Cost-Effectiveness of Genetic Testing for All Women Diagnosed with Breast Cancer in China
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Model and Genetic Testing Strategy
2.2. Probabilities
2.3. Relatives: Number and Age Distribution
2.4. Costs
2.5. Life-Years
2.6. Quality-Adjusted Life-Years (QALYs)
2.7. Analysis
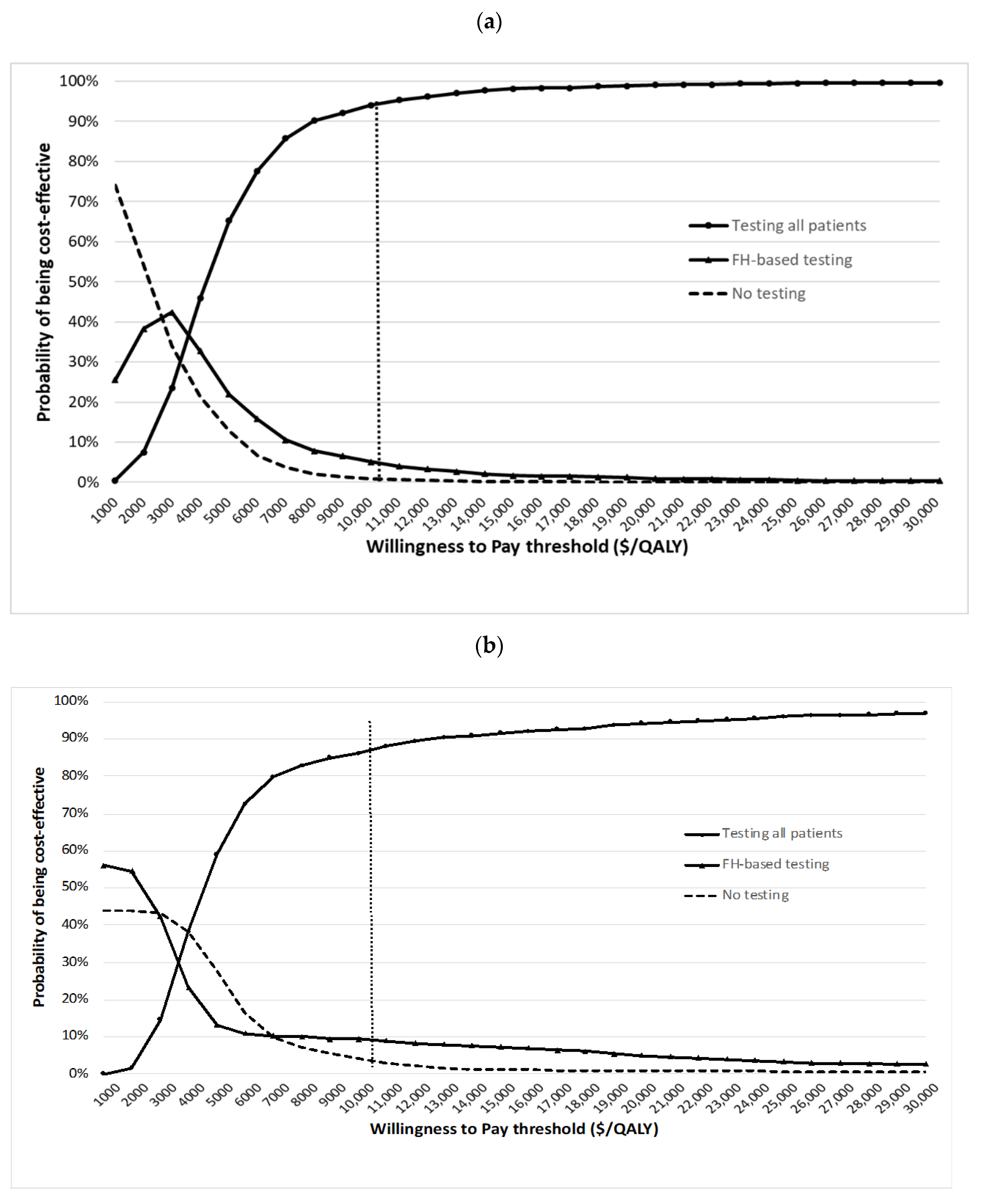
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NICE. Familial Breast Cancer: Classification, Care and Managing Breast Cancer and Related Risks in People with a Family History of Breast Cancer; NICE Clinical Guideline CG164, Updated 2017 ed.; National Institute for Health and Care Excellence: London, UK, 2017; Available online: https://www.nice.org.uk/guidance/cg164 (accessed on 10 June 2021).
- Manchanda, R.; Burnell, M.; Abdelraheim, A.; Johnson, M.; Sharma, A.; Benjamin, E.; Brunell, C.; Saridogan, E.; Gessler, S.; Oram, D.; et al. Factors influencing uptake and timing of risk reducing salpingo-oophorectomy in women at risk of familial ovarian cancer: A competing risk time to event analysis. BJOG 2012, 119, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, K.A.; Eisen, A.; Poll, A.; Candib, A.; McCready, D.; Cil, T.; Wright, F.; Demsky, R.; Mancuso, T.; Sun, P.; et al. Frequency of Contralateral Prophylactic Mastectomy in Breast Cancer Patients with a Negative BRCA1 and BRCA2 Rapid Genetic Test Result. Ann. Surg. Oncol. 2021, 28, 4967–4973. [Google Scholar] [CrossRef]
- Childers, C.P.; Childers, K.K.; Maggard-Gibbons, M.; Macinko, J. National Estimates of Genetic Testing in Women With a History of Breast or Ovarian Cancer. J. Clin. Oncol. 2017, 35, 3800–3806. [Google Scholar] [CrossRef] [PubMed]
- Kurian, A.W.; Ward, K.C.; Howlader, N.; Deapen, D.; Hamilton, A.S.; Mariotto, A.; Miller, D.; Penberthy, L.S.; Katz, S.J. Genetic Testing and Results in a Population-Based Cohort of Breast Cancer Patients and Ovarian Cancer Patients. J. Clin. Oncol. 2019, 37, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Strasser-Weippl, K.; Li, J.-J.; Louis, J.S.; Finkelstein, D.M.; Yu, K.-D.; Chen, W.-Q.; Shao, Z.-M.; Goss, P.E. Breast Cancer in China. Lancet Oncol. 2014, 15, e279–e289. [Google Scholar]
- Li, J.; Zhang, B.N.; Fan, J.H.; Pang, Y.; Zhang, P.; Wang, S.L.; Zheng, S.; Zhang, B.; Yang, H.J.; Xie, X.M.; et al. A nation-wide multicenter 10-year (1999–2008) retrospective clinical epidemiological study of female breast cancer in China. BMC Cancer 2011, 11, 364. [Google Scholar] [CrossRef]
- Fan, L.; Strasser-Weippl, K.; Li, J.J.; St Louis, J.; Finkelstein, D.M.; Yu, K.D.; Chen, W.Q.; Shao, Z.M.; Goss, P.E. Breast cancer in China. Lancet. Oncol. 2014, 15, e279–e289. [Google Scholar] [CrossRef]
- Sun, L.; Brentnall, A.; Patel, S.; Buist, D.S.M.; Bowles, E.J.A.; Evans, D.G.R.; Eccles, D.; Hopper, J.; Li, S.; Southey, M.; et al. A Cost-effectiveness Analysis of Multigene Testing for All Patients With Breast Cancer. JAMA Oncol. 2019, 5, 1718–1730. [Google Scholar] [CrossRef]
- National Bureau of Statistics. Main Data of the Seventh National Population Census. Available online: http://www.stats.gov.cn/tjsj/zxfb/202105/t20210510_1817176.html (accessed on 31 October 2021).
- Sun, J.; Meng, H.; Yao, L.; Lv, M.; Bai, J.; Zhang, J.; Wang, L.; Ouyang, T.; Li, J.; Wang, T.; et al. Germline Mutations in Cancer Susceptibility Genes in a Large Series of Unselected Breast Cancer Patients. Clin. Cancer Res. 2017, 23, 6113–6119. [Google Scholar] [CrossRef]
- Beitsch, P.D.; Whitworth, P.W.; Hughes, K.; Patel, R.; Rosen, B.; Compagnoni, G.; Baron, P.; Simmons, R.; Smith, L.A.; Grady, I.; et al. Underdiagnosis of Hereditary Breast Cancer: Are Genetic Testing Guidelines a Tool or an Obstacle? J. Clin. Oncol. 2019, 37, 453–460. [Google Scholar] [CrossRef]
- Metcalfe, K.A.; Eisen, A.; Poll, A.; Candib, A.; McCready, D.; Cil, T.; Wright, F.; Lerner-Ellis, J.; McCuaig, J.; Graham, T.; et al. Rapid Genetic Testing for BRCA1 and BRCA2 Mutations at the Time of Breast Cancer Diagnosis: An Observational Study. Ann. Surg. Oncol. 2021, 28, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.C.; Casadei, S.; Heikkinen, T.; Barrowdale, D.; Pylkas, K.; Roberts, J.; Lee, A.; Subramanian, D.; De Leeneer, K.; Fostira, F.; et al. Breast-cancer risk in families with mutations in PALB2. N. Engl. J. Med. 2014, 371, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Leslie, G.; Doroszuk, A.; Schneider, S.; Allen, J.; Decker, B.; Dunning, A.M.; Redman, J.; Scarth, J.; Plaskocinska, I.; et al. Cancer Risks Associated with Germline PALB2 Pathogenic Variants: An International Study of 524 Families. J. Clin. Oncol. 2019, 38, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Asphaug, L.; Melberg, H.O. The Cost-Effectiveness of Multigene Panel Testing for Hereditary Breast and Ovarian Cancer in Norway. MDM Policy Pract. 2019, 4, 2381468318821103. [Google Scholar] [CrossRef] [PubMed]
- Koldehoff, A.; Danner, M.; Civello, D.; Rhiem, K.; Stock, S.; Müller, D. Cost-Effectiveness of Targeted Genetic Testing for Breast and Ovarian Cancer: A Systematic Review. Value Health 2021, 24, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Manahan, E.R.; Kuerer, H.M.; Sebastian, M.; Hughes, K.S.; Boughey, J.C.; Euhus, D.M.; Boolbol, S.K.; Taylor, W.A. Consensus Guidelines on Genetic‘ Testing for Hereditary Breast Cancer from the American Society of Breast Surgeons. Ann. Surg. Oncol. 2019, 26, 3025–3031. [Google Scholar] [CrossRef] [PubMed]
- Easton, D.F.; Pharoah, P.D.; Antoniou, A.C.; Tischkowitz, M.; Tavtigian, S.V.; Nathanson, K.L.; Devilee, P.; Meindl, A.; Couch, F.J.; Southey, M.; et al. Gene-panel sequencing and the prediction of breast-cancer risk. N. Engl. J. Med. 2015, 372, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Haddow, J.; Palomaki, G. ACCE: A Model Process for Evaluating Data on Emerging Genetic Tests. In Human Genome Epidemiology: A Scientific Foundation for Using Genetic Information to Improve Health and Prevent Disease; Khoury, M., Little, J., Burke, W., Eds.; Oxford University Press: Oxford, UK, 2003; pp. 217–233. [Google Scholar]
- International Agency for Research on Cancer. Estimated Number of New Cases in 2018, Worldwide, Females, All Ages. Available online: http://gco.iarc.fr/today/online-analysis-table (accessed on 21 June 2021).
- Van Marcke, C.; Collard, A.; Vikkula, M.; Duhoux, F.P. Prevalence of pathogenic variants and variants of unknown significance in patients at high risk of breast cancer: A systematic review and meta-analysis of gene-panel data. Crit. Rev. Oncol. Hematol. 2018, 132, 138–144. [Google Scholar] [CrossRef]
- Mersch, J.; Brown, N.; Pirzadeh-Miller, S.; Mundt, E.; Cox, H.C.; Brown, K.; Aston, M.; Esterling, L.; Manley, S.; Ross, T. Prevalence of Variant Reclassification Following Hereditary Cancer Genetic Testing. JAMA 2018, 320, 1266–1274. [Google Scholar] [CrossRef]
- Chai, X.; Domchek, S.; Kauff, N.; Rebbeck, T.; Chen, J. RE: Breast Cancer Risk After Salpingo-Oophorectomy in Healthy BRCA1/2 Mutation Carriers: Revisiting the Evidence for Risk Reduction. J. Natl. Cancer Inst. 2015, 107, djv217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Domchek, S.M.; Friebel, T.M.; Singer, C.F.; Evans, D.G.; Lynch, H.T.; Isaacs, C.; Garber, J.E.; Neuhausen, S.L.; Matloff, E.; Eeles, R.; et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010, 304, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Rebbeck, T.R.; Kauff, N.D.; Domchek, S.M. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J. Natl. Cancer Inst. 2009, 101, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk-Gerritsen, B.A.; Seynaeve, C.; van Asperen, C.J.; Ausems, M.G.; Collee, J.M.; van Doorn, H.C.; Gomez Garcia, E.B.; Kets, C.M.; van Leeuwen, F.E.; Meijers-Heijboer, H.E.; et al. Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: Revisiting the evidence for risk reduction. J. Natl. Cancer Inst. 2015, 107, djv033. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Treatment—National Cancer Institute. Available online: https://www.cancer.gov/types/breast/hp/breast-treatment-pdq (accessed on 22 November 2021).
- Parker, W.H.; Feskanich, D.; Broder, M.S.; Chang, E.; Shoupe, D.; Farquhar, C.M.; Berek, J.S.; Manson, J.E. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet. Gynecol. 2013, 121, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.M.; Grossardt, B.R.; Rhodes, D.J.; Brown, R.D., Jr.; Roger, V.L.; Melton, L.J., 3rd; Rocca, W.A. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause 2009, 16, 15–23. [Google Scholar] [CrossRef]
- Edejer, T.; Baltussen, R.; Adam, T.; Hutubessy, R.; Acharya, A.; Evans, D.; Murray, C. WHO Guide to Cost-Effectiveness Analysis; WHO, Ed.; World Health Organisation: Geneva, Switzerland, 2003. [Google Scholar]
- United Nations Department of Economic and Social Affairs. World Population Prospects 2019. Available online: https://population.un.org/wpp/ (accessed on 10 June 2021).
- Chen, H.; Chen, Y.; Cui, B. The association of multimorbidity with healthcare expenditure among the elderly patients in Beijing, China. Arch. Gerontol. Geriatr. 2018, 79, 32–38. [Google Scholar] [CrossRef]
- The World Bank. PPP Conversion Factor, GDP. The World Bank: 2019. Available online: https://data.worldbank.org/indicator/PA.NUS.PPP (accessed on 15 June 2021).
- Peking University; Fudan University; China Pharmaceutical University; Tianjin University; Ministry of Human Resources and Social Security; PLA 306 Hospital. China Guidelines for Pharmacoeconomic Evaluations. Available online: http://www.ispor.org/peguidelines/index.asp (accessed on 9 August 2021).
- Sanders, G.D.; Neumann, P.J.; Basu, A.; Brock, D.W.; Feeny, D.; Krahn, M.; Kuntz, K.M.; Meltzer, D.O.; Owens, D.K.; Prosser, L.A.; et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 2016, 316, 1093–1103. [Google Scholar] [CrossRef]
- World Health Organisation. Life Tables; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Evans, D.G.; Lalloo, F.; Ashcroft, L.; Shenton, A.; Clancy, T.; Baildam, A.D.; Brain, A.; Hopwood, P.; Howell, A. Uptake of risk-reducing surgery in unaffected women at high risk of breast and ovarian cancer is risk, age, and time dependent. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2318–2324. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Niksic, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Esteve, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Hammerschmidt, T.; Goertz, A.; Wagenpfeil, S.; Neiss, A.; Wutzler, P.; Banz, K. Validation of health economic models: The example of EVITA. Value Health 2003, 6, 551–559. [Google Scholar] [CrossRef] [PubMed]
- The World Bank. GDP per Capita (Current US$). Available online: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD (accessed on 15 June 2021).
- Health Economics Resource Center. U.S. Department of Veterans Affairs. Cost-Effectiveness Analysis. Available online: https://www.herc.research.va.gov/include/page.asp?id=cost-effectiveness-analysis (accessed on 16 February 2021).
- Briggs, A. Probabilistic analysis of cost-effectiveness models: Statistical representation of parameter uncertainty. Value Health 2005, 8, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hart, S.N.; Gnanaolivu, R.; Huang, H.; Lee, K.Y.; Na, J.; Gao, C.; Lilyquist, J.; Yadav, S.; Boddicker, N.J.; et al. A Population-Based Study of Genes Previously Implicated in Breast Cancer. N. Engl. J. Med. 2021, 384, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Association, C.; Dorling, L.; Carvalho, S.; Allen, J.; Gonzalez-Neira, A.; Luccarini, C.; Wahlstrom, C.; Pooley, K.A.; Parsons, M.T.; Fortuno, C.; et al. Breast Cancer Risk Genes—Association Analysis in More than 113,000 Women. N. Engl. J. Med. 2021, 384, 428–439. [Google Scholar] [CrossRef]
- NICE. NICE Health Technology Evaluations: The Manual. Available online: https://www.nice.org.uk/process/pmg36/chapter/introduction-to-health-technology-evaluation (accessed on 16 February 2022).
- Metcalfe, K.A.; Birenbaum-Carmeli, D.; Lubinski, J.; Gronwald, J.; Lynch, H.; Moller, P.; Ghadirian, P.; Foulkes, W.D.; Klijn, J.; Friedman, E.; et al. International variation in rates of uptake of preventive options in BRCA1 and BRCA2 mutation carriers. Int. J. Cancer 2008, 122, 2017–2022. [Google Scholar] [CrossRef]
- Kong, Y.; Yang, L.; Tang, H.; Lv, N.; Xie, X.; Li, J.; Guo, J.; Li, L.; Wu, M.; Gao, J.; et al. A nation-wide multicenter retrospective study of the epidemiological, pathological and clinical characteristics of breast cancer in situ in Chinese women in 1999–2008. PLoS ONE 2013, 8, e81055. [Google Scholar] [CrossRef]
- Xuan, Q.; Gao, K.; Song, Y.; Zhao, S.; Dong, L.; Zhang, Z.; Zhang, Q.; Wang, J. Adherence to Needed Adjuvant Therapy Could Decrease Recurrence Rates for Rural Patients With Early Breast Cancer. Clin. Breast Cancer 2016, 16, e165–e173. [Google Scholar] [CrossRef]
- Payne, K.; Gavan, S.P.; Wright, S.J.; Thompson, A.J. Cost-effectiveness analyses of genetic and genomic diagnostic tests. Nat. Rev. Genet. 2018, 19, 235–246. [Google Scholar] [CrossRef]
- Lowry, K.P.; Geuzinge, H.A.; Stout, N.K.; Alagoz, O.; Hampton, J.; Kerlikowske, K.; de Koning, H.J.; Miglioretti, D.L.; van Ravesteyn, N.T.; Schechter, C.; et al. Breast Cancer Screening Strategies for Women With ATM, CHEK2, and PALB2 Pathogenic Variants: A Comparative Modeling Analysis. JAMA Oncol. 2022. [Google Scholar] [CrossRef]
- Francken, A.B.; Schouten, P.C.; Bleiker, E.M.; Linn, S.C.; Rutgers, E.J. Breast cancer in women at high risk: The role of rapid genetic testing for BRCA1 and -2 mutations and the consequences for treatment strategies. Breast 2013, 22, 561–568. [Google Scholar] [CrossRef]
- Pal, T.; Agnese, D.; Daly, M.; La Spada, A.; Litton, J.; Wick, M.; Klugman, S.; Esplin, E.D.; Jarvik, G.P.; Professional, P.; et al. Points to consider: Is there evidence to support BRCA1/2 and other inherited breast cancer genetic testing for all breast cancer patients? A statement of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2020, 22, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, P.; Tao, X.; Zhong, N. Genetic services and testing in China. J. Community Genet. 2013, 4, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, D.; Sobocan, M.; Blyuss, O.; Miller, R.E.; Evans, O.; Crusz, S.M.; Mills-Baldock, T.; Sun, L.; Hammond, R.F.L.; Gaba, F.; et al. Implementation of Multigene Germline and Parallel Somatic Genetic Testing in Epithelial Ovarian Cancer: SIGNPOST Study. Cancers 2021, 13, 4344. [Google Scholar] [CrossRef] [PubMed]

| Interventions | Health Effects | Costs (USD) | ICER (Cost/QALY) | ||||
|---|---|---|---|---|---|---|---|
| LYGs | QALYs | Payer | Societal | Payer | Societal | ||
| Testing all BC patients | 14.164 | 13.483 | 4686 | 6808 | Testing all BC patients vs. testing based on FH/clinical criteria | 6848 | 4152 |
| Testing based on FH/clinical criteria | 14.149 | 13.470 | 4596 | 6753 | Testing all BC patients vs. no testing | 8340 | 5416 |
| No testing | 14.144 | 13.465 | 4554 | 6726 | - | - | - |
| Testing all BC Patients | No Testing | ICER | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Health Effects | Costs (USD) | Health Effects | Costs (USD) | Cost/LYG | Cost/QALY | ||||||
| LYGs | QALYs | Payer | Societal | LYGs | QALYs | Payer | Societal | Payer | Societal | Payer | Societal |
| Baseline | |||||||||||
| 14.164 | 13.483 | 4686 | 6808 | 14.144 | 13.465 | 4554 | 6726 | 6509 | 4037 | 7266 | 4506 |
| Scenario: No reduction in BC risk from RRSO b | |||||||||||
| 14.164 | 13.483 | 4686 | 6808 | 14.144 | 13.465 | 4554 | 6726 | 6508 | 4060 | 7308 | 4558 |
| Scenario: No HRT Adherence c | |||||||||||
| 14.163 | 13.483 | 4687 | 6809 | 14.144 | 13.465 | 4554 | 6726 | 6730 | 4201 | 7576 | 4729 |
| Scenario: Half RRM uptake in unaffected relatives d | |||||||||||
| 14.164 | 13.483 | 4687 | 6811 | 14.144 | 13.465 | 4554 | 6726 | 6546 | 4198 | 7449 | 4777 |
| Scenario: Half RRSO uptake in unaffected relatives e | |||||||||||
| 14.163 | 13.482 | 4682 | 6807 | 14.144 | 13.465 | 4554 | 6726 | 6425 | 4090 | 7439 | 4735 |
| Scenario: Half RRM and half RRSO uptake in unaffected relatives f | |||||||||||
| 14.164 | 13.482 | 4685 | 6813 | 14.144 | 13.465 | 4554 | 6726 | 6514 | 4342 | 7802 | 5201 |
| Scenario: Half CPM uptake in patients g | |||||||||||
| 14.160 | 13.481 | 4683 | 6812 | 14.144 | 13.465 | 4554 | 6726 | 7857 | 5243 | 8310 | 5546 |
| Scenario: Half RRSO uptake in patients h | |||||||||||
| 14.160 | 13.481 | 4672 | 6800 | 14.144 | 13.465 | 4554 | 6726 | 7014 | 4412 | 7588 | 4773 |
| Scenario: Lower uptake rate of genetic testing in patients and relatives i (70%) | |||||||||||
| 14.158 | 13.477 | 4644 | 6787 | 14.144 | 13.465 | 4554 | 6726 | 6229 | 4233 | 7575 | 5148 |
| Scenario: Lower uptake rate of genetic testing in patients and relatives i (50%) | |||||||||||
| 14.153 | 13.473 | 4607 | 6762 | 14.144 | 13.465 | 4554 | 6726 | 5449 | 3731 | 6922 | 4739 |
| Scenario: No VUS management j | |||||||||||
| 14.162 | 13.479 | 4629 | 6766 | 14.144 | 13.465 | 4554 | 6726 | 3943 | 2097 | 5355 | 2848 |
| IMPACT | Testing All BC Patients | Testing Based on Family History | No Testing | Difference (Testing All vs. No Testing) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Patients | Relatives | Patients | Relatives | Patients | Relatives | Patients | Relatives | Total | |
| Germline BC cases | 2075 a | 7658 | 3806 a | 10,493 | 4515 a | 11,576 | 2440 | 3918 | 6358 |
| Germline OC cases | 737 | 2144 | 1263 | 2640 | 1487 | 2904 | 750 | 760 | 1510 |
| Germline BC/OC deaths | 4873 | 3679 | 7237 | 4968 | 8247 | 5469 | 3374 | 1790 | 5164 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Cui, B.; Wei, X.; Sadique, Z.; Yang, L.; Manchanda, R.; Legood, R. Cost-Effectiveness of Genetic Testing for All Women Diagnosed with Breast Cancer in China. Cancers 2022, 14, 1839. https://doi.org/10.3390/cancers14071839
Sun L, Cui B, Wei X, Sadique Z, Yang L, Manchanda R, Legood R. Cost-Effectiveness of Genetic Testing for All Women Diagnosed with Breast Cancer in China. Cancers. 2022; 14(7):1839. https://doi.org/10.3390/cancers14071839
Chicago/Turabian StyleSun, Li, Bin Cui, Xia Wei, Zia Sadique, Li Yang, Ranjit Manchanda, and Rosa Legood. 2022. "Cost-Effectiveness of Genetic Testing for All Women Diagnosed with Breast Cancer in China" Cancers 14, no. 7: 1839. https://doi.org/10.3390/cancers14071839
APA StyleSun, L., Cui, B., Wei, X., Sadique, Z., Yang, L., Manchanda, R., & Legood, R. (2022). Cost-Effectiveness of Genetic Testing for All Women Diagnosed with Breast Cancer in China. Cancers, 14(7), 1839. https://doi.org/10.3390/cancers14071839








