Simple Summary
Neuroendocrine refers to the cells that synthesize and secrete messenger chemicals such as neuropeptides and amines. Neuroendocrine neoplasms (NENs) are aggressive tumors arising from neuroendocrine cells, with an annual incidence of 6.98/100,000 and a prevalence of 170,000 in the United States. Primary gynecologic NENs constitute ≤2% of female reproductive tumors. NENs of the gynecologic tract are associated with high recurrence rates and dismal prognosis, making their treatment challenging. This article focuses on the updated staging classifications, clinicopathological characteristics, imaging, and management of NENs of the gynecological tract.
Abstract
Gynecological tract neuroendocrine neoplasms (NEN) are rare, aggressive tumors from endocrine cells derived from the neuroectoderm, neural crest, and endoderm. The primary gynecologic NENs constitute 2% of gynecologic malignancies, and the cervix is the most common site of NEN in the gynecologic tract. The updated WHO classification of gynecologic NEN is based on the Ki-67 index, mitotic index, and tumor characteristics such as necrosis, and brings more uniformity in the terminology of NENs like other disease sites. Imaging plays a crucial role in the staging, triaging, restaging, and surveillance of NENs. The expression of the somatostatin receptors on the surface of neuroendocrine cells forms the basis of increasing evaluation with functional imaging modalities using traditional and new tracers, including 68Ga-DOTA-Somatostatin Analog-PET/CT. Management of NENs involves a multidisciplinary approach. New targeted therapies could improve the paradigm of care for these rare malignancies. This article focuses on the updated staging classifications, clinicopathological characteristics, imaging, and management of gynecologic NENs of the cervix, ovary, endometrium, vagina, and vulva, emphasizing the relatively common cervical neuroendocrine carcinomas among these entities.
1. Introduction
Neuroendocrine neoplasms (NENs) are rare tumors with an annual incidence of 6.98/100,000 and a prevalence of 170,000 in the United States [1,2,3]. They were discovered in the late 19th century by Langhans and Lubarsch [4]. They include a heterogeneous group of neoplasms from the endocrine cells derived from the neuroectoderm, neural crest, and endoderm [4,5]. Neuroendocrine refers to cells that synthesize and secrete messenger chemicals, such as neuropeptides and amines [4]. Neuroendocrine cells express somatostatin receptors on the surface, forming functional imaging modalities, including 68Ga-DOTA-Somatostatin Analog-PET/CT. In addition, they express neuroendocrine markers such as synaptophysin and chromogranin A, which are stored in vesicles and granules, respectively [6]. The most frequent locations of NENs are the gastrointestinal tract (62–70%), followed by the tracheobronchial system (25%) [4,6,7].
Primary gynecologic NENs constitute ≤2% of female reproductive tumors [8]. In a study by Crane et al., the origin of these neoplasms was 54% in the cervix, 24% in the uterine corpus, 16% in the ovary/fallopian tube, 5% in the vagina, and 1% in the vulva of the study participants [9]. Along the gynecologic tract, the ovary is the most common site for low-grade NETs. In contrast, the cervix is the most common site for aggressive, poorly differentiated neuroendocrine carcinoma (NEC) [10,11,12]. The presenting symptoms are non-specific and depend on the organ of origin. Gibbs et al. reported the extrauterine spread in 83.6% of uterine, 83.5% of ovarian, and 67% of cervical NENs upon diagnosis [13]. A few patients may manifest paraneoplastic syndromes due to the secretion of peptides from these neoplasms [14]. Overall, NENs of the gynecologic tract are uncommon and aggressive, with high recurrence rates and dismal prognosis, making their treatment challenging. Imaging evaluation of these neoplasms is similar to other histologic types, with added benefits of functional somatostatin receptor (SSTR) imaging. There are no reliable guidelines for either the diagnosis or management of these cases. The revised International Federation of Gynecology and Obstetrics (FIGO) Staging System remains the preferred staging system for gynecologic NENs [14].
The article focuses on the updated staging classifications, clinicopathological characteristics, imaging, and management of NENs of the cervix, ovary, endometrium, vagina, and vulva, emphasizing the relatively common cervical NECs among these entities.
2. Nomenclature
In the early 20th century, NENs were termed carcinoids by Oberndorfer, which refers to their less-aggressive behavior with small and multiple foci of undifferentiated cellular formations, well-defined borders, and absent metastatic potential [4,15]. Later, the nomenclature was subjected to numerous revisions until the updated WHO terminology. A dedicated consensus conference was held in November 2017 at the International Agency for Research on Cancer (IARC), Lyon, under the auspices of the World Health Organization (WHO) Classification of Tumors Group. The consensus meeting framed the uniform classification for NENs, published in the 2018 WHO classification guide. The updated fifth edition WHO classification of gynecologic NEN is delineated in Table 1 and in-general grading criteria for NENs in Table 2 [8]. Grading of tumors is based on the Ki-67 index, mitotic index, and tumor characteristics such as necrosis [16]. In terms of gynecologic tract NENs, grades 1 and 2 represent well-differentiated low-grade NETs, while grade 3 represents poorly differentiated high-grade NECs. Grading is only performed for NETs (grades 1 and 2). Although there are some reports of the entity, NET G3 is still not officially included in the recently updated WHO classification [17], in contrast to the new NET G3 category for gastroenteropancreatic (GEP) NENs [18]. There is also no further differentiation of grade 1 versus grade 2 NETs in the ovaries. NENs of the ovary are classified into ovarian carcinoids (grade 1) and ovarian NEC (grade 3). Except for the ovaries, the terms carcinoid and atypical carcinoid were removed from the nomenclature; instead, these correspond to NETs in the updated classification [8,19].

Table 1.
WHO terminology for NEN of gynecologic tract, fifth edition.

Table 2.
In general, Grading criteria for neuroendocrine neoplasms.
3. Histology and Immunohistochemistry of NEN
Well-differentiated NETs comprise small uniform cells in nesting, trabecular, or gyriform/serpentine growth patterns. They demonstrate round to oval nuclei, inconspicuous nucleoli, fine to coarsely granular chromatin described as “salt and pepper”, and a pale to moderately eosinophilic cytoplasm [7,10]. NEC tumor cells are pleomorphic, which proliferate in a “sheet-like” pattern and contain irregular nuclei, high mitotic index, a few cytoplasmic secretory granules, and necrosis. Although NENs have characteristic histology, immunohistochemistry is essential to confirm the diagnosis. Neuroendocrine biomarkers, including synaptophysin, chromogranin A, CD56, CD57, neuron-specific enolase, synaptic vesicle protein2 (SV2), and protein gene product 9.5 (PGP 9.5), are used to identify NENs. The positive staining for these markers is observed in one or more NEN variants. The majority of the studies on the effectiveness of biomarkers are performed on GEP-NEN. An increased chromogranin A level is a widely accepted biomarker with 60–90% sensitivity. However, it is non-specific (specificity 10–35%), expressed in healthy tissues, inflammatory diseases, and other tumors such as prostate cancer, small cell lung cancer, and pancreatic cancer [20]. A high cut-off level of chromogranin A is considered to overcome these limitations. A study by Campana et al. reported that the chromogranin A level of 84–87 U/l has a sensitivity and specificity of 55% and 95%, respectively [21]. Elevated neuron-specific enolase ranging from 154–370 U/l has been observed in the literature depending on the stage of the disease and the tested subjects [22,23]. Studies have reported that the levels of neuron-specific enolase were decreased with treatment, yet recurrences were observed within a year [19,24,25]. The sensitivity and specificity of neuron-specific enolase in differentiating neuroendocrine from non-neuroendocrine tumors are 39–43% and 65–73%, respectively [26]. Although elevated neuron-specific enolase may indicate poorly differentiated tumors, it is no longer routinely used in clinical practice, as it is not superior to chromogranin A [27,28].
Nevertheless, no biomarker is highly accurate in determining the diagnosis and prognosis of the NEN. Hence a consensus on biomarkers for NEN suggested that a combination of imaging and circulating biomarkers should be considered to provide additional information on tumor behavior. Table 3 describes an overview of the imaging and circulating biomarkers studied in GEP-NEN [26,29]. However, the linear correlations between the imaging, biomarker, and final tissue-derived data have not been established [29]. The miRNAs are the non-coding RNAs that regulate the gene expression by degrading mRNAs or by inhibiting the translation [30]. The consensus by Oberg et al. also concluded that the multianalyte biomarkers such as mRNA have the highest sensitivity and specificity in identifying the minimal disease, predicting the efficacy of the treatment and prognosis (Figure 1) [29].

Table 3.
Overview of biomarkers in NEN.
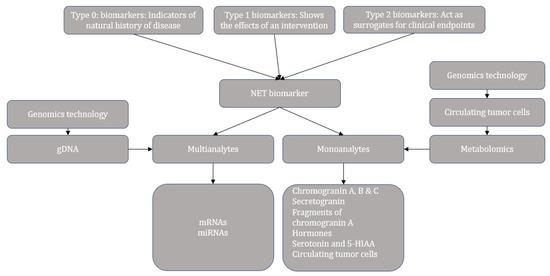
Figure 1.
Categories and progress overview of neuroendocrine tumor biomarkers.
Immunohistochemistry can help to distinguish between primary and metastatic NENs. For instance, positive thyroid transcription factor-1 staining indicates the primary NEN in the thyroid and lung, ISL-1, and PDX-1 in the pancreas, PAX8 in the thyroid, and CDX-2 and villin in the gastrointestinal tract [11,31]. Recently, a new marker, insulinoma-associated protein 1 (INSM1), was reported in 90% of patients with high-grade genitourinary NEC on immunostaining by Chen et al. [32]. The sensitivity of INSM1 (90%) was similar to chromogranin (87%), synaptophysin (92%), and CD56 (85%), while the specificity was 97.4% in the report by Chen et al. [32]. According to Zou et al., the specificity of INSM1 in high-grade gynecologic NEC is 95% compared to chromogranin (82%), synaptophysin (81%), and CD56 (75%) [33]. Figure 2 illustrates the different grades and classifications of NENs.
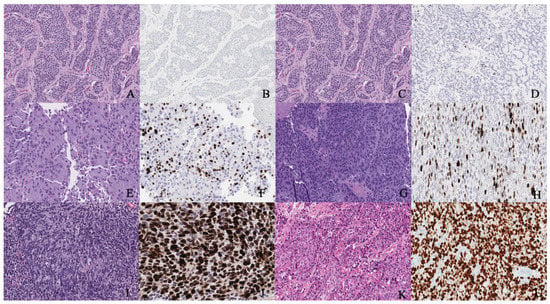
Figure 2.
(A,B) Well-differentiated NET grade 1. (A) Well-differentiated neuroendocrine tumor grade 1 (low grade) with an organoid pattern, with a meshwork of thin fibrovascular septa surrounding nests of tumor cells. Tumor cells are uniform with a polygonal shape, round to oval nuclei with salt and pepper chromatin, inconspicuous nucleoli, and moderate to abundant eosinophilic cytoplasm. (B) The Ki67 immunohistochemical stain shows a proliferation rate of 2%. (C,D) Well-differentiated NET grade 2. (C) Neuroendocrine cells in a well-differentiated neuroendocrine tumor, grade 2. Tumor cells are relatively uniform and round. The nuclear chromatin is finely granular. (D) The Ki67 immunohistochemical stain shows a proliferation rate of 10%. (E,F) Well-differentiated NET grade 3. (E) Neuroendocrine cells in a well-differentiated neuroendocrine tumor, grade 3. Tumor cells are relatively uniform and round with eosinophilic cytoplasm. The nuclear chromatin is granular. (F) The Ki67 immunohistochemical stain shows a proliferation rate of 30–40%. (G,H) Poorly differentiated neuroendocrine carcinoma. (G) The tumor shows solid nests of poorly differentiated epithelioid cells with dense chromatin. (H) Ki67 immunohistochemical stain shows a proliferation rate of 80%. (I,J) Small cell carcinoma. (I) Sheets of oval blue cells with minimal cytoplasm. The chromatin is dense. Nuclei demonstrate molding and smudging. (J) Ki67 shows a proliferation index of 80%. (K,L) Large cell neuroendocrine carcinoma. (K) Tumor cells with sheets of large, epithelioid cells. Cytologic features show abundant cytoplasm, coarse chromatin, nuclear pleomorphism, and prominent nucleoli. (L) Ki67 is greater than 90%.
4. Cervical NEN
The cervix is the most common site of NENs in the gynecologic tract, and 0.9–1.5% of cervical neoplasms belong to the NEN category [34,35] (Figure 3 and Figure 4). Around 200 cases of cervical NECs are diagnosed every year [36]. Cervical NENs is classified into cervical NETs and high-grade cervical NECs based on Ki-67 and the mitotic index. Low-grade (G1) tumors have ≤3 Ki-67 and 2/10 HPF mitotic indices, and include typical carcinoids, while intermediate-grade (G2) NETs (Ki-67 >3% and mitotic index 2–20/10HPF) include prior atypical carcinoid variants. In contrast, high-grade NECs have >20 Ki and >20/10 HPF mitotic indices, and include small and large cell NECs [8]. The small cell variant is the most common (80%) variant among cervical NENs, followed by large-cell NEC (12%) and differentiated (G1–G2) cervical NETs. Only a few cases of lower-grade cervical NETs have been reported in the literature [35]. NECs may also co-exist with adenocarcinoma or squamous cell carcinoma, and the clinical behavior of such cases is determined by the neuroendocrine component [37].
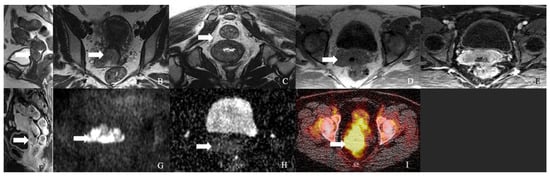
Figure 3.
A 62-year-old female with neuroendocrine carcinoma of the cervix. (A) Sagittal T2 weighted image, (B) axial T2 weighted image, (C) oblique T2 weighted image, (D) axial pre-contrast T1 weighted image, (E) axial fat-saturated post-contrast T1 weighted image, (F) sagittal fat-saturated post-contrast T1 weighted image, (G) axial diffusion-weighted images (B-100), (H) axial apparent diffusion coefficient MRI images, and (I) Axial Ga-68 dotatate PET/CT image demonstrate a FDG avid enhancing mass in the cervix (arrow) with restricted diffusion and no parametrium involvement. The mass biopsy reported a small cell neuroendocrine carcinoma.
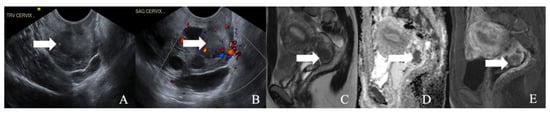
Figure 4.
A 52-year-old female with a neuroendocrine tumor of the cervix uteri. (A) Transverse and (B) sagittal ultrasound image of the cervix demonstrates a heterogenous cervical mass (arrow) measuring about 14 cm. (C) Sagittal T2 weighted image, (D) sagittal apparent diffusion coefficient map, and (E) sagittal fat-saturated post-contrast T1 weighted MRI images demonstrate a mass in the cervix uteri (arrow) with restricted diffusion. The mass biopsy reported a small cell neuroendocrine tumor of grade G3.
The incidence of cervical NENs peaks around the fourth decade of life. Patients may present with abnormal vaginal bleeding (22–73%), vaginal discharge, post-coital bleeding, and pelvic pain/pressure (8%) [35]. Given the aggressive nature of the neoplasm and extensive lymphatic invasion (37–57%), distant metastasis can be expected at the initial presentation. For example, stage IB1 cervical NEC is associated with positive pelvic lymph nodes in 40% of cases compared to 10–15% with the same stage (IB1) cervical squamous cell carcinomas [36]. The reported correlation between human papillomavirus (HPV) infection and cervical NECs in the literature prompts considering a prophylactic HPV vaccine to prevent NECs [36,38]. In a study by Castle et al., HPV was associated with 85% of small-cell and 88% of large-cell cervical NECs; of which, HPV-16 was detected in 10% and 29%, and HPV-18 was detected in 51% and 30% of small cell and large cell cervical NECs, respectively [39].
4.1. Molecular Characterization and Immunohistochemistry of Cervical NEC
At a molecular level, cervical NECs is associated with mutations in the PIK3CA gene (18%), KRAS gene (14%), and TP53 (11%) [40]. Hillman et al. reported that cervical NECs are genetically more similar to non-NEC cervical cancers than extra-cervical NECs of the lungs and bladder [41]. The coexistence of the PIK3CA mutation can explain this similarity in one of the APOBEC motifs in cervical NEC and non-NEC cancers [41,42]. Identifying genetic alterations in small-cell cervical NEC allows for the application of targeted therapies, including angiogenesis inhibitors, cell signaling pathway inhibitors, apoptosis promoters, and immunotherapies in managing small-cell cervical NEC. Xing et al. reported that the targeted next-generation sequencing of small-cell cervical NECs showed genetic alterations in the MAPK, TP53/BRCA, and PI3K/AKT/mTOR pathways. Such alterations allowed the tumor to be amenable to targeted therapies [43]. Similarly, a study by Cho et al. described recurring mutations in ATRX, EBRR4, and AKT/mTOR pathway genes in the pathogenesis of small-cell cervical NEC [44]. Schultheis et al. characterized genomes and discovered highly recurring Tp53 and Rb1 alterations in lung NEC and p53 and RB tumor suppressor protein inactivation in cervical NEC, indicating small-cell NECs are convergent phenotypes [45].
Immunohistochemistry has been implemented as an ancillary tool to diagnose high-grade cervical NEC. In a study by McCluggage et al., PGP9.5, chromogranin, synaptophysin, and CD56 were positive in 43%, 57%, 90%, and 90%, respectively, of cervical NEC [46]. CD56 and synaptophysin are the most sensitive markers; however, CD56 lacks specificity due to its wide occurrence in cervical neuroendocrine and non-neuroendocrine neoplasms [46]. Chromogranin is the most specific marker for cervical NEN [8]. Other markers studied in the literature include TTF1, p16, and insulinoma-associated protein-1 (INSM1). McCluggage et al. reported 71% positivity of TTF1 in cases of cervical NEC [46]. According to Maria et al., 86% of patients with cervical NEN overexpressed p16 upon immunostaining, in concordance with Alejo et al. [38]. Overexpression of p16, a cyclin-dependent kinase inhibitor, serves as a biomarker of HPV infection, with 97% of cervical squamous cell carcinomas and 50% of adenocarcinomas staining positive for p16 [36,38]. Hence, p16 cannot be relied on to make an NEN diagnosis. To distinguish from cervical squamous cell carcinoma, p63 staining is more valuable, as most cervical NENs stain negative for p63. Evolving markers such as insulinoma-associated protein-1 (INSM1) are noted to be specific, with positive staining in >95% of cases [47].
4.2. Prognostic Factors
NENs are associated with a worse prognosis than non-NEN cervical malignancies [48]. The most frequently related prognostic factors for early-stage disease are FIGO stage [49,50,51,52], lymph node metastasis [49,52,53,54], parametrial invasion, tumor size [55,56,57,58], lymphovascular space involvement, histological heterogeneity, older age [56,59,60,61,62], and smoking [56,63,64]. Of all these, Ishikawa et al. reported LVSI as the most significant prognostic factor for overall and disease-free survival. At the same time, pelvic lymph node metastasis was the critical prognostic factor for disease-free survival [63]. Early-stage disease without lymph node metastasis is a favorable prognostic factor [65]. Disease recurrence was noted in 50% of early-stage small cell cervical NEC patients included in a study by Pei et al. Distant organs were involved in most of the recurrences [66]. Hence, the study recommended adjuvant systemic chemotherapy for early-stage small cell cervical NEC.
Several studies show that advanced age is a poor prognostic factor, regardless of the tumor stage. Chen et al. reported that ages of ≤45 and >45 years are associated with overall survival of 62.5% and 35%, respectively (p = 0.04) [56]. Age of >60 years was associated with decreased survival in early-stage disease in a study by Intaraphet et al. (HR = 4.9; p = 0.007) (45), whereas the overall survival for advanced-stage disease were determined by both age at diagnosis (age > 60 years: HR = 9.9; p < 0.001; versus age < 45 years: HR = 3.4; p = 0.035) and FIGO stage IV (HR = 7.4; p = 0.024) [61]. Lin et al. reported that the threshold age of 50 years determines the overall survival in small cell cervical NEC (HR: 1.561; p = 0.034) [67]. Similarly, age < 50 years was associated with prolonged overall survival in a study by Hoskins et al. (p = 0.02) [68].
FIGO staging is an essential independent prognostic criterion for cervical NEC. The five-year overall survival rate is 20–50% for stage I–II and 2–15% for stage III–IV tumors [8]. In a study by Chen et al., the five-year overall survival rates of stages I–IV cervical NEC were 75%, 56%, 41%, and 0%, respectively, and the 5-year progression-free survival rates were 64%, 54.5%, 31%, and 0%, respectively [56]. Specific to small-cell cervical NEC, Wang et al. reported a five-year survival rate of 52% and 25% in stages I–IIA and IIB–IVB, respectively [66,69]. In a similar study by Intaraphet et al., the five-year cancer-specific survival was better in early-stage compared to advanced-stage disease (62.6% vs. 18.1%; p < 0.001) [61] in patients with cervical small cell NECs.
Tumors < 2 cm size have been found to be associated with better survival rates than the tumors > 2 cm (HR: 1.92; p = 0.055) [64]. In a study by Sheets et al., tumors < 2 cm had a more prolonged progression-free survival rate than tumors > 2 cm [70]. Chen et al. described that tumors < 4 cm size exhibit better overall survival than tumors ≥ 4 cm (76% vs. 39%; p = 0.013) [56]. Similarly, larger tumor size (≥4 cm) was associated with poor prognosis in a study by Liao et al. (HR: 2.4; p = 0.006) [58], and tumors < 4 cm had better five-year overall survival rates in a study by Bermudez et al. (76% vs. 18%; p < 0.05) [71].
NENs are aggressive and have a high incidence of lymph node involvement even during early-stage disease. In a study by Chen et al., positive and negative lymph nodes were associated with a progression-free survival of 6.7% and 45.9%, respectively (p = 0.014) [56]. They also reported that the negative and positive lymph node metastasis showed a significant difference in five-year overall survival (68% vs. 42%; p = 0.002) and five-year progression-free survival rates (62.6% vs. 29.3%, respectively; p = 0.019) [56]. In another study involving patients with small cell cervical NEC, lymph node positivity had a worse cancer-specific survival (HR: 8.832; p < 0.001) and overall survival (HR: 8.462; p < 0.001) [62].
Smoking is significantly associated with a worse prognosis of cervical NEC, especially for early-stage disease. In a study by Chan et al., the relationship was statistically significant in early-stage disease with a hazard ratio of 2.08 (p = 0.037) and was not statistically significant in combined I–IV stages with a hazard ratio of 1.82 (p = 0.069) [64]. The increased association between smoking and HPV infection can also explain the interrelation between smoking and cervical NEC.
Cervical NEC represents an aggressive group of tumors, unlike treatable squamous cell carcinoma and adenocarcinoma. According to a multivariable analysis, cervical small cell NEC is associated with worse survival than adenocarcinoma (stage III: HR = 2.9 and Stage IV-HR = 4.5) and squamous cell carcinoma (Stage III: HR = 1.7 and Stage IV: HR: 3.7) of similar stages. In a study by Chen et al., the five-year survival rates of small cell carcinoma (35.7%) were worse when compared to squamous cell carcinoma (60.5%, HR: 0.55; 95% CI: 0.43–0.69%) and adenocarcinoma (69.7%, HR: 0.48; 95% CI: 0.37–0.61%) patients with early-stage and node-negative disease [60]. However, large-cell and small-cell carcinoma are managed similarly and are associated with variable survival. According to Stecklein et al., large-cell cervical NEC has better outcomes in event-free survival and overall survival than small cell NEC [65].
4.3. Imaging of Cervical NEN
Imaging is critical for tumor staging, while histology is essential for diagnosis but not for assessing the extent of the tumor spread [72]. Hence, imaging modalities including computed tomography (CT), magnetic resonance imaging (MRI), and 18F fluoro-deoxy-glucose positron emission tomography/computed tomography (18F-FDG PET/CT) should be considered to evaluate and stage the NEN. Currently, the imaging of NENs is based on a combination of anatomic and functional imaging.
Although cervical NEC shows enhancement on contrast-enhanced CT, the role of CT is limited due to the small size of these lesions and the low contrast resolution. Nonetheless, combined PET and CT enable the use of low-dose CT to correlate findings on PET scans in addition to attenuation correction. In addition, the advantage of a whole-body survey in a single scan makes 18-FDG PET/CT superior to other imaging modalities. As small lesions (<5 mm) can be missed on PET scans, accompanying contrast-enhanced CT in the venous phase is preferred to improve the visualization [73]. As small cell cervical, NEC has a high predilection for lymphatic and hematogenous spread; the role of FDG PET/CT is crucial to rule out distant metastasis. The metastases from cervical NEC exhibit an avid uptake of 18F-FDG tracer and show SUVmax values from 2.2 to 9.6. The diagnostic accuracy, sensitivity, and specificity of PET/CT for recurrent cervical NEC are 87.9%, 94.7%, and 83.7%, respectively [35]. PET/CT also assesses the metabolic changes before the morphologic changes in response to treatment. In addition, the presence or absence of response guides the further treatment plan and predicts the prognosis.
The surface of the neoplastic cells comprising NENs, express somatostatin receptors (SSTR), through which the somatostatin hormone acts. [74]. Octreotide and lanreotide, like somatostatin analogs, act through these receptors and help slow tumor growth and inhibit hormone secretion. Their radionuclide-labeled analogs help identify this SSTR on the tumor cells and are helpful for the diagnosis, staging, and restaging of these neoplasms. Although various radionuclides were available, such as 111In, 123I, and 99mTc, 111In-DTPA (diethylene-triaminepentaacetic acid) octreotide (octreoscan) was the only FDA approved label for SRI until recently. Somatostatin receptor imaging (SRI) was first performed in patients with carcinoids and endocrine pancreatic tumors with a gamma camera. SPECT/CT fusion technique was used to improve the anatomical localization. Despite the approval of octreoscan application, the main disadvantage is its increased physiologic uptake limiting the visualization of smaller lesions, high radiation dose, and poor resolution.
With the enhanced application of PET scans in clinical practice, somatostatin analogs are labeled with different positron-emitting radioisotopes such as Gallium-68 and Copper-64, which the FDA has recently approved. PET/CT can provide functional (PET) and anatomic (CT) details of the tumors together [73]. PET/CT with tracer 68 Gallium DOTA-(Tyr3)-octreotide (68Ga-DOTATATE PET/CT) is 96% sensitive and 100% specific for SSTR positive cervical NEC [75,76]. Before SSTR-PET, Octreoscan used to be the standard imaging for tumor staging and characterization [77]. Pfeifer et al. reported that Octreoscan has a sensitivity of 87–88%, and 64-Cu DOTATATE PET/CT is 97% sensitive for detecting pathologically proven NENs. PET scans using 68Ga-DOTA-Somatostatin Analogs (68Ga-DOTANOC, 68Ga-DOTATOC, and 68Ga-DOTATATE) aid in the diagnosis and staging of NENs [78]. While 68Ga-DOTATATE utilizes octreotate as a peptide, 68Ga-DOTATOC and -NOC utilize octreotide. As a radioisotope is used in the scan, 68-Gallium DOTATATE PET/CT should be avoided during pregnancy, breastfeeding, and patients < 18 years [79]. Somatostatin receptor-based 68 Ga-tetra-azacyclododecane-tetraacetic acid (DOTA)-peptide PET/CT has also shown exciting advantages over conventional imaging in the diagnosis of NENs. Here, the somatostatin analog peptide binds to somatostatin receptors 2 and 5 with high affinity. Studies reported that 68-Gallium DOTA-peptide PET/CT is very sensitive, and 68-Ga DOTATATE PET/CT is very accurate in detecting initial or recurrent NENs [73].
For neoplasms > 10 mm and confined to the cervix, pelvic MRI is the imaging modality of choice to assess the size and local extension of the lesion [36]. It detects cervical NEC with a positive (PPV) and negative predictive value (NPV) of 74% and 100%, respectively, compared to PET scan, which has 44% and 44% PPV and NPV, respectively [35]. Table 4 summarizes the MRI pelvis protocol for the evaluation of gynecological tumors. The imaging features of cervical NEC on MRI are non-specific and include irregular shape and margin, adjacent structural infiltration, and lymph node involvement. Upon T2 weighted imaging, the tumor demonstrates homogeneous signal intensity alongside homogeneous enhancement compared to non-NEC cervical carcinomas. In conjunction with aggressive tumor behavior, these imaging features suggest the diagnosis of cervical NEC [48].

Table 4.
MRI of the pelvis protocol.
MR imaging stages cervical cancer with an accuracy ranging from 75–96%. According to a study by Xiaohui et al., the accuracy of MRI is 85.7% in staging cervical NEC [48]. It identifies vaginal and parametrial invasion with 83% and 88–97% accuracy, respectively [48]. MR imaging can ascertain parametrial involvement with 69% and 93% sensitivity and specificity, respectively [35]. Of cervical NEC cases, 37–57% involve lymph nodes, and MRI assesses the nodal involvement with a sensitivity, specificity, and accuracy of 37–90%, 71–100%, and 67–95%, respectively [35,48]. Ultrasmall supra-paramagnetic iron oxide (USPIO) particles can enhance the sensitivity of MR imaging to 93% [35]. The decreased uptake of USPIO by the lymph nodes implies metastasis. Considering 0.900 as the ADC (×10−3 mm2/s) cutoff value, diffusion-weighted imaging allows for differentiation of NEC from other cervical carcinomas with a sensitivity and specificity of 63.3% and 95%, respectively [48].
Positron emission tomography/magnetic resonance imaging (PET/MRI) is a hybrid imaging modality that allows for morphological evaluation of the tumor and metabolic information by the PET component [80,81]. In a recent metanalysis (n = 12), the sensitivity rate, specificity rate, diagnostic odds ratio, and area under the receiver operating characteristic curve pooled for 18F-FDG PET/MRI in the diagnosis of gynecological malignancies in a patient-based analysis were 74.2% (95% confidence interval, 66.2–80.8%), 89.8% (95% CI, 82.2–94.3%), 26 (95% CI, 10–67%), and 0.834, respectively, and upon lesion-based analysis were 87.5% (95% CI, 75.8–94.0%), 88.2% (95% CI, 84.2–91.3%), 50 (95% CI, 23–111%), and 0.922, respectively [82]. Another metanalysis compared the diagnostic performance of PET/CT with PET/MRI for gynecological malignancies. The study reported a slightly better diagnostic performance of 18F-FDG PET/MRI to that of 18F-FDG PET/CT at the lesion level (44 vs. 26, p = 0.4), as well as in the patient-level analysis (28 vs. 17, p = 0.48) [83].
4.4. Management of Cervical NEN
The treatment regimen for cervical NEC is derived from the trial designs of small cell carcinoma of the lung, as the clinical trials of the former are limited due to its low incidence. Unlike cervical SCC and adenocarcinoma, which are relatively curable even at advanced stages, NEC is highly aggressive with poor outcomes, even when detected at early stages. Hence, most treatment algorithms aim at a multimodality approach combining surgery, platinum-based chemotherapy, and radiotherapy. Table 5 and Figure 5 summarize FIGO staging, and Figure 6 shows the stage-based treatment algorithm for cervical neuroendocrine carcinoma.

Table 5.
FIGO 2018 staging of cervical cancer.
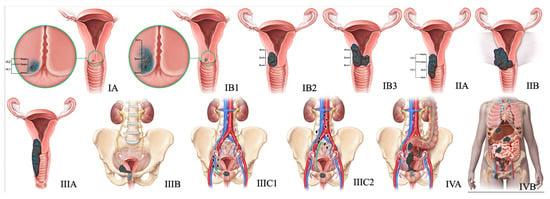
Figure 5.
2018 FIGO staging system for cervical cancer. Stage I, confined to the cervix. Stage IA, ≤5 mm depth. Stage IA1, ≤3 mm depth. Stage IA2, 3 mm and ≤5 mm depth. Stage IB, >5 mm depth. Stage IB1, ≤2 cm maximum diameter. Stage IB2, >2 cm and ≤4 cm maximum diameter. Stage IB3, >4 cm maximum diameter. Stage II, beyond the uterus but not involving the lower one-third of the vagina or pelvic sidewall. Stage IIA, upper two-thirds of the vagina. Stage IIA1, upper two-thirds of the vagina and ≤4 cm. Stage IIA2, Upper two-thirds of the vagina and >4 cm. Stage IIB, parametrial invasion. Stage III, lower vagina, pelvic sidewall, ureters, and lymph nodes. Stage IIIA, lower one-third of the vagina. Stage IIIB, pelvic sidewall. Stage IIIC, pelvic, and para-aortic lymph node involvement. Stage IIIC1, pelvic lymph node involvement. Stage IIIC2, para-aortic lymph node involvement. Stage IV, adjacent and distant organs. Stage IVA, rectal or bladder involvement. Stage IVB, distant organs outside the pelvis.
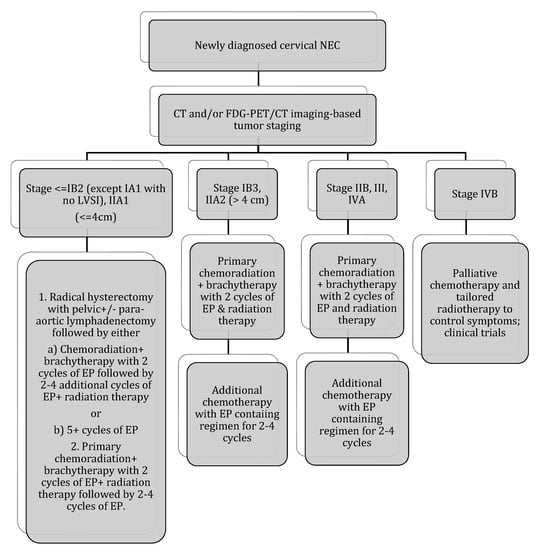
Figure 6.
Stage-based treatment algorithm for cervical neuroendocrine carcinoma [2]. NEC: neuroendocrine carcinoma; LVSI: lymphovascular space invasion; EP: etoposide and cisplatin; chemotherapy with EP alone: cisplatin 60–80 mg/m2 on day 1 every 3 weeks and etoposide 80–120 mg/m2 on days 1–3 every 3 weeks (with growth factor support); chemotherapy with EP + radiation therapy: 2 cycles of EP q3 weeks (cisplatin 60 mg/m2 and etoposide 100 mg/m2 given on day 1 of 21 day cycle) during radiation therapy followed by an additional 2–4 cycles of EP alone (recommended regimen). Radiotherapy: 40–45 Gy external beam radiotherapy ±40–45 Gy brachytherapy (modified from Winer et al.).
4.4.1. Early-Stage Tumor: IA, IB (Except IB3), and IIA1
The primary treatment, as recommended by Gynecologic Cancer InterGroup (GCIG) and the Society of Gynecologic Oncology (SGO), is radical surgery for early-stage disease and chemoradiation for an advanced-stage disease [55,84]. The optimal management for an early-stage tumor (≤4 cm) with negative lymph nodes upon imaging is radical hysterectomy and pelvic lymphadenectomy followed by chemotherapy [36]. Cohen et al. studied individuals with stages I–IIA small-cell cervical NEC and reported a significant five-year overall survival rate of 38.2% and 23.8% in individuals who did and did not undergo radical hysterectomy, respectively [85]. On the contrary, Wang et al. reported a worse five-year failure-free survival (41% vs. 60.5%; p = 0.086) in patients with stages IA–IIB disease who underwent surgery as primary treatment compared to those who experienced prior chemotherapy and radiotherapy [69]. As almost half of patients with small cell cervical NEC have lymph node involvement, pelvic lymphadenectomy (PLN) is included alongside radical hysterectomy. Boruta et al. and Pei et al. even recommended the routine inclusion of para-aortic lymphadenectomy in early-stage small-cell cervical NEC [54,66].
Adjuvant chemotherapy is recommended in early-stage disease after complete surgical resection [55]. Etoposide and cisplatin (EP) and vincristine, adriamycin, and cyclophosphamide (VAC) are the two regimens that have been advocated with improved survival compared to other regimens [54]. However, EP is preferred due to the higher toxicity of VAC. Ishikawa et al. suggested that etoposide-platinum or irinotecan-platinum are the best chemotherapy for stages I and II cervical NEC, as the response rates were 43.8% compared to 12.9% for the taxane-platinum regimen [63,86]. The number of chemotherapy cycles is also crucial for preventing the recurrence of the tumor. For instance, ≥five cycles of chemotherapy with an EP regimen following radical surgery was shown to improve five-year recurrence-free survival compared to other regimens (67.6% vs. 20.9%; p < 0.001) [66]. However, Yaun et al. reported improved outcomes after >4 cycles of chemotherapy, regardless of regimen, although these were statistically insignificant [51]. The SGO proposed that neoadjuvant chemotherapy (NAC) be considered when tumors are larger than 4 cm [55,56]. However, this needs validation in more and larger cohort studies. Chen et al. reported similar five-year overall survival (61% vs. 57%; p = 0.559) and five-year progression-free survival rates (53.5% vs. 52%; p = 0.511) in individuals who received and did not receive NAC [56]. Similarly, the benefit of radiotherapy in contrast to surgery is controversial for early-stage tumors. According to a study by Ishikawa et al., the hazard ratio of death was 4.74 among patients who underwent definitive radiotherapy than radical surgery [63]. In a study by Lee et al., the overall survival in patients after radical surgery alone was better than in patients after radical surgery plus adjuvant radiation (54% vs. 40%) [59]. However, the relapse rate was higher in the former group (33.3%) than in the latter (13%) in a study by Viswanathan et al. [87].
4.4.2. Advanced-Stage Tumor
The SGO guidelines recommend a combination of chemotherapy and radiation in patients with locally advanced diseases and non-surgical individuals [55]. Chen et al. reported that five-year overall survival and five-year progression-free survival rates were 42.4% and 41.3% in patients who received primary radiotherapy, and 50.6% and 34.7% in patients who received primary surgery in stages IIB–IIIC2 disease, respectively [56].
Some studies have also validated the efficacy of chemotherapy among individuals with stages IIB–IV cervical NEC. Cohen et al. reported an improved three-year survival rate in individuals who did receive (17.8%) compared to those who did not receive chemotherapy (12%) either as a primary or adjuvant or with concurrent radiation [85]. An adequate number of chemotherapy cycles is essential for stage IV disease patients. Chen et al. recommended at least six cycles of chemotherapy, as it is associated with better two-year overall survival (83.3% vs. 9%, p < 0.001) and two-year progression-free survival (57% vs. 0%, p = 0.01) compared to fewer than six cycles of chemotherapy [56]. Wang et al. reported a better five-year CSS with at least five etoposide/platinum regimen cycles in 75% of stages IIB–IVB patients included in their study [69]. Immunotherapies were observed to have a therapeutic effect in high-grade extragenital NET, while their efficacy in cervical NEC has not been extensively studied. In a study including 40 specimens of cervical NEC, the authors concluded that cervical NEC is unlikely to be responsive to PD-L1 inhibitors due to the negative PD-L1 expression [88]. As the majority of cervical NECs expressed PARP-1 (poly adenosine diphosphate-ribose polymerase-1), PARP inhibitors may be included in the management plan [88].
5. Ovarian NEN
Ovarian NENs constitute 1–2% of ovarian tumors. The differences in the ovarian NEN 2014 and 2020 WHO classifications are outlined in Table 6.

Table 6.
Differences between WHO’s 2014 and 2020 classification of ovarian neuroendocrine tumors.
5.1. Ovarian Carcinoid Tumors
Most ovarian NENs are benign carcinoid tumors that arise from dermoid cysts [11] (Figure 7). Ovarian carcinoids can be a primary or metastatic tumor. Primary ovarian carcinoids (POC) are more common than metastatic tumors, constituting 0.1% of the ovarian neoplasms [89]. Most POC manifest as a small, lobulated, solid, and unilateral soft tissue lesion in a dermoid cyst. Upon gross inspection, a larger POC may be visible as a yellow nodule [11]. Metastatic ovarian carcinoids can be frequently observed in patients with a history of carcinoid tumors in the midgut. They often present as bilateral, nodular masses with extensive lymphovascular and extra-ovarian involvement [11]. The five-year survival rate of ovarian carcinoid is 84% and 94% in patients with and without dermoid, respectively [90]. Morphologically POC comprises insular, trabecular, stromal, and mucinous carcinoid variants. The pathological features of these variants are described in Table 7 [11].
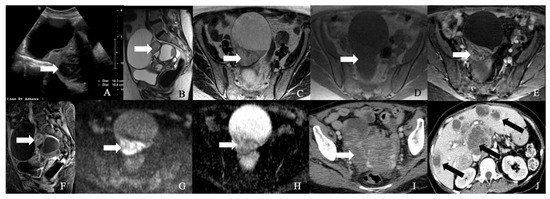
Figure 7.
52-year-old female with a neuroendocrine tumor of the ovary. (A) Transverse ultrasound image of the pelvis demonstrates a heterogeneous mass in the pelvis (arrow) measuring about 14 cm. (B) Sagittal T2 weighted image, (C) axial T2 weighted image, (D) axial pre-contrast T1 weighted image, (E) axial fat-saturated post-contrast T1 weighted image, (F) sagittal fat-saturated post-contrast T1 weighted image, (G) axial diffusion-weighted images (B-100), (H) axial apparent diffusion coefficient images demonstrate a multiloculated mass with enhancing nodule (arrow) and restricted diffusion, (I) axial post-contrast CT image of the pelvis shows the heterogenous mass (arrow), and (J) axial post-contrast CT image of the abdomen demonstrates the multiple hepatic metastases (arrow). The mass was resected, and a biopsy reported neuroendocrine tumor of the ovary with synaptophysin, CD56, CK7, and CDX-2 were positive, and chromogranin was focally positive, with a Ki67 index of less than 5%.

Table 7.
Variants of ovarian carcinoid tumors and their pathological and immunohistochemical features.
5.2. Ovarian NEC
Small cell ovarian carcinoma has two types—small carcinoma of the ovary, pulmonary type (SCCOPT), and small cell carcinoma of the ovary, hypercalcemic type (SCCOHT). Of the two types, SCCOPT is included under the category of miscellaneous tumors of the ovary according to the WHO 2014 classification and belongs to the NEN family, while SCCOHT is not categorized under the NEN family. It is more related to the rhabdoid-like tumor [91]. The differentiating features of SCCOPT and SCCOHT are outlined in Table 8 [91]. Small cell NECs are rare, and to date, only around 40 cases have been reported in the literature [8].

Table 8.
Differentiating features between ovarian small cell carcinoma of pulmonary and hypercalcemic type.
Large cell ovarian NECs are synonymous with undifferentiated non-small cell NECs of the ovary [92,93]. They are the rarest among ovarian NENs and aggressive tumors, with a median survival of only ten-months [93,94]. Even when diagnosed at stage-I, the average overall survival is 42 months [8,92]. Fewer than 60 cases of large-cell ovarian NECs have been reported in the literature, of which 18 patients were of the pure large-cell type [8,93]. Often, they are combined with the other germ cell and ovarian epithelial tumors. The composition of neuroendocrine components ranges from 10–90% in combined large cell NEC [95]. Histologically, they are composed of intermediate to large, round to oval pleomorphic cells arranged in solid, trabecular, or nested patterns with extensive mitoses and necrosis [95].
5.3. Management
Due to the rarity of ovarian NENs, the literature consists of case reports and small case series. The majority of small-cell NEC presented in peri- or post-menopausal women underwent hysterectomy with bilateral salpingo-oophorectomy and debulking as a part of the management [8]. Table 9 and Figure 8 describe FIGO staging, and Figure 9 and Figure 10 illustrate the diagnostic and treatment algorithms for ovarian NENs. In a study by Pang et al., the five-year overall survival and cancer-specific survival after surgery were 97% and 97%, respectively, in patients with early-stage ovarian NENs [94]. In cases of advanced disease, comprehensive treatment comprising surgery, chemotherapy, and radiotherapy was associated with an improved five-year overall (73%) and cancer-specific (70%) survival [94]. In a study including 11 case reports, age, AJCC stage, mode of treatment, and histological type were the significant prognostic factors for ovarian NENs [94].

Table 9.
The International Federation of Gynecology and Obstetrics (FIGO) staging of ovarian cancer (2014).
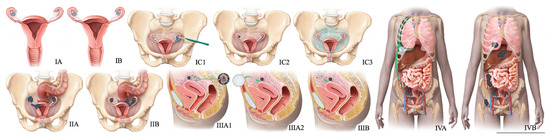
Figure 8.
FIGO staging system for ovarian cancer. In stage IA cancer, the tumor is limited to one ovary or fallopian tube. The tumor is limited to both ovaries or fallopian tubes in stage IB cancer. Stage IC1 cancer results from the intraoperative spill. In stage IC3 cancer, malignant cells are found in ascites or peritoneal washings. In stage IIA, the tumor extends to or is implanted on (or both) uterus or fallopian tubes (or both). Stage IIIA1 cancer shows positive retroperitoneal lymph nodes. Stage IIIA2 cancer, microscopic, extrapelvic (above pelvic brim) peritoneal involvement is seen with or without positive retroperitoneal lymph nodes. Stage IIIB or stage IIIC cancer, macroscopic, extrapelvic (above pelvic brim) peritoneal involvement is seen with or without positive retroperitoneal lymph nodes (≤2 cm for stage IIIB and >2 cm for stage IIIC). Stage IVA cancer indicates pleural effusion with positive cytology. Stage IVB cancer, hepatic or splenic parenchymal metastasis is seen (or both) and metastasis to extra-abdominal organs.
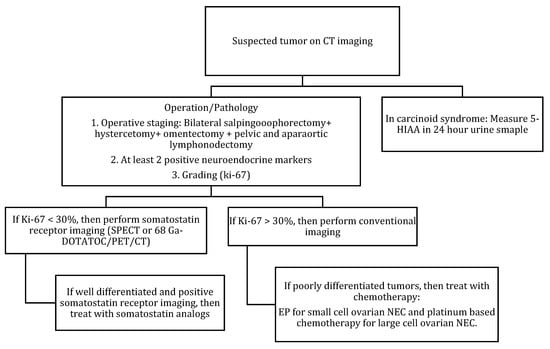
Figure 9.
Diagnostic algorithm for ovarian NENs.
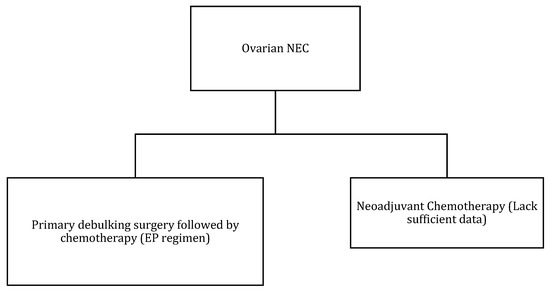
Figure 10.
Treatment algorithm of ovarian NEC. If non-surgical or metastatic disease: palliative chemotherapy (EP regimen) or enrollment into clinical trials.
6. Endometrial NEN
NENs are a rare group of tumors constituting 0.8% of endometrial malignancies [96] (Figure 11). They are rare and usually adenocarcinoma or small cell cancers with a neuroendocrine differentiation [14]. Around 56% of patients present with advanced disease with abnormal uterine bleeding or metastatic symptoms [96]. The diagnosis is based on histology and imaging, and dilatation and curettage provide adequate biopsy tissue to determine histology. Like cervical carcinoma, non-specific imaging features of endometrial NENs do not allow for histological distinction. Most endometrial cancers have similar imaging characteristics: low to moderate signal on T2WI and hypointensity relative to the hyperintense enhancing myometrium on dynamic contrast-enhanced MRI sequences. With tumor growth, the histological appearance of endometrial NEC becomes identical to FIGO grade 3 endometroid carcinoma and undifferentiated carcinoma. In indeterminate imaging and histology cases, immunohistochemistry aids in the differentiation of endometrial malignancies. Most endometrial NEC demonstrates a diffuse expression of ≥2 markers such as neuron-specific enolase, synaptophysin, chromogranin, or CD56, whereas endometroid and undifferentiated carcinomas exhibit focal positivity for neuroendocrine markers. Hence, to diagnose NEC, more than 20% of the tumor cells need to be positive for at least two of the markers [97]. The expression of p16, p53, and TTF1 by both NEC and endometroid carcinoma makes these markers unreliable and requires further studies to determine their accuracy.
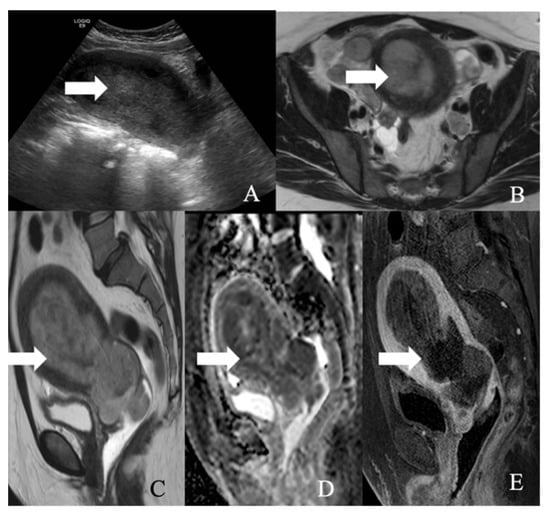
Figure 11.
A 52-year-old female with a large cell neuroendocrine tumor of the endometrium. (A) Transverse ultrasound image of the uterus demonstrates thickened endometrium (arrow), (B) axial T2 weighted image, (C) sagittal T2 weighted image, (D) sagittal apparent diffusion coefficient map, and (E) sagittal fat-saturated post-contrast T1 weighted MRI images demonstrate a hypoenhancing tumor in the endometrium (arrow) with restricted diffusion.
Nonetheless, when a highly aggressive endometrial tumor is diagnosed, NENs should be suspected. Table 10 and Figure 12 describe the FIGO staging, while Figure 13 demonstrates the treatment algorithm of endometrial NEN. Endometrial NECs are aggressive carcinomas associated with an overall survival of 22 and 12 months in stages I–II and III–IV, respectively [57]. According to Schlechtweg et al., the five-year and median survival for patients with endometrial NEC are 38.3% and 17 months from diagnosis. Compared to other endometrial carcinomas, NEC has an increased risk of death with a hazard ratio of 2.32 (95% CI: 1.88–2.88%) [96].

Table 10.
2009 FIGO staging for endometrial cancer.
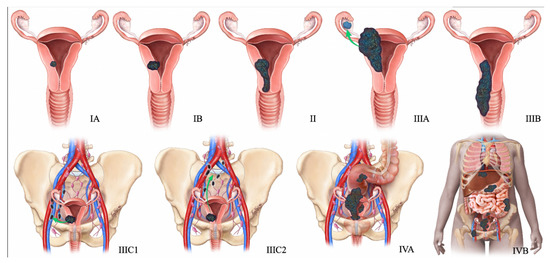
Figure 12.
2009 FIGO staging system for endometrial cancer. Stage I tumor confined to the uterus. Stage IA < 50% myometrial invasion. Stage IB ≥ 50% myometrial invasion. Stage II Cervical stromal invasion. Stage IIIA tumor invasion into serosa or adnexa. Stage IIIB vaginal or parametrial involvement. Stage IIIC1 pelvic node involvement. Stage IIIC2 paraaortic node involvement. Stage IVA tumor invasion into bladder or bowel mucosa. Stage IVB distant metastases (including abdominal metastases) or inguinal lymph node involvement.
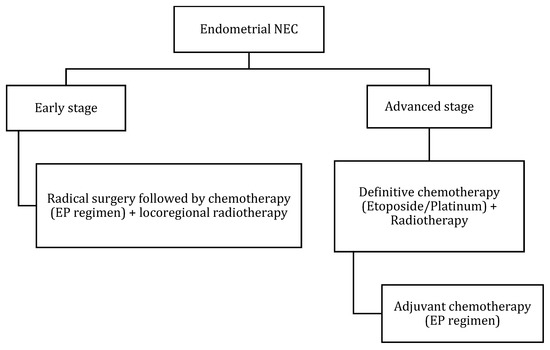
Figure 13.
Treatment algorithm of endometrial NEC. If non-surgical or metastatic disease: palliative chemotherapy with EP regimen or enrollment into clinical trials.
7. Vaginal NEN
Primary vaginal NEC is an extremely rare malignancy and was first reported by Scully et al. in 1984 [98]. The mean age of presentation is around 59 years, and the symptoms include vaginal discharge, leucorrhea, or metastatic symptoms [99]. Approximately 85% of patients with vaginal small cell NEC died within one year of diagnosis in a study by Gardner et al. [55]. Diagnosis requires the exclusion of metastases from other common sites of small cell NEC, such as the lungs and cervix [100]. Bing et al. reported that primary small cell vaginal NEC is histologically, immunohistochemically, and ultra-structurally similar to the pulmonary counterpart [101]. Histological findings include small, round, or oval cells with a scanty cytoplasm, fine granular unclear chromatin, absent or inconspicuous nucleoli, nuclear molding, and ill-defined cell borders.
Table 11 and Figure 14 outline the FIGO staging, and Table 12 summarizes AJCC staging for vaginal cancer. The treatment regime lacks consensus due to the rarity of the tumor, and hence case-based therapy is recommended, similar to small cell carcinoma of the lungs (Figure 15). Chemoradiation alone or in combination with surgical excision is the routinely suggested management. Cisplatin and etoposide have been described as effective in previous studies [102]. Patients with localized upper one-third vaginal NEC may need a radical hysterectomy, partial vaginectomy, and lymphadenectomy [102]. Advanced primary small cell vaginal NEC management is through concurrent chemoradiation alongside anterior and posterior chemotherapy [103]. Currently, evidence is limited to the case reports and case series. Hence more extensive studies are required to emphasize the specific diagnosis and management plan. Figure 15 describes the treatment algorithm of vaginal/vulvar NEN.

Table 11.
2009 FIGO staging of vaginal cancer.
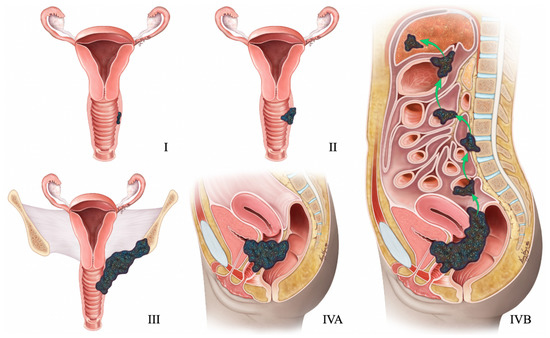
Figure 14.
FIGO Staging of vaginal tumors. Stage I is limited to the vaginal wall. Stage II is beyond the vaginal wall without pelvic sidewall involvement. Stage III extends to the pelvic sidewall. Stage IVA infiltrates bladder or rectum or the tumor extending beyond the pelvis, with any nodal metastasis. Stage IVB has distant metastasis.

Table 12.
American Joint Committee on Cancer (AJCC) staging of vaginal cancer.
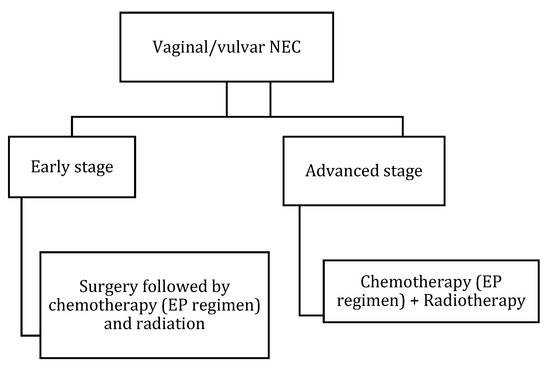
Figure 15.
Treatment algorithm of vaginal/vulvar NEC. If non-surgical or metastatic disease: palliative chemotherapy with EP regimen or enrollment in clinical trials.
8. Vulvar NEC
Primary vulvar small cell NEC resembles Merkel cell carcinoma (MCC) histologically; however, it differs in the immunohistochemistry [91]. Small cell vulvar NEC demonstrates TTF-1 uptake and MCC stains for neurofilament and CK20. Around 90% of MCC express CK20 and 30% of small vulvar cell NEC express TTF-1 uptake [104]. In addition, the detection of polyomavirus helps as a differentiating feature. With the literature being limited, most of the reported cases were found admixed with other histological types, most commonly squamous cell carcinoma and vulvar intraepithelial neoplasia [105]. FIGO staging of vulvar NEN is described in Table 13 and Figure 16. Surgical excision with or without lymphadenectomy and adjuvant chemoradiation is recommended for patients with early-stage disease, and definitive chemoradiation is recommended for advanced-stage disease [10,11] (Figure 15).

Table 13.
New (2021) FIGO staging for carcinoma of the vulva.
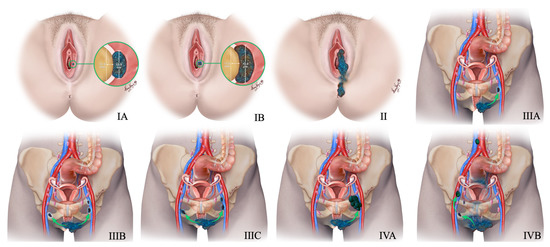
Figure 16.
New 2021 FIGO Staging of vulvar tumors. Stage-I tumor confined to the vulva. Stage IA tumor size less than equal to 2 cm and stromal invasion less than equal to 1 mm. Stage IB tumor size more than 2 cm and stromal invasion more than 1 mm. Stage II tumor of any size with extension to lower one-third of the urethra, lower one-third of the vagina, lower one-third of the anus with negative nodes. Stage III tumor of any size with extension to the upper part of adjacent perineal structures or with any number of nonfixed, nonulcerated lymph nodes. Stage IIIA tumor of any size with disease extension to the upper two-thirds of the urethra, upper two-thirds of the vagina, bladder mucosa, rectal mucosa, or regional lymph node metastases less than equal to 5 mm. Stage IIIB regional lymph node metastases more than 5 mm. Stage IIIC regional lymph node metastases with extracapsular spread. Stage IV tumor of any size fixed to bone or fixed, ulcerated lymph node metastases, or distant metastases. Stage IVA disease fixed to the pelvic bone or fixed or ulcerated regional lymph node metastases. Stage IVB distant metastases.
9. Future Perspective
Novel targeted therapies could play a vital role in treating gynecological tract NENs. Immunotherapeutic and targeted agents have been effective in other high-grade NENs [106,107,108]. In addition, the immune checkpoint inhibitors (ICIs) for programmed cell death-1 (PD-1) or programmed death-ligand 1 (PD-L1) have reported favorable response results in gynecological malignancies [109,110,111]. Other molecular targeted therapeutic agents, such as mTOR, MEK, and PTEN inhibitors, and anti-antiangiogenic inhibitors, have demonstrated encouraging results in other non-gynecological NECs [112]. Nevertheless, their part in treating gynecologic tract NENs is limited due to low incidence or insufficient clinical trials data. A comprehensive study by Mahdi et al. reported that gynecological NEC demonstrated frequent mutations in KMT2C and KMT2D genes involved in gene enhancers regulation [113]. They also found that DDR (DNA damage response) gene mutations are high in gynecological NEC, making them susceptible to PARP inhibitors. In addition, mutations in CCDC6 suggest promising benefits from PARP inhibitors [113]. Further studies are required to evaluate the efficacy of targeted immunotherapy against HMGB1/TIM-3 and CD47/SIRPα.
10. Conclusions
Gynecologic tract NENs are diverse tumors and often involve a multidisciplinary management regime. The small cell NEC of the cervix and the carcinoid of the ovary are the frequent gynecologic NENs. The imaging feature of non-specific for diagnosis; nonetheless, imaging is quintessential in baseline staging, treatment response assessment, and surveillance. The adapted staging system for gynecologic tract NENs is the Revised International Federation of Gynecology and Obstetrics (FIGO) Staging System remains. There is a lack of consensus on standard treatment guidelines for gynecologic tract NENs. The treatment regimens are usually based on other gynecologic and non-gynecologic malignancies. Clinical trials, tumor genomic decoding for target therapies, accurate pathology grading, tumor bases registries, and referral to experience centers are critical elements to improve the outcome in these tumors.
Author Contributions
Conceptualization, P.B., A.C.M. and C.L.; validation, P.B., A.C.M., C.L., D.R.G. and R.W.; writing—original draft preparation, M.V. and S.S.V.; writing—review and editing, P.B., A.C.M., C.L., D.R.G., S.K. and R.W.; supervision, P.B. and A.C.M.; project administration, M.V. and S.S.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank Kelly Kage for the beautiful illustrations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Galgano, S.J.; Sharbidre, K.; Morgan, D.E. Multimodality imaging of neuroendocrine tumors. Radiol. Clin. 2020, 58, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Migut, A.E.; Kaur, H.; Avritscher, R. Neuroendocrine tumors: Imaging of treatment and follow-up. Radiol. Clin. 2020, 58, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Heller, M.T.; Shah, A.B. Imaging of neuroendocrine tumors. Radiol. Clin. 2011, 49, 529–548. [Google Scholar] [CrossRef] [PubMed]
- Tempfer, C.B.; Tischoff, I.; Dogan, A.; Hilal, Z.; Schultheis, B.; Kern, P.; Rezniczek, G.A. Neuroendocrine carcinoma of the cervix: A systematic review of the literature. BMC Cancer 2018, 18, 530. [Google Scholar] [CrossRef] [Green Version]
- Klöppel, G. Neuroendocrine neoplasms: Dichotomy, origin and classifications. Visc. Med. 2017, 33, 324–330. [Google Scholar] [CrossRef]
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing but NET: A review of neuroendocrine tumors and carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef]
- Winer, I.; Kim, C.; Gehrig, P. Neuroendocrine tumors of the gynecologic tract update. Gynecol. Oncol. 2021, 162, 210–219. [Google Scholar] [CrossRef]
- Crane, E.K.; Ramos, P.; Farley, J.H.; Naumann, R.W.; Tait, D.L.; Higgins, R.V.; Brown, J. Molecular profiling in a large cohort of gynecologic neuroendocrine tumors. Gynecol. Oncol. 2020, 159, 262. [Google Scholar] [CrossRef]
- KeFelİ, M.; Usubütün, A. An update of neuroendocrine tumors of the female reproductive system. Turk Patoloji Derg. 2015, 31, 128–144. [Google Scholar] [CrossRef] [Green Version]
- Howitt, B.E.; Kelly, P.; McCluggage, W.G. Pathology of neuroendocrine tumours of the female genital tract. Curr. Oncol. Rep. 2017, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.; Mei, S.; Economos, K.; Lee, Y.-C.; Kanis, M.J. Clinicopathologic features, incidence, and survival trends of gynecologic neuroendocrine tumors: A SEER database analysis. Am. J. Obstet. Gynecol. 2019, 221, 53.e51–53.e56. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.L.; Cunha, T.M.; Gomes, F.V.; Callé, C.; Félix, A. Neuroendocrine tumours of the female genital tract: A case-based imaging review with pathological correlation. Insights Imaging 2015, 6, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Williams, E.; Sandler, M. The classification of carcinoid tumours. Lancet 1963, 281, 238–239. [Google Scholar] [CrossRef]
- Rindi, G.; Inzani, F. Neuroendocrine neoplasm update: Toward universal nomenclature. Endocr.-Relat. Cancer 2020, 27, R211–R218. [Google Scholar] [CrossRef]
- Katafuchi, T.; Kawakami, F.; Iwagoi, Y.; Saito, F.; Mikami, Y. “Neuroendocrine Tumor Grade 3 (NET G3)” of the Uterine Cervix: A Report of 2 Cases. Int. J. Gynecol. Pathol. 2021. [Google Scholar] [CrossRef]
- Saleh, M.; Bhosale, P.R.; Yano, M.; Itani, M.; Elsayes, A.K.; Halperin, D.; Bergsland, E.K.; Morani, A.C. New frontiers in imaging including radiomics updates for pancreatic neuroendocrine neoplasms. Abdom. Radiol. 2020. [Google Scholar] [CrossRef]
- Georgescu, T.A.; Bohiltea, R.E.; Munteanu, O.; Furtunescu, F.; Lisievici, A.C.; Grigoriu, C.; Gherghiceanu, F.; Vlădăreanu, E.M.; Berceanu, C.; Ducu, I.; et al. Emerging Therapeutic Concepts and Latest Diagnostic Advancements Regarding Neuroendocrine Tumors of the Gynecologic Tract. Medicina 2021, 57, 1338. [Google Scholar] [CrossRef]
- Modlin, I.M.; Oberg, K.; Taylor, A.; Drozdov, I.; Bodei, L.; Kidd, M. Neuroendocrine tumor biomarkers: Current status and perspectives. Neuroendocrinology 2014, 100, 265–277. [Google Scholar] [CrossRef]
- Campana, D.; Nori, F.; Piscitelli, L.; Morselli-Labate, A.M.; Pezzilli, R.; Corinaldesi, R.; Tomassetti, P. Chromogranin A: Is it a useful marker of neuroendocrine tumors? J. Clin. Oncol. 2007, 25, 1967–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Wu, C.; Juang, G.; Wang, J.; Chen, T.-M.; Hsieh, C.-Y. Serum neuron-specific enolase levels in patients with small cell carcinoma of the uterine cervix. J. Formos. Med. Assoc. Taiwan Yi Zhi 1994, 93, 81–83. [Google Scholar] [PubMed]
- Pang, L.; Yang, H.; Ning, Y.; Zheng, C. Retrospective analysis of clinicopathological features and prognosis of gynecological small-cell carcinoma. Cancer Manag. Res. 2021, 13, 4529. [Google Scholar] [CrossRef] [PubMed]
- Shahabi, S.; Pellicciotta, I.; Hou, J.; Graceffa, S.; Huang, G.S.; Samuelson, R.N.; Goldberg, G.L. Clinical utility of chromogranin A and octreotide in large cell neuro endocrine carcinoma of the uterine corpus. Rare Tumors 2011, 3, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Roda, H.; Abella, L.; Simó, P.; Maazouzi, Y.; Medina, M.; Escrig, S. Ovarian Neuroendocrine Carcinoma-Case Report. Obstet. Gynecol. Int. J. 2016, 4, 1–3. [Google Scholar]
- Hofland, J.; Zandee, W.T.; de Herder, W.W. Role of biomarker tests for diagnosis of neuroendocrine tumours. Nat. Rev. Endocrinol. 2018, 14, 656–669. [Google Scholar] [CrossRef]
- Baudin, E.; Gigliotti, A.; Ducreux, M.; Ropers, J.; Comoy, E.; Sabourin, J.; Bidart, J.; Cailleux, A.; Bonacci, R.; Ruffié, P. Neuron-specific enolase and chromogranin A as markers of neuroendocrine tumours. Br. J. Cancer 1998, 78, 1102–1107. [Google Scholar] [CrossRef]
- Modlin, I.M.; Bodei, L.; Kidd, M. Neuroendocrine tumor biomarkers: From monoanalytes to transcripts and algorithms. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 59–77. [Google Scholar] [CrossRef]
- Oberg, K.; Modlin, I.M.; De Herder, W.; Pavel, M.; Klimstra, D.; Frilling, A.; Metz, D.C.; Heaney, A.; Kwekkeboom, D.; Strosberg, J. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol. 2015, 16, e435–e446. [Google Scholar] [CrossRef] [Green Version]
- Sansone, A.; Lauretta, R.; Vottari, S.; Chiefari, A.; Barnabei, A.; Romanelli, F.; Appetecchia, M. Specific and non-specific biomarkers in neuroendocrine gastroenteropancreatic tumors. Cancers 2019, 11, 1113. [Google Scholar] [CrossRef] [Green Version]
- Caruso, G.; Sassu, C.M.; Tomao, F.; Di Donato, V.; Perniola, G.; Fischetti, M.; Panici, P.B.; Palaia, I. The puzzle of gynecologic neuroendocrine carcinomas: State of the art and future directions. Crit. Rev. Oncol./Hematol. 2021, 162, 103344. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-F.; Yang, C.; Sun, Y.; Cao, D. Expression of novel neuroendocrine marker insulinoma-associated protein 1 (INSM1) in genitourinary high-grade neuroendocrine carcinomas: An immunohistochemical study with specificity analysis and comparison to chromogranin, synaptophysin, and CD56. Pathol.-Res. Pract. 2020, 216, 152993. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Zhang, L.; Cheng, Z.; Guo, X.; Cao, D. INSM1 Is Less Sensitive But More Specific Than Synaptophysin in Gynecologic High-grade Neuroendocrine Carcinomas: An Immunohistochemical Study of 75 Cases with Specificity Test and Literature Review. Am. J. Surg. Pathol. 2021, 45, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Carinelli, S.; Aletti, G. Neuroendrocrine tumors of the uterine cervix: A therapeutic challenge for gynecologic oncologists. Gynecol. Oncol. 2017, 144, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Elsherif, S.; Odisio, E.G.; Faria, S.; Javadi, S.; Yedururi, S.; Frumovitz, M.; Ramalingam, P.; Bhosale, P. Imaging and staging of neuroendocrine cervical cancer. Abdom. Radiol. 2018, 43, 3468–3478. [Google Scholar] [CrossRef] [PubMed]
- Salvo, G.; Martin, A.G.; Gonzales, N.R.; Frumovitz, M. Updates and management algorithm for neuroendocrine tumors of the uterine cervix. Int. J. Gynecol. Cancer 2019, 29, 986–995. [Google Scholar] [CrossRef]
- Lee, D.Y.; Chong, C.; Lee, M.; Kim, J.W.; Park, N.H.; Song, Y.S.; Park, S.Y. Prognostic factors in neuroendocrine cervical carcinoma. Obstet. Gynecol. Sci. 2016, 59, 116–122. [Google Scholar] [CrossRef]
- Alejo, M.; Alemany, L.; Clavero, O.; Quiros, B.; Vighi, S.; Seoud, M.; Cheng-Yang, C.; Garland, S.M.; Juanpere, N.; Lloreta, J. Contribution of human papillomavirus in neuroendocrine tumors from a series of 10,575 invasive cervical cancer cases. Papillomavirus Res. 2018, 5, 134–142. [Google Scholar] [CrossRef]
- Castle, P.E.; Pierz, A.; Stoler, M.H. A systematic review and meta-analysis on the attribution of human papillomavirus (HPV) in neuroendocrine cancers of the cervix. Gynecol. Oncol. 2018, 148, 422–429. [Google Scholar] [CrossRef]
- Frumovitz, M.; Burzawa, J.K.; Byers, L.A.; Lyons, Y.A.; Ramalingam, P.; Coleman, R.L.; Brown, J. Sequencing of mutational hotspots in cancer-related genes in small cell neuroendocrine cervical cancer. Gynecol. Oncol. 2016, 141, 588–591. [Google Scholar] [CrossRef] [Green Version]
- Hillman, R.T.; Cardnell, R.; Fujimoto, J.; Lee, W.-C.; Zhang, J.; Byers, L.A.; Ramalingam, P.; Leitao, M.; Swisher, E.; Futreal, P.A. Comparative genomics of high grade neuroendocrine carcinoma of the cervix. PLoS ONE 2020, 15, e0234505. [Google Scholar] [CrossRef] [PubMed]
- Revathidevi, S.; Murugan, A.K.; Nakaoka, H.; Inoue, I.; Munirajan, A.K. APOBEC: A molecular driver in cervical cancer pathogenesis. Cancer Lett. 2021, 496, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Zheng, G.; Schoolmeester, J.K.; Li, Z.; Pallavajjala, A.; Haley, L.; Conner, M.G.; Vang, R.; Hung, C.-F.; Wu, T.-C. Next-generation sequencing reveals recurrent somatic mutations in small cell neuroendocrine carcinoma of the uterine cervix. Am. J. Surg. Pathol. 2018, 42, 750. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Choi, M.; Ban, H.-J.; Lee, C.H.; Park, S.; Kim, H.; Kim, Y.-S.; Lee, Y.S.; Lee, J.-Y. Cervical small cell neuroendocrine tumor mutation profiles via whole exome sequencing. Oncotarget 2017, 8, 8095. [Google Scholar] [CrossRef] [PubMed]
- Schultheis, A.M.; de Bruijn, I.; Selenica, P.; Macedo, G.S.; da Silva, E.M.; Piscuoglio, S.; Jungbluth, A.A.; Park, K.J.; Klimstra, D.S.; Wardelmann, E. Genomic characterization of small cell carcinomas of the uterine cervix. Mol. Oncol. 2022, 16, 833–845. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.G.; Kennedy, K.; Busam, K.J. An immunohistochemical study of cervical neuroendocrine carcinomas: Neoplasms that are commonly TTF1 positive and which may express CK20 and P63. Am. J. Surg. Pathol. 2010, 34, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Kuji, S.; Watanabe, R.; Sato, Y.; Iwata, T.; Hirashima, Y.; Takekuma, M.; Ito, I.; Abe, M.; Nagashio, R.; Omae, K. A new marker, insulinoma-associated protein 1 (INSM1), for high-grade neuroendocrine carcinoma of the uterine cervix: Analysis of 37 cases. Gynecol. Oncol. 2017, 144, 384–390. [Google Scholar] [CrossRef]
- Duan, X.; Ban, X.; Zhang, X.; Hu, H.; Li, G.; Wang, D.; Wang, C.Q.; Zhang, F.; Shen, J. MR imaging features and staging of neuroendocrine carcinomas of the uterine cervix with pathological correlations. Eur. Radiol. 2016, 26, 4293–4302. [Google Scholar] [CrossRef]
- Huang, R.; Gan, Q.; Cheng, J. Prognostic factors and local treatment modalities of small-cell carcinoma of the cervix: An analysis according to the international federation of gynecology and obstetrics stage. Cancer Manag. Res. 2020, 12, 3445. [Google Scholar] [CrossRef]
- Kawamura, M.; Koide, Y.; Murai, T.; Ishihara, S.; Takase, Y.; Murao, T.; Okazaki, D.; Yamaguchi, T.; Uchiyama, K.; Itoh, Y. The importance of choosing the right strategy to treat small cell carcinoma of the cervix: A comparative analysis of treatments. BMC Cancer 2021, 21, 1046. [Google Scholar] [CrossRef]
- Yuan, L.; Jiang, H.; Lu, Y.; Guo, S.-W.; Liu, X. Prognostic factors of surgically treated early-stage small cell neuroendocrine carcinoma of the cervix. Int. J. Gynecol. Cancer 2015, 25, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhang, M.; Shui, R. Prognostic factors and treatment comparison in early-stage small cell carcinoma of the uterine cervix. Oncol. Lett. 2012, 3, 125–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Lin, J.-X.; Yu, Y.-H.; Zhang, M.-Y.; Wang, H.-Y.; Zheng, M. Downregulation of six microRNAs is associated with advanced stage, lymph node metastasis and poor prognosis in small cell carcinoma of the cervix. PLoS ONE 2012, 7, e33762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boruta, D.M., II; Schorge, J.O.; Duska, L.A.; Crum, C.P.; Castrillon, D.H.; Sheets, E.E. Multimodality therapy in early-stage neuroendocrine carcinoma of the uterine cervix. Gynecol. Oncol. 2001, 81, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Gardner, G.J.; Reidy-Lagunes, D.; Gehrig, P.A. Neuroendocrine tumors of the gynecologic tract: A Society of Gynecologic Oncology (SGO) clinical document. Gynecol. Oncol. 2011, 122, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, Y.; Chen, L.; Zang, L.; Lin, C.; Lu, Y.; Lin, L.; Lin, A.; Dan, H.; Chen, Y. Prognostic factors and treatment of neuroendocrine tumors of the uterine cervix based on the FIGO 2018 staging system: A single-institution study of 172 patients. PeerJ 2021, 9, e11563. [Google Scholar] [CrossRef]
- Atienza-Amores, M.; Guerini-Rocco, E.; Soslow, R.A.; Park, K.J.; Weigelt, B. Small cell carcinoma of the gynecologic tract: A multifaceted spectrum of lesions. Gynecol. Oncol. 2014, 134, 410–418. [Google Scholar] [CrossRef]
- Liao, L.-M.; Zhang, X.; Ren, Y.-F.; Sun, X.-Y.; Di, N.; Zhou, N.; Pan, R.-K.; Ma, S.-H.; Zhou, L.-X. Chromogranin A (CgA) as poor prognostic factor in patients with small cell carcinoma of the cervix: Results of a retrospective study of 293 patients. PLoS ONE 2012, 7, e33674. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-M.; Lee, K.-B.; Nam, J.-H.; Ryu, S.-Y.; Bae, D.-S.; Park, J.-T.; Kim, S.-C.; Cha, S.-D.; Kim, K.-R.; Song, S.-Y. Prognostic factors in FIGO stage IB–IIA small cell neuroendocrine carcinoma of the uterine cervix treated surgically: Results of a multi-center retrospective Korean study. Ann. Oncol. 2008, 19, 321–326. [Google Scholar] [CrossRef]
- Chen, J.; Macdonald, O.K.; Gaffney, D.K. Incidence, mortality, and prognostic factors of small cell carcinoma of the cervix. Obstet. Gynecol. 2008, 111, 1394–1402. [Google Scholar] [CrossRef]
- Intaraphet, S.; Kasatpibal, N.; Siriaunkgul, S.; Chandacham, A.; Sukpan, K.; Patumanond, J. Prognostic factors for small cell neuroendocrine carcinoma of the uterine cervix: An institutional experience. Int. J. Gynecol. Cancer 2014, 24, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, H.Y.; Wu, S.G.; He, Z.Y.; Lin, H.X.; Sun, J.Y.; Li, Q.; Guo, Z.W. The local treatment modalities in FIGO stage I-II small-cell carcinoma of the cervix are determined by disease stage and lymph node status. Cancer Med. 2016, 5, 1108–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, M.; Kasamatsu, T.; Tsuda, H.; Fukunaga, M.; Sakamoto, A.; Kaku, T.; Nakanishi, T.; Hasumi, Y.; Iwata, T.; Baba, T. Prognostic factors and optimal therapy for stages I–II neuroendocrine carcinomas of the uterine cervix: A multi-center retrospective study. Gynecol. Oncol. 2018, 148, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Loizzi, V.; Burger, R.A.; Rutgers, J.; Monk, B.J. Prognostic factors in neuroendocrine small cell cervical carcinoma: A multivariate analysis. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2003, 97, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Stecklein, S.R.; Jhingran, A.; Burzawa, J.; Ramalingam, P.; Klopp, A.H.; Eifel, P.J.; Frumovitz, M. Patterns of recurrence and survival in neuroendocrine cervical cancer. Gynecol. Oncol. 2016, 143, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Xiang, L.; Ye, S.; He, T.; Cheng, Y.; Yang, W.; Wu, X.; Yang, H. Cycles of cisplatin and etoposide affect treatment outcomes in patients with FIGO stage I-II small cell neuroendocrine carcinoma of the cervix. Gynecol. Oncol. 2017, 147, 589–596. [Google Scholar] [CrossRef]
- Lin, L.M.; Lin, Q.; Liu, J.; Chu, K.X.; Huang, Y.X.; Zhang, Z.K.; Li, T.; Dai, Y.Q.; Li, J.L. Prognostic factors and treatment comparison in small cell neuroendocrine carcinoma of the uterine cervix based on population analyses. Cancer Med. 2020, 9, 6524–6532. [Google Scholar] [CrossRef]
- Hoskins, P.; Swenerton, K.; Pike, J.; Lim, P.; Aquino-Parsons, C.; Wong, F.; Lee, N. Small-cell carcinoma of the cervix: Fourteen years of experience at a single institution using a combined-modality regimen of involved-field irradiation and platinum-based combination chemotherapy. J. Clin. Oncol. 2003, 21, 3495–3501. [Google Scholar] [CrossRef]
- Wang, K.-L.; Chang, T.-C.; Jung, S.-M.; Chen, C.-H.; Cheng, Y.-M.; Wu, H.-H.; Liou, W.-S.; Hsu, S.-T.; Ou, Y.-C.; Yeh, L.-S. Primary treatment and prognostic factors of small cell neuroendocrine carcinoma of the uterine cervix: A Taiwanese Gynecologic Oncology Group study. Eur. J. Cancer 2012, 48, 1484–1494. [Google Scholar] [CrossRef]
- Sheets, E.E.; Berman, M.L.; Hrountas, C.K.; Liao, S.; DiSAIA, P.J. Surgically treated, early-stage neuroendocrine small-cell cervical carcinoma. Obstet. Gynecol. 1988, 71, 10–14. [Google Scholar]
- Bermúdez, A.; Vighi, S.; Garcı, A.; Sardi, J. Neuroendocrine cervical carcinoma: A diagnostic and therapeutic challenge. Gynecol. Oncol. 2001, 82, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Kihara, T.; Kawanaka, Y.; Kido, A.; Yoshida, K.; Mizumoto, Y.; Tomiyama, A.; Okuda, S.; Jinzaki, M.; Kato, F. Neuroendocrine carcinoma of uterine cervix findings shown by MRI for staging and survival analysis—Japan multicenter study. Oncotarget 2020, 11, 3675. [Google Scholar] [CrossRef] [PubMed]
- Shell, J.; Keutgen, X.M.; Millo, C.; Nilubol, N.; Patel, D.; Sadowski, S.; Boufraqech, M.; Yang, L.; Merkel, R.; Atallah, C. 68-Gallium DOTATATE scanning in symptomatic patients with negative anatomic imaging but suspected neuroendocrine tumor. Int. J. Endocr. Oncol. 2018, 5, IJE04. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanli, Y.; Garg, I.; Kandathil, A.; Kendi, T.; Zanetti, M.J.B.; Kuyumcu, S.; Subramaniam, R.M. Neuroendocrine tumor diagnosis and management: 68Ga-DOTATATE PET/CT. Am. J. Roentgenol. 2018, 211, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Damian, A.; Lago, G.; Rossi, S.; Alonso, O.; Engler, H. Early Detection of Bone Metastasis in Small Cell Neuroendocrine Carcinoma of the Cervix by 68Ga-DOTATATE PET/CT Imaging. Clin. Nucl. Med. 2017, 42, 216–217. [Google Scholar] [CrossRef]
- Yang, J.; Kan, Y.; Ge, B.H.; Yuan, L.; Li, C.; Zhao, W. Diagnostic role of Gallium-68 DOTATOC and Gallium-68 DOTATATE PET in patients with neuroendocrine tumors: A meta-analysis. Acta Radiol. 2014, 55, 389–398. [Google Scholar] [CrossRef]
- Hope, T.A.; Bergsland, E.K.; Bozkurt, M.F.; Graham, M.; Heaney, A.P.; Herrmann, K.; Howe, J.R.; Kulke, M.H.; Kunz, P.L.; Mailman, J. Appropriate use criteria for somatostatin receptor PET imaging in neuroendocrine tumors. J. Nucl. Med. 2018, 59, 66–74. [Google Scholar] [CrossRef]
- Pfeifer, A.; Knigge, U.; Binderup, T.; Mortensen, J.; Oturai, P.; Loft, A.; Berthelsen, A.K.; Langer, S.W.; Rasmussen, P.; Elema, D. 64Cu-DOTATATE PET for neuroendocrine tumors: A prospective head-to-head comparison with 111In-DTPA-octreotide in 112 patients. J. Nucl. Med. 2015, 56, 847–854. [Google Scholar] [CrossRef] [Green Version]
- Virgolini, I.; Ambrosini, V.; Bomanji, J.B.; Baum, R.P.; Fanti, S.; Gabriel, M.; Papathanasiou, N.D.; Pepe, G.; Oyen, W.; De Cristoforo, C. Procedure guidelines for pet/ct tumour imaging with 68 Ga-dota-conjugated peptides: 68 Ga-dota-toc, 68 Ga-dota-noc, 68 Ga-dota-tate. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2004–2010. [Google Scholar] [CrossRef]
- Virarkar, M.; Ganeshan, D.; Gulati, A.T.; Palmquist, S.; Iyer, R.; Bhosale, P. Diagnostic performance of PET/CT and PET/MR in the management of ovarian carcinoma—A literature review. Abdom. Radiol. 2021, 46, 2323–2349. [Google Scholar] [CrossRef]
- Virarkar, M.; Viswanathan, C.; Iyer, R.; de Castro Faria, S.; Morani, A.; Carter, B.; Ganeshan, D.; Elsherif, S.; Bhosale, P.R. The Role of Positron Emission Tomography/Magnetic Resonance Imaging in Gynecological Malignancies. J. Comput. Assist. Tomogr. 2019, 43, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Virarkar, M.; Devine, C.; Bassett, R., Jr.; Javadi, S.; Faria, S.C.; Bhosale, P. Update on Diagnostic Performance of PET/MRI in Gynecological Malignancies: A Systematic Review and Meta-Analysis. J. Belg. Soc. Radiol. 2020, 104, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virarkar, M.; Ganeshan, D.; Devine, C.; Bassett, R., Jr.; Kuchana, V.; Bhosale, P. Diagnostic value of PET/CT versus PET/MRI in gynecological malignancies of the pelvis: A meta-analysis. Clin. Imaging 2020, 60, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Takei, Y.; Treilleux, I.; Devouassoux-Shisheboran, M.; Ledermann, J.; Viswanathan, A.N.; Mahner, S.; Provencher, D.M.; Mileshkin, L.; Åvall-Lundqvist, E. Gynecologic Cancer InterGroup (GCIG) consensus review for small cell carcinoma of the cervix. Int. J. Gynecol. Cancer 2014, 24, S102–S108. [Google Scholar] [CrossRef]
- Cohen, J.G.; Kapp, D.S.; Shin, J.Y.; Urban, R.; Sherman, A.E.; Chen, L.-M.; Osann, K.; Chan, J.K. Small cell carcinoma of the cervix: Treatment and survival outcomes of 188 patients. Am. J. Obstet. Gynecol. 2010, 203, 347.e341–347.e346. [Google Scholar] [CrossRef]
- Ishikawa, M.; Kasamatsu, T.; Tsuda, H.; Fukunaga, M.; Sakamoto, A.; Kaku, T.; Kato, T.; Takahashi, K.; Ariyoshi, K.; Suzuki, K. A multi-center retrospective study of neuroendocrine tumors of the uterine cervix: Prognosis according to the new 2018 staging system, comparing outcomes for different chemotherapeutic regimens and histopathological subtypes. Gynecol. Oncol. 2019, 155, 444–451. [Google Scholar] [CrossRef]
- Viswanathan, A.N.; Deavers, M.T.; Jhingran, A.; Ramirez, P.T.; Levenback, C.; Eifel, P.J. Small cell neuroendocrine carcinoma of the cervix: Outcome and patterns of recurrence. Gynecol. Oncol. 2004, 93, 27–33. [Google Scholar] [CrossRef]
- Carroll, M.R.; Ramalingam, P.; Salvo, G.; Fujimoto, J.; Soto, L.M.S.; Phoolcharoen, N.; Hillman, R.T.; Cardnell, R.; Byers, L.; Frumovitz, M. Evaluation of PARP and PDL-1 as potential therapeutic targets for women with high-grade neuroendocrine carcinomas of the cervix. Int. J. Gynecol. Cancer 2020, 30, 1303–1307. [Google Scholar] [CrossRef]
- Katabathina, V.S.; Vikram, R.; Olaoya, A.; Paspulati, R.M.; Nicolas, M.M.; Rao, P.; Zaheer, A.; Prasad, S.R. Neuroendocrine neoplasms of the genitourinary tract in adults: Cross-sectional imaging spectrum. Abdom. Radiol. 2017, 42, 1472. [Google Scholar] [CrossRef]
- Soga, J.; Osaka, M.; Yakuwa, Y. Carcinoids of the ovary: An analysis of 329 reported cases. J. Exp. Clin. Cancer Res. CR 2000, 19, 271–280. [Google Scholar]
- Patibandla, J.R.; Fehniger, J.E.; Levine, D.A.; Jelinic, P. Small cell cancers of the female genital tract: Molecular and clinical aspects. Gynecol. Oncol. 2018, 149, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, J.; Dong, R. Pathological features, clinical presentations and prognostic factors of ovarian large cell neuroendocrine carcinoma: A case report and review of published literature. J. Ovarian Res. 2019, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Bagga, R.; Rai, B.; Srinivasan, R. Primary pure large cell neuroendocrine carcinoma of the ovary: Histopathologic and immunohistochemical analysis with review of the literature. Int. J. Clin. Exp. Pathol. 2021, 14, 1000. [Google Scholar] [PubMed]
- Pang, L.; Guo, Z. Primary neuroendocrine tumors of the ovary: Management and outcomes. Cancer Med. 2021, 10, 8558–8569. [Google Scholar] [CrossRef]
- Veras, E.; Deavers, M.T.; Silva, E.G.; Malpica, A. Ovarian nonsmall cell neuroendocrine carcinoma: A clinicopathologic and immunohistochemical study of 11 cases. Am. J. Surg. Pathol. 2007, 31, 774–782. [Google Scholar] [CrossRef]
- Schlechtweg, K.; Chen, L.; Clair, C.M.S.; Tergas, A.I.; Khoury-Collado, F.; Hou, J.Y.; Melamed, A.; Neugut, A.I.; Hershman, D.L.; Wright, J.D. Neuroendocrine carcinoma of the endometrium: Disease course, treatment, and outcomes. Gynecol. Oncol. 2019, 155, 254–261. [Google Scholar] [CrossRef]
- Bartosch, C.; Lopes, J.M.; Oliva, E. Endometrial carcinomas: A review emphasizing overlapping and distinctive morphological and immunohistochemical features. Adv. Anat. Pathol. 2011, 18, 415–437. [Google Scholar] [CrossRef]
- Scully, R.E.; Aguirre, P.; DeLellis, R.A. Argyrophilia, serotonin, and peptide hormones in the female genital tract and its tumors. Int. J. Gynecol. Pathol. 1984, 3, 51–70. [Google Scholar] [CrossRef]
- Kostamo, K.; Peart, M.; McKenzie, N.; Holloman, C.; Carlan, S.; Ge, L.; Maksem, J. Novel treatment of small-cell neuroendocrine of the vagina. Case Rep. Oncol. Med. 2018, 2018, 9157036. [Google Scholar] [CrossRef] [Green Version]
- Kaminski, J.M.; Anderson, P.R.; Han, A.C.; Mitra, R.K.; Rosenblum, N.G.; Edelson, M.I. Primary small cell carcinoma of the vagina. Gynecol. Oncol. 2003, 88, 451–455. [Google Scholar] [CrossRef]
- Bing, Z.; Levine, L.; Lucci, J.A.; Hatch, S.S.; Eltorky, M.A. Primary small cell neuroendocrine carcinoma of the vagina: A clinicopathologic study. Arch. Pathol. Lab. Med. 2004, 128, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.; Bócoli, M.C.; Saldanha, J.C.; Murta, E.F.C.; Nomelini, R.S. Primary small cell carcinoma of the vagina. Case Rep. Obstet. Gynecol. 2013, 2013, 827037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kombathula, S.H.; Rapole, P.S.; Prem, S.S.; Badhe, B. Primary small cell carcinoma of the vagina: A rare instance of prolonged survival. BMJ Case Rep. CP 2019, 12, e227100. [Google Scholar] [CrossRef] [PubMed]
- Bobos, M.; Hytiroglou, P.; Kostopoulos, I.; Karkavelas, G.; Papadimitriou, C.S. Immunohistochemical distinction between Merkel cell carcinoma and small cell carcinoma of the lung. Am. J. Dermatopathol. 2006, 28, 99–104. [Google Scholar] [CrossRef]
- Matsumoto, R.; Bito, T.; Washio, K.; Ikeda, T.; Oka, M. Primary cutaneous small cell carcinoma of the vulva arising from squamous cell carcinoma. Br. J. Dermatol. 2011, 165, 1147–1148. [Google Scholar] [CrossRef]
- Herrera-Martínez, A.D.; Hofland, J.; Hofland, L.J.; Brabander, T.; Eskens, F.A.; Moreno, M.A.G.; Luque, R.M.; Castaño, J.P.; de Herder, W.W.; Feelders, R.A. Targeted systemic treatment of neuroendocrine tumors: Current options and future perspectives. Drugs 2019, 79, 21–42. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.; Chan, D.L.; Wong, M.H.; Li, B.T.; Lumba, S.; Clarke, S.J.; Samra, J.; Pavlakis, N. Systematic review of the role of targeted therapy in metastatic neuroendocrine tumors. Neuroendocrinology 2017, 104, 209–222. [Google Scholar] [CrossRef] [Green Version]
- Cives, M.; Quaresmini, D.; Rizzo, F.M.; Tucci, M.; Silvestris, F. The tumor microenvironment in neuroendocrine tumors: Biology and therapeutic implications. Neuroendocrinology 2019, 109, 83–99. [Google Scholar] [CrossRef]
- Grywalska, E.; Sobstyl, M.; Putowski, L.; Roliński, J. Current possibilities of gynecologic cancer treatment with the use of immune checkpoint inhibitors. Int. J. Mol. Sci. 2019, 20, 4705. [Google Scholar] [CrossRef] [Green Version]
- Kurnit, K.C.; Reid, P.; Moroney, J.W.; Fleming, G.F. Immune checkpoint inhibitors in women with gynecologic cancers: Practical considerations. Gynecol. Oncol. 2020, 158, 531–537. [Google Scholar] [CrossRef]
- Menderes, G.; Hicks, C.; Black, J.D.; Schwab, C.L.; Santin, A.D. Immune checkpoint inhibitors in gynecologic cancers with lessons learned from non-gynecologic cancers. Expert Opin. Biol. Ther. 2016, 16, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Manchana, T.; Ittiwut, C.; Mutirangura, A.; Kavanagh, J.J. Targeted therapies for rare gynaecological cancers. Lancet Oncol. 2010, 11, 685–693. [Google Scholar] [CrossRef]
- Mahdi, H.; Joehlin-Price, A.; Elishaev, E.; Dowlati, A.; Abbas, A. Genomic analyses of high-grade neuroendocrine gynecological malignancies reveal a unique mutational landscape and therapeutic vulnerabilities. Mol. Oncol. 2021, 15, 3545. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).