Quantifying Tumor Heterogeneity via MRI Habitats to Characterize Microenvironmental Alterations in HER2+ Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Animal Model
2.2. Longitudinal Tumor Growth Study
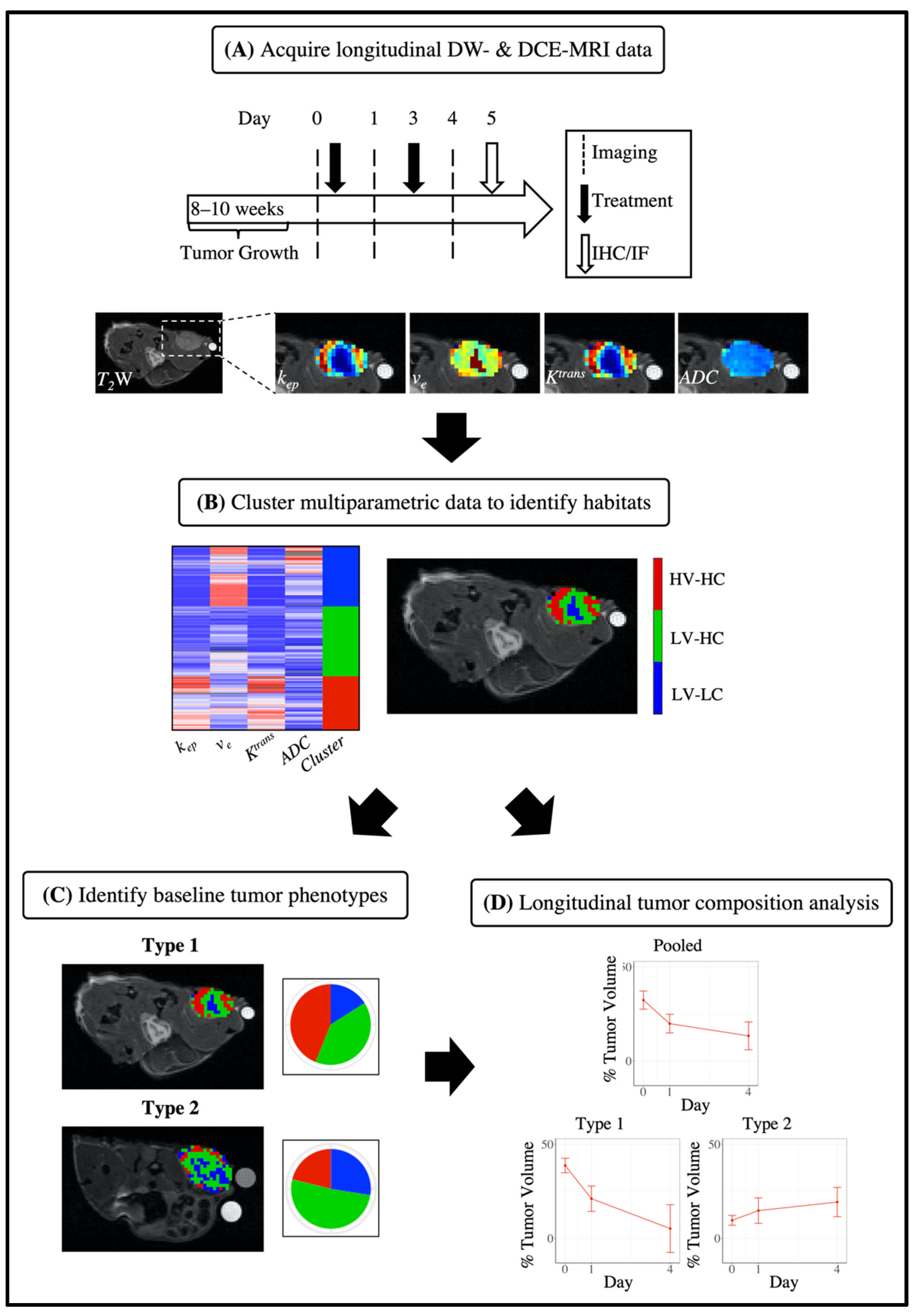
2.3. Magnetic Resonance Imaging
2.4. Immunohistochemistry and Immunofluorescence
2.5. Identification of MRI Tumor Habitats
2.6. Discovery of Tumor Imaging Phenotypes
2.7. Quantifying Longitudinal Alterations in Tumor Composition
2.8. Statistical Analysis
3. Results
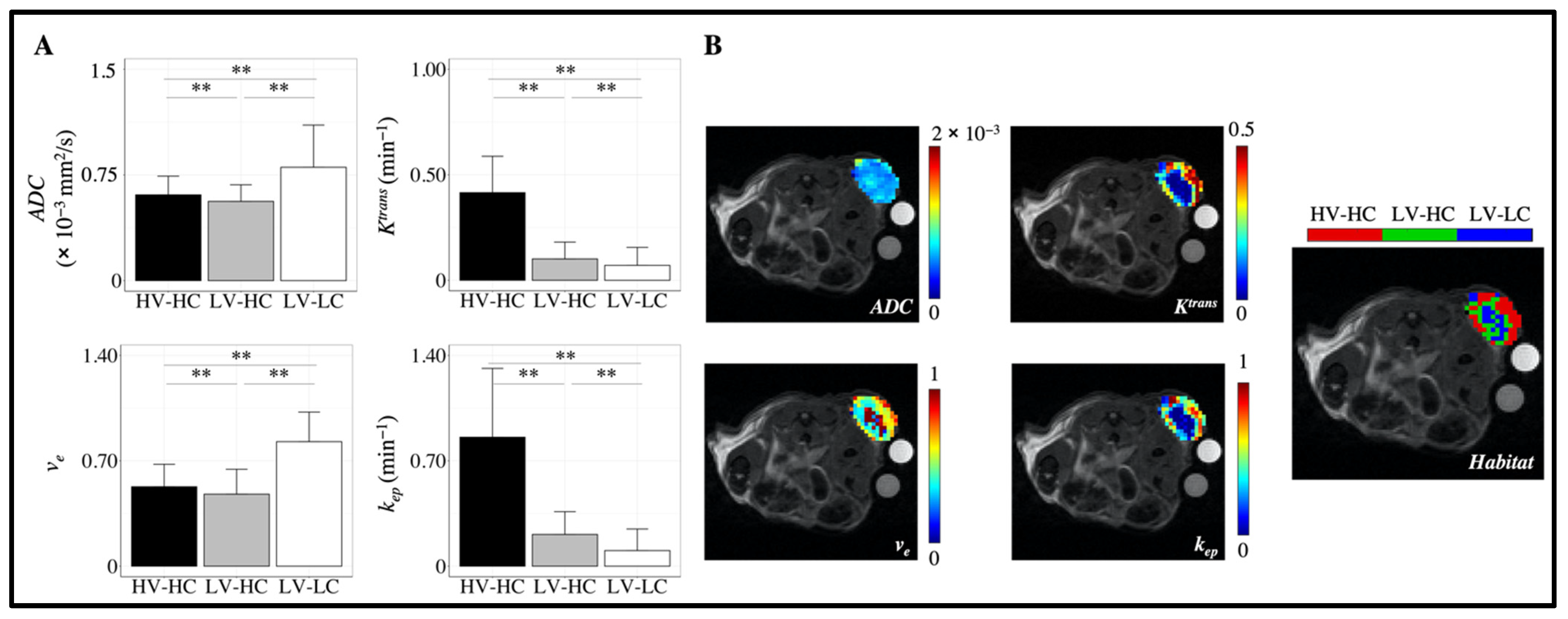
3.1. Characterization of MRI Tumor Habitats
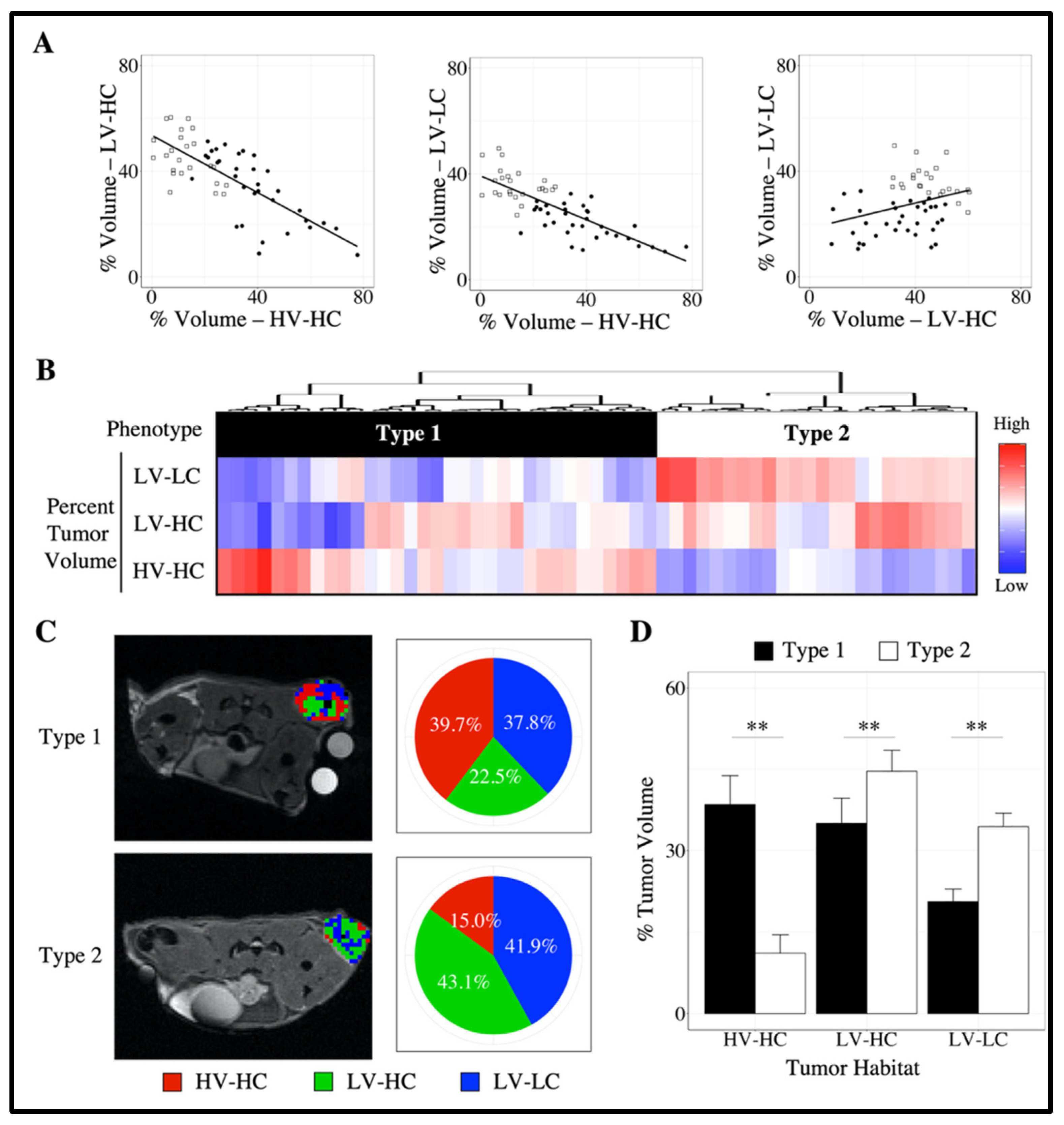
3.2. Characterization of Tumor Imaging Phenotypes
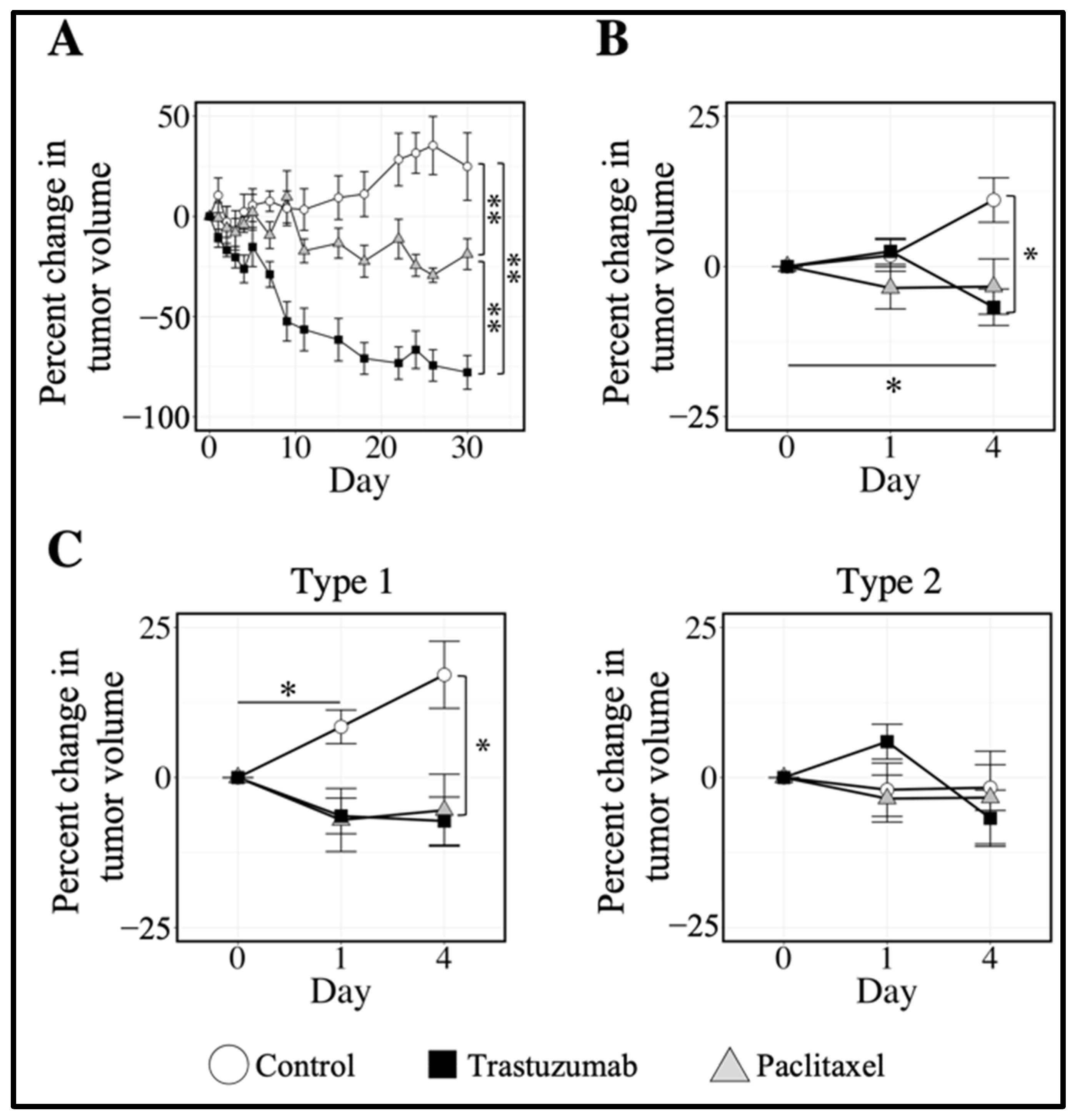
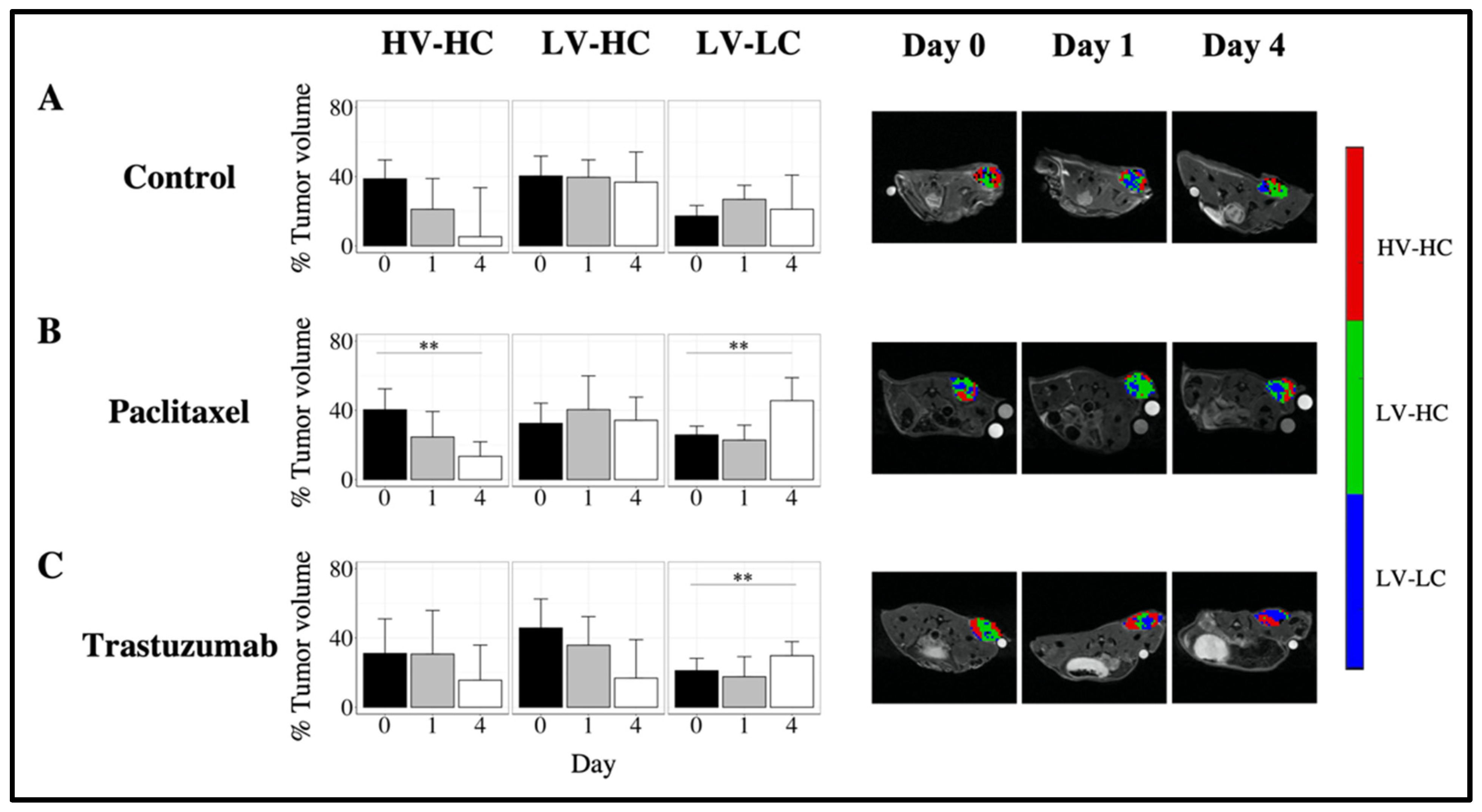
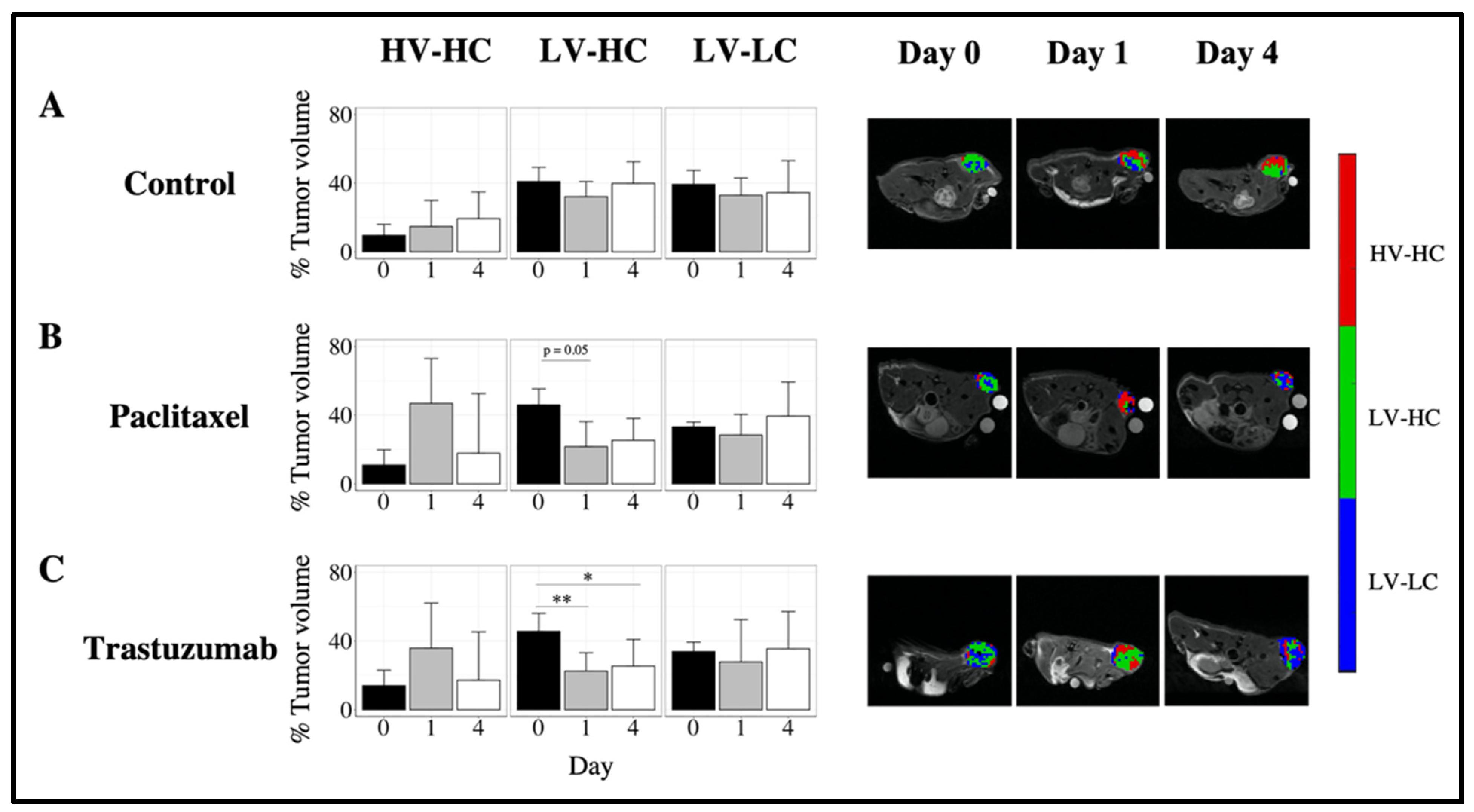
3.3. Quantifying Longitudinal Alterations in Tumor Volume
3.4. Quantifying Longitudinal Alterations in Tumor Composition
3.5. Correlation between MRI Habitats and Histology Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2019. CA A Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Dean-Colomb, W.; Esteva, F.J. Her2-Positive Breast Cancer: Herceptin and Beyond. Eur. J. Cancer 2008, 44, 2806–2812. [Google Scholar] [CrossRef]
- Buzdar, A.U.; Ibrahim, N.K.; Francis, D.; Booser, D.J.; Thomas, E.S.; Theriault, R.L.; Pusztai, L.; Green, M.C.; Arun, B.K.; Giordano, S.H.; et al. Significantly Higher Pathologic Complete Remission Rate After Neoadjuvant Therapy with Trastuzumab, Paclitaxel, and Epirubicin Chemotherapy: Results of a Randomized Trial in Human Epidermal Growth Factor Receptor 2–Positive Operable Breast Cancer. J. Clin. Oncol. 2005, 23, 3676–3685. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A.; Romond, E.H.; Suman, V.J.; Jeong, J.-H.; Sledge, G.; Geyer, C.E.; Martino, S.; Rastogi, P.; Gralow, J.; Swain, S.M.; et al. Trastuzumab Plus Adjuvant Chemotherapy for Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: Planned Joint Analysis of Overall Survival From NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 2014, 32, 3744–3752. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Martin-Castillo, B.; Oliveras-Ferraros, C.; Vazquez-Martin, A.; Cufí, S.; Moreno, J.M.; Corominas-Faja, B.; Urruticoechea, A.; Martín, Á.G.; López-Bonet, E.; Menendez, J.A. Basal/HER2 Breast Carcinomas: Integrating Molecular Taxonomy with Cancer Stem Cell Dynamics to Predict Primary Resistance to Trastuzumab (Herceptin). Cell Cycle 2013, 12, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Zardavas, D.; Irrthum, A.; Swanton, C.; Piccart, M. Clinical Management of Breast Cancer Heterogeneity. Nat. Rev. Clin. Oncol. 2015, 12, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Seo, A.N.; Kim, E.J.; Jang, M.H.; Suh, K.J.; Ryu, H.S.; Kim, Y.J.; Kim, J.H.; Im, S.-A.; Gong, G.; et al. HER2 Heterogeneity Affects Trastuzumab Responses and Survival in Patients With HER2-Positive Metastatic Breast Cancer. Am. J. Clin. Pathol. 2014, 142, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, A.M.; Hormuth, D.A.; Adhikarla, V.; Sahoo, P.; Abler, D.; Tumyan, L.; Schmolze, D.; Mortimer, J.; Rockne, R.C.; Yankeelov, T.E. Towards Integration of 64 Cu-DOTA-Trastuzumab PET-CT and MRI with Mathematical Modeling to Predict Response to Neoadjuvant Therapy in HER2 + Breast Cancer. Sci. Rep. 2020, 10, 20518. [Google Scholar] [CrossRef] [PubMed]
- Onsum, M.D.; Geretti, E.; Paragas, V.; Kudla, A.J.; Moulis, S.P.; Luus, L.; Wickham, T.J.; McDonagh, C.F.; MacBeath, G.; Hendriks, B.S. Single-Cell Quantitative HER2 Measurement Identifies Heterogeneity and Distinct Subgroups within Traditionally Defined HER2-Positive Patients. Am. J. Pathol. 2013, 183, 1446–1460. [Google Scholar] [CrossRef] [PubMed]
- Rye, I.H.; Trinh, A.; Sætersdal, A.B.; Nebdal, D.; Lingjærde, O.C.; Almendro, V.; Polyak, K.; Børresen-Dale, A.-L.; Helland, Å.; Markowetz, F.; et al. Intratumor Heterogeneity Defines Treatment-Resistant HER2+ Breast Tumors. Mol. Oncol. 2018, 12, 1838–1855. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, M.; Massicano, A.V.F.; Song, P.N.; Mansur, A.; Heinzman, K.A.; Larimer, B.M.; Lapi, S.E.; Sorace, A.G. [89Zr]-Pertuzumab PET Imaging Reveals Paclitaxel Treatment Efficacy Is Positively Correlated with HER2 Expression in Human Breast Cancer Xenograft Mouse Models. Molecules 2021, 26, 1568. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; Tarpey, P.; et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Yankeelov, T.E.; Abramson, R.G.; Quarles, C.C. Quantitative Multimodality Imaging in Cancer Research and Therapy. Nat. Rev. Clin. Oncol. 2014, 11, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Arlinghaus, L.R.; Yankeelov, T.E. Diffusion-Weighted MRI. In Quantitative MRI in Cancer; Imaging in Medical Diagnosis and Therapy; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-1-4398-2058-2. [Google Scholar]
- Yankeelov, T.; Gore, J. Dynamic Contrast Enhanced Magnetic Resonance Imaging in Oncology:Theory, Data Acquisition, Analysis, and Examples. Curr. Med. Imaging Rev. 2007, 3, 91–107. [Google Scholar] [CrossRef]
- Barnes, S.L.; Sorace, A.G.; Whisenant, J.G.; McIntyre, J.O.; Kang, H.; Yankeelov, T.E. DCE- and DW-MRI as Early Imaging Biomarkers of Treatment Response in a Preclinical Model of Triple Negative Breast Cancer. NMR Biomed. 2017, 30, e3799. [Google Scholar] [CrossRef]
- Sorace, A.G.; Quarles, C.C.; Whisenant, J.G.; Hanker, A.B.; McIntyre, J.O.; Sanchez, V.M.; Yankeelov, T.E. Trastuzumab Improves Tumor Perfusion and Vascular Delivery of Cytotoxic Therapy in a Murine Model of HER2+ Breast Cancer: Preliminary Results. Breast Cancer Res. Treat. 2016, 155, 273–284. [Google Scholar] [CrossRef]
- Virostko, J.; Hainline, A.; Kang, H.; Arlinghaus, L.R.; Abramson, R.G.; Barnes, S.L.; Blume, J.D.; Avery, S.; Patt, D.; Goodgame, B.; et al. Dynamic Contrast-Enhanced Magnetic Resonance Imaging and Diffusion-Weighted Magnetic Resonance Imaging for Predicting the Response of Locally Advanced Breast Cancer to Neoadjuvant Therapy: A Meta-Analysis. J. Med. Imaging 2018, 5, 11011. [Google Scholar] [CrossRef]
- Fangberget, A.; Nilsen, L.B.; Hole, K.H.; Holmen, M.M.; Engebraaten, O.; Naume, B.; Smith, H.-J.; Olsen, D.R.; Seierstad, T. Neoadjuvant Chemotherapy in Breast Cancer-Response Evaluation and Prediction of Response to Treatment Using Dynamic Contrast-Enhanced and Diffusion-Weighted MR Imaging. Eur. Radiol. 2011, 21, 1188–1199. [Google Scholar] [CrossRef][Green Version]
- Galons, J.-P.; Altbach, M.I.; Paine-Murrieta, G.D.; Taylor, C.W.; Gillies, R.J. Early Increases in Breast Tumor Xenograft Water Mobility in Response to Paclitaxel Therapy Detected by Non-Invasive Diffusion Magnetic Resonance Imaging. Neoplasia 1999, 1, 113–117. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Nia, H.T.; Munn, L.L.; Jain, R.K. Physical Traits of Cancer. Science 2020, 370, eaaz0868. [Google Scholar] [CrossRef]
- Martin, J.D.; Seano, G.; Jain, R.K. Normalizing Function of Tumor Vessels: Progress, Opportunities, and Challenges. Annu. Rev. Physiol. 2019, 81, 505–534. [Google Scholar] [CrossRef] [PubMed]
- Zemek, R.M.; Chin, W.L.; Nowak, A.K.; Millward, M.J.; Lake, R.A.; Lesterhuis, W.J. Sensitizing the Tumor Microenvironment to Immune Checkpoint Therapy. Front. Immunol. 2020, 11, 223. [Google Scholar] [CrossRef] [PubMed]
- Jardim-Perassi, B.V.; Martinez, G.; Gillies, R. Habitat Imaging of Tumor Evolution by Magnetic Resonance Imaging (MRI). In Radiomics and Radiogenomics; Li, R., Xing, L., Napel, S., Rubin, D.L., Eds.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2019; pp. 115–120. ISBN 978-1-351-20827-7. [Google Scholar]
- Syed, A.K.; Whisenant, J.G.; Barnes, S.L.; Sorace, A.G.; Yankeelov, T.E. Multiparametric Analysis of Longitudinal Quantitative MRI Data to Identify Distinct Tumor Habitats in Preclinical Models of Breast Cancer. Cancers 2020, 12, 1682. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Norton, L.; Albanell, J.; Kim, Y.-M.; Mendelsohn, J. Recombinant Humanized Anti-HER2 Antibody (HerceptinTM) Enhances the Antitumor Activity of Paclitaxel and Doxorubicin against HER2/Neu Overexpressing Human Breast Cancer Xenografts. Cancer Res. 1998, 58, 2825–2831. [Google Scholar] [PubMed]
- Bloom, M.J.; Song, P.; Virostko, J.; Yankeelov, T.E.; Sorace, A.G. Quantifying the Effects of Combination Trastuzumab and Radiation Therapy in Human Epidermal Growth Factor Receptor 2 Positive Breast Cancer. Preprint. Available online: https://www.researchsquare.com/article/rs-30208/v1 (accessed on 1 June 2020).
- Jarrett, A.M.; Bloom, M.J.; Godfrey, W.; Syed, A.K.; Ekrut, D.A.; Ehrlich, L.I.; Yankeelov, T.E.; Sorace, A.G. Mathematical Modelling of Trastuzumab-Induced Immune Response in an in Vivo Murine Model of HER2+ Breast Cancer. Math. Med. Biol. J. IMA 2019, 36, 381–410. [Google Scholar] [CrossRef] [PubMed]
- Kzhyshkowska, J.; Riabov, V.; Gudima, A.; Wang, N.; Orekhov, A.; Mickley, A. Role of Tumor Associated Macrophages in Tumor Angiogenesis and Lymphangiogenesis. Front. Physiol. 2014, 5, 75. [Google Scholar]
- Chaudhury, B.; Zhou, M.; Farhidzadeh, H.; Goldgof, D.B.; Hall, L.O.; Gatenby, R.A.; Gillies, R.J.; Weinfurtner, R.J.; Drukteinis, J.S. Predicting Ki67% Expression from DCE-MR Images of Breast Tumors Using Textural Kinetic Features in Tumor Habitats. In Proceedings of the Medical Imaging 2016: Computer-Aided Diagnosis, San Diego, CA, USA, 27 February–3 March 2016; Volume 9785, p. 97850T. [Google Scholar]
- Katiyar, P.; Divine, M.R.; Kohlhofer, U.; Quintanilla-Martinez, L.; Schölkopf, B.; Pichler, B.J.; Disselhorst, J.A. A Novel Unsupervised Segmentation Approach Quantifies Tumor Tissue Populations Using Multiparametric MRI: First Results with Histological Validation. Mol. Imaging Biol. 2017, 19, 391–397. [Google Scholar] [CrossRef]
- Martin, J.D.; Fukumura, D.; Duda, D.G.; Boucher, Y.; Jain, R.K. Reengineering the Tumor Microenvironment to Alleviate Hypoxia and Overcome Cancer Heterogeneity. Cold Spring Harb. Perspect. Med. 2016, 6, a027094. [Google Scholar] [CrossRef] [PubMed]
- Sorace, A.G.; Syed, A.K.; Barnes, S.L.; Quarles, C.C.; Sanchez, V.; Kang, H.; Yankeelov, T.E. Quantitative [18F]FMISO PET Imaging Shows Reduction of Hypoxia Following Trastuzumab in a Murine Model of HER2+ Breast Cancer. Mol. Imaging Biol. 2017, 19, 130–137. [Google Scholar] [CrossRef]
- McCormack, D.R.; Walsh, A.J.; Sit, W.; Arteaga, C.L.; Chen, J.; Cook, R.S.; Skala, M.C. In Vivo Hyperspectral Imaging of Microvessel Response to Trastuzumab Treatment in Breast Cancer Xenografts. Biomed. Opt. Express 2014, 5, 2247–2261. [Google Scholar] [CrossRef] [PubMed]
- Hardee, M.E.; Eapen, R.J.; Rabbani, Z.N.; Dreher, M.R.; Marks, J.; Blackwell, K.L.; Dewhirst, M.W. Her2/Neu Signaling Blockade Improves Tumor Oxygenation in a Multifactorial Fashion in Her2/Neu+ Tumors. Cancer Chemother. Pharmacol. 2009, 63, 219–228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, S.; Franc, B.L.; Harnish, R.J.; Liu, G.; Mitra, D.; Copeland, T.P.; Arasu, V.A.; Kornak, J.; Jones, E.F.; Behr, S.C.; et al. Exploration of PET and MRI Radiomic Features for Decoding Breast Cancer Phenotypes and Prognosis. NPJ Breast Cancer 2018, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chitalia, R.D.; Rowland, J.; McDonald, E.S.; Pantalone, L.; Cohen, E.A.; Gastounioti, A.; Feldman, M.; Schnall, M.; Conant, E.; Kontos, D. Imaging Phenotypes of Breast Cancer Heterogeneity in Preoperative Breast Dynamic Contrast Enhanced Magnetic Resonance Imaging (DCE-MRI) Scans Predict 10-Year Recurrence. Clin. Cancer Res. 2020, 26, 862–869. [Google Scholar] [CrossRef]
- Wu, J.; Cui, Y.; Sun, X.; Cao, G.; Li, B.; Ikeda, D.M.; Kurian, A.W.; Li, R. Unsupervised Clustering of Quantitative Image Phenotypes Reveals Breast Cancer Subtypes with Distinct Prognoses and Molecular Pathways. Clin. Cancer Res. 2017, 23, 3334–3342. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, A.G.V.; Gibbs, P.; Saccarelli, C.R.; Daimiel, I.; Gullo, R.L.; Fox, M.J.; Thakur, S.; Pinker, K.; Morris, E.A.; Morrow, M.; et al. MRI-Based Machine Learning Radiomics Can Predict HER2 Expression Level and Pathologic Response after Neoadjuvant Therapy in HER2 Overexpressing Breast Cancer. eBioMedicine 2020, 61, 103042. [Google Scholar] [CrossRef] [PubMed]
- Sorace, A.G.; Wu, C.; Barnes, S.L.; Jarrett, A.M.; Avery, S.; Patt, D.; Goodgame, B.; Luci, J.J.; Kang, H.; Abramson, R.G.; et al. Repeatability, Reproducibility, and Accuracy of Quantitative Mri of the Breast in the Community Radiology Setting. J. Magn. Reson. Imaging 2018, 48, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Yankeelov, T.E.; Mankoff, D.A.; Schwartz, L.H.; Lieberman, F.S.; Buatti, J.M.; Mountz, J.M.; Erickson, B.J.; Fennessy, F.M.M.; Huang, W.; Kalpathy-Cramer, J.; et al. Quantitative Imaging in Cancer Clinical Trials. Clin. Cancer Res. 2016, 22, 284–290. [Google Scholar] [CrossRef]
- Luxburg, U.V. Clustering Stability: An Overview; Now Publishers Inc.: Hanover, MA, USA, 2010; ISBN 978-1-60198-344-2. [Google Scholar]
- Wehrli, F.W. Magnetic Resonance of Calcified Tissues. J. Magn. Reson. 2013, 229, 35–48. [Google Scholar] [CrossRef]
- Yankeelov, T.E.; Rooney, W.D.; Li, X.; Springer, C.S. Variation of the Relaxographic “Shutter-Speed” for Transcytolemmal Water Exchange Affects the CR Bolus-Tracking Curve Shape. Magn. Reson. Med. 2003, 50, 1151–1169. [Google Scholar] [CrossRef]
- Yankeelov, T.E.; Rooney, W.D.; Huang, W.; Dyke, J.P.; Li, X.; Tudorica, A.; Lee, J.-H.; Koutcher, J.A.; Springer, C.S. Evidence for Shutter-Speed Variation in CR Bolus-Tracking Studies of Human Pathology. NMR Biomed. 2005, 18, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Kety, S.S. The Theory and Applications of the Exchange of Inert Gas at the Lungs and Tissues. Pharmacol. Rev. 1951, 3, 1–41. [Google Scholar] [PubMed]
- Tofts, P.S. Modeling Tracer Kinetics in Dynamic Gd-DTPA MR Imaging. J. Magn. Reson. Imaging 1997, 7, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Loveless, M.E.; Halliday, J.; Liess, C.; Xu, L.; Dortch, R.D.; Whisenant, J.; Waterton, J.C.; Gore, J.C.; Yankeelov, T.E. A Quantitative Comparison of the Influence of Individual versus Population-Derived Vascular Input Functions on Dynamic Contrast Enhanced-MRI in Small Animals. Magn. Reson. Med. 2012, 67, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rooney, W.D.; Várallyay, C.G.; Gahramanov, S.; Muldoon, L.L.; Goodman, J.A.; Tagge, I.J.; Selzer, A.H.; Pike, M.M.; Neuwelt, E.A.; et al. Dynamic Contrast Enhanced-MRI with Extravasating Contrast Reagent: Rat Cerebral Glioma Blood Volume Determination. J. Magn. Reson. San Diego Calif 1997 2010, 206, 190–199. [Google Scholar] [CrossRef]
- Wu, C.; Pineda, F.; Hormuth, D.A.; Karczmar, G.S.; Yankeelov, T.E. Quantitative Analysis of Vascular Properties Derived from Ultrafast DCE-MRI to Discriminate Malignant and Benign Breast Tumors. Magn. Reson. Med. 2019, 81, 2147–2160. [Google Scholar] [CrossRef]
- Syed, A.K.; Woodall, R.; Whisenant, J.G.; Yankeelov, T.E.; Sorace, A.G. Characterizing Trastuzumab-Induced Alterations in Intratumoral Heterogeneity with Quantitative Imaging and Immunohistochemistry in HER2+ Breast Cancer. Neoplasia 2019, 21, 17–29. [Google Scholar] [CrossRef]
- Vangestel, C.; de Wiele, C.V.; Damme, N.V.; Staelens, S.; Pauwels, P.; Reutelingsperger, C.P.M.; Peeters, M. 99mTc-(CO)3 His-Annexin A5 Micro-SPECT Demonstrates Increased Cell Death by Irinotecan During the Vascular Normalization Window Caused by Bevacizumab. J. Nucl. Med. 2011, 52, 1786–1794. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazerouni, A.S.; Hormuth, D.A., II; Davis, T.; Bloom, M.J.; Mounho, S.; Rahman, G.; Virostko, J.; Yankeelov, T.E.; Sorace, A.G. Quantifying Tumor Heterogeneity via MRI Habitats to Characterize Microenvironmental Alterations in HER2+ Breast Cancer. Cancers 2022, 14, 1837. https://doi.org/10.3390/cancers14071837
Kazerouni AS, Hormuth DA II, Davis T, Bloom MJ, Mounho S, Rahman G, Virostko J, Yankeelov TE, Sorace AG. Quantifying Tumor Heterogeneity via MRI Habitats to Characterize Microenvironmental Alterations in HER2+ Breast Cancer. Cancers. 2022; 14(7):1837. https://doi.org/10.3390/cancers14071837
Chicago/Turabian StyleKazerouni, Anum S., David A. Hormuth, II, Tessa Davis, Meghan J. Bloom, Sarah Mounho, Gibraan Rahman, John Virostko, Thomas E. Yankeelov, and Anna G. Sorace. 2022. "Quantifying Tumor Heterogeneity via MRI Habitats to Characterize Microenvironmental Alterations in HER2+ Breast Cancer" Cancers 14, no. 7: 1837. https://doi.org/10.3390/cancers14071837
APA StyleKazerouni, A. S., Hormuth, D. A., II, Davis, T., Bloom, M. J., Mounho, S., Rahman, G., Virostko, J., Yankeelov, T. E., & Sorace, A. G. (2022). Quantifying Tumor Heterogeneity via MRI Habitats to Characterize Microenvironmental Alterations in HER2+ Breast Cancer. Cancers, 14(7), 1837. https://doi.org/10.3390/cancers14071837





