656–668. [Google Scholar] [CrossRef]
- Gao, J.; Jung, M.; Mayoh, C.; Venkat, P.; Hannan, K.M.; Fletcher, J.I.; Kamili, A.; Gifford, A.J.; Kusnadi, E.P.; Pearson, R.B.; et al. Suppression of ABCE1-Mediated mRNA Translation Limits N-MYC–Driven Cancer Progression. Cancer Res. 2020, 80, 3706–3718. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.J.; Haber, M.; Porro, A.; Munoz, M.A.; Iraci, N.; Xue, C.; Norris, M.D. ABCC multidrug transporters in childhood neuroblastoma: Clinical and biological effects independent of cytotoxic drug efflux. JNCI 2011, 103, 1236–1251. [Google Scholar] [CrossRef] [PubMed]
- Sodek, K.L.; Ringuette, M.J.; Brown, T.J. Compact spheroid formation by ovarian cancer cells is associated with contractile behavior and an invasive phenotype. Int. J. Cancer 2009, 124, 2060–2070. [Google Scholar] [CrossRef]
- Lee, J.M.; Mhawech-Fauceglia, P.; Lee, N.; Parsanian, L.C.; Lin, Y.G.; Gayther, S.A.; Lawrenson, K. A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro. Lab. Investig. 2013, 93, 528–542. [Google Scholar]
- Heredia-Soto, V.; Redondo, A.; Berjón, A.; Miguel-Martín, M.; Díaz, E.; Crespo, R.; Mendiola, M. High-throughput 3-dimensional culture of epithelial ovarian cancer cells as preclinical model of disease. Oncotarget 2018, 9, 21893–21903. [Google Scholar] [CrossRef]
- Kaneko, T.; Kanno, C.; Ichikawa-Tomikawa, N.; Kashiwagi, K.; Yaginuma, N.; Ohkoshi, C.; Chiba, H. Liver X receptor reduces proliferation of human oral cancer cells by promoting cholesterol efflux via up-regulation of ABCA1 expression. Oncotarget 2015, 6, 33345–33357. [Google Scholar] [CrossRef][Green Version]
- Sekine, Y.; Demosky, S.J.; Stonik, J.A.; Furuya, Y.; Koike, H.; Suzuki, K.; Remaley, A.T. High-Density Lipoprotein Induces Proliferation and Migration of Human Prostate Androgen–Independent Cancer Cells by an ABCA1-Dependent Mechanism. Mol. Cancer Res. 2010, 8, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Huang, C.H.; Houlihan, S.L.; Regunath, K.; Freed-Pastor, W.A.; Morris, J.P.; Tschaharganeh, D.F.; Kastenhuber, E.R.; Barsotti, A.M.; Culp-Hill, R.; et al. p53 Represses the Mevalonate Pathway to Mediate Tumor Suppression. Cell 2019, 176, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.L.; Huang, R.L.; Shay, J.; Chen, L.Y.; Lin, S.J.; Yan, P.S.; Chao, W.T.; Lai, Y.H.; Lai, Y.L.; Chao, T.K.; et al. Hypermethylation of the TGF-beta target, ABCA1 is associated with poor prognosis in ovarian cancer patients. Clin. Epigenetics 2015, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Bornfeldt, K.E. Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ. Res. 2016, 118, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.M.; Tabas, I. Free Cholesterol Loading of Macrophages Is Associated with Widespread Mitochondrial Dysfunction and Activation of the Mitochondrial Apoptosis Pathway. J. Biol. Chem. 2001, 276, 42468–42476. [Google Scholar] [CrossRef]
- Kritharides, L.; Kus, M.; Brown, A.J.; Jessup, W.; Dean, R.T. Hydroxypropyl-beta-cyclodextrin-mediated efflux of 7-ketocholesterol from macrophage foam cells. JBC 1996, 271, 27450–27455. [Google Scholar] [CrossRef]
- Gajate, C.; Mollinedo, F. The antitumor ether lipid ET-18-OCH3 induces apoptosis through translocation and capping of Fas/CD95 into membrane rafts in human leukemic cells. Blood 2001, 98, 3860–3863. [Google Scholar] [CrossRef]
- Hueber, A.O.; Bernard, A.M.; Hérincs, Z.; Couzinet, A.; He, H.T. An essential role for membrane rafts in the initiation of Fas/CD95-triggered cell death in mouse thymocytes. EMBO Rep. 2002, 3, 190–196. [Google Scholar] [CrossRef]
- Tsukano, H.; Gotoh, T.; Endo, M.; Miyata, K.; Tazume, H.; Kadomatsu, T.; Oike, Y. The endoplasmic reticulum stress-C/EBP homologous protein pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques. Arter. Thromb. Vasc. Biol. 2010, 30, 1925–1932. [Google Scholar] [CrossRef]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Et Biophys. Acta (BBA) Mol. Cell Res. 2013, 1833, 3460–3470. [Google Scholar] [CrossRef]
- Goossens, P.; Rodriguez-Vita, J.; Etzerodt, A.; Masse, M.; Rastoin, O.; Gouirand, V.; Ulas, T.; Papantonopoulou, O.; Van Eck, M.; Auphan-Anezin, N.; et al. Membrane Cholesterol Efflux Drives Tumor-Associated Macrophage Reprogramming and Tumor Progression. Cell Metab. 2019, 29, 1376–1389. [Google Scholar] [CrossRef] [PubMed]
- Key, C.C.; Liu, M.; Kurtz, C.L.; Chung, S.; Boudyguina, E.; Dinh, T.A.; Bashore, A.; Phelan, P.E.; Freedman, B.I.; Osborne, T.F.; et al. Hepatocyte ABCA1 Deletion Impairs Liver Insulin Signaling and Lipogenesis. Cell Rep. 2017, 19, 2116–2129. [Google Scholar] [CrossRef] [PubMed]
- Quazi, F.; Molday, R.S. Differential Phospholipid Substrates and Directional Transport by ATP-binding Cassette Proteins ABCA1, ABCA7, and ABCA4 and Disease-causing Mutants. J. Biol. Chem. 2013, 288, 34414–34426. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Walsh, M.T.; Hammad, S.M.; Cuchel, M.; Rader, D.J.; Hussain, M.M. ATP binding cassette family A protein 1 determines hexosylceramide and sphingomyelin levels in human and mouse plasma. J. Lipid Res. 2018, 59, 2084–2097. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Jung, M.; Williams, R.T.; Hui, D.; Russell, A.J.; Naim, A.J.; Kamili, A.; Clifton, M.; Bongers, A.; Mayoh, C.; et al. Suppression of the ABCA1 Cholesterol Transporter Impairs the Growth and Migration of Epithelial Ovarian Cancer. Cancers 2022, 14, 1878. https://doi.org/10.3390/cancers14081878
Gao J, Jung M, Williams RT, Hui D, Russell AJ, Naim AJ, Kamili A, Clifton M, Bongers A, Mayoh C, et al. Suppression of the ABCA1 Cholesterol Transporter Impairs the Growth and Migration of Epithelial Ovarian Cancer. Cancers. 2022; 14(8):1878. https://doi.org/10.3390/cancers14081878
Chicago/Turabian StyleGao, Jixuan, MoonSun Jung, Rebekka T. Williams, Danica Hui, Amanda J. Russell, Andrea J. Naim, Alvin Kamili, Molly Clifton, Angelika Bongers, Chelsea Mayoh, and et al. 2022. "Suppression of the ABCA1 Cholesterol Transporter Impairs the Growth and Migration of Epithelial Ovarian Cancer" Cancers 14, no. 8: 1878. https://doi.org/10.3390/cancers14081878
APA StyleGao, J., Jung, M., Williams, R. T., Hui, D., Russell, A. J., Naim, A. J., Kamili, A., Clifton, M., Bongers, A., Mayoh, C., Ho, G., Scott, C. L., Jessup, W., Haber, M., Norris, M. D., Henderson, M. J., & on behalf of Australian Ovarian Cancer Study. (2022). Suppression of the ABCA1 Cholesterol Transporter Impairs the Growth and Migration of Epithelial Ovarian Cancer. Cancers, 14(8), 1878. https://doi.org/10.3390/cancers14081878





