N, LNR or LODDS: Which Is the Most Appropriate Lymph Node Classification Scheme for Patients with Radically Resected Pancreatic Cancer?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Procedures
2.2. Tumor Staging and Lymph Node Classification
2.3. Statistical Analysis
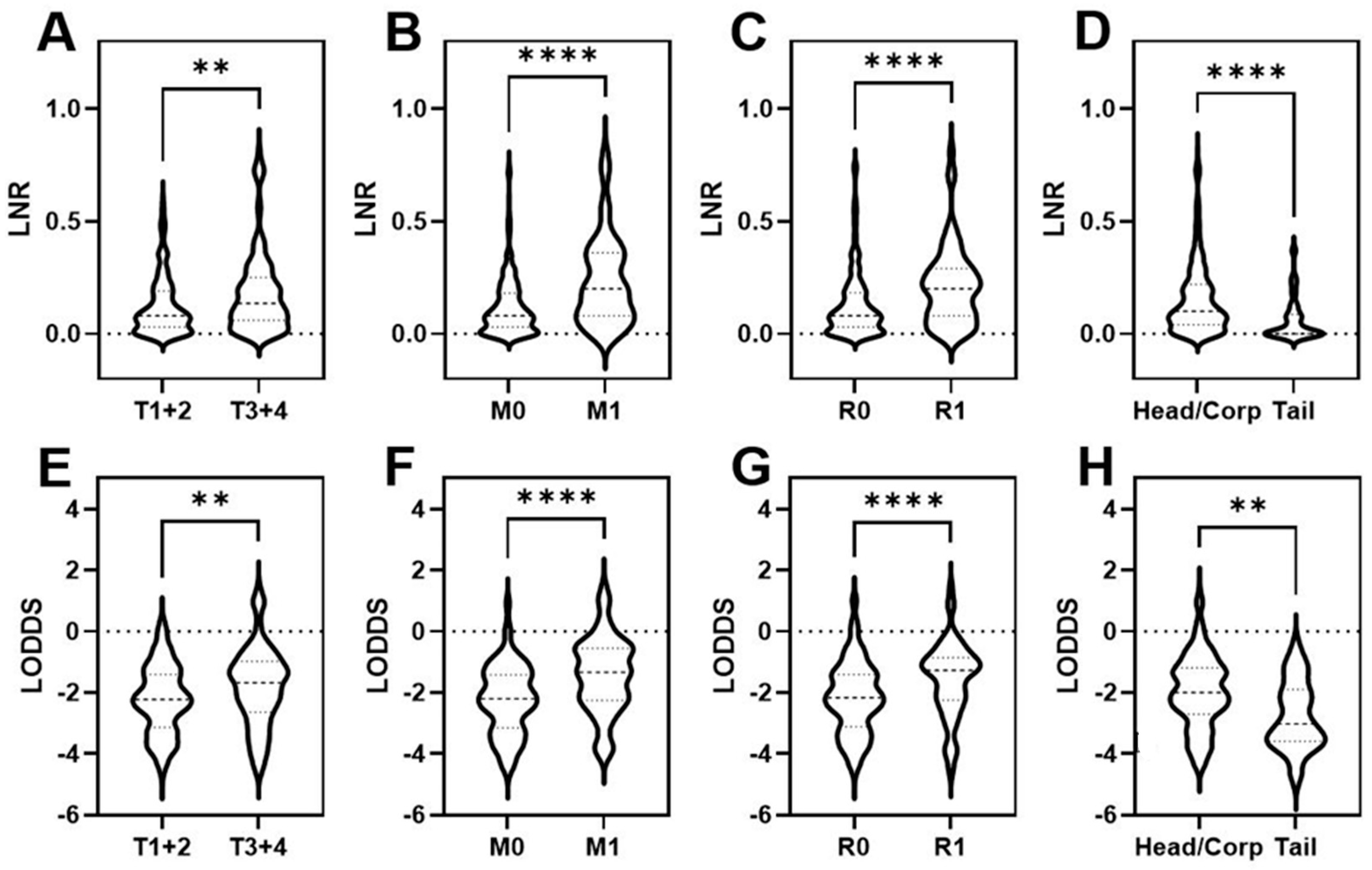
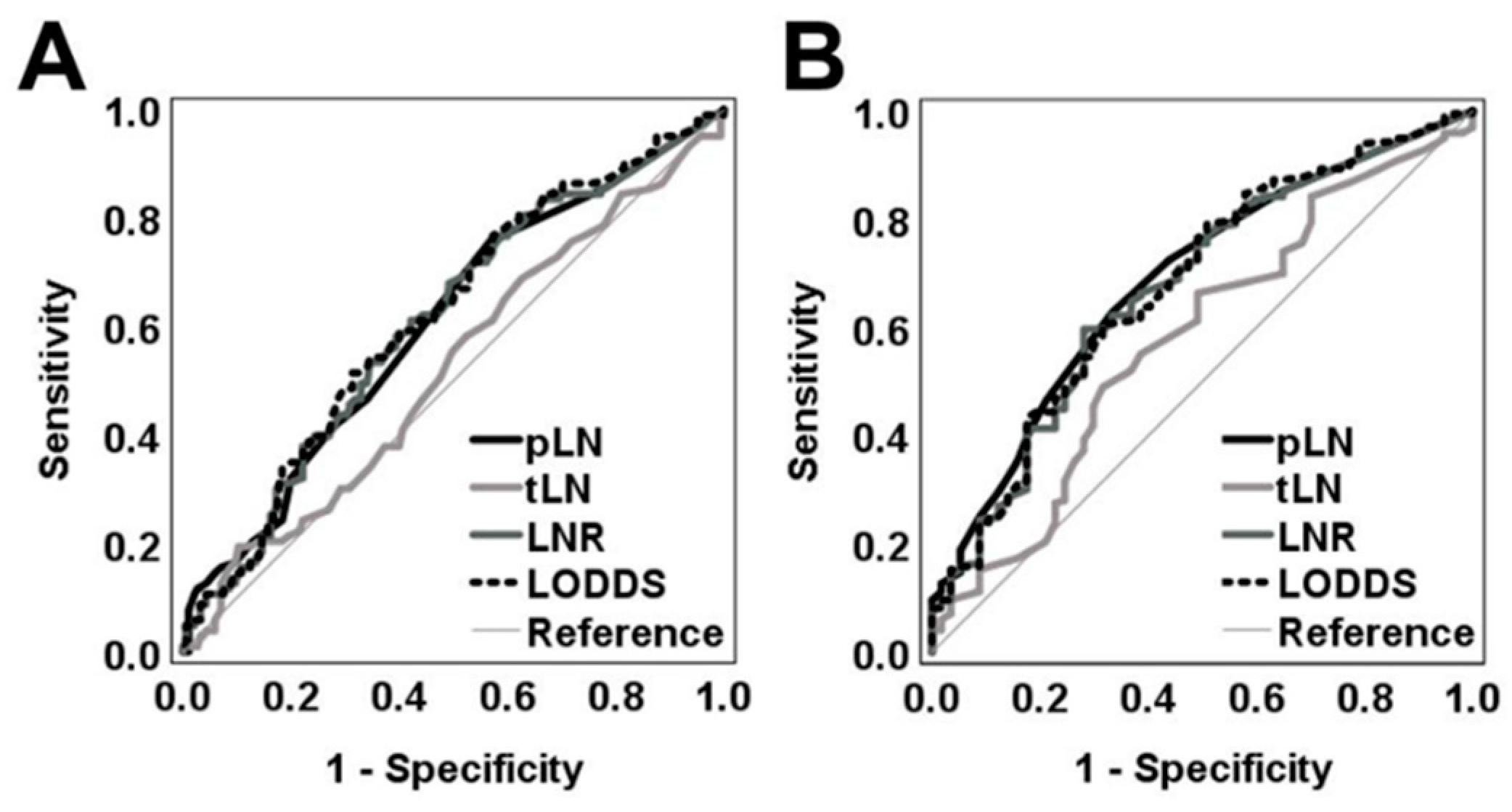
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, J.; He, W.; Ye, W. Burden of pancreatic cancer along with attributable risk factors in Europe between 1990 and 2019, and projections until 2039. Int. J. Cancer 2021, 149, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- O’Connell, J.B.; Manunga, J., Jr.; Tomlinson, J.S.; Reber, H.A.; Ko, C.Y.; Hines, O.J. Survival after resection of ampullary carcinoma: A national population-based study. Ann. Surg. Oncol. 2008, 15, 1820–1827. [Google Scholar] [CrossRef] [PubMed]
- Warschkow, R.; Köhn, N.; Erdem, S.; Schmied, B.; Nussbaum, D.P.; Gloor, B.; Müller, S.A.; Blazer, D.; Worni, M. Role of lymphadenectomy, adjuvant chemotherapy, and treatment at high-volume centers in patients with resected pancreatic cancer-a distinct view on lymph node yield. Langenbecks Arch. Surg. 2020, 405, 43–54. [Google Scholar] [CrossRef] [PubMed]
- AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: New York, NY, USA, 2017.
- TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Oxford, UK; Hoboken, NJ, USA, 2017.
- Agalar, C.; Unek, T.; Egeli, T.; Ozbilgin, M.; Akturk, N.; Semiz, H.S.; Unek, T.; Akarsu, M.; Soyturk, M.; Ellidokuz, H.; et al. The Role of Log Odds of Positive Lymph Nodes in Predicting the Survival after Resection for Ampullary Adenocarcinoma. Pathol. Oncol. Res. 2020, 26, 467–473. [Google Scholar] [CrossRef]
- Aoyama, T.; Kamiya, M.; Murakawa, M.; Tamagawa, H.; Sawazaki, S.; Numata, M.; Shiozawa, M.; Kobayashi, S.; Ueno, M.; Morimoto, M.; et al. The Lymph Node Ratio Is an Independent Prognostic Factor in Pancreatic Cancer Patients Who Receive Curative Resection Followed by Adjuvant Chemotherapy. Anticancer Res. 2018, 38, 4877–4882. [Google Scholar] [CrossRef] [PubMed]
- Tol, J.A.; Bassi, C.; Dervenis, C.; Montorsi, M.; Adham, M.; Andrén-Sandberg, A.; Asbun, H.J.; Bockhorn, M.; Büchler, M.W.; Conlon, K.C.; et al. International Study Group on Pancreatic Surgery. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: A consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014, 156, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Takaori, K.; Bassi, C.; Biankin, A.; Brunner, T.B.; Cataldo, I.; Campbell, F.; Cunningham, D.; Falconi, M.; Frampton, A.E.; Furuse, J.; et al. IAP/EPC study group on the clinical managements of pancreatic cancer. International Association of Pancreatology (IAP)/European Pancreatic Club (EPC) consensus review of guidelines for the treatment of pancreatic cancer. Pancreatology 2016, 16, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Callery, M.P.; Chang, K.J.; Fishman, E.K.; Talamonti, M.S.; William Traverso, L.; Linehan, D.C. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: Expert consensus statement. Ann. Surg. Oncol. 2009, 16, 1727–1733. [Google Scholar] [CrossRef]
- Knottnerus, A.; Tugwell, P. STROBE—A checklist to Strengthen the Reporting of Observational Studies in Epidemiology. J. Clin. Epidemiol. 2008, 61, 323. [Google Scholar] [CrossRef]
- Agnes, A.; Biondi, A.; Cananzi, F.; Rausei, S.; Reddavid, R.; Laterza Galli, F.; Quagliuolo, V.; Degiuli, M.; D’Ugo, D.; Persiani, R. Ratio-based staging systems are better than the 7th and 8th editions of the TNM in stratifying the prognosis of gastric cancer patients: A multicenter retrospective study. J. Surg. Oncol. 2019, 119, 948–957. [Google Scholar] [CrossRef]
- Arslan, N.; Sokmen, S.; Canda, A.; Terzi, C.; Sarioglu, S. The prognostic impact of the log odds of positive lymph nodes in colon cancer. Colorectal Dis. 2014, 16, O386–O392. [Google Scholar] [CrossRef]
- Bagante, F.; Tran, T.; Spolverato, G.; Ruzzenente, A.; Buttner, S.; Ethun, C.; Groot Koerkamp, B.; Conci, S.; Idrees, K.; Isom, C.A.; et al. Perihilar Cholangiocarcinoma: Number of Nodes Examined and Optimal Lymph Node Prognostic Scheme. J. Am. Coll. Surg. 2016, 222, 750–759. [Google Scholar] [CrossRef]
- Calero, A.; Escrig-Sos, J.; Mingol, F.; Arroyo, A.; Martinez-Ramos, D.; de Juan, M.; Salvador-Sanchis, J.L.; Garcia-Granero, E.; Calpena, R.; Lacueva, F.J. Usefulness of the log odds of positive lymph nodes to predict and discriminate prognosis in gastric carcinomas. J. Gastrointest. Surg. 2015, 19, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Tang, Z.; Yu, Z.; Wang, Q.; Li, Z.; Lu, Q.; Wu, Y. Comparison of the 8th union for international cancer control lymph node staging system for gastric cancer with two other lymph node staging systems. Oncol. Lett. 2019, 17, 1299–1305. [Google Scholar]
- Chang, Y.; Chang, Y.; Chen, L.; Chung, K.; Lai, M. Evaluation of lymph nodes in patients with colon cancer undergoing colon resection: A population-based study. World J. Surg. 2012, 36, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Conci, S.; Ruzzenente, A.; Sandri, M.; Bertuzzo, F.; Campagnaro, T.; Bagante, F.; Capelli, P.; D’Onofrio, M.; Piccino, M.; Dorna, A.E.; et al. What is the most accurate lymph node staging method for perihilar cholangiocarcinoma? Comparison of UICC/AJCC pN stage, number of metastatic lymph nodes, lymph node ratio, and log odds of metastatic lymph nodes. Eur. J. Surg. Oncol. 2017, 43, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Yang, H.; He, Z.; Zhao, H.; Fu, Z.; Zhou, F.; Zhou, Y. Log odds of positive lymph nodes is superior to the number- and ratio-based lymph node classification systems for colorectal cancer patients undergoing curative (R0) resection. Mol. Clin. Oncol. 2017, 6, 782–788. [Google Scholar] [CrossRef][Green Version]
- Fortea-Sanchis, C.; Martínez-Ramos, D.; Escrig-Sos, J. The lymph node status as a prognostic factor in colon cancer: Comparative population study of classifications using the logarithm of the ratio between metastatic and nonmetastatic nodes (LODDS) versus the pN-TNM classification and ganglion ratio systems. BMC Cancer 2018, 18, 1208. [Google Scholar] [CrossRef]
- Huang, B.; Chen, C.; Ni, M.; Mo, S.; Cai, G.; Cai, S. Log odds of positive lymph nodes is a superior prognostic indicator in stage III rectal cancer patients: A retrospective analysis of 17,632 patients in the SEER database. Int. J. Surg. 2016, 32, 24–30. [Google Scholar] [CrossRef]
- Jian-Hui, C.; Shi-Rong, C.; Hui, W.; Si-le, C.; Jian-Bo, X.; Er-Tao, Z.; Chuang-Qi, C.; Yu-Long, H. Prognostic value of three different lymph node staging systems in the survival of patients with gastric cancer following D2 lymphadenectomy. Tumor Biol. 2016, 37, 11105–11113. [Google Scholar] [CrossRef]
- Negi, S.; Singh, A.; Chaudhary, A. Lymph nodal involvement as prognostic factor in gallbladder cancer: Location, count or ratio? J. Gastrointest. Surg. 2011, 15, 1017–1025. [Google Scholar] [CrossRef]
- La Torre, M.; Nigri, G.; Petrucciani, N.; Cavallini, M.; Aurello, P.; Cosenza, G.; Balducci, G.; Ziparo, V.; Ramacciato, G. Prognostic assessment of different lymph node staging methods for pancreatic cancer with R0 resection: pN staging, lymph node ratio, log odds of positive lymph nodes. Pancreatology 2014, 14, 289–294. [Google Scholar] [CrossRef]
- Lee, J.; Ali, B.; Park, C.; Song, K. Different lymph node staging systems in patients with gastric cancer from Korean. What is the best prognostic assessment tool? Med. Baltim. 2016, 95, e3860. [Google Scholar] [CrossRef]
- Liu, H.; Deng, J.; Zhang, R.; Hao, X.; Jiao, X.; Liang, H. The RML of lymph node metastasis was superior to the LODDS for evaluating the prognosis of gastric cancer. Int. J. Surg. 2013, 11, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Malleo, G.; Maggino, L.; Capelli, P.; Gulino, F.; Segattini, S.; Scarpa ABassi, C.; Butturini, G.; Salvia, R. Reappraisal of Nodal Staging and Study of Lymph Node Station Involvement in Pancreaticoduodenectomy with the Standard International Study Group of Pancreatic Surgery Definition of Lymphadenectomy for Cancer. J. Am. Coll. Surg. 2015, 221, 367–379. [Google Scholar] [CrossRef]
- Riediger, H.; Kulemann, B.; Wittel, U.; Adam, U.; Sick, O.; Neeff, H.; Höppner, J.; Hopt, U.T.; Makowiec, F. Prognostic Role of Log Odds of Lymph Nodes After Resection of Pancreatic Head Cancer. J. Gastrointest. Surg. 2016, 20, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R.; Friederichs, J.; Schuster, T.; Gertler, R.; Maak, M.; Becker, K.; Grebner, A.; Ulm, K.; Höfler, H.; Nekarda, H.; et al. Prognosis of patients with colorectal cancer is associated with lymph node ratio: A single-center analysis of 3026 patients over a 25-year time period. Ann. Surg. 2008, 248, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Nelson, R.; Schwarz, R. A Comparison of Five Competing Lymph Node Staging Schemes in a Cohort of Resectable Gastric Cancer Patients. Ann. Surg. Oncol. 2014, 21, 875–882. [Google Scholar] [CrossRef]
- Song, Y.; Gao, P.; Wang, Z.; Tong, L.; Xu, Y.; Sun, Z.; Xing, C.Z.; Xu, H.M. Which is the most suitable classification for colorectal cancer, log odds, the number or the ratio of positive lymph nodes? PLoS ONE 2011, 6, e28937. [Google Scholar] [CrossRef]
- Sun, Z.; Xu, Y.; Li, D.; Wang, Z.; Zhu, G.; Huang, B.; Li, K.; Xu, H.M. Log Odds of Positive Lymph Nodes A Novel Prognostic Indicator Superior to the Number-Based and the Ratio-Based N Category for Gastric Cancer Patients With R0 Resection. Cancer 2010, 116, 2571–2580. [Google Scholar] [CrossRef]
- Wang, J.; Hassett, J.; Dayton, M.; Kulaylat, M. The Prognostic Superiority of Log Odds of Positive Lymph Nodes in Stage III Colon Cancer. J. Gastrointest. Surg. 2008, 12, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, D.; Li, Y.; Guan, Y.; Sun, X.; Chen Kesari, R.; Huang, C.Y.; Li, W.; Zhan, Y.Q.; Zhou, Z.W. Tumor-ratio-metastasis staging system as an alternative to the 7th edition UICC TNM system in gastric cancer after D2 resection—Results of a single-institution study of 1343 Chinese patients. Ann. Oncol. 2011, 22, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cao, J.; Wang, L.; Wang, Z.; Wang, Y.; Wu, Y.; Lv, W.; Hu, J. Prognostic performance of three lymph node staging schemes for patients with Siewert type II adenocarcinoma of esophagogastric junction. Sci. Rep. 2017, 7, 10123. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, J.; Sun, H.; Liao, Y.; Liao, W. Comparison of three lymph node classifications for survival prediction in distant metastatic gastric cancer. Int. J. Surg. 2016, 35, 165–171. [Google Scholar] [CrossRef]
- Amini, N.; Kim, Y.; Gupta, R.; Margonis, G.A.; Ejaz, A.; Pawlik, T.M. Lymph node status after resection for gallbladder adenocarcinoma: Prognostic implications of different nodal staging/scoring systems. J. Surg. Oncol. 2015, 111, 299–305. [Google Scholar] [CrossRef]
- Amini, N.; Kim, Y.; Wilson, A.; Margonis, G. Prognostic Implications of Lymph Node Status for Patients With Gallbladder Cancer: A Multi-Institutional Study. Ann. Surg. Oncol. 2016, 23, 3016–3023. [Google Scholar] [CrossRef]
- Cao, J.; Ma, H.; Ye, P.; Wang, Y.; Yuan, X.; Bao, F.; Lv, W.; Hu, J. Log Odds of Positive Lymph Nodes Predicts Survival in Patients After Resection for Esophageal Cancer. Ann. Thorac. Surg. 2016, 102, 424–432. [Google Scholar] [CrossRef]
- He, C.; Mao, Y.; Wang, J.; Huang, X.; Lin, X.; Li, S. Surgical management of periampullary adenocarcinoma: Defining an optimal prognostic lymph node stratification schema. J. Cancer 2018, 9, 1667–1679. [Google Scholar] [CrossRef]
- Persiani, R.; Cananzi, F.C.; Biondi, A.; Paliani, G.; Tufo, A.; Ferrara, F.; Vigorita, V.; D’Ugo, D. Log Odds of Positive Lymph Nodes in Colon Cancer: A Meaningful Ratio-based Lymph Node Classification System. World J. Surg. 2012, 36, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Ramacciato, G.; Nigri, G.; Petrucciani, N.; Pinna, A.D.; Ravaioli, M.; Jovine, E.; Minni, F.; Grazi, G.L.; Chirletti, P.; Tisone, G.; et al. Prognostic role of nodal ratio, LODDS, pN in patients with pancreatic cancer with venous involvement. BMC Surg. 2017, 17, 109. [Google Scholar] [CrossRef] [PubMed]
- Tóth, D.; Bíró, A.; Varga, Z.; Török, M.; Árkosy, P. Comparison of different lymph node staging systems in prognosis of gastric cancer: A bi-institutional study from Hungary. Chin. J. Cancer Res. 2017, 29, 323–332. [Google Scholar] [CrossRef]
- Wang, X.; Appleby, D.; Zhang, X.; Gan, L.; Wang, J.; Wan, F. Comparison of three lymph node staging schemes for predicting outcome in patients with gastric cancer. Br. J. Surg. 2013, 100, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.G.; Sun, J.Y.; Yang, L.C.; Zhou, J.; Li, F.Y.; Li, Q.; Lin, H.X.; Lin, Q.; He, Z.Y. Prognosis of patients with esophageal squamous cell carcinoma after esophagectomy using the log odds of positive lymph nodes. Oncotarget 2015, 6, 36911–36922. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bian, Y.; Jin, X.; Cao, H. Prognostic assessment of different metastatic lymph node staging methods for gastric cancer after D2 resection. World J. Gastroenterol. 2013, 19, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, H.; Ma, Z.; Gong, L.; Chen, C.; Ren, P.; Shang, X.; Tang, P.; Jiang, H.; Yu, Z. Log odds of positive lymph nodes is a novel prognostic indicator for advanced ESCC after surgical resection. J. Thorac. Dis. 2017, 9, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Prassas, D.; Pavljak, C.; Rehders, A.; Krieg, S.; Luedde, T.; Knoefel, W.T.; Krieg, A. Prognostic Discrimination of Alternative Lymph Node Classification Systems for Patients with Radically Resected Non-Metastatic Colorectal Cancer: A Cohort Study from a Single Tertiary Referral Center. Cancers 2021, 13, 3898. [Google Scholar] [CrossRef] [PubMed]
- Prassas, D.; Kounnamas, A.; Cupisti, K.; Schott, M.; Knoefel, W.T.; Krieg, A. Prognostic Performance of Alternative Lymph Node Classification Systems for Patients with Medullary Thyroid Cancer: A Single Center Cohort Study. Ann. Surg. Oncol. 2021, 29, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://CRANR-projectorg (accessed on 4 May 2020).
- Xie, Y. Dynamic Documents with R and Knitr, 2nd ed.; Chapman and Hall: Boca Raton, FL, USA, 2015. [Google Scholar]
- A Package for Survival Analysis in R. R Package Version 3.1-12. Available online: https://CRAN.R-project.org/package=survival (accessed on 4 May 2020).
- Harrell, F., Jr. rms: Regression Modeling Strategies; CRAN: Nashville, TN, USA, 2021. [Google Scholar]
- Morales-Oyarvide, V.; Rubinson, D.A.; Dunne, R.F.; Kozak, M.M.; Bui, J.L.; Yuan, C.; Qian, Z.R.; Babic, A.; Da Silva, A.; Nowak, J.A.; et al. Lymph node metastases in resected pancreatic ductal adenocarcinoma: Predictors of disease recurrence and survival. Br. J. Cancer 2017, 117, 1874–1882. [Google Scholar] [CrossRef]
- You, M.S.; Lee, S.H.; Choi, Y.H.; Shin, B.S.; Paik, W.H.; Ryu, J.K.; Kim, Y.T.; Jang, D.K.; Lee, J.K.; Kwon, W.; et al. Lymph node ratio as valuable predictor in pancreatic cancer treated with R0 resection and adjuvant treatment. BMC Cancer 2019, 19, 952. [Google Scholar] [CrossRef]
- Contreras, C.M.; Oster, R.A.; Reddy, S.; Wang, T.; Vickers, S.; Heslin, M. Increased pancreatic cancer survival with greater lymph node retrieval in the National Cancer Data Base. Am. J. Surg. 2017, 214, 442–449. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Van Laethem, J.L.; Hammel, P.; Mornex, F.; Azria, D.; Van Tienhoven, G.; Vergauwe, P.; Peeters, M.; Polus, M.; Praet, M.; Mauer, M.; et al. Adjuvant gemcitabine alone versus gemcitabine-based chemoradiotherapy after curative resection for pancreatic cancer: A randomized EORTC-40013-22012/FFCD-9203/GERCOR phase II study. J. Clin. Oncol. 2010, 28, 4450–4456. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Guirguis, A.; Ashamalla, H. The Value of Total Lymph Nodes Examined and Number of Positive Lymph Nodes in Determining the Role of Adjuvant Radiation in Pancreatic Cancer Patients. Pancreas 2020, 49, 435–441. [Google Scholar] [CrossRef] [PubMed]


| Variable | All Patients (%) | PPPD (%) | Distal Pancreatectomy (%) | Total Pancreatectomy (%) |
|---|---|---|---|---|
| Number of subjects | 319 | 274 | 28 | 17 |
| Age | ||||
| Median (range) | 68 (17–95) | 69 (42–95) | 61.5 (17–80) | 70 (55–83) |
| Sex | ||||
| Male | 174 (55) | 150 (54.7) | 16 (57.1) | 8 (47.1) |
| Female | 145 (45) | 124 (45.3) | 12 (42.9) | 9 (52.9) |
| Localization | ||||
| Head/corpus | 291 (91) | |||
| Tail | 28 (8.8) | |||
| Extend of tumor (T) | ||||
| T1 | 25 (7.8) | 21 (7.7) | 4 (14.3) | 0 |
| T2 | 166 (52) | 152 (55.2) | 9 (32.1) | 5 (29.4) |
| T3 | 120 (37.6) | 95 (34.7) | 15 (53.6) | 10 (58.8) |
| T4 | 8 (2.6) | 6 (2.2) | 0 | 2 (11.8) |
| Lymph node metastasis (N) | ||||
| N0 | 62 (19.4) | 45 (16.4) | 15 (53.6) | 2 (11.8) |
| N1 | 241 (75.5) | 219 (79.9) | 13 (46.4) | 9 (52.9) |
| N2 | 16 (5.1) | 10 (3.6) | 0 | 6 (35.3) |
| tLN median (range) | 27 (1–95) | 27 (6–95) | 17.5 (1–58) | 42 (13–68) |
| pLN median (range) | 3 (0–43) | 3 (0–43) | 0 (0–13) | 4 (0–20) |
| Distant metastasis (M) | ||||
| M0 | 248 (78) | 218 (79.6) | 20 (71.4) | 10 (58.8) |
| M1 | 71 (22) | 56 (20.4) | 8 (28.6) | 7 (41.2) |
| Resection margin (R) | ||||
| R0 | 254 (70) | 220 (80.3) | 24 (85.7) | 10 (58.8) |
| R1 | 65 (20) | 54 (19.7) | 4 (14.3) | 7 (41.2) |
| Differentiation (G) | ||||
| G1 | 4 (1.3) | 1 (0.4) | 3 (10.7) | 0 |
| G2 | 180 (56.4) | 153 (55.8) | 16 (57.1) | 11 (64.7) |
| G3 | 135 (42.3) | 120 (43.8) | 9 (32.1) | 6 (35.3) |
| Neoadj. therapy | ||||
| Yes | 16 (5) | 8 (2.9) | 6 (21.4) | 2 (11.8) |
| No | 303 (95) | 266 (97.1) | 22 (78.6) | 15 (88.2) |
| Adjuvant therapy | ||||
| Yes | 263/277 (94.9) | 224/237 (94.5) | 25/26 (96.1) | 14 (82.4) |
| No | 14/277 (5.1) | 10/237 (5.5) | 1/26 (3.9) | 3 (17.6) |
| Unknown | 42 (13.2) | 40 (14.6) | 2 (7.1) | 0 |
| Clinicopathological Variable | HR (95% CI) | p Value |
|---|---|---|
| Sex | ||
| Female | 1.00 (reference) | 0.458 |
| Male | 1.11 (0.85–1.44) | |
| Age | ||
| <68 | 1.00 (reference) | 0.052 |
| ≥68 | 1.31 (1.00–1.72) | |
| Localization | ||
| Tail | 1.00 (reference) | 0.003 |
| Head/corpus | 2.50 (1.37–4.58) | |
| Neoadjuvant therapy | ||
| No | 1.00 (reference) | 0.344 |
| Yes | 1.41 (0.69–2.88) | |
| Extend of tumor | ||
| T1 + 2 | 0.92 (0.70–1.22) | 0.566 |
| T3 + 4 | 1.00 (reference) | |
| Distant metastasis | ||
| M0 | 1.00 (reference) | 0.003 |
| M1 | 1.63 (1.19–2.24) | |
| Differentiation | ||
| G1 + 2 | 0.65 (0.50–0.85) | 0.002 |
| G3 + 4 | 1.00 (reference) | |
| Resection margins | ||
| R0 | 1.00 (reference) | 0.024 |
| R1 | 1.44 (1.05–1.99) |
| All Cases | Head/Corpus | Tail | M(0) | M(+) | R(0) | R(+) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LNR | pc <0.05 | pHR <0.05 | Incr. HR | pc <0.05 | pHR <0.05 | Incr. HR | pc <0.05 | pHR <0.05 | Incr. HR | pc <0.05 | pHR <0.05 | Incr. HR | pc <0.05 | pHR <0.05 | Incr. HR | pc <0.05 | pHR <0.05 | Incr. HR | pc <0.05 | pHR <0.05 | Incr. HR |
| Agnes [13] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | YES | NO | NO |
| Arslan [14] | YES | YES | YES | YES | YES | YES | NO | NO | NO | NO | YES | YES | NO | NO | NO | YES | YES | YES | NO | NO | NO |
| Bagante [15] | NO | NO | YES | NO | NO | YES | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Calero [16] | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Cao [17] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Chang [18] | NO | YES | NO | YES | YES | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO |
| Conci [19] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | NO | YES | NO |
| Fang [20] | NO | YES | YES | NO | YES | YES | NO | NO | NO | NO | YES | YES | NO | NO | NO | NO | YES | YES | NO | NO | NO |
| Fortea-S. [21] | NO | NO | YES | NO | NO | YES | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | YES | NO | NO | NO |
| Huang [22] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Jian-Hui [23] | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | YES | NO | NO | NO |
| Kim [24] | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | YES | YES |
| La Torre [25] | NO | NO | YES | NO | NO | YES | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Lee [26] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Liu [27] | NO | NO | YES | NO | NO | YES | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | YES | NO | NO |
| Malleo [28] | NO | NO | YES | NO | NO | YES | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | YES | NO | NO |
| Riediger [29] | NO | YES | NO | NO | YES | YES | NO | NO | NO | NO | YES | YES | NO | NO | NO | NO | YES | NO | NO | NO | NO |
| Rosenberg [30] | NO | NO | YES | NO | NO | YES | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | YES | NO | NO |
| Smith [31] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Song [32] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Sun [33] | NO | NO | YES | NO | NO | YES | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | NO | YES | NO |
| Wang [34] | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | YES | YES | NO | NO | NO | NO | NO | YES | NO | NO | NO |
| Wang [35] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Xu [36] | NO | NO | YES | NO | NO | YES | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | YES | NO | NO |
| Zhou [37] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| All Cases | Head/Corpus | Tail | M(0) | M(+) | R(0) | R(+) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LODDS | pc <0.05 | pHR <0.05 | Incr. HR | pc <0.05 | pHR <0.05 | Incr. HR | pc <0.05 | pHR <0.05 | Incr. HR | pc <0.05 | pHR <0.05 | Incr. HR | pc <0.05 | pHR <0.05 | Incr. HR | pc <0.05 | pHR <0.05 | Incr. HR | pc <0.05 | pHR <0.05 | Incr. HR |
| Amini [38] | NO | YES | YES | NO | YES | YES | NO | NO | NO | NO | YES | YES | NO | NO | NO | NO | YES | YES | NO | NO | NO |
| Amini [39] | NO | YES | YES | NO | YES | YES | NO | NO | NO | NO | YES | YES | NO | NO | NO | NO | YES | YES | NO | NO | NO |
| Bagante [15] | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | YES | YES | NO | NO | NO |
| Calero [16] | YES | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | YES | NO | NO | NO | YES | YES | YES | NO | NO | NO |
| Cao [40] | YES | NO | NO | YES | YES | YES | NO | NO | NO | YES | YES | YES | NO | NO | NO | YES | YES | YES | NO | NO | NO |
| Cao [17] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Chang [18] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Conci [19] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Fang [20] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Fortea-S. [21] | NO | YES | YES | NO | YES | YES | NO | NO | YES | NO | YES | YES | NO | NO | NO | NO | YES | YES | NO | NO | NO |
| He [41] | YES | NO | NO | YES | NO | NO | NO | NO | NO | YES | NO | YES | NO | NO | NO | YES | NO | YES | NO | NO | NO |
| Huang [22] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Jian-Hui [23] | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Lee [26] | NO | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | YES | NO | NO | NO | YES | NO | NO | NO | NO | NO |
| Persiani [42] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Ramacciato [43] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Riediger [29] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Song [32] | NO | NO | NO | NO | NO | NO | NO | NO | NO | YES | YES | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO |
| Sun [33] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Toth [44] | NO | NO | YES | NO | NO | YES | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Wang [45] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | YES | YES | NO | NO | NO | YES | YES | YES | NO | NO | NO |
| Wang [35] | NO | NO | YES | NO | NO | YES | NO | NO | YES | NO | NO | YES | NO | NO | NO | NO | NO | YES | NO | NO | NO |
| Wu [46] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | YES | NO | NO | NO |
| Xu [36] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Xu [47] | NO | YES | YES | NO | YES | YES | NO | NO | NO | YES | NO | YES | NO | NO | NO | YES | YES | YES | NO | NO | NO |
| Yang [48] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| Zhou [37] | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| All Cases | Head/Corpus | Tail | M(0) | M(+) | R(0) | R(+) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pc <0.05 | pHR <0.05 | Incr. HR | pc <0.05 | pHR <0.05 | Incr. HR | pc <0.05 | pHR <0.05 | Incr. HR | pc <0.05 | pHR <0.05 | Incr. HR | pc <0.05 | pHR <0.05 | Incr. HR | pc <0.05 | pHR <0.05 | Incr. HR | pc <0.05 | pHR <0.05 | Incr. HR | |
| N category | (Ref.) | NO | NO | (Ref.) | NO | NO | (Ref.) | NO | YES | (Ref.) | NO | NO | (Ref.) | NO | NO | (Ref.) | NO | NO | (Ref.) | NO | YES |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prassas, D.; Safi, S.A.; Stylianidi, M.C.; Telan, L.A.; Krieg, S.; Roderburg, C.; Esposito, I.; Luedde, T.; Knoefel, W.T.; Krieg, A. N, LNR or LODDS: Which Is the Most Appropriate Lymph Node Classification Scheme for Patients with Radically Resected Pancreatic Cancer? Cancers 2022, 14, 1834. https://doi.org/10.3390/cancers14071834
Prassas D, Safi SA, Stylianidi MC, Telan LA, Krieg S, Roderburg C, Esposito I, Luedde T, Knoefel WT, Krieg A. N, LNR or LODDS: Which Is the Most Appropriate Lymph Node Classification Scheme for Patients with Radically Resected Pancreatic Cancer? Cancers. 2022; 14(7):1834. https://doi.org/10.3390/cancers14071834
Chicago/Turabian StylePrassas, Dimitrios, Sami Alexander Safi, Maria Chara Stylianidi, Leila Anne Telan, Sarah Krieg, Christoph Roderburg, Irene Esposito, Tom Luedde, Wolfram Trudo Knoefel, and Andreas Krieg. 2022. "N, LNR or LODDS: Which Is the Most Appropriate Lymph Node Classification Scheme for Patients with Radically Resected Pancreatic Cancer?" Cancers 14, no. 7: 1834. https://doi.org/10.3390/cancers14071834
APA StylePrassas, D., Safi, S. A., Stylianidi, M. C., Telan, L. A., Krieg, S., Roderburg, C., Esposito, I., Luedde, T., Knoefel, W. T., & Krieg, A. (2022). N, LNR or LODDS: Which Is the Most Appropriate Lymph Node Classification Scheme for Patients with Radically Resected Pancreatic Cancer? Cancers, 14(7), 1834. https://doi.org/10.3390/cancers14071834









