Simple Summary
The current routine treatment for glioblastoma (GB), the most lethal high-grade brain tumor in adults, aims to induce DNA damage in the tumor. However, the tumor cells might be able to repair that damage, which leads to therapy resistance. Fortunately, DNA repair defects are common in GB cells, and their survival is often based on a sole backup repair pathway. Hence, targeted drugs inhibiting essential proteins of the DNA damage response have gained momentum and are being introduced in the clinic. This review gives a perspective on the use of radiopharmaceuticals targeting DDR kinases for imaging in order to determine the DNA repair phenotype of GB, as well as for effective radionuclide therapy. Finally, four new promising radiopharmaceuticals are suggested with the potential to lead to a more personalized GB therapy.
Abstract
Despite numerous innovative treatment strategies, the treatment of glioblastoma (GB) remains challenging. With the current state-of-the-art therapy, most GB patients succumb after about a year. In the evolution of personalized medicine, targeted radionuclide therapy (TRT) is gaining momentum, for example, to stratify patients based on specific biomarkers. One of these biomarkers is deficiencies in DNA damage repair (DDR), which give rise to genomic instability and cancer initiation. However, these deficiencies also provide targets to specifically kill cancer cells following the synthetic lethality principle. This led to the increased interest in targeted drugs that inhibit essential DDR kinases (DDRi), of which multiple are undergoing clinical validation. In this review, the current status of DDRi for the treatment of GB is given for selected targets: ATM/ATR, CHK1/2, DNA-PK, and PARP. Furthermore, this review provides a perspective on the use of radiopharmaceuticals targeting these DDR kinases to (1) evaluate the DNA repair phenotype of GB before treatment decisions are made and (2) induce DNA damage via TRT. Finally, by applying in-house selection criteria and analyzing the structural characteristics of the DDRi, four drugs with the potential to become new therapeutic GB radiopharmaceuticals are suggested.
1. Introduction
Treatment challenges posed by malignant gliomas remain considerable, and many derive from the molecular and cellular heterogeneity inherent to these tumor variants [1,2]. New treatment strategies for glioblastomas (GB), known as the most malignant gliomas (grade IV), are urgently warranted. For newly diagnosed GB patients with overall good health status, the standard of care includes maximal surgical resection, combined external beam radiation therapy (RT), and temozolomide (TMZ), followed by maintenance TMZ [3]. However, even with an optimal treatment protocol and recent advances in targeted therapies, survival has only slightly improved, and almost all tumors recur [1,4]. Molecular biomarkers play an increasing role in treatment decisions and response prediction. For example, the methylation status of the O6-methylguanine-DNA methyltransferase (MGMT) promoter is a major cause of TMZ resistance [5,6]. The focus for advancing GB therapy lies in the field of personalized, targeted therapy with the ultimate aim to selectively eradicate GB cells without damaging the surrounding healthy brain tissue [4,7,8]. To achieve this, mechanisms that induce therapy resistance and strategies to induce selective cell death in GB cells need to be explored and exploited. GB is recognized as being highly radioresistant and influenced by the presence of glioma stem cells (GSCs), cellular hypoxia, high cell heterogeneity, and aberrant activation of DNA damage response (DDR) proteins [9,10]. The dysregulation of the DDR in GB allows cancer cells to repair DNA damage and results in resistance to the current state-of-the-art therapies. In contrast to normal cells, components of the DDR pathway are frequently compromised in tumor cells, and their survival is often based on a sole backup pathway. Hence, targeted strategies against essential components of the DDR offer the possibility to promote cell death in cancer cells and increase the tumor’s sensitivity to cancer therapies based on the principle of ‘synthetic lethality’ (Figure 1) [10,11,12,13]. In order to sensitize GB cells to DNA damaging agents, two approaches can be adopted. First, directly targeting key DNA damage signaling kinases such as phosphatidylinositol 3′ kinase (PI3K)-related kinases (PIKKs) and PIKK-regulated downstream kinases. These include DNA damage sensor and repair proteins, e.g., ataxia-telangiectasia mutated (ATM), ATM-RAD3-related (ATR) protein, and DNA-dependent protein kinase (DNA-PK) [14]. Second, they interfere with cell cycle checkpoint proteins, which monitor DNA integrity before cell division (G2–M checkpoint) and DNA replication (G1–S checkpoint) [15]. Interestingly, ‘replication stress’ present in cancer cells could further be enhanced following these therapies through further loosening the remaining checkpoints and inducing failure of further proliferation [16].
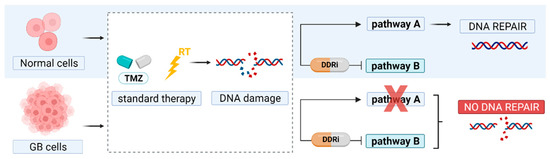
Figure 1.
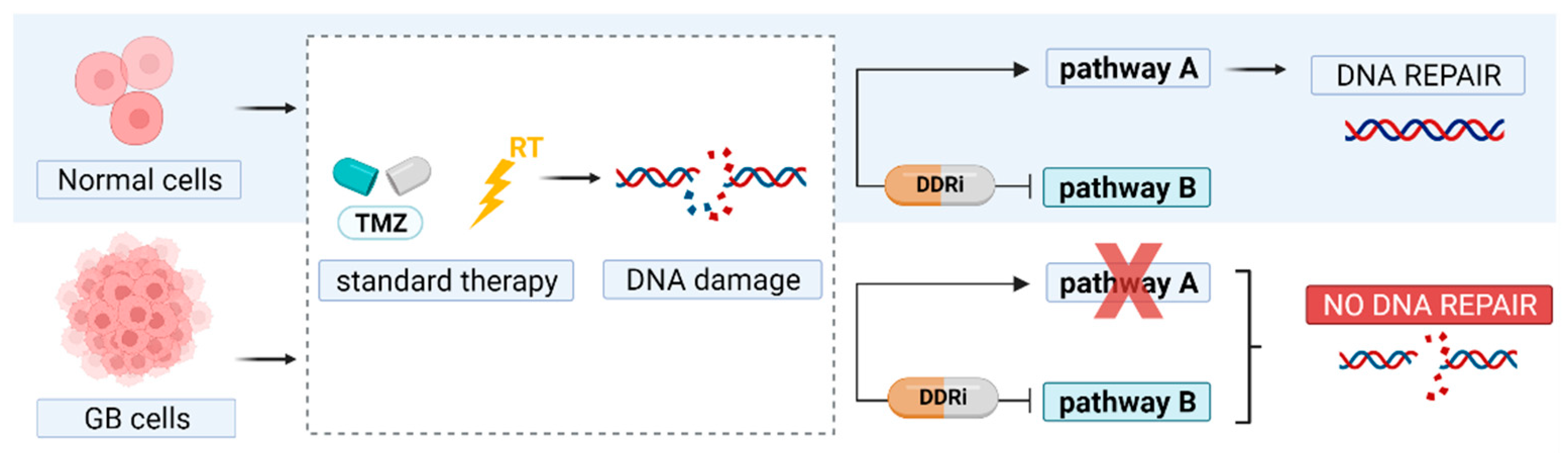
Principle of synthetic lethality. In glioblastoma (GB) therapy, DNA damage is induced by temozolomide (TMZ) and radiation therapy (RT). DNA repair pathways are often disrupted (pathway A) and therefore GB cells solely depend on a back-up pathway to repair DNA damage. Inhibitors of essential DNA damage response kinases (DDRi) can block this rescue pathway to promote GB cell death.
In this review, the rationale and current status of targeted drugs that inhibit essential DDR kinases (DDRi) for the therapy of GB are given. Secondly, a perspective is given on radiopharmaceuticals for nuclear imaging and targeted radionuclide therapy (TRT) targeting DDR kinases. The ability to monitor DNA repair processes using nuclear imaging may be an asset for personalized GB therapy and for monitoring the response to DNA damaging treatments and DDRi. Finally, selection criteria have been applied to reveal candidate compounds that have the potential to become radiopharmaceuticals targeting DDR kinases [17].
2. Targeting DDR Pathways in GB
Under physiological conditions, the DDR protects the human genome by removing errors and avoiding the insurgence of mutations. However, in tumors treated with DNA damaging agents, DNA repair systems contribute to treatment failure [10,13,18,19]. Depending on the type of damage and cell cycle phase of the tumor cells, different actors of the DDR pathway are activated; these aspects have been previously reviewed [10,13,20]. Most of the subtle changes to DNA, such as oxidative lesions and single-strand breaks (SSBs), are repaired through different pathways such as base excision repair (BER), nucleotide excision repair (NER), or mismatch repair (MMR) [19]. SSB repair is activated following TMZ chemotherapy [21]. DNA double-strand breaks (DSBs), induced by RT, appear to be primarily repaired by non-homologous end-joining (NHEJ) or the error-free homologous repair (HR) pathway [19]. Surveillant sensor kinases in the DDR pathways, e.g., ATM, ATR, poly (ADP-ribose) polymerase-1 and -2 (PARP1 and PARP2) or the DNA-PK catalytic subunit (DNA-PKcs), recognize DNA damage and are recruited to the sites of SSBs and DSBs (Figure 2). Moreover, sensor kinases are responsible for the formation of protein complexes such as the MRN complex (Nbs1/hMre11/hRad50) or DNA-PK (Ku70/Ku80/DNA-PKcs). The DNA-PK and MRN complexes assemble and compete at sites of DNA DSBs where they act as damage sensors and initiate cell cycle dependent NHEJ or HR, respectively [22]. After sensor proteins detect DNA damage, transducer proteins with kinase activity, e.g., checkpoint kinase-1 (CHK1) and -2 (CHK2), trigger the activity of downstream effectors that can influence and/or direct a variety of cellular responses, including transcription processes, cell cycle regulation, DNA repair processes and apoptosis initiation [13].
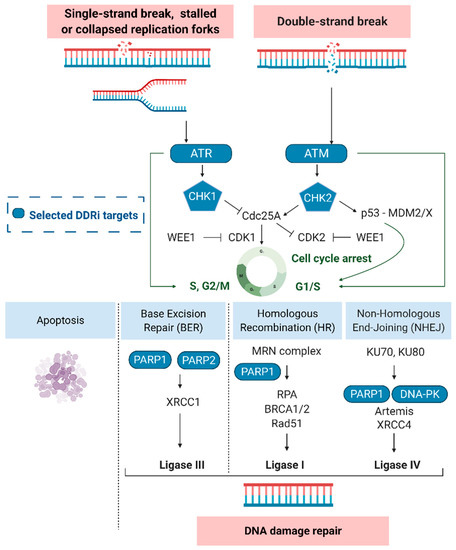
Figure 2.
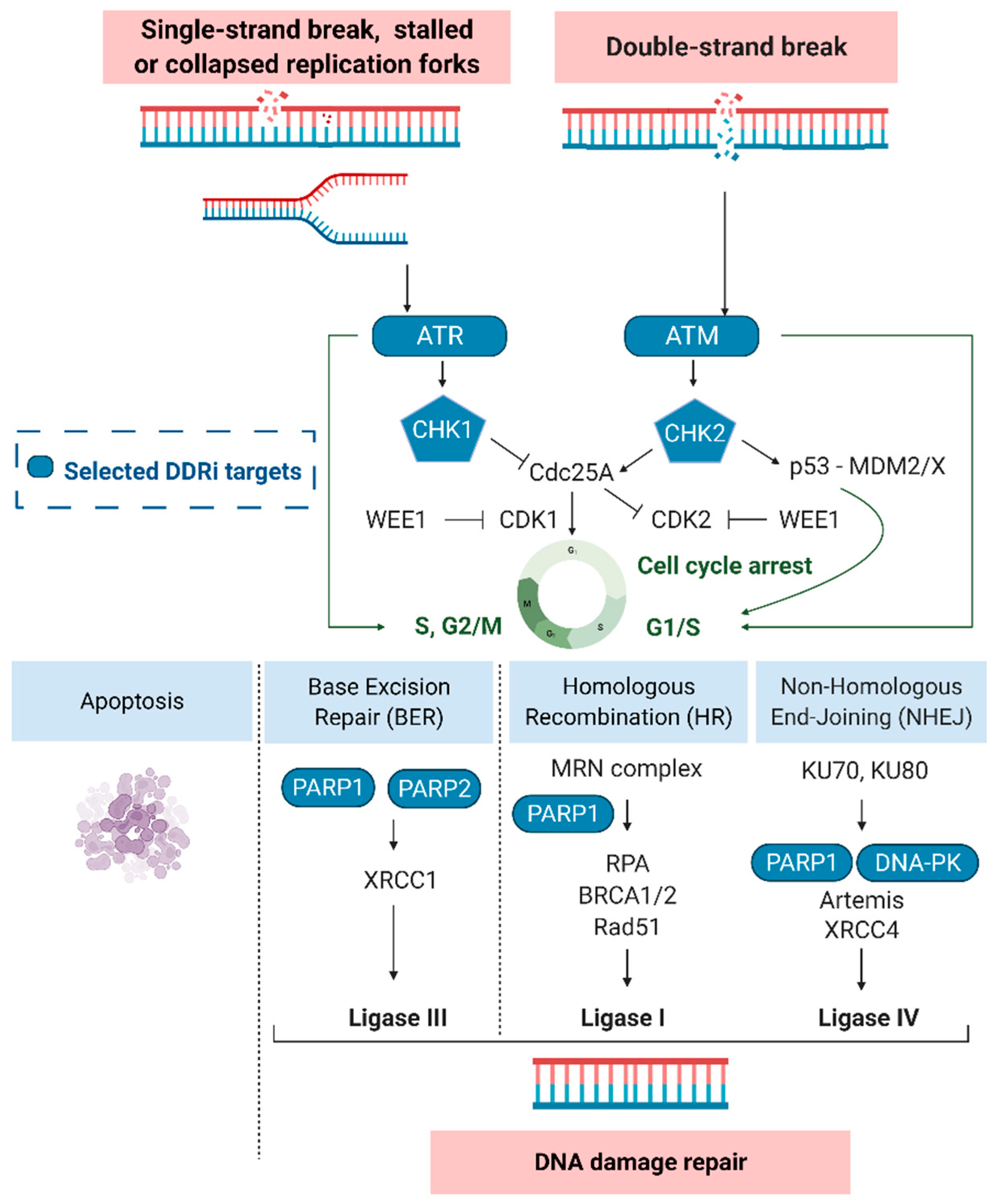
The DNA damage response and selected targets (blue). Ataxia-telangiectasia mutated (ATM), ATM-RAD3-related protein (ATR), cyclin-dependent kinase 1 (CDK1/2), checkpoint kinase-1 and -2 (CHK1/2), DNA-dependent protein kinase (DNA-PK), Mouse double minute 2/X homolog (MDM2/X), Nbs1/hMre11/hRad50 (MRN complex), poly (ADP-ribose) polymerase-1 and -2 (PARP), replication protein A (RPA), X-ray repair cross-complementing protein 4 (XRCC4).
Aberrant activation of these DDR kinases (ATM, ATR, DNA-PK, CHK1, CHK2, and PARP) in cancer is strongly correlated with resistance to genotoxic cancer therapies, including in GB [10,23]. Defects in the ATM-CHK2-p53 pathway promote GB formation and play a role in the response of glioma to ionizing radiation (IR) [24]. Mutations in isocitrate dehydrogenase 1 (IDH1) are frequently found in gliomas and are associated with better therapeutic outcomes. Interestingly, co-mutations in DDR kinases could play a role. In IDH1 mutated astrocytoma patients, TP53 (63%) and ATRX (27%) are the top two genes that display a higher frequency of mutations. An association between IDH1 mutations and reduced ATRX expression has also been shown. Mutations in CHK2 are instead associated with an IDH1-wildtype astrocytoma [25]. Núñez et al. discovered that mutant IDH1 helps maintain genomic stability in tumors by enhancing the DDR [26]. Glioma stem cells (GSC) have been shown to promote radioresistance by preferential activation of the DDR pathway through increased cell cycle checkpoint activation. This contributes to an increased DNA repair capacity and results in greater survival [9]. Since the standard therapy of GB includes TMZ and RT, which both aim to damage the DNA, DDR inhibition is being explored as a way to increase treatment efficacy [10]. For example, the inhibition of phosphatase and tensin homolog (PTEN) phosphorylation at Y240 sensitizes GB to IR by preventing enhanced DNA repair [27]. The standard TMZ chemotherapy modifies DNA or RNA at N7-guanine, O6-guanine, and N3-adenine by the addition of methyl groups. Methylated O6-guanine sites are usually repaired by MGMT. Since MGMT is often upregulated in GB, TMZ-resistance may occur. However, as TMZ also introduces N-site alkylations, which are normally repaired by BER, GB cells can be sensitized to TMZ by inhibiting key proteins of the BER pathway (such as PARP) [28]. A number of DDRi have already reached the clinic for the treatment of GB, including the PARP inhibitors (PARPi) olaparib and veliparib (Figure 3) [11,29].
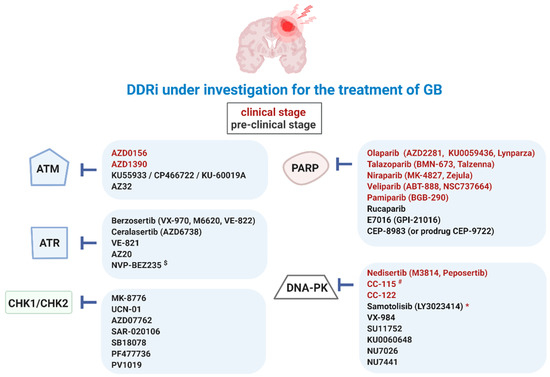
Figure 3.
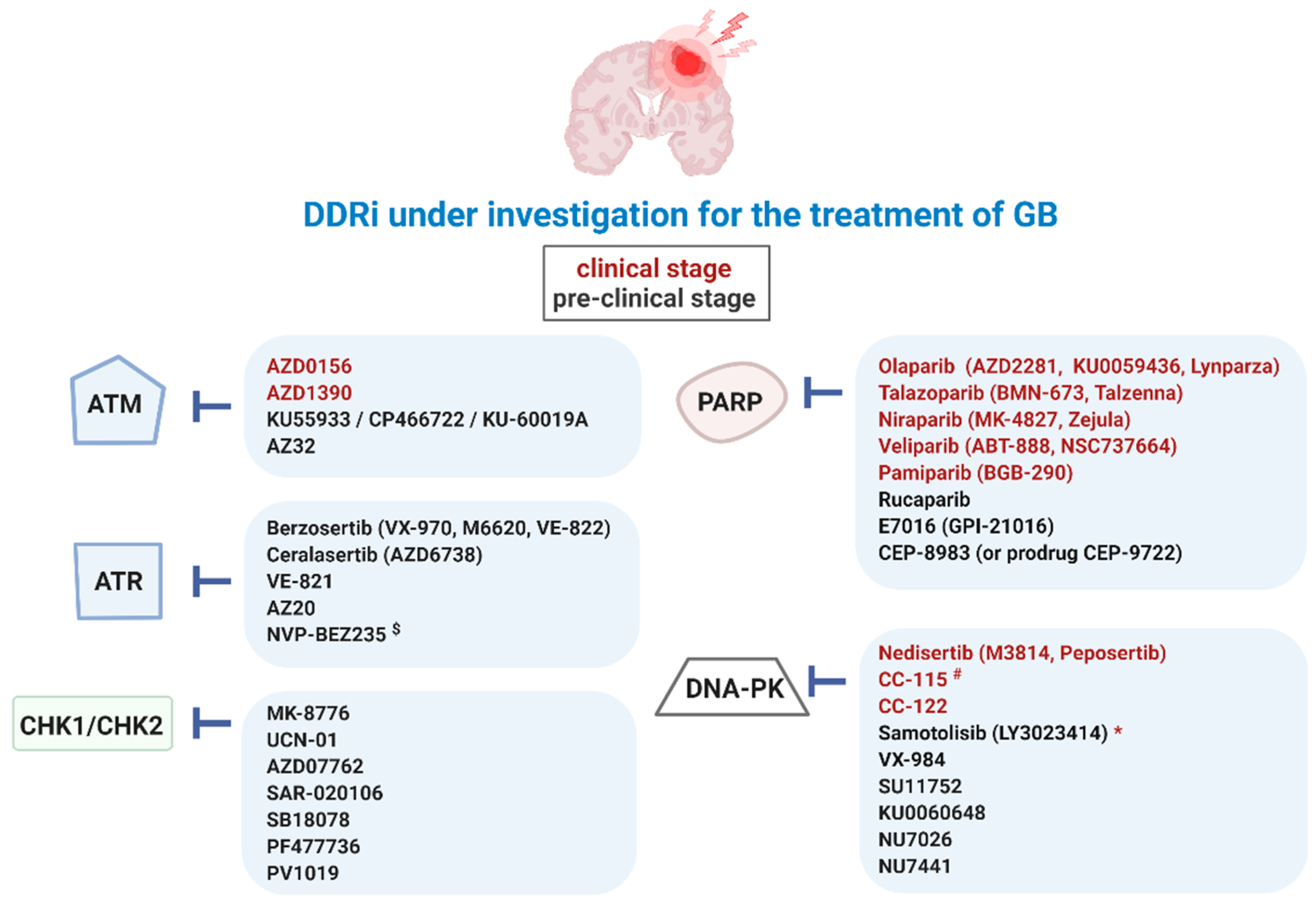
Overview of DDRi for targeted therapy of GB. Ataxia-telangiectasia mutated (ATM), ATM-RAD3-related protein (ATR), checkpoint kinase-1 and -2 (CHK1/2), DDR kinase inhibitor (DDRi), DNA-dependent protein kinase (DNA-PK), poly (ADP-ribose) polymerase-1 and -2 (PARP). $ also targets phosphatidylinositol-3-kinase (PI3K)/Akt and the mammalian target of rapamycin (mTOR) signaling pathways (PI3K/mTOR), * paediatric central nervous system tumors, # also targets mTOR.
Sensitivity towards DDRi is dependent on specific biomarkers, and the identification of treatment-responsive patients constitutes one of the key challenges associated with the clinical use of DDRi. Recent work has identified genomic and functional DNA repair assays that provide the identification of predictive and pharmacodynamic DDRi biomarkers (Figure 4) [30]. Mutational signatures associated with robust HR deficiency (HRD) primarily include alterations affecting BRCA1, BRCA2, PALB2, and two canonical RAD51 paralog genes (RAD51B, RAD51C). A more complex “BRCAness” signature has been defined to denote HRD tumors that share molecular features of BRCA1/2-mutant tumors, which are likely to benefit from DDRi [31,32,33]. The most promising biomarkers of BRCAness in GB relate to IDH1/2, epidermal growth factor receptor (EGFR), PTEN, MYC proto-oncogene, and estrogen receptors beta (ERβ) signatures [34]. For example, the anti-tumor effect of TMZ with ATMi or PARPi is enhanced in IDH1 mutant gliomas, and TMZ increases ATRi sensitivity in MGMT-deficient GB cells [35,36,37].
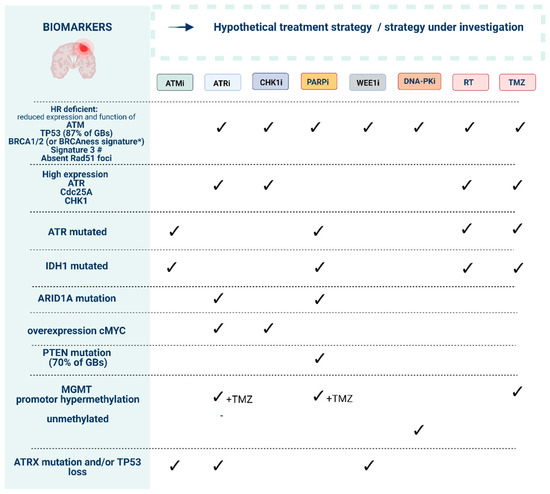
Figure 4.
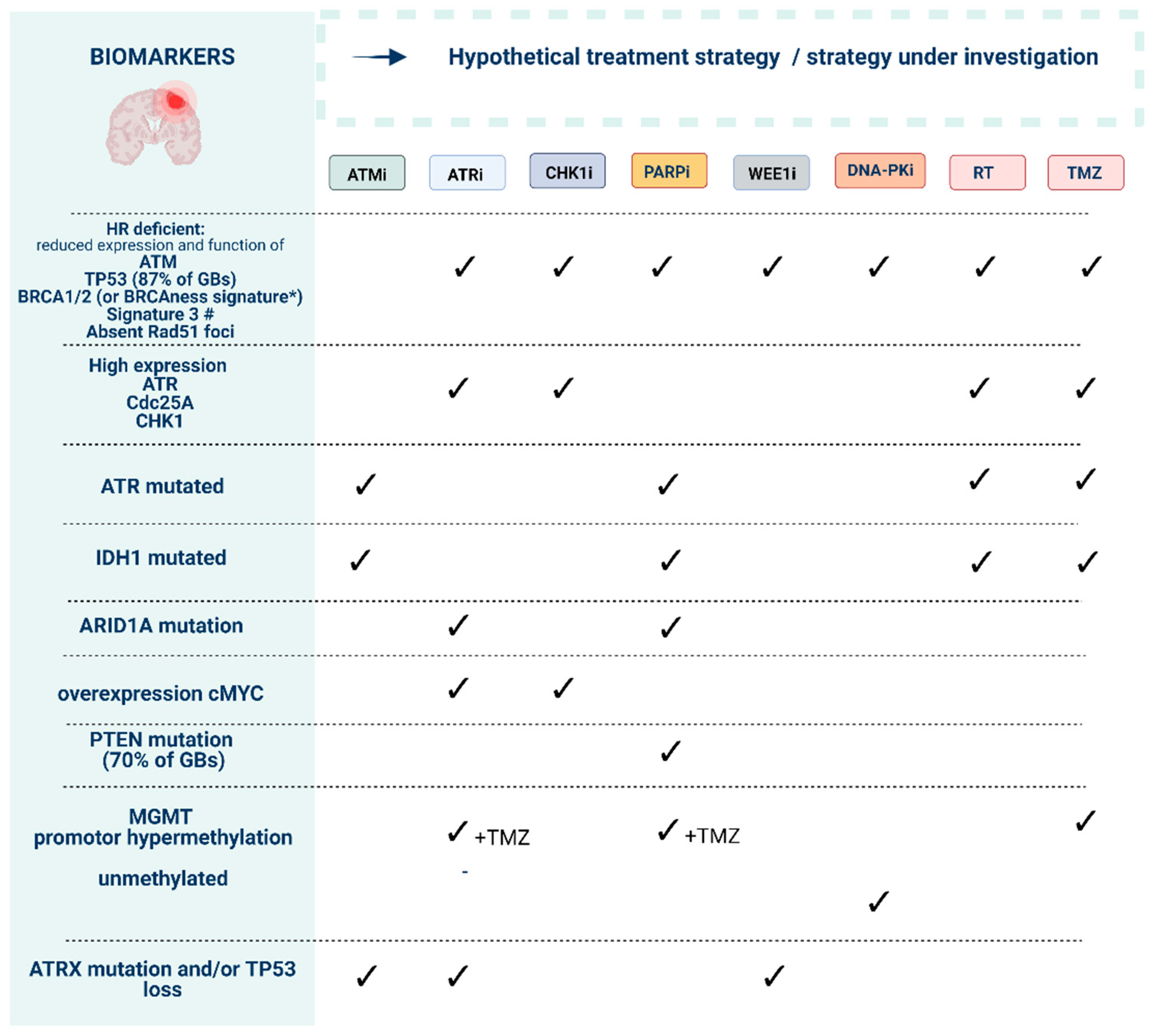
Preliminary biomarkers to guide the use of DNA damage response (DDR) inhibitors in order to reach synthetic lethality. Ataxia-telangiectasia mutated (ATM), ATM-RAD3-related protein (ATR), checkpoint kinase-1 and -2 (CHK1/2), DDR kinase inhibitor (DDRi), DNA-dependent protein kinase (DNA-PK), poly (ADP-ribose) polymerase-1 and -2 (PARP). * “BRCAness” signature can include mutations in ATM, ATR, BAP1, BRCA1, BRCA2, CDK12, CHK1, CHK2, FANCA, FANCC, FANCD2, FANCE, FANCF, PALB2, NGS1, WRN, RAD50, RAD51B, RAD51C, RAD51D, MRE11A, BLM, BRIP1. # Mutational signature found in human cancers characterized by defective homologous-recombination-based DNA double-strand break repair [33].
Strategies to define the mutational status of these genes include immunohistochemistry (IHC) and next-generation sequencing techniques. The requirement of whole-genome or whole-exome sequencing for the identification of selected gene signatures limits its widespread clinical utilization as a biomarker. However, new computational tools such as signature multivariate analysis and combinations of genomic analyses with single-cell imaging may increase the number of patients to be considered for treatments targeting HRD [38,39].
3. DDR (Radio)Pharmaceuticals
Single-photon emission computed tomography (SPECT) and positron emission tomography (PET) imaging could be utilized to identify patients that would benefit from DDRi therapy. Radiopharmaceuticals targeting DDR kinases have potential for the assessment of DDR target engagement (e.g., PARP activity) and may assist in monitoring response to DDRi or other DNA damaging treatments [40]. In addition, given the well-understood involvement of DDR during tumorigenesis, the ability to monitor these repair processes using PET or SPECT may facilitate the detection of earlier stages of carcinogenesis [41].
Upon confirming the expression of the DDR kinase in the GB tumor, therapeutic DDR targeting radiopharmaceuticals could be administered prior to or in combination with other DNA-damaging agents (e.g., TMZ and external beam RT), ultimately causing a synergistic anti-tumor response. Noteworthy, DDRi induced toxicity to healthy tissue might be limited due to intact DDR pathways in healthy cells (Figure 1) [42]. Interestingly, TRT agents targeting DDR kinases offer increased cytotoxicity compared to cold DDRi due to the additional radiation-induced DNA damage. Our group recently published a perspective on TRT for the treatment in GB, with a special focus on radiopharmaceutical requirements, including target and radionuclide selection, blood–brain barrier (BBB) passage, toxicity, validation, and combined therapy strategies [43].
The nature of the induced DNA damage in TRT is dependent on the specific radiation characteristics of the used isotopes. Most GB research has investigated the cellular and physical effects of IR in the context of external beam RT, radiation effects which are significantly different compared to TRT radiation effects [20]. Lutetium-177, iodine-131, rhenium-186, rhenium-188, or yttrium-90, are commonly utilized for TRT of GB, featuring β--particle emissions with relatively low linear energy transfer (LET) (0.2–2 keV/μm) and a low relative biological effectiveness (RBE). As a result, the β--emission induced damage consists of some DSBs but mostly repairable SSBs, which could result in sublethal damage repair [44]. Targeted α-particle therapy (TAT) using astatine-211, actinium-225, or bismuth-213, is gaining attention due to the higher LET (50–230 keV/μm) and RBE inducing more complex DNA damage and a lower dependency on the tumor oxygenation status [45,46]. Complex DNA damage significantly contributes to exceeding the cellular capabilities of DNA repair, thereby forcing cells towards cell death [20]. The first positive clinical trials on TAT have emerged, and TAT was suggested as a facilitator to overcome tumoral resistance to chemotherapy [47,48]. A nice example is the astatine-211 radiolabeled PARPi, which induced cellular lethality by targeting alpha-emitters directly to the nucleus, with high sensitivity in neuroblastoma in vitro and in vivo. The [211At]-PARPi was 10,000 times more potent than talazoparib, indicating that the likely mechanism of cell killing does not rely on pharmacological PARP inhibition but rather on alpha-particle induced DNA damage [49,50]. Lastly, the short penetration range and LET (4–26 keV/μm) of Auger electron emitters make them suitable candidates for inducing damage to a specific target with dimensions comparable to the DNA, leading to complex, lethal DNA damage [51].
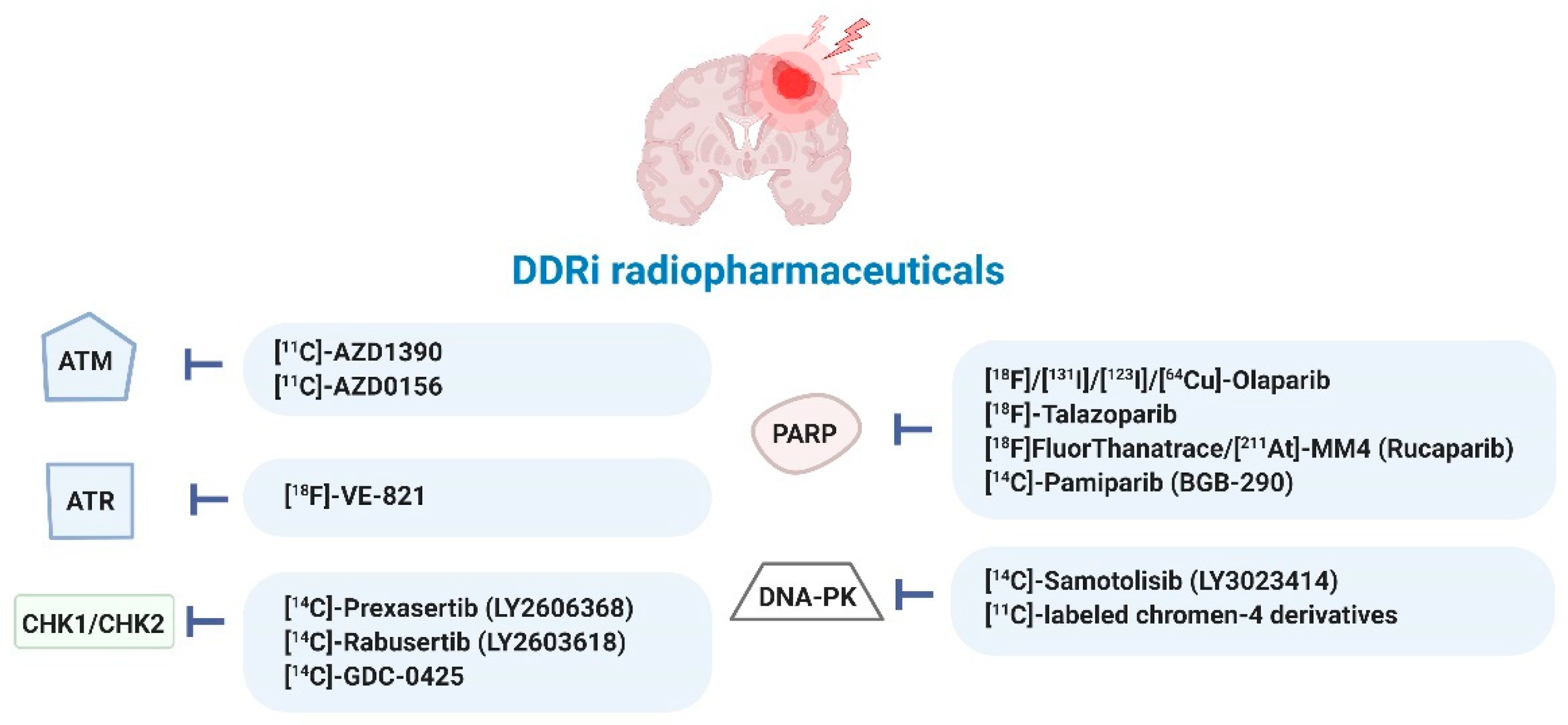
A current list of radiopharmaceuticals targeting DDR processes in various cancer types is provided in Table S2 and summarized in Figure 5. So far, most DDRi have been directly radiolabeled with 123I-, 131I-, 18F- and 211At-radionuclides. Only one analog of olaparib was 64Cu-radiolabeled following conjugation of a 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) moiety, allowing for PET imaging of mesothelioma [29,40,49,50,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91].
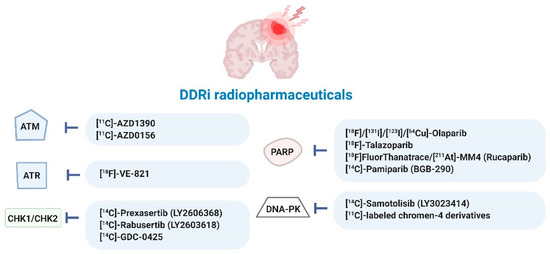
Figure 5.
Radiopharmaceuticals targeting DDR kinases studied in various cancer types (for details see Table S2). Ataxia-telangiectasia mutated (ATM), ATM-RAD3-related protein (ATR), checkpoint kinase-1 and -2 (CHK1/2), DDR kinase inhibitor (DDRi), DNA-dependent protein kinase (DNA-PK), poly (ADP-ribose) polymerase-1 and -2 (PARP) [29,40,49,50,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91].
Interestingly, it was also hypothesized that ‘cold’ DDRi could increase the effectiveness of TRT agents. This hypothesis has already been confirmed in ovarian cancer xenografts, where the synergistic effect of a mesothelin-targeted 227Th-conjugate in combination with ATMi, ATRi, DNA-PKi, and PARPi was investigated [92]. In prostate and neuroendocrine cancer, multiple clinical trials are still running, combining PARPi with [177Lu]-DOTATATE, [177Lu]-PSMA-617 or [223Ra]-dichloride (ClinicalTrials.gov Identifiers: NCT03874884, NCT03317392, NCT03076203, NCT04086485, NCT04375267) [29]. Unfortunately, studies have not been initiated in GB yet.
3.1. ATM/ATR Inhibitors
3.1.1. Targeting ATM/ATR as an Anti-GB Strategy
ATM and ATR are members of the PIKK family of serine/threonine protein kinases, which are crucial in the initiation of cell cycle arrest and apoptosis (Figure 1). ATM is the main kinase in the cellular response to DNA DSBs, while ATR is activated by single-stranded DNA structures, which may arise upon SSB induction and stalled or collapsed replication forks. Although ATM and ATR are activated by different types of DNA damage, their signaling cascades are partially overlapping [93,94,95]. For example, CHK1/2 is a downstream target in both pathways. However, ATM plays a crucial role in the activation of the G1/S cell cycle checkpoint while ATR enforces the intra-S-phase and G2/M cell cycle checkpoint (Figure 1) [96]. Notably, the list of substrates undergoing ATM-dependent phosphorylation is still growing [97].
Hypersensitivity of ATM-defective cells to IR and the critical function of the ATR pathway for the survival of tumor cells has led to considerable interest in ATM and ATR as therapeutic targets for cancer therapy [93,98,99]. Glioma cells, especially GSCs, exhibit increased resistance to IR, which is mediated by an upregulation of DDR targets such as ATM, ATR, PARP1, and CHK1. This results in a rapid G2/M cell cycle checkpoint activation and enhanced DNA repair [9,100]. However, tumor cells often suffer from defects in ATM function through mutation of the ATM protein itself or its associated downstream targets, particularly p53. Such mutated cells must maintain functional S and G2/M cell cycle checkpoints mediated by ATR/CHK1 to avoid premature mitotic entry [101]. The genomic characterization of human GB genes and their core pathways showed that p53 signaling was altered in 87% of GB cases [102]. Therefore, ATR/CHK1 inhibition shows great potential to induce synthetic lethality [12,102,103]. Treatment with ATM- or ATR-inhibitors (ATMi/ATRi) may thus selectively sensitize glioma cells and GSCs to IR and/or TMZ [103,104,105].
Possible determinants of ATRi sensitivity include high levels of ATR, Cdc25A, and CHK1. Multiple predictive biomarkers have also been incorporated into early phase trials: alternative lengthening of telomeres, reduced expression/loss of function of ATM, BRCA1/2, TP53, ARID1A, and overexpression of CCNE1, APOBEC, and MYC (Table S1 and Figure 4) [106,107,108]. Especially DDRi combined with IR could provide a therapeutic strategy for IDH1R132H glioma patients who also harbor p53- and ATRX-inactivating mutations [26]. Alpha Thalassemia/Mental Retardation Syndrome X-Linked (ATRX)-deficient glioma displaying p53 loss of function could also benefit from ATRi therapy [109,110]. In p53-deficient settings, suppression of ATM dramatically sensitized cells to chemotherapy, whereas, conversely, ATM suppression had the opposite effect in the presence of functional p53 [101]. ATM kinase inhibition combined with low dose radiation was also selectively toxic to glioma with mutant p53 through the induction of mitotic catastrophe and apoptosis [111]. Overexpression of cMYC has previously been shown to cause replicative stress and to confer sensitivity to CHK1i and ATR knockdown. Mutations in ARID1A predict response to ATRi and PARPi since ARID1A-deficient cells rely on ATR checkpoint activity to prevent apoptosis [112]. Lastly, pRAD50 has been identified as a novel and clinically applicable pharmacodynamic biomarker of sensitivity to ATM/ATR inhibition [113].
3.1.2. Current Status of ATM/ATR Targeted Therapy in GB
An overview of oncological clinical trials investigating ATMi and ATRi and their relevant biomarker selection criteria are summarized in Table 1 and Table S2. There are currently four ATMi (KU-60019, AZD0156, AZD1390, and M3541) evaluated in clinical trials, of which two (AZD1390 and AZD0156) include glioma patients (Table 1) [103]. One of the first-generation ATMi includes the small molecule ATP-competitive inhibitor 2-morpholin-4-yl-6-thianthren-1-yl-pyran-4-one (KU55933) and its ameliorated derivatives KU-60019 and CP466722 [114,115,116]. Therapy with these ATMi has resulted in chemo- and radiosensitization of GB cells and a significant two- to three-fold increased survival when KU-60019 was administered intratumorally in GB models combined with external beam IR. Particularly, a signature of IDH mutations combined with a low expression of TP53 or MGMT and high expression of phosphatidylinositol-3-kinase (PI3K) has been identified as a biomarker for more effective ATM-based therapy [35,117,118,119,120,121]. Interestingly, KU-60019 limited glioma cell growth in co-culture with human astrocytes, with the latter seemingly unaffected by the same treatment [104,115,118,120]. The last generation ATMi AZ32 and AZD1390 have been specifically designed to effectively cross the BBB and showed radiosensitizing effects in GB both in vitro and in vivo [75,93,103,111]. This led to a phase I study of AZD1390 in combination with RT in patients with brain cancer (ClinicalTrials.gov Identifier: NCT03423628). The ATMi AZD0156 has shown potential in multiple cancer types, including synergism with PARPi [29,122,123,124]. In a phase I trial combining AZD0156 with olaparib, hematologic toxicity appears to be the treatment-limiting toxicity in advanced malignancies (including glioma), although efficient doses could be reached [125]. Data on ATMi KU-59403 in GB are awaited [126]. Finally, besides employing small molecule ATMi, silencing of ATM or ATR using siRNA has also been shown to increase glioma cell chemo- and radiosensitivity [21,127,128].

Table 1.
Relevant clinical trials concerning DDRi therapy in glioma patients.
ATRi has demonstrated significant therapeutic potential in cancer treatment, with anti-tumor activity when administered as monotherapy but also when combined with conventional chemotherapy, RT, or immunotherapy [99]. The ATRi currently in clinical trials are VX-970 (also known as VE-822, M6620, or berzosertib, Merck®, Darmstadt, Germany), VX-803 (M4344, Merck®), BAY1895344 (elimusertib, Bayer®, Leverkusen, Germany), M1774, RP-3500, and AZD6738 (ceralasertib, AstraZeneca®, Cambridge, UK). Notably, some of these trials considered biomarkers for patient stratification (Table S1). Unfortunately, no trials have been initiated in GB so far [29,107,133,134].
VX-970, for which 15 trials are now active, reached the clinic first [29,124,135,136]. Radiation and chemosensitization effects have been shown, but efflux pump mechanisms limit brain accumulation of VX-970 [37,137,138,139]. However, prolonged survival was noted in rats with intracranial GB tumors that were treated with RT combined with VX-970. Survival was even more improved upon the combination with PARPi [140,141]. The synthetic lethal interaction of VX-970 might be enhanced by selecting another ATR/CHK1 downstream target, such as WEE1. WEE1 inhibitors (WEE1i) have recently attracted attention with multiple phase I/II studies investigating this synergy (Figure 1) [29,142,143]. WEE1 promotes S and G2/M cell cycle arrest by blocking cyclin-dependent kinase 1 and 2 (CDK1/2) and allowing DNA repair, as shown in Figure 2 [144]. The most studied WEE1i is adavosertib (MK1775, AZD1175), with 23 active trials, including a phase I trial in GB patients (ClinicalTrials.gov Identifier: NCT01849146) [29]. In addition, 27 clinical trials are currently actively evaluating the selective ATRi AZD6738 (Table S1) after promising preclinical results [29,99,145,146]. Notably, no significant radiosensitizing effect was found in an orthotopic GB animal model despite effective AZD6738 brain penetration [147].
NVP-BEZ235, a dual PI3K/mammalian target of rapamycin (mTOR) inhibitor, was identified as a potent inhibitor of ATR and ATR homologs, ATM and DNA-PK [148]. NVP-BEZ235 effectively crosses the BBB with GB radiosensitization and TMZ sensitization effects, but toxicity was shown upon introduction in the clinic [29,147,149,150,151,152,153,154,155,156,157]. Several ATRi have been abandoned in the development stage before reaching the clinic, including Schisandrin B, NU6027, ETP-46464, VE-821 (later optimized to VE-822/VX-970), and AZ20 (later optimized to AZD6738) [99].
3.1.3. ATM/ATR Radiopharmaceuticals
Two ATMi have been 11C-radiolabelled: AZD1390 and AZD0156. In macaque monkeys, intravenous administration revealed superior permeability and BBB penetrating properties of [11C]-AZD1390 compared to [11C]-AZD0156 [75]. A first clinical trial in healthy volunteers analyzed the brain distribution of [11C]-AZD1390 and confirmed BBB penetration [59]. These findings support the use of radiolabeled AZD1390 for therapy and/or diagnostics in patients with central nervous system (CNS) malignancies, including GB. Only one ATRi, VE-821, a less potent precursor of the ATRi VE-822, has been 18F-radiolabelled. This VE-821 analog (termed ‘[18F]-ATRi’) was put forward as a clinically relevant PET imaging agent in an in vivo study by Carlucci et al., and specific target binding was confirmed using a U251 MG GB animal model [40].
3.2. CHK1/2 Inhibitors
3.2.1. Current Status of CHK1/2 Targeted Therapy in GB
CHK1/2 are cell cycle checkpoint kinases that prevent cell cycle progression when DNA damage is detected and being repaired, as shown in Figure 2 [158,159].CHK1 is activated by ATR phosphorylation on Ser317 and Ser345, and CHK2 is activated by ATM phosphorylation on Thr68. CHK2 phosphorylates p53, preventing its interaction with MDM2, and subsequently, p53 drives the expression of genes involved in apoptosis induction and cell cycle checkpoint activation, such as p21/CDKN1 [96]. CHK1 plays an important role in intra-S-phase and G2/M cell cycle checkpoint progression mediated by phosphorylation and inhibition of Cdc25A and Cdc25C [93,159]. Inhibited Cdc25 proteins are no longer able to activate their CDK proteins substrates and thereby fail to induce cell cycle arrest [160].
CHK1/2 upregulation has been shown in GB, and inhibition is of interest, particularly in GBs with aberrations in other cell cycle regulating factors, such as p53, since these tumors rely on the remaining checkpoints to repair DNA damage. Approximately 50% of GB patients with CHK2 alterations also carry defects in the p53 signaling pathway, while this is only 10–13% for DDR components ATM, ATR, or CHK1 [9,24,161]. In GSCs, the basal expression of CHK1 and Cdc25C has also shown to be much higher compared to differentiated GB cells [100,161].
CHK1/2 inhibition has been extensively explored clinically in various cancer types but not yet in GB, likely because numerous CHK1/2i were discontinued before phase III, such as UCN-01 (7-hydroxystaurosporine), rabusertib (LY2603618) and MK-8776 (SCH 900776) [162,163,164,165,166,167,168]. AZD7762, for instance, showed severe cardiac toxicities in patients with advanced solid tumors (AST) [169]. Clinical trials are currently ongoing for CHK1-selective inhibitors CCT245737 (SRA737), GDC0575 (ARRY-575, RG7741), and the CDK1/2 inhibitor prexasertib (LY2606368). Prexasertib-related neutropenia has been identified as an adverse effect but warrants further development with clinical activity in ovarian cancer, squamous cell carcinoma, and advanced cancer types [170,171,172,173,174]. The CHK1i GDC-0425 or GDC-0575, given in combination with gemcitabine to solid tumor patients, both warrant further investigation [175,176].
CHK1i therapy of GB has remained in the preclinical setting. Treatment with gemcitabine and the CHK1i MK-8776 effectively permeated the BBB and inhibited glioma growth in vivo [177]. Moreover, UCN-01, although in itself non-toxic, increased the cytotoxicity of TMZ by five-fold in U87MG (p53 wild-type or deficient) glioma cells by accumulating the number of cells bypassing G2-M arrest and thereby undergoing mitotic catastrophe [178]. UCN-01 also inhibited GSC growth in vitro, and AZD7762 radiosensitized p53-mutated GB cell lines (confirmed in GB in vivo models) [179,180,181]. SAR-020106 sensitized human GB cells to RT, TMZ, and decitabine treatment [182]. The impact of CHK1 inhibition on GB cells was also studied using SB18078 and PF477736, confirming an influence on colony and tumor sphere formation, as well as cell proliferation. Khanna et al. also confirmed that CHK1 acts via protein phosphatase 2A in promoting GB cell growth [183]. Unfortunately, in AST, a phase I study on PF477736 combined with gemcitabine was terminated due to business reasons (NCT00437203) [29]. Interestingly, targeting the CHK1 gene in GSCs, using, for example, lentivirus-delivered short hairpin RNA (shRNA), also showed the potential to increase radiosensitivity via apoptosis induction [184].
Less research is performed on CHK2i in GB. It should be noted that while knockdown of CHK1 expression enhanced radiosensitivity of human GSCs, this was not the case upon CHK2 inhibition [184]. TMZ-induced cell death was also more prominently enhanced by pharmacologic inhibition of CHK1 compared to CHK2 inhibition [128]. However, the CHK2i PV1019 radiosensitized U251 glioma cells [12,185]. As an alternative to CHK1/2 inhibition, inhibition of their downstream targets CDK1/2 or Cdc25A protein phosphatase has been studied [186]. In our opinion, the multi-targeted MAPK inhibitor MEK162, which also inhibits CDK1/CDK2/WEE1/p-ATM besides CHK2, should be further explored since it downregulated and radiosensitized spheroidal and orthotopic GB xenografts [15].
3.2.2. CHK1/2 Radiopharmaceuticals
Therapeutic effects of CHK1/2 inhibition can be visualized using molecular imaging techniques such as PET or MRI. For example, radiosensitization effects after CHK1/2i therapy were visualized using diffusion-weighted MRI in GB models [181]. The proliferation PET tracer 3′-deoxy-3′-[18F]fluoro-thymidine ([18F]FLT) was also able to visualize antiproliferative effects in xenograft rodents following PF00477736 therapy [187]. Unfortunately, radiolabeled CHK1/2i are scarce. Both CHK1/2i prexasertib and CHK1i LY2603618 (rabusertib) were radiolabeled with carbon-14 to study their metabolism in advanced/metastatic solid tumor patients [60,91]. Also, [14C]-GDC-0425 was used to evaluate safety concerns of thiocyanate arising from GDC-0425 administration, but these proved to be negligible [90].
3.3. PARP Inhibitors
3.3.1. Current Status of PARP Targeted Therapy in GB
PARPi have shown significant promise in a variety of malignancies with deficiencies in HR signaling [34,188]. In GB, the BRCAness phenotype leads to impairment of HR and thus PARPi sensitivity [34]. Glioma biomarkers of predictive value for PARPi therapeutic efficacy include IDH1/2 mutations, a low BRCA1 expression, aberrant ATM or ATR signaling, MYC overexpression, and inactivation of mismatch repair genes, especially MSH6 [36,123,189,190,191,192,193,194]. PTEN mutations, present in 70% of GB tumors, have shown to increase the level of DSBs upon PARP inhibition, though some studies contradict this [195,196,197,198]. MGMT promoter hyper methylation is also being studied as a potentially predictive biomarker for PARPi-mediated TMZ sensitization [189]. TMZ-induced damage can be repaired by either direct repair (in case of O6-methylguanine lesions) or BER (in case of N7-methylguanine and N3-methyladenine lesions). Thus, inhibiting PARP-mediated SSB repair (BER) leads to the accumulation of DNA DSBs, thereby enhancing cytotoxicity [199,200]. This way, glioma patients may still benefit from alkylating chemotherapy, regardless of their MGMT promotor status [200,201,202]. Another mechanism of PARPi TMZ sensitization is allosteric PARP trapping (leading to instability of stalled replication forks), as well as BRCA1 and RAD51 depletion (leading to compromised fork protection) [189,203]. For more info on the combination effects of PARPi and chemotherapeutics, we refer the reader to [204]. Interestingly, cancers with BRCA-deficiency and PARPi resistance could also benefit from a combined therapy including CHKi and PARPi [174,205,206]. CHK2 inhibition might also provide a strategy to alleviate hematologic toxicity from PARPi [207].
Currently, four PARPi have been FDA-approved: olaparib (AZD2281 or KU0059436, Lynparza®, AstraZeneca); rucaparib (Rubraca®, Clovis Oncology, Boulder, CO, USA); talazoparib (Talzenna®, Pfizer, Manhattan, NY, USA); and lastly, niraparib (Zejula®, Tesaro, Waltham, MA, USA). A fifth PARPi, veliparib (ABT-888, Abbott Laboratories, Abbott Park, IL, USA), is expected to obtain approval in the near future, following promising phase III trial results in metastatic breast cancer [29,188]. In-depth reviews of the current status of PARPi as mono- or combination therapies for cancer were previously published [162,188,208].
Preclinically, olaparib delayed GB recurrence when combined with RT and sensitized IDH1-mutated tumor cells when combined with TMZ, leading to clinical trials in GB patients (Table 1) [29,36,209,210]. The phase I OPARATIC trial in recurrent GB patients confirmed that olaparib could be safely combined with daily TMZ if intermittent dosing was applied. Additionally, drug penetration into the entire tumor specimen was confirmed [129]. A phase I/II study in GB of olaparib combined TMZ/RT is currently recruiting [29,211,212].
The radiosensitizing effect of the PARPi veliparib (ABT-888) has been shown preclinically in GB, despite two studies proving otherwise [195,200,210,213,214,215,216,217,218,219,220]. The addition of veliparib to TMZ also prevented TMZ resistance, although this may not be achievable in a tolerable dosing regimen [191,221,222,223]. In recurrent GB previously treated with bevacizumab, the TMZ/veliparib combination did not significantly improve six-month progression-free survival [131,224]. Administering veliparib in combination with standard RT/TMZ was also not tolerable in GB patients and did not provide clinical benefit in unmethylated MGMT GB patients [130]. The MGMT-methylated GB patient population will be addressed in the Alliance A071102 trial (ClinicalTrials.gov Identifier: NCT02152982) [29]. Inhibition of ABCB1 and ABCG2 (drug efflux transporters expressed at the BBB) by elacridar may improve the efficacy of TMZ/veliparib therapy [196]. Combined veliparib/RT/TMZ is also being explored in malignant glioma patients without H3 K27M or BRAFV600 mutations (Table 1) [29].
Rucaparib has shown anti-GB effects in vitro, which were ameliorated when combined with BKM120 (PI3K inhibitor) or when conjugated to IR-786 (heptamethine cyanine dye) [225,226]. In combination with TMZ, rucaparib prolonged the time to tumor regrowth by 40% in heterotopic GB xenografts. However, this could not be confirmed in orthotopic GB models, most likely due to limited drug delivery [227]. Despite being FDA-approved for various cancer types, rucaparib has not yet been investigated in clinical trials for GB patients. In AST, however, rucaparib/TMZ was well-tolerated and showed proof-of-principle [29,228].
The PARPi talazoparib is FDA-approved for breast cancer, and a phase II trial on the talazoparib/carboplatin combination is currently recruiting recurrent high-grade glioma patients with DDR deficiency [29]. The combination of high and low LET radiation qualities with talazoparib led to promising preclinical results when administered to GSCs. Moreover, EFGR amplification might increase their sensitivity [229,230,231]. In vivo, talazoparib combined with TMZ prolonged GB stasis, but this could not be confirmed in orthotopic GB models, most likely due to BBB efflux mechanisms [232].
Niraparib (MK-4827) is currently being investigated in recurrent GB, either combined with RT or with tumor-treating fields (TTFs). TTFs are expected to reduce BRCA1 signaling and thereby reduce DNA repair capacity, causing PARPi-assisted synthetic lethality [29]. The first results on the niraparib/TMZ combination indicated tolerance and efficiency in patients with advanced cancer [132]. Notably, niraparib penetrated intracranial tumors in breast cancer models [233].
Other PARPi under investigation in GB include pamiparib (BGB-290, Partruvix™; BeiGene Ltd., Changping Qu, Beijing, China), E7016 and CEP-8983 (prodrug CEP-9722) [215,234,235]. Preclinically, pamiparib has shown strong anti-tumor synergism with TMZ and improved BBB penetration compared to other PARPi, which led to clinical trials, as outlined in Table 1 [29,197]. In a phase I trial in patients with solid tumors, CEP-9722 showed limited clinical activity [236].
Finally, it was shown that combined inhibition of PARPi and ATRi in GSCs resulted in a profound radiosensitization, which exceeded the effect of a single ATRi [100,141]. Multiple clinical trials are exploring this combination (olaparib/ceralasertib), including a phase II trial in IDH mutant solid tumors (ClinicalTrials.gov Identifier: NCT03878095) [29].
3.3.2. PARP Radiopharmaceuticals
Radiolabeled versions of PARPi strongly gained momentum in the last years due to their potential to directly and non-invasively image PARP expression, quantify the biodistribution of a PARPi and its tumor uptake, define treatment response and stratify patients likely to respond to PARPi therapy [199]. Due to the nuclear sub-cellular location of PARP and confirmed overexpression in GB, with overall low expression in healthy brain tissue, PARP-1 is also a near-ideal target to develop radiotherapeutics [56,188]. In addition to eliciting synthetic lethality, promoting genomic instability, and enhancing cytotoxicity of a subsequently administered DNA-damaging agent, PARP-TRT could also cause DNA damage [237]. In response to DNA damage, the expression of PARP-1 also increases, which may result in an increased target availability for the therapeutic radiopharmaceutical [50].
Most radiopharmaceuticals targeting PARP are structurally similar to small molecules olaparib ([18F]-BO, [18F]-PARPi, [18F]-20, [123I/131I]-PARPi, [18F]-PARPi-FL, [64Cu]-PARPi, [18F]-olaparib, [18F]-AZD2461, [18F]-AZD2281, [11C]-PJ34) or rucaparib ([18F]FTT), [18F]-WC-DZ-F, [18F]FE-LS-75, [125I]-KX1, [125I]-KX-02–019, [14C]-rucaparib, [211At]-MM4) [40,50,53,56,64,69,78,87,188]. These radiopharmaceuticals were recently reviewed [188,237,238]. Three of these PARP radiopharmaceuticals, namely [18F]-PARPi, [18F]FTT, and [14C]-rucaparib have reached the clinical setting (Table S2) [61,69,76,85].
[18F]-PARPi, was deemed well tolerable and safe in patients with head-and-neck cancer [61]. In GB mouse models, [18F]-PARPi and a bimodal fluorescence/PET imaging agent succeeded in visualizing the tumor [40,53,54]. Additionally, [18F]-PARPi has shown potential in discriminating active brain cancer from treatment-related changes in a murine model of radiation necrosis. This was confirmed in brain cancer patients, including three patients with IDH wild-type primary GB [76,77]. [18F]-PARPi-PET/MRI is currently being evaluated in a pilot study in recurrent brain tumors (ClinicalTrials.gov Identifier: NCT04173104) [29]. [18F]FTT is currently being investigated in phase I studies in various cancer types, including GB (Table S2) [29,85]. [18F]FTT-PET was, for example, performed to measure PARP-1 expression pre- and post-treatment with TTF and niraparib. Additionally, [18F]FTT uptake was correlated with HR deficiency status [29]. Unfortunately, early clinical results of [18F]FTT report low brain penetration and high uptake values in the liver and spleen [86]. In addition, regrettable results have also been reported for other olaparib-based radiopharmaceuticals. An absorption, metabolism, and excretion (ADME) analysis of [14C]-rucaparib, reported no brain uptake, and development of [18F]-20 was halted due to substantial defluorination [53,69].
PARP radiopharmaceuticals in a preclinical phase that are worth exploring in GB include [14C]-pamiparib and [64Cu]-DOTA-PARPi. The ADME of [14C]-pamiparib was evaluated in four patients with advanced cancer and indicated near-complete absorption and low renal clearance of the parent drug [74]. [64Cu]-DOTA-PARPi showed potential in mesothelioma-bearing animal models [64]. Notably, fluorinated radiopharmaceuticals based on talazoparib have been evaluated in a prostate cancer model and indicated TRT potential [65].
Therapeutic radiopharmaceuticals targeting PARP have been studied in GB preclinically with promising results. The first Auger-based theranostic PARPi, the Iodine-123 Meitner-Auger PARP1 inhibitor, successfully delivered a lethal payload within a 50 Å distance of the DNA of GB cancer cells and demonstrated a survival benefit in mouse models of GB [57,239,240]. [123I] -I2-PARPi retained within GB xenograft tumors and correlated with PARP expression [55]. Jannetti et al. developed [131I] -PARPi, a 1(2H)-phthalazinone with a similar structure to olaparib. Convection enhanced delivery of [131I] -PARPi led to increased survival of mice with orthotopic brain tumors [56]. Selective binding of 131I- and 124I-labelled I2-PARPi was also confirmed in GB models [58]. A particularly promising PARP-TRT agent is [211At]-MM4, a rucaparib derivative, due to its high cytotoxicity and favorable half-life (7.2 h). In neuroblastoma models, this compound resulted in increased survival [50].
3.4. DNA-PK Inhibitors
3.4.1. Current Status of DNA-PK Targeted Therapy in GB
DNA-PK consists of a heterodimer (Ku70/80) and a large catalytic subunit, known as DNA-PKcs [12,241]. This complex initiates NHEJ by binding to the DSB, leading to subsequent phosphorylation and activation of DNA-binding proteins, ultimately causing ligation of DSB ends (Figure 1) [10]. In GB, high DNA-PK levels correlate with poor survival and increased GSC stability [242,243]. DNA-PK has been shown to mediate GSC radioresistance and glioma progression in vivo, suggesting DNA-PK/RAD50 as promising targets for GSC eradication [244]. To date, research on biomarkers demonstrating DNA-PK inhibition sensitivity is only preclinical, but HR deficiency could theoretically predict sensitivity to DNA-PKi given the increased reliance of HR deficient cells on NHEJ [162]. Sun et al. identified p53 as a potential predictive biomarker of response to the combination of DNA-PKi and RT [245].
Small molecule inhibitors of DNA-PK, from the discovery of the first identified inhibitors (wortmannin and its derivatives PX-866 and PWT-458) to more selective DNA-PKi, have been reviewed [13,246,247]. The DNA-PKi VX-984 (Vertex, now licensed to Merck KGaA, Darmstadt, Germany as M9831), nedisertib (M3814, peposertib, MSC2490484A, Merck KGaA), and the recently discovered AZD7648, have entered clinical trials. Only nedisertib combined with RT/TMZ is currently under investigation for GB patients with unmethylated MGMT promotor status, following preclinical evidence of a radiosensitizing upon NHEJ inhibition [29,248]. In phase I trials for AST, this combination was well tolerated and demonstrated modest efficacy [249]. VX-984 has shown promising radiosensitizing effects in GB in vitro and in vivo with confirmed BBB crossing [250]. Interestingly, an inability to resolve γ-H2AX foci in the presence of VX-984 could be induced in T98G cells only [251]. Results on the safety of VX-984 administered in AST patients are still pending [29]. AZD7648 is undergoing clinical evaluation in AST after it showed RT/TMZ sensitizing effects and synergism with olaparib. However, this needs to be confirmed in GB [29,252,253].
Less selective DNA-PKi co-targeting mTOR include CC-115, avadomide (CC-122), samotolisib (LY3023414), and NVP-BEZ235. In GB patients, CC-115 was well tolerated, and 21% achieved a stable disease status with proven drug distribution in GB tissue [254,255]. CC-115 has shown a synergistically lethal effect with functional ATM loss and is included in one of the three experimental arms of the ongoing Individualized Screening Trial of Innovative GB Therapy (INSIGhT) trial [254,256]. Avadomide (CC-122) has recently been deemed safe in various cancer types, and applicability in CNS-related cancers was suggested. A phase I trial on avadomide in patients with advanced tumors unresponsive to standard therapies, including GB, is still active (ClinicalTrials.gov Identifier: NCT01421524) [257]. Samotolisib (LY3023414) had single-agent activity in advanced cancer patients and is being investigated further in pediatric CNS tumors. To be noted, BBB penetration remains a stumbling block [29,258,259,260].
Finally, based on preclinical data alone, SU11752, KU0060648, NU7026, and NU7441 have been put forward as glioma targeted drugs, either as single agents or in combination regimens (RT/TMZ/topoisomerase II inhibitors) [261,262,263,264,265].
3.4.2. DNA-PK Radiopharmaceuticals
Radiopharmaceuticals targeting DNA-PK are scarce. In healthy subjects, the disposition of the samotolisib derivative [14C]-LY3023414 following oral administration was studied; however, results are pending (ClinicalTrials.gov Identifier: NCT02575703) [29]. It should be noted that the uptake of radiolabeled LY3023414 would not be DNA-PK specific because it also targets PI3K/mTOR. Additionally, the radiosynthesis protocols for 11C-labelled chromen-4 derivatives as new potential DNA-PK-PET imaging radiopharmaceuticals were published by Gao et al. but have not yet been validated in vivo [73].
4. Development of Other DDR Radiopharmaceuticals
Besides DDRi radiopharmaceuticals themselves, radiotracers enabling the visualization and quantification of the amount of DNA damage induced after TRT would be extremely valuable, e.g., to assess the radiobiological treatment response of the tumor. This category includes γH2AX radiotracers: 89Zr-/111In-labelled anti-γH2AX-TAT. Anti-γH2AX antibodies are routinely used in ex vivo assays to quantify the number of γH2AX foci or DNA DSBs within cell populations, but a cell-penetrating peptide is required for in vivo applications [41,266]. For example, the extent of DNA damage response after [177Lu]-DOTATATE therapy was evaluated using [111In]-anti-γH2AX-TAT SPECT imaging [267].
5. Challenges and Risks of DDRi (Radio)Pharmaceuticals
Exploiting synthetic lethal interactions has attracted considerable attention as an anticancer strategy; however, the development of such approaches to selectively target cancer cells while sparing health tissues remains challenging [158]. Major hurdles include tumor biology, heterogeneity, and complexity; an inadequate understanding of synthetic lethal interactions; drug resistance, and the challenges regarding screening and clinical translation. Hence, there is an urgent need to develop improved efforts aiming to identify and understand synthetic lethal interactions, as well as validate new screening tools and biomarkers, including DDR radiopharmaceuticals. Improved genetic perturbation techniques, including CRISPR/Cas9 gene editing, are also promising prospects concerning synthetic lethal effects in cancer [268].
DDRi induced toxicity to healthy tissue can be limited due to the innate DDR pathways in healthy cells (Figure 1). The phenomenon of “replication stress”, unique to fast proliferating cancer cells, enforces this statement [16,42]. Unspecific cellular toxicity may occur since most DNA repair pathways overlap in terms of DNA repair proteins. This could lead to unwanted DNA damage to normal tissue, increasing the risk for late toxicity [158]. For example, ATM inhibition showed a greater radiosensitizing effect in p53-deficient tumors, but effects were also observed in p53 wild-type cells [111]. This might be an important consideration for proliferating cells of the CNS, where a p53-dependent G1/S checkpoint would stay at least partially activated in the presence of an ATMi (via ATR), thereby inducing cell cycle arrest and preventing apoptosis. In neurons, ATM seems to be a requirement for apoptosis. Hence, transient brain exposure to an ATMi might not be extremely toxic [111]. Upon PARP inhibition, toxicity to the normal brain is expected to be minimal since PARP-1 expression has not been detected in normal neurons [269]. Moreover, early-phase clinical trial data indicates that the radiosensitizing properties of PARPi are most pronounced in rapidly proliferating cells [212]. Hence, due to the non-dividing nature of neuronal tissue in the brain, it is assumed that the addition of a PARPi to RT would have a relatively larger effect on highly proliferative GB cells compared to normal brain cells [200]. The toxicity of PARPi is also related to their PARP trapping capacity, and reactivities differ with different combination partners and the DNA damage mutations present [204,270]. For example, the combination of PARPi with chemotherapy is hampered by overlapping toxicities, thereby limiting their administrable dose. Interestingly, hematologic toxicity seems more pronounced in germline BRCA carriers [270,271].
In the context of TRT, the combined toxicity of the cold DDRi with the radionuclide is important to consider. TRT toxicity can be related to targeting efficiency, radionuclide stability and the nuclear recoil effect, physical properties of the radionuclides, dosimetry, immunogenicity, and administration route [43]. Confirming the presence of the DDR target using an imaging DDR radiopharmaceutical (SPECT/PET) and evaluating its distribution throughout the body (before selecting TRT as a treatment strategy) is essential. Increased toxicity might be expected in case multiple DNA damaging strategies are combined. However, it should be noted that the concentration of the DDRi for targeted therapy will be markedly higher than the prospective dosage given of a radiolabeled DDRi during nuclear imaging or TRT. Following PARP-TRT, normal tissue toxicities in the spleen and bone marrow are projected due to PARP-1 expression in normal tissues. Other potential sites for toxicity include the liver and gastrointestinal tract if involved in the biological clearance of the compound [50].
Nuclear imaging strategies have shown the ability to measure expression levels of DDR kinases in vivo. However, when compared to the tumor uptake of radiopharmaceuticals targeting cancer biomarkers situated on the cell surface, uptake of these agents is generally low. Factors such as the transient nature of DDR protein activation (e.g., following RT, the expression levels of many biomarkers, including PARP-1 and γH2AX, disappear within days), the inefficient drug internalization/nuclear translocation, and specifically for GB applications, BBB crossing play a role [41]. In the case of alpha emitters, sub-cellular delivery to cell nuclei will increase the cytotoxicity due to the high probability that both the alpha particle and its atomic parent nuclei recoil radiation will traverse the cell nucleus [50]. As can be seen in Table S2, most of the DDRi investigated for TRT have been radiolabeled with halogens. Reaching the nucleus might be difficult upon radiometal chelation; however, some preclinical results showed promise. A Cu-64-radiolabeled olaparib analog containing a DOTA moiety resulted in clear tumor uptake in mesothelioma [64]. The nuclear uptake of a [177Lu]-DOTA-labeled DNA intercalator in Raji cells was deemed sufficient, although it was lower compared to total cellular uptake [272].
Treatment resistance is a bothersome limitation for the application of DDRi and DDR-based TRT. GB tumors relying on one DNA repair pathway for their survival may additionally hold mutations that cause resistance to certain DDRi [158]. For example, PARPi resistance may be induced by HR restoration or mitigation of replication stress. Identified biomarkers for PARPi resistance include loss of 53BP1/ARID1A, low level of Schlafen 11 (SLFN11) or GBP1, and genomic reversion of BRCA1/2. In addition, DNA replication fork protection (PTIP/EZH2) or genetic mutations that result in the activation of a drug efflux pump play a role. This highlights the need for functional biomarkers that can assess HR proficiency, predict DDRi effectiveness, and the need for a combined treatment strategy (e.g., PARPi with other DDRi or TKIs) [30,162,208,273,274]. Unfortunately, due to the limited number of clinical trials involving ATMi/ATRi/CHK1i/DNA-PKi, biomarkers indicating resistance to these DDRi are largely unknown. A few examples include: PGBD5 and Cdc25A depletion have been associated with ATRi resistance, and overexpression of ATP-binding cassette G2 (ABCG2) increased CC-115 resistance [275,276,277,278].
6. Selection of New GB Radiopharmaceuticals Targeting the DDR
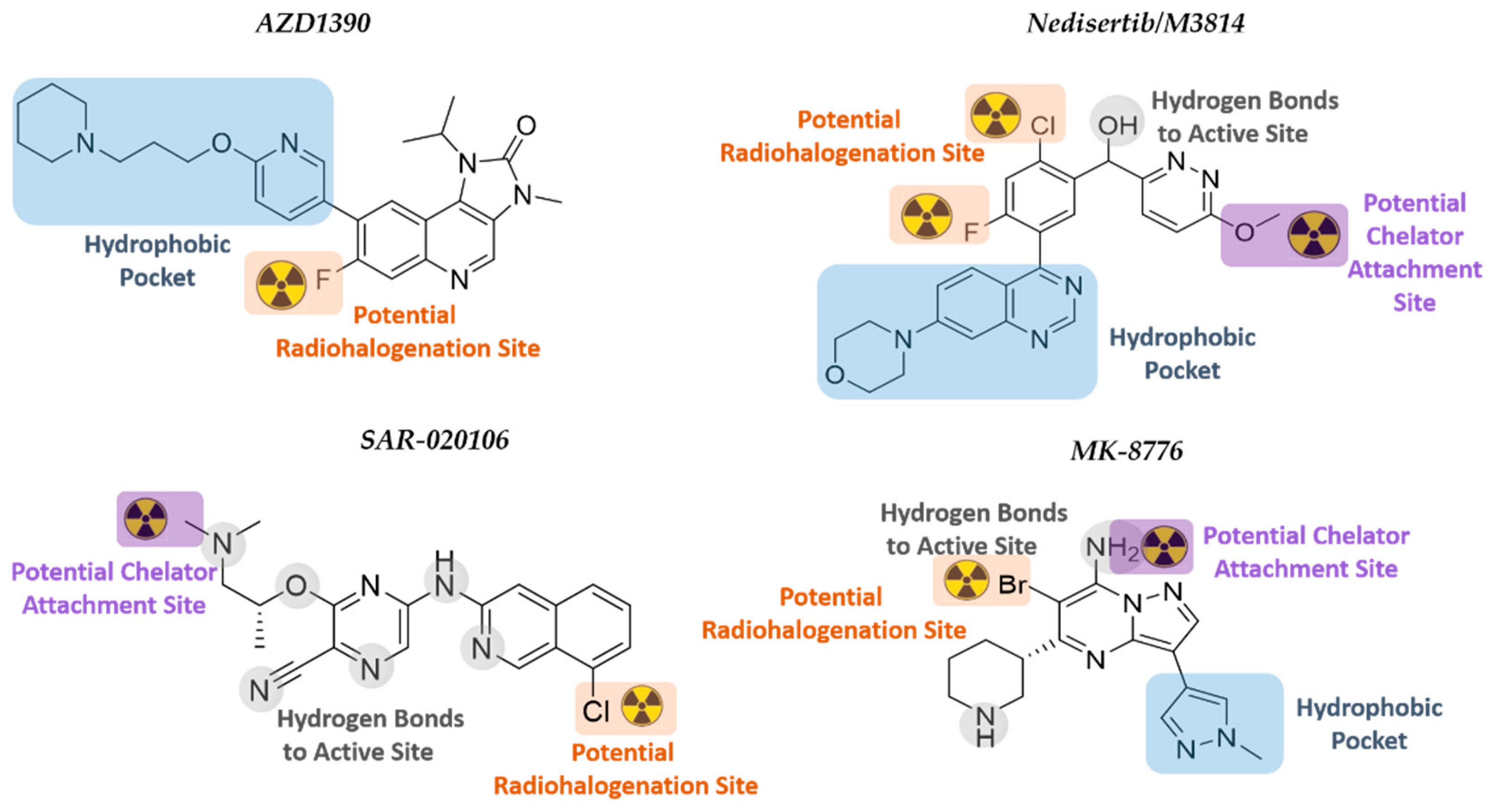
In order to select suitable candidate radiopharmaceuticals capable of targeting DDR kinases for GB imaging and therapy, several factors need to be considered, such as biochemical and pharmacological characteristics, radiolabeling, and radionuclide half-life options, and the ability to cross the BBB. The latter is affected by the molecular weight, lipophilicity, polar surface area, and hydrogen bond donors of the inhibitor [43]. In order to identify those DDRi that have the potential to become suitable GB TRT agents, in-house selection criteria were applied to all the above mentioned DDRi studied in GB (listed in Table 2). Thereby four DDRi are suggested that could potentially be converted into novel TRT radiopharmaceuticals: AZD1390, Nedisertib (M3814), SAR-020106, and MK8776 (Figure 6).

Table 2.
Selection criteria for assessment of candidate GB DDRi TRT agents.
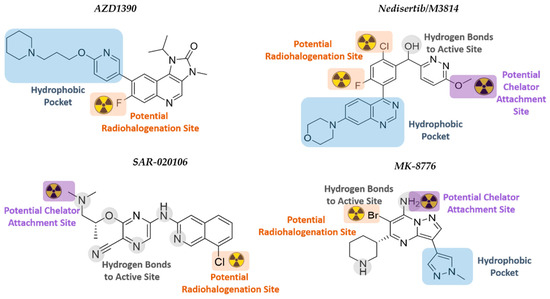
Figure 6.
Chemical structures of the selected DDRi. The structure–activity relationship and potential radionuclide attachment sites are indicated.
These DDRi contain a halogen in an aryl position that could be a designated location for radiohalogenation, for example, using iodine-125 (Auger emitter), iodine-131 (beta emitter), or astatine-211 (alpha-particle emitter); and/or qualify for insertion of a chelator substituent to harbor a therapeutic radiometal.
Nucleophilic halogen exchange (iodine for iodine) reactions are regularly used for the incorporation of radioiodine into organic molecules, with inorganic salts (ammonium sulfate) or copper (II) salts often being added to catalyze the iodine exchange. Notably, a naturally stable isotope of astatine does not exist, and, therefore, halogen exchange using astatine-211 would require the iodo- or bromo- derivatives [279]. However, this approach is unable to yield a pure, astatinated product since the unreacted iodo- or bromo-starting compounds cannot be removed. Therefore, astatination reactions generally occur through electrophilic substitution reactions in the presence of oxidants, with a new method developed using the substitution of a dihydroxyboryl group [279,280]. When radiohalogenating the abovementioned DDRi, the effect that the larger halogen will have on the modified molecule may also result in altered biological properties.
The consideration of using a chelator would be that the increase in size, molecular weight, and the possible change in overall charge of the inhibitor could affect pharmacological properties (lipophilicity, metabolism, biological half-life, target binding), and especially for GB targeting, the BBB crossing [43]. Attachment of chelators to biomolecules is generally carried out through a nucleophilic reaction between a bifunctional chelating agent and a primary amine. Insertion of a chelator into the structure of a DDRi would require the replacement of the substituent on an N- or O-atom with a functionalized chelating agent through a variety of available reactions.
6.1. ATMi AZD1390
AZD1390, developed by AstraZeneca, is a highly potent ATMi (10.000-fold more specific for ATM than for other PIKK members) that blocks ATM autophosphorylation at Ser-1981 and phosphorylation of KAP1 at Ser-824 [281]. AZD1390 has been converted to a 11C-radiolabeled drug that showed good BBB penetration (1% ID at Tmax[brain] = 21 min) in healthy volunteers. Results on the aspects of safety, tolerability, and pharmacokinetics of [11C]-AZD1390 in combination with RT are expected by 2024 [29,59]. The fast localization of AZD1390 to the brain limits the use of AZD1390 in TRT to those therapeutic radionuclides that match its biodistribution characteristics. AZD1390 has a piperidine moiety, an isopropyl moiety, and fluorine at the ortho-position of ring two. There are no crystal structures of ATM reported to date, and the ATM model developed by Degorce et al. was used in this review for SAR rationalization [282]. SAR studies have reported the need for the 4-amino and 3-carboxamide derivative within the structure, as well as the importance of the internal hydrogen bond that is formed between this moiety and the bioactive conformation of ATM [282]. It has a potential radiohalogenation site at the fluoride atom at the ortho-position of ring two. Direct radiohalogenation can potentially add a therapeutic radioiodine or radioastatine to that position. A chelator could possibly replace the piperidinyl moiety; however, it sits within the hydrophobic pocket and would most likely affect binding [75,283].
6.2. DNA-PKi Nedisertib (M3814)
Nedisertib (M3814, Peposertib, MSC2490484A), developed by BioVision Inc., Milpitas, CA, USA is an orally bioavailable, highly potent, and selective DNA-PKi. Nedisertib was well-tolerated as monotherapy in AST patients, and two clinical trials are currently evaluating nedisertib (peposertib) in combination with chemo/RT. The maximum systemic concentration of nedisertib occurred between 1–2 h after administration. The BBB penetration capabilities are still under investigation (CilinicalTrials.gov Identifier: NCT04555577). Based on the structural interactions between nedisertib and the active site of the DNA-PK, both the quinazoline and morpholino moieties bind into the hydrophobic pocket, while the pyridazine ring rotates to have π-π interactions with the quinazoline plane [284]. The chloro-fluorobenzene ring in the active site is directed towards the N-lobe, thus potentially allowing radiohalogenation at positions one and three of the ring. However, it is noted that the fluorine points towards the hydrophobic pocket, and thus, radioiodination or radioastatination at this position might not be feasible. The binding model further indicates that the methoxy group on the pyridazine ring is orientated outwards towards the solvent region. The methyl group could potentially be amended to a longer alkyl chain that will extend further into the solvent area and be functionalized with a chelator group in the terminal position. A chelator would be able to complex metallic radioisotopes for TRT.
6.3. CHK1i SAR-020106 and MK-8776 (SCH900776)
The kinase domain of CHK1/2 consists of an N- and C-terminal lobe with a hinge region connecting the two lobes. The hinge forms the ATP-binding pocket, and the majority of CHK1i will compete with ATP for binding to this site. Inhibitors bind through hydrogen bonding to peptides (typically Glu-85, Tyr-86, and Cys-87), as well as peptide-bound water within the active site. Generally, polar substituents of the inhibitors are orientated into the ribose pocket, with more lipophilic groups being directed toward the surface where the hinge cleft opens to the solvent. A substituent projecting into the solvent area could be modified with more hydrophilic groups in order to improve inhibitor pharmacokinetics [163].
SAR-020106 is a highly selective and potent inhibitor of CHK1 (IC50 of 13 nmol/L; >7 000-fold selectivity over CHK2) that is still in its preclinical phase. Although SAR-0020106 is highly bound (94%) to plasma proteins, the tumor drug accumulation within 24 h is significant, with tumor/plasma ratios of 47:1 and 85:1 after 6 h and 24 h, respectively [285]. SAR-020106 is structurally classified into the ‘pyrazine scaffold’ inhibitor group, with an ether-linked ethylamine substituent on a cyanopyrazine ring connected to a chlorinated isoquinoline [163]. The cyanopyrazine group significantly interacts with Lys-38 and the protein-bound water network within the active site, while the isoquinoline nitrogen and secondary amine connect with Cys-87 and Glu-85, respectively. The chloro-group on the isoquinoline indicates a potential position for radiohalogenation using radioiodine or –astatine since this chlorine atom is not involved in active site interaction. The nitrogen atom of the tertiary amine side chain of SAR-020106 also binds to water within the protein active site, but this amine has two methyl groups, one of which could potentially be substituted with a longer alkyl chain extending into the solvent region. A lengthened alkyl chain should not drastically affect the hydrogen bonding of the amine and would potentially allow for the insertion of a chelating group at the end of the hydrocarbon chain. The chelator could then be used for the complexation of therapeutic metal isotopes, such as lutetium-177, for TRT.
MK-8776 (SCH900776), developed by Merck KGaA, is another highly selective and potent inhibitor of CHK1 (IC50 of 3 nmol/L) that is currently in phase I/II clinical trials for various cancers but has only been tested preclinically for GB therapy [286,287]. These studies have indicated that MK-8776 enhances cellular susceptibility to chemotherapeutic agents, such as gemcitabine and hydroxyurea [288]. The BBB penetration of MK-8776 is currently unknown, but the drug is 49% plasma protein bound with a plasma half-life of 5.6–9.8 h [164]. The structural scaffold for MK-8776 is pyrazolo[1,5-a]pyrimidine functionalized with a piperidine and 1-methyl-pyrazole ring [163,289]. MK-8776 binds to the hinge region of the kinase ATP-binding site through N1 and C7-NH2 of the pyrazolo[1,5-a]pyrimidine core, while the nitrogen of the 1-methyl pyrazole is within the interior pocket bound to water. The piperidine nitrogen atom is hydrogen-bonded to Glu-91 and the amide carbonyl of Glu-134 in the ribose pocket. Position C6 of the pyrazolo[1,5-a]pyrimidine is functionalized with bromine which could potentially be converted to a therapeutic radiohalogen. Although the C7 primary amine of MK-8776 is involved in binding to the active site, similar compounds with a secondary amine in this position that were investigated prior to the development of the clinical candidate also indicated very selective and strong inhibition of CHK1 [163]. Therefore, alkylation of the C7-amine with a chelator-functionalized alkyl chain (to harbor therapeutic metal radionuclides) will convert MK-8776 into a TRT-radiopharmaceutical.
7. Conclusions
DDR kinases are attractive targets to promote DNA damage and DNA replication stress and to render GB cells more vulnerable to RT and TMZ, following the principle of synthetic lethality. The current DDRi targeting ATM/ATR, PARP, CHK1/2, and DNA-PK for the treatment of GB and a perspective and overview on potential radiolabeling options for those small molecules are presented. Despite the hurdles of GB heterogeneity and drug resistance, radiopharmaceuticals targeting DDR kinases have the potential to stratify patients for DDRi therapy, predict response to DNA damaging treatments and guide TRT agents to the nucleus of GB cells, ultimately increasing therapeutic effectiveness. This review revealed that only a limited number of developed DDRi have been explored for their TRT potential. Through the application of relevant selection criteria, four DDRi compounds were identified that could potentially be converted into novel TRT radiopharmaceuticals: AZD1390, Nedisertib (M3814), SAR-020106, and MK8776. Radiopharmaceutical development of these candidates may greatly influence a more tailored and personalized GB therapy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14071821/s1, Table S1: Overview of cancer clinical trials of ATMi and ATRi, Table S2: Cancer radiopharmaceuticals targeting DDR kinases.
Author Contributions
Conceptualization, J.B.; writing—original draft preparation, L.E., J.B., S.N., C.H.S.D., T.E. and C.V.; writing—review and editing, J.B., L.E., C.V., T.E., S.N., C.H.S.D., M.M.S. and I.G.; final approval of the version published, L.E., J.B., S.N., C.H.S.D., T.E., M.M.S., I.G. and C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in the article and Supplementary Material.
Acknowledgments
Biorender (https://biorender.com/, accessed on 5 January 2022 was used for creating the images.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Caragher, S.P.; Sachdev, S.; Ahmed, A. Radiotherapy and Glioma Stem Cells: Searching for Chinks in Cellular Armor. Curr. Stem Cell Rep. 2017, 3, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Huse, J.T.; Holland, E.C. Targeting brain cancer: Advances in the molecular pathology of malignant glioma and medulloblastoma. Nat. Rev. Cancer 2010, 10, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. A Critical Overview of Targeted Therapies for Glioblastoma. Front. Oncol. 2018, 8, 419. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Tonn, J.C.; Stupp, R.; Preusser, M.; Cohen-Jonathan-Moyal, E.; Henriksson, R.; Le Rhun, E.; Balana, C.; Chinot, O.; et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017, 18, e315–e329. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef]
- Touat, M.; Idbaih, A.; Sanson, M.; Ligon, K.L. Glioblastoma targeted therapy: Updated approaches from recent biological insights. Ann. Oncol. 2017, 28, 1457–1472. [Google Scholar] [CrossRef]
- Le Rhun, E.; Preusser, M.; Roth, P.; Reardon, D.A.; van den Bent, M.; Wen, P.; Reifenberger, G.; Weller, M. Molecular targeted therapy of glioblastoma. Cancer Treat. Rev. 2019, 80, 101896. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Ferri, A.; Stagni, V.; Barilà, D. Targeting the DNA Damage Response to Overcome Cancer Drug Resistance in Glioblastoma. Int. J. Mol. Sci. 2020, 21, 4910. [Google Scholar] [CrossRef]
- Li, L.-Y.; Guan, Y.-D.; Chen, X.-S.; Yang, J.-M.; Cheng, Y. DNA Repair Pathways in Cancer Therapy and Resistance. Front. Pharmacol. 2021, 11, 2520. [Google Scholar] [CrossRef] [PubMed]
- Velic, D.; Couturier, A.M.; Ferreira, M.T.; Rodrigue, A.; Poirier, G.G.; Fleury, F.; Masson, J.Y. DNA Damage Signalling and Repair Inhibitors: The Long-Sought-After Achilles’ Heel of Cancer. Biomolecules 2015, 5, 3204–3259. [Google Scholar] [CrossRef]
- Huang, R.X.; Zhou, P.K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal. Transduct. Target. Ther. 2020, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Lanz, M.C.; Dibitetto, D.; Smolka, M.B. DNA damage kinase signaling: Checkpoint and repair at 30 years. EMBO J. 2019, 38, e101801. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.S.; Gasol, A.; Slangen, P.L.G.; Cornelissen, F.M.G.; Lagerweij, T.; Veldman, H.; Dik, R.; van den Berg, J.; Slotman, B.J.; Würdinger, T.; et al. Identification of MEK162 as a Radiosensitizer for the Treatment of Glioblastoma. Mol. Cancer Ther. 2018, 17, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dai, Q.; Park, D.; Deng, X. Targeting DNA Replication Stress for Cancer Therapy. Genes 2016, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Ou, A.; Yung, W.K.A.; Majd, N. Molecular Mechanisms of Treatment Resistance in Glioblastoma. Int. J. Mol. Sci. 2020, 22, 351. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. The DNA damage response and cancer therapy. Nature 2012, 481, 287–294. [Google Scholar] [CrossRef]
- Reuvers, T.G.A.; Kanaar, R.; Nonnekens, J. DNA Damage-Inducing Anticancer Therapies: From Global to Precision Damage. Cancers 2020, 12, 2098. [Google Scholar] [CrossRef]
- Zhang, J.; Stevens, M.F.; Bradshaw, T.D. Temozolomide: Mechanisms of action, repair and resistance. Curr. Mol. Pharmacol. 2012, 5, 102–114. [Google Scholar] [CrossRef]
- Shibata, A.; Jeggo, P.; Löbrich, M. The pendulum of the Ku-Ku clock. DNA Repair 2018, 71, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Helleday, T.; Petermann, E.; Lundin, C.; Hodgson, B.; Sharma, R.A. DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer 2008, 8, 193–204. [Google Scholar] [CrossRef]
- Squatrito, M.; Brennan, C.W.; Helmy, K.; Huse, J.T.; Petrini, J.H.; Holland, E.C. Loss of ATM/Chk2/p53 pathway components accelerates tumor development and contributes to radiation resistance in gliomas. Cancer Cell 2010, 18, 619–629. [Google Scholar] [CrossRef]
- Pappula, A.L.; Rasheed, S.; Mirzaei, G.; Petreaca, R.C.; Bouley, R.A. A Genome-Wide Profiling of Glioma Patients with an IDH1 Mutation Using the Catalogue of Somatic Mutations in Cancer Database. Cancers 2021, 13, 4299. [Google Scholar] [CrossRef]
- Núñez, F.J.; Mendez, F.M.; Kadiyala, P.; Alghamri, M.S.; Savelieff, M.G.; Garcia-Fabiani, M.B.; Haase, S.; Koschmann, C.; Calinescu, A.-A.; Kamran, N.; et al. IDH1-R132H acts as a tumor suppressor in glioma via epigenetic up-regulation of the DNA damage response. Sci. Transl. Med. 2019, 11, eaaq1427. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Benitez, J.A.; Li, J.; Miki, S.; Ponte de Albuquerque, C.; Galatro, T.; Orellana, L.; Zanca, C.; Reed, R.; Boyer, A.; et al. Inhibition of Nuclear PTEN Tyrosine Phosphorylation Enhances Glioma Radiation Sensitivity through Attenuated DNA Repair. Cancer Cell 2019, 35, 504–518. [Google Scholar] [CrossRef]
- Kaina, B.; Ochs, K.; Grösch, S.; Fritz, G.; Lips, J.; Tomicic, M.; Dunkern, T.; Christmann, M. BER, MGMT, and MMR in defense against alkylation-induced genotoxicity and apoptosis. Prog. Nucleic. Acid Res. Mol. Biol. 2001, 68, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov. Available online: https://clinicaltrials.gov/ct2/home (accessed on 7 December 2021).
- Cleary, J.M.; Aguirre, A.J.; Shapiro, G.I.; D’Andrea, A.D. Biomarker-Guided Development of DNA Repair Inhibitors. Mol. Cell 2020, 78, 1070–1085. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. BRCAness revisited. Nat. Rev. Cancer 2016, 16, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Knijnenburg, T.A.; Wang, L.; Zimmermann, M.T.; Chambwe, N.; Gao, G.F.; Cherniack, A.D.; Fan, H.; Shen, H.; Way, G.P.; Greene, C.S.; et al. Genomic and Molecular Landscape of DNA Damage Repair Deficiency across The Cancer Genome Atlas. Cell Rep. 2018, 23, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Xavier, M.A.; Rezende, F.; Titze-de-Almeida, R.; Cornelissen, B. BRCAness as a Biomarker of Susceptibility to PARP Inhibitors in Glioblastoma Multiforme. Biomolecules 2021, 11, 1188. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Cai, J.; Tan, Z.; Meng, X.; Li, R.; Li, Y.; Jiang, C. Mutant IDH1 Enhances Temozolomide Sensitivity via Regulation of the ATM/CHK2 Pathway in Glioma. Cancer Res. Treat. 2021, 53, 367–377. [Google Scholar] [CrossRef]
- Lu, Y.; Kwintkiewicz, J.; Liu, Y.; Tech, K.; Frady, L.N.; Su, Y.T.; Bautista, W.; Moon, S.I.; MacDonald, J.; Ewend, M.G.; et al. Chemosensitivity of IDH1-Mutated Gliomas Due to an Impairment in PARP1-Mediated DNA Repair. Cancer Res. 2017, 77, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Noorbakhsh, S.I.; Sundaram, R.K.; Kalathil, A.N.; Ganesa, S.; Jia, L.; Breslin, H.; Burgenske, D.M.; Gilad, O.; Sarkaria, J.N.; et al. Temozolomide Sensitizes MGMT-Deficient Tumor Cells to ATR Inhibitors. Cancer Res. 2019, 79, 4331–4338. [Google Scholar] [CrossRef] [PubMed]
- Färkkilä, A.; Gulhan, D.C.; Casado, J.; Jacobson, C.A.; Nguyen, H.; Kochupurakkal, B.; Maliga, Z.; Yapp, C.; Chen, Y.A.; Schapiro, D.; et al. Immunogenomic profiling determines responses to combined PARP and PD-1 inhibition in ovarian cancer. Nat. Commun. 2020, 11, 1459. [Google Scholar] [CrossRef]
- Gulhan, D.C.; Lee, J.J.; Melloni, G.E.M.; Cortés-Ciriano, I.; Park, P.J. Detecting the mutational signature of homologous recombination deficiency in clinical samples. Nat. Genet. 2019, 51, 912–919. [Google Scholar] [CrossRef]
- Carlucci, G.; Carney, B.; Sadique, A.; Vansteene, A.; Tang, J.; Reiner, T. Evaluation of [(18)F]-ATRi as PET tracer for in vivo imaging of ATR in mouse models of brain cancer. Nucl. Med. Biol. 2017, 48, 9–15. [Google Scholar] [CrossRef]
- Knight, J.C.; Koustoulidou, S.; Cornelissen, B. Imaging the DNA damage response with PET and SPECT. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Vidal, A.; Bonnemaire, C.; Kraeber-Bodéré, F.; Chérel, M.; Pallardy, A.; Rousseau, C.; Garcion, E.; Lacoeuille, F.; Hindré, F.; et al. Potential for Nuclear Medicine Therapy for Glioblastoma Treatment. Front Pharmacol. 2019, 10, 772. [Google Scholar] [CrossRef] [PubMed]
- Bolcaen, J.; Kleynhans, J.; Nair, S.; Verhoeven, J.; Goethals, I.; Sathekge, M.; Vandevoorde, C.; Ebenhan, T. A perspective on the radiopharmaceutical requirements for imaging and therapy of glioblastoma. Theranostics 2021, 11, 7911–7947. [Google Scholar] [CrossRef] [PubMed]
- Puttemans, J.; Lahoutte, T.; D’Huyvetter, M.; Devoogdt, N. Beyond the Barrier: Targeted Radionuclide Therapy in Brain Tumors and Metastases. Pharmaceutics 2019, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Ersahin, D.; Doddamane, I.; Cheng, D. Targeted radionuclide therapy. Cancers 2011, 3, 3838–3855. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Roeske, J.C.; McDevitt, M.R.; Palm, S.; Allen, B.J.; Fisher, D.R.; Brill, A.B.; Song, H.; Howell, R.W.; Akabani, G.; et al. MIRD Pamphlet No. 22 (abridged): Radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J. Nucl. Med. 2010, 51, 311–328. [Google Scholar] [CrossRef]
- Zalutsky, M.R.; Reardon, D.A.; Akabani, G.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; McLendon, R.E.; Wong, T.Z.; Bigner, D.D. Clinical experience with alpha-particle emitting 211At: Treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J. Nucl. Med. 2008, 49, 30–38. [Google Scholar] [CrossRef]
- Królicki, L.; Bruchertseifer, F.; Kunikowska, J.; Koziara, H.; Pawlak, D.; Kuliński, R.; Rola, R.; Merlo, A.; Morgenstern, A. Dose escalation study of targeted alpha therapy with [(225)Ac]Ac-DOTA-substance P in recurrence glioblastoma-safety and efficacy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3595–3605. [Google Scholar] [CrossRef]
- Reilly, S.W.; Makvandi, M.; Xu, K.; Mach, R.H. Rapid Cu-Catalyzed [(211)At]Astatination and [(125)I]Iodination of Boronic Esters at Room Temperature. Org. Lett. 2018, 20, 1752–1755. [Google Scholar] [CrossRef]
- Makvandi, M.; Lee, H.; Puentes, L.N.; Reilly, S.W.; Rathi, K.S.; Weng, C.C.; Chan, H.S.; Hou, C.; Raman, P.; Martinez, D.; et al. Targeting PARP-1 with Alpha-Particles Is Potently Cytotoxic to Human Neuroblastoma in Preclinical Models. Mol. Cancer Ther. 2019, 18, 1195–1204. [Google Scholar] [CrossRef]
- Pirovano, G.; Wilson, T.C.; Reiner, T. Auger: The future of precision medicine. Nucl. Med. Biol. 2021, 96, 50–53. [Google Scholar] [CrossRef]
- Carlucci, G.; Carney, B.; Brand, C.; Kossatz, S.; Irwin, C.P.; Carlin, S.D.; Keliher, E.J.; Weber, W.; Reiner, T. Dual-Modality Optical/PET Imaging of PARP1 in Glioblastoma. Mol. Imaging Biol. 2015, 17, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Carney, B.; Carlucci, G.; Salinas, B.; Di Gialleonardo, V.; Kossatz, S.; Vansteene, A.; Longo, V.A.; Bolaender, A.; Chiosis, G.; Keshari, K.R.; et al. Non-invasive PET Imaging of PARP1 Expression in Glioblastoma Models. Mol. Imaging Biol. 2016, 18, 386–392. [Google Scholar] [CrossRef]
- Zmuda, F.; Blair, A.; Liuzzi, M.C.; Malviya, G.; Chalmers, A.J.; Lewis, D.; Sutherland, A.; Pimlott, S.L. An (18)F-Labeled Poly(ADP-ribose) Polymerase Positron Emission Tomography Imaging Agent. J. Med. Chem. 2018, 61, 4103–4114. [Google Scholar] [CrossRef] [PubMed]
- Zmuda, F.; Malviya, G.; Blair, A.; Boyd, M.; Chalmers, A.J.; Sutherland, A.; Pimlott, S.L. Synthesis and Evaluation of a Radioiodinated Tracer with Specificity for Poly(ADP-ribose) Polymerase-1 (PARP-1) in Vivo. J. Med. Chem. 2015, 58, 8683–8693. [Google Scholar] [CrossRef] [PubMed]
- Jannetti, S.A.; Carlucci, G.; Carney, B.; Kossatz, S.; Shenker, L.; Carter, L.M.; Salinas, B.; Brand, C.; Sadique, A.; Donabedian, P.L.; et al. PARP-1-Targeted Radiotherapy in Mouse Models of Glioblastoma. J. Nucl. Med. 2018, 59, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Pirovano, G.; Jannetti, S.A.; Carter, L.M.; Sadique, A.; Kossatz, S.; Guru, N.; Demétrio De Souza França, P.; Maeda, M.; Zeglis, B.M.; Lewis, J.S.; et al. Targeted Brain Tumor Radiotherapy Using an Auger Emitter. Clin. Cancer Res. 2020, 26, 2871–2881. [Google Scholar] [CrossRef]
- Salinas, B.; Irwin, C.P.; Kossatz, S.; Bolaender, A.; Chiosis, G.; Pillarsetty, N.; Weber, W.A.; Reiner, T. Radioiodinated PARP1 tracers for glioblastoma imaging. EJNMMI Res. 2015, 5, 123. [Google Scholar] [CrossRef]
- Jucaite, A.; Stenkrona, P.; Cselényi, Z.; De Vita, S.; Buil-Bruna, N.; Varnäs, K.; Savage, A.; Varrone, A.; Johnström, P.; Schou, M.; et al. Brain exposure of the ATM inhibitor AZD1390 in humans-a positron emission tomography study. Neuro Oncol. 2021, 23, 687–696. [Google Scholar] [CrossRef]
- Wickremsinhe, E.R.; Hynes, S.M.; Payne, C.D.; Guo, Y.; Cassidy, K.C. Disposition of [(14)C]LY2606368 following intravenous administration in patients with advanced and/or metastatic solid tumours. Xenobiotica 2020, 50, 793–804. [Google Scholar] [CrossRef]
- Schöder, H.; França, P.D.S.; Nakajima, R.; Burnazi, E.; Roberts, S.; Brand, C.; Grkovski, M.; Mauguen, A.; Dunphy, M.P.; Ghossein, R.A.; et al. Safety and Feasibility of PARP1/2 Imaging with (18)F-PARPi in Patients with Head and Neck Cancer. Clin. Cancer Res. 2020, 26, 3110–3116. [Google Scholar] [CrossRef]
- Guibbal, F.; Hopkins, S.L.; Pacelli, A.; Isenegger, P.G.; Mosley, M.; Torres, J.B.; Dias, G.M.; Mahaut, D.; Hueting, R.; Gouverneur, V.; et al. [(18)F]AZD2461, an Insight on Difference in PARP Binding Profiles for DNA Damage Response PET Imaging. Mol. Imaging Biol. 2020, 22, 1226–1234. [Google Scholar] [CrossRef]
- Reilly, S.W.; Puentes, L.N.; Schmitz, A.; Hsieh, C.J.; Weng, C.C.; Hou, C.; Li, S.; Kuo, Y.M.; Padakanti, P.; Lee, H.; et al. Synthesis and evaluation of an AZD2461 [(18)F]PET probe in non-human primates reveals the PARP-1 inhibitor to be non-blood-brain barrier penetrant. Bioorg. Chem. 2019, 83, 242–249. [Google Scholar] [CrossRef]
- Huang, T.; Hu, P.; Banizs, A.B.; He, J. Initial evaluation of Cu-64 labeled PARPi-DOTA PET imaging in mice with mesothelioma. Bioorg. Med. Chem. Lett. 2017, 27, 3472–3476. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Chen, H.; Mpoy, C.; Afrin, S.; Rogers, B.E.; Garbow, J.R.; Katzenellenbogen, J.A.; Xu, J. Radiosynthesis and Evaluation of Talazoparib and Its Derivatives as PARP-1-Targeting Agents. Biomedicines 2021, 9, 565. [Google Scholar] [CrossRef] [PubMed]
- Sander Effron, S.; Makvandi, M.; Lin, L.; Xu, K.; Li, S.; Lee, H.; Hou, C.; Pryma, D.A.; Koch, C.; Mach, R.H. PARP-1 Expression Quantified by [(18)F]FluorThanatrace: A Biomarker of Response to PARP Inhibition Adjuvant to Radiation Therapy. Cancer Biother. Radiopharm 2017, 32, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, C.E.; Makvandi, M.; Lieberman, B.P.; Xu, K.; Zeng, C.; Li, S.; Hou, C.; Lee, H.; Greenberg, R.A.; Mankoff, D.A.; et al. [(18)F]FluorThanatrace uptake as a marker of PARP1 expression and activity in breast cancer. Am J. Nucl. Med. Mol. Imaging 2016, 6, 94–101. [Google Scholar] [PubMed]
- Lee, H.; Riad, A.; Martorano, P.; Mansfield, A.; Samanta, M.; Batra, V.; Mach, R.H.; Maris, J.M.; Pryma, D.A.; Makvandi, M. PARP-1-Targeted Auger Emitters Display High-LET Cytotoxic Properties In Vitro but Show Limited Therapeutic Utility in Solid Tumor Models of Human Neuroblastoma. J. Nucl. Med. 2020, 61, 850–856. [Google Scholar] [CrossRef]
- Liao, M.; Watkins, S.; Nash, E.; Isaacson, J.; Etter, J.; Beltman, J.; Fan, R.; Shen, L.; Mutlib, A.; Kemeny, V.; et al. Evaluation of absorption, distribution, metabolism, and excretion of [(14)C]-rucaparib, a poly(ADP-ribose) polymerase inhibitor, in patients with advanced solid tumors. Investig. New Drugs 2020, 38, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, M.; Xu, K.; Lieberman, B.P.; Anderson, R.C.; Effron, S.S.; Winters, H.D.; Zeng, C.; McDonald, E.S.; Pryma, D.A.; Greenberg, R.A.; et al. A Radiotracer Strategy to Quantify PARP-1 Expression In Vivo Provides a Biomarker That Can Enable Patient Selection for PARP Inhibitor Therapy. Cancer Res. 2016, 76, 4516–4524. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.; Makvandi, M.; Xu, K.; Lieberman, B.P.; Zeng, C.; Pryma, D.A.; Mach, R.H. Iodinated benzimidazole PARP radiotracer for evaluating PARP1/2 expression in vitro and in vivo. Nucl. Med. Biol. 2016, 43, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Riss, P.J.; Soskic, V.; Schrattenholz, A.; Roesch, F. Synthesis and radiosynthesis of N5-[18F]fluoroethyl-pirenzepine and its metabolite N5-[18F]fluoroethyl-LS 75. J. Label. Compd. Radiopharm 2009, 52, 576–579. [Google Scholar] [CrossRef]
- Gao, M.; Wang, M.; Miller, K.D.; Zheng, Q.H. Simple synthesis of carbon-11-labeled chromen-4-one derivatives as new potential PET agents for imaging of DNA-dependent protein kinase (DNA-PK) in cancer. Appl. Radiat Isot. 2012, 70, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Palmer, D.; Fitzgerald, R.; Andreu-Vieyra, C.; Zhang, H.; Tang, Z.; Su, D.; Sahasranaman, S. Human Mass Balance and Metabolite Profiling of [(14) C]-Pamiparib, a Poly (ADP-Ribose) Polymerase Inhibitor, in Patients With Advanced Cancer. Clin. Pharmacol. Drug Dev. 2021, 10, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Durant, S.T.; Zheng, L.; Wang, Y.; Chen, K.; Zhang, L.; Zhang, T.; Yang, Z.; Riches, L.; Trinidad, A.G.; Fok, J.H.L.; et al. The brain-penetrant clinical ATM inhibitor AZD1390 radiosensitizes and improves survival of preclinical brain tumor models. Sci. Adv. 2018, 4, eaat1719. [Google Scholar] [CrossRef]
- Young, R.J.; Demétrio De Souza França, P.; Pirovano, G.; Piotrowski, A.F.; Nicklin, P.J.; Riedl, C.C.; Schwartz, J.; Bale, T.A.; Donabedian, P.L.; Kossatz, S.; et al. Preclinical and first-in-human-brain-cancer applications of [(18)F]poly (ADP-ribose) polymerase inhibitor PET/MR. Neurooncol. Adv. 2020, 2, vdaa119. [Google Scholar] [CrossRef]
- Donabedian, P.L.; Kossatz, S.; Engelbach, J.A.; Jannetti, S.A.; Carney, B.; Young, R.J.; Weber, W.A.; Garbow, J.R.; Reiner, T. Discriminating radiation injury from recurrent tumor with [(18)F]PARPi and amino acid PET in mouse models. EJNMMI Res. 2018, 8, 59. [Google Scholar] [CrossRef]
- Reiner, T.; Keliher, E.J.; Earley, S.; Marinelli, B.; Weissleder, R. Synthesis and in vivo imaging of a 18F-labeled PARP1 inhibitor using a chemically orthogonal scavenger-assisted high-performance method. Angew. Chem. Int. Ed. 2011, 50, 1922–1925. [Google Scholar] [CrossRef]
- Laird, J.; Lok, B.H.; Carney, B.; Kossatz, S.; de Stanchina, E.; Reiner, T.; Poirier, J.T.; Rudin, C.M. Positron-Emission Tomographic Imaging of a Fluorine 18-Radiolabeled Poly(ADP-Ribose) Polymerase 1 Inhibitor Monitors the Therapeutic Efficacy of Talazoparib in SCLC Patient-Derived Xenografts. J. Thorac. Oncol. 2019, 14, 1743–1752. [Google Scholar] [CrossRef]
- Carney, B.; Kossatz, S.; Lok, B.H.; Schneeberger, V.; Gangangari, K.K.; Pillarsetty, N.V.K.; Weber, W.A.; Rudin, C.M.; Poirier, J.T.; Reiner, T. Target engagement imaging of PARP inhibitors in small-cell lung cancer. Nat. Commun. 2018, 9, 176. [Google Scholar] [CrossRef]
- Demétrio de Souza França, P.; Roberts, S.; Kossatz, S.; Guru, N.; Mason, C.; Zanoni, D.K.; Abrahão, M.; Schöder, H.; Ganly, I.; Patel, S.G.; et al. Fluorine-18 labeled poly (ADP-ribose) polymerase1 inhibitor as a potential alternative to 2-deoxy-2-[(18)F]fluoro-d-glucose positron emission tomography in oral cancer imaging. Nucl. Med. Biol. 2020, 84, 80–87. [Google Scholar] [CrossRef]
- Tang, J.; Salloum, D.; Carney, B.; Brand, C.; Kossatz, S.; Sadique, A.; Lewis, J.S.; Weber, W.A.; Wendel, H.G.; Reiner, T. Targeted PET imaging strategy to differentiate malignant from inflamed lymph nodes in diffuse large B-cell lymphoma. Proc. Natl. Acad. Sci. USA 2017, 114, e7441–e7449. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.C.; Xavier, M.A.; Knight, J.; Verhoog, S.; Torres, J.B.; Mosley, M.; Hopkins, S.L.; Wallington, S.; Allen, P.D.; Kersemans, V.; et al. PET Imaging of PARP Expression Using (18)F-Olaparib. J. Nucl. Med. 2019, 60, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.L.; Friis, S.D.; Audrain, H.; Nordeman, P.; Antoni, G.; Skrydstrup, T. Efficient 11C-carbonylation of isolated aryl palladium complexes for PET: Application to challenging radiopharmaceutical synthesis. J. Am Chem. Soc. 2015, 137, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, M.; Pantel, A.; Schwartz, L.; Schubert, E.; Xu, K.; Hsieh, C.J.; Hou, C.; Kim, H.; Weng, C.C.; Winters, H.; et al. A PET imaging agent for evaluating PARP-1 expression in ovarian cancer. J. Clin. Investig. 2018, 128, 2116–2126. [Google Scholar] [CrossRef]
- Michel, L.S.; Dyroff, S.; Brooks, F.J.; Spayd, K.J.; Lim, S.; Engle, J.T.; Phillips, S.; Tan, B.; Wang-Gillam, A.; Bognar, C.; et al. PET of Poly (ADP-Ribose) Polymerase Activity in Cancer: Preclinical Assessment and First In-Human Studies. Radiology 2017, 282, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Xu, J.; Mpoy, C.; Chu, W.; Kim, S.H.; Li, H.; Rogers, B.E.; Katzenellenbogen, J.A. Preliminary evaluation of a novel (18)F-labeled PARP-1 ligand for PET imaging of PARP-1 expression in prostate cancer. Nucl. Med. Biol. 2018, 66, 26–31. [Google Scholar] [CrossRef]
- Zhou, D.; Chu, W.; Xu, J.; Jones, L.A.; Peng, X.; Li, S.; Chen, D.L.; Mach, R.H. Synthesis, [18F] radiolabeling, and evaluation of poly (ADP-ribose) polymerase-1 (PARP-1) inhibitors for in vivo imaging of PARP-1 using positron emission tomography. Bioorg. Med. Chem. 2014, 22, 1700–1707. [Google Scholar] [CrossRef]
- Riad, A.; Gitto, S.B.; Lee, H.; Winters, H.D.; Martorano, P.M.; Hsieh, C.J.; Xu, K.; Omran, D.K.; Powell, D.J., Jr.; Mach, R.H.; et al. PARP Theranostic Auger Emitters Are Cytotoxic in BRCA Mutant Ovarian Cancer and Viable Tumors from Ovarian Cancer Patients Enable Ex-Vivo Screening of Tumor Response. Molecules 2020, 25, 6029. [Google Scholar] [CrossRef]
- Takahashi, R.H.; Halladay, J.S.; Siu, M.; Chen, Y.; Hop, C.E.; Khojasteh, S.C.; Ma, S. Novel Mechanism of Decyanation of GDC-0425 by Cytochrome P450. Drug Metab. Dispos. 2017, 45, 430–440. [Google Scholar] [CrossRef]
- Wickremsinhe, E.R.; Hynes, S.M.; Palmieri, M.D.; Mitchell, M.I.; Abraham, T.L.; Rehmel, J.F.; Chana, E.; Jost, L.M.; Cassidy, K.C. Disposition and metabolism of LY2603618, a Chk-1 inhibitor following intravenous administration in patients with advanced and/or metastatic solid tumors. Xenobiotica 2014, 44, 827–841. [Google Scholar] [CrossRef]
- Wickstroem, K.; Hagemann, U.B.; Cruciani, V.; Wengner, A.M.; Kristian, A.; Ellingsen, C.; Siemeister, G.; Bjerke, R.M.; Karlsson, J.; Ryan, O.B.; et al. Synergistic Effect of a Mesothelin-Targeted (227)Th Conjugate in Combination with DNA Damage Response Inhibitors in Ovarian Cancer Xenograft Models. J. Nucl. Med. 2019, 60, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.M.; Ryan, A.J. ATM and ATR as therapeutic targets in cancer. Pharmacol. Ther. 2015, 149, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Helt, C.E.; Cliby, W.A.; Keng, P.C.; Bambara, R.A.; O’Reilly, M.A. Ataxia telangiectasia mutated (ATM) and ATM and Rad3-related protein exhibit selective target specificities in response to different forms of DNA damage. J. Biol. Chem. 2005, 280, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Paull, T.T. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science 2005, 308, 551–554. [Google Scholar] [CrossRef]
- Hafner, A.; Bulyk, M.L.; Jambhekar, A.; Lahav, G. The multiple mechanisms that regulate p53 activity and cell fate. Nat. Rev. Mol. Cell Biol. 2019, 20, 199–210. [Google Scholar] [CrossRef]
- Bennetzen, M.V.; Larsen, D.H.; Bunkenborg, J.; Bartek, J.; Lukas, J.; Andersen, J.S. Site-specific phosphorylation dynamics of the nuclear proteome during the DNA damage response. Mol. Cell. Proteom. 2010, 9, 1314–1323. [Google Scholar] [CrossRef]
- Taylor, A.M.; Harnden, D.G.; Arlett, C.F.; Harcourt, S.A.; Lehmann, A.R.; Stevens, S.; Bridges, B.A. Ataxia telangiectasia: A human mutation with abnormal radiation sensitivity. Nature 1975, 258, 427–429. [Google Scholar] [CrossRef]
- Barnieh, F.M.; Loadman, P.M.; Falconer, R.A. Progress towards a clinically-successful ATR inhibitor for cancer therapy. Curr. Res. Pharmacol. Drug Dis. 2021, 2, 100017. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.U.; Carruthers, R.; Gilmour, L.; Yildirim, S.; Watts, C.; Chalmers, A.J. Selective Inhibition of Parallel DNA Damage Response Pathways Optimizes Radiosensitization of Glioblastoma Stem-like Cells. Cancer Res. 2015, 75, 4416–4428. [Google Scholar] [CrossRef]
- Jiang, H.; Reinhardt, H.C.; Bartkova, J.; Tommiska, J.; Blomqvist, C.; Nevanlinna, H.; Bartek, J.; Yaffe, M.B.; Hemann, M.T. The combined status of ATM and p53 link tumor development with therapeutic response. Genes Dev. 2009, 23, 1895–1909. [Google Scholar] [CrossRef]
- Cancer Genome Atlas (TCGA) Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Frosina, G.; Marubbi, D.; Marcello, D.; Vecchio, D.; Daga, A. The efficacy and toxicity of ATM inhibition in glioblastoma initiating cells-driven tumor models. Crit. Rev. Oncol. Hematol. 2019, 138, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Golding, S.E.; Rosenberg, E.; Adams, B.R.; Wignarajah, S.; Beckta, J.M.; O’Connor, M.J.; Valerie, K. Dynamic inhibition of ATM kinase provides a strategy for glioblastoma multiforme radiosensitization and growth control. Cell Cycle 2012, 11, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Quek, H.; Offenhäuser, C.; Fazry, S.; Boyd, A.; Lavin, M.; Roberts, T.; Day, B. ATM inhibition prevents interleukin-6 from contributing to the proliferation of glioblastoma cells after ionizing radiation. J. Neurooncol. 2018, 138, 509–518. [Google Scholar] [CrossRef]
- Bradbury, A.; Hall, S.; Curtin, N.; Drew, Y. Targeting ATR as Cancer Therapy: A new era for synthetic lethality and synergistic combinations? Pharmacol. Ther. 2020, 207, 107450. [Google Scholar] [CrossRef]
- Gorecki, L.; Andrs, M.; Rezacova, M.; Korabecny, J. Discovery of ATR kinase inhibitor berzosertib (VX-970, M6620): Clinical candidate for cancer therapy. Pharmacol. Ther. 2020, 210, 107518. [Google Scholar] [CrossRef]
- Yap, T.A.; Tolcher, A.W.; Plummer, E.R.; Becker, A.; Fleuranceau-Morel, P.; Goddemeier, T.; Locatelli, G.; Gounaris, I.; Bono, J.S.D. A first-in-human phase I study of ATR inhibitor M1774 in patients with solid tumors. J. Clin. Oncol. 2021, 39, TPS3153. [Google Scholar] [CrossRef]
- Nandakumar, P.; Mansouri, A.; Das, S. The Role of ATRX in Glioma Biology. Front. Oncol. 2017, 7, 236. [Google Scholar] [CrossRef]
- Garbarino, J.; Eckroate, J.; Sundaram, R.K.; Jensen, R.B.; Bindra, R.S. Loss of ATRX confers DNA repair defects and PARP inhibitor sensitivity. Transl. Oncol. 2021, 14, 101147. [Google Scholar] [CrossRef]
- Karlin, J.; Allen, J.; Ahmad, S.F.; Hughes, G.; Sheridan, V.; Odedra, R.; Farrington, P.; Cadogan, E.B.; Riches, L.C.; Garcia-Trinidad, A.; et al. Orally Bioavailable and Blood-Brain Barrier-Penetrating ATM Inhibitor (AZ32) Radiosensitizes Intracranial Gliomas in Mice. Mol. Cancer Ther. 2018, 17, 1637–1647. [Google Scholar] [CrossRef]
- Williamson, C.T.; Miller, R.; Pemberton, H.N.; Jones, S.E.; Campbell, J.; Konde, A.; Badham, N.; Rafiq, R.; Brough, R.; Gulati, A.; et al. ATR inhibitors as a synthetic lethal therapy for tumours deficient in ARID1A. Nat. Commun. 2016, 7, 13837. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.N.; Rooney, C.; Griffin, N.; Roudier, M.; Young, L.A.; Garcia-Trinidad, A.; Hughes, G.D.; Whiteaker, J.R.; Wilson, Z.; Odedra, R.; et al. pRAD50: A novel and clinically applicable pharmacodynamic biomarker of both ATM and ATR inhibition identified using mass spectrometry and immunohistochemistry. Br. J. Cancer 2018, 119, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Hickson, I.; Zhao, Y.; Richardson, C.J.; Green, S.J.; Martin, N.M.; Orr, A.I.; Reaper, P.M.; Jackson, S.P.; Curtin, N.J.; Smith, G.C. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004, 64, 9152–9159. [Google Scholar] [CrossRef] [PubMed]
- Golding, S.E.; Rosenberg, E.; Valerie, N.; Hussaini, I.; Frigerio, M.; Cockcroft, X.F.; Chong, W.Y.; Hummersone, M.; Rigoreau, L.; Menear, K.A.; et al. Improved ATM kinase inhibitor KU-60019 radiosensitizes glioma cells, compromises insulin, AKT and ERK prosurvival signaling, and inhibits migration and invasion. Mol. Cancer Ther. 2009, 8, 2894–2902. [Google Scholar] [CrossRef] [PubMed]
- Rainey, M.D.; Charlton, M.E.; Stanton, R.V.; Kastan, M.B. Transient inhibition of ATM kinase is sufficient to enhance cellular sensitivity to ionizing radiation. Cancer Res. 2008, 68, 7466–7474. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, A.; Shrivastav, M.; Mladek, A.C.; Schwingler, P.M.; Grogan, P.T.; Chen, J.; Sarkaria, J.N. ATM inhibitor KU-55933 increases the TMZ responsiveness of only inherently TMZ sensitive GBM cells. J. Neurooncol. 2012, 110, 349–357. [Google Scholar] [CrossRef]
- Tang, S.; Li, Z.; Yang, L.; Shen, L.; Wang, Y. A potential new role of ATM inhibitor in radiotherapy: Suppressing ionizing Radiation-Activated EGFR. Int. J. Radiat. Biol. 2020, 96, 461–468. [Google Scholar] [CrossRef]
- Carruthers, R.; Ahmed, S.U.; Strathdee, K.; Gomez-Roman, N.; Amoah-Buahin, E.; Watts, C.; Chalmers, A.J. Abrogation of radioresistance in glioblastoma stem-like cells by inhibition of ATM kinase. Mol. Oncol. 2015, 9, 192–203. [Google Scholar] [CrossRef]
- Vecchio, D.; Daga, A.; Carra, E.; Marubbi, D.; Baio, G.; Neumaier, C.E.; Vagge, S.; Corvò, R.; Pia Brisigotti, M.; Louis Ravetti, J.; et al. Predictability, efficacy and safety of radiosensitization of glioblastoma-initiating cells by the ATM inhibitor KU-60019. Int. J. Cancer 2014, 135, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Biddlestone-Thorpe, L.; Sajjad, M.; Rosenberg, E.; Beckta, J.M.; Valerie, N.C.; Tokarz, M.; Adams, B.R.; Wagner, A.F.; Khalil, A.; Gilfor, D.; et al. ATM kinase inhibition preferentially sensitizes p53-mutant glioma to ionizing radiation. Clin. Cancer Res. 2013, 19, 3189–3200. [Google Scholar] [CrossRef]
- Riches, L.C.; Trinidad, A.G.; Hughes, G.; Jones, G.N.; Hughes, A.M.; Thomason, A.G.; Gavine, P.; Cui, A.; Ling, S.; Stott, J.; et al. Pharmacology of the ATM Inhibitor AZD0156: Potentiation of Irradiation and Olaparib Responses Preclinically. Mol. Cancer Ther. 2020, 19, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Mak, J.P.Y.; Ma, H.T.; Poon, R.Y.C. Synergism between ATM and PARP1 Inhibition Involves DNA Damage and Abrogating the G(2) DNA Damage Checkpoint. Mol. Cancer Ther. 2020, 19, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; O’Carrigan, B.; Penney, M.S.; Lim, J.S.; Brown, J.S.; de Miguel Luken, M.J.; Tunariu, N.; Perez-Lopez, R.; Rodrigues, D.N.; Riisnaes, R.; et al. Phase I Trial of First-in-Class ATR Inhibitor M6620 (VX-970) as Monotherapy or in Combination with Carboplatin in Patients With Advanced Solid Tumors. J. Clin. Oncol. 2020, 38, 3195–3204. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Bang, Y.J.; Carter, L.; Azaro, A.; Krebs, M.; Im, S.-A.; Chen, Y.; Buil-Bruna, N.; Li, Y.; Eaton, D.; et al. Abstract A094: Phase I modular study of AZD0156, a first-in-class oral selective inhibitor of ataxia telangiectasia mutated protein kinase (ATM), in combination with olaparib (AToM Study, Module 1). Mol. Cancer Ther. 2018, 17, A094. [Google Scholar] [CrossRef]
- Batey, M.A.; Zhao, Y.; Kyle, S.; Richardson, C.; Slade, A.; Martin, N.M.; Lau, A.; Newell, D.R.; Curtin, N.J. Preclinical evaluation of a novel ATM inhibitor, KU59403, in vitro and in vivo in p53 functional and dysfunctional models of human cancer. Mol. Cancer Ther. 2013, 12, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Li, B.; Wu, Z.; Wu, Y.; Wang, Y.; Jin, F.; Li, D.; Ma, H.; Wang, D. Silencing of ataxia-telangiectasia mutated by siRNA enhances the in vitro and in vivo radiosensitivity of glioma. Oncol. Rep. 2016, 35, 3303–3312. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eich, M.; Roos, W.P.; Nikolova, T.; Kaina, B. Contribution of ATM and ATR to the resistance of glioblastoma and malignant melanoma cells to the methylating anticancer drug temozolomide. Mol. Cancer Ther. 2013, 12, 2529–2540. [Google Scholar] [CrossRef] [PubMed]
- Hanna, C.; Kurian, K.M.; Williams, K.; Watts, C.; Jackson, A.; Carruthers, R.; Strathdee, K.; Cruickshank, G.; Dunn, L.; Erridge, S.; et al. Pharmacokinetics, safety, and tolerability of olaparib and temozolomide for recurrent glioblastoma: Results of the phase I OPARATIC trial. Neuro Oncol. 2020, 22, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Sim, H.W.; McDonald, K.L.; Lwin, Z.; Barnes, E.H.; Rosenthal, M.; Foote, M.C.; Koh, E.S.; Back, M.; Wheeler, H.; Sulman, E.P.; et al. A randomized phase II trial of veliparib, radiotherapy and temozolomide in patients with unmethylated MGMT glioblastoma: The VERTU study. Neuro Oncol. 2021, 32, 1736–1749. [Google Scholar] [CrossRef]
- Robins, H.I.; Zhang, P.; Gilbert, M.R.; Chakravarti, A.; de Groot, J.F.; Grimm, S.A.; Wang, F.; Lieberman, F.S.; Krauze, A.; Trotti, A.M.; et al. A randomized phase I/II study of ABT-888 in combination with temozolomide in recurrent temozolomide resistant glioblastoma: An NRG oncology RTOG group study. J. Neurooncol. 2016, 126, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Kurzrock, R.; Galanis, E.; Johnson, D.R.; Kansra, V.; Wilcoxen, K.; Mcclure, T.; Martell, R.E.; Agarwal, S. A phase I study of niraparib in combination with temozolomide (TMZ) in patients with advanced cancer. J. Clin. Oncol. 2014, 32, 2092. [Google Scholar] [CrossRef]
- Mei, L.; Zhang, J.; He, K. Ataxia telangiectasia and Rad3-related inhibitors and cancer therapy: Where we stand. J. Hematol. Oncol. 2019, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Krebs, M.G.; Postel-Vinay, S.; El-Khouiery, A.; Soria, J.C.; Lopez, J.; Berges, A.; Cheung, S.Y.A.; Irurzun-Arana, I.; Goldwin, A.; et al. Ceralasertib (AZD6738), an oral ATR kinase inhibitor, in combination with carboplatin in patients with advanced solid tumors: A Phase I study. Clin. Cancer Res. 2021, 27, 5213–5224. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Redon, C.E.; Sciuto, L.; Padiernos, E.; Ji, J.; Lee, M.J.; Yuno, A.; Lee, S.; Zhang, Y.; Tran, L.; et al. Phase I Study of ATR Inhibitor M6620 in Combination With Topotecan in Patients With Advanced Solid Tumors. J. Clin. Oncol. 2018, 36, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Middleton, M.R.; Dean, E.; Evans, T.R.J.; Shapiro, G.I.; Pollard, J.; Hendriks, B.S.; Falk, M.; Diaz-Padilla, I.; Plummer, R. Phase 1 study of the ATR inhibitor berzosertib (formerly M6620, VX-970) combined with gemcitabine ± cisplatin in patients with advanced solid tumours. Br. J. Cancer 2021, 125, 510–519. [Google Scholar] [CrossRef]
- Hall, A.B.; Newsome, D.; Wang, Y.; Boucher, D.M.; Eustace, B.; Gu, Y.; Hare, B.; Johnson, M.A.; Milton, S.; Murphy, C.E.; et al. Potentiation of tumor responses to DNA damaging therapy by the selective ATR inhibitor VX-970. Oncotarget 2014, 5, 5674–5685. [Google Scholar] [CrossRef]
- Fokas, E.; Prevo, R.; Pollard, J.R.; Reaper, P.M.; Charlton, P.A.; Cornelissen, B.; Vallis, K.A.; Hammond, E.M.; Olcina, M.M.; Gillies McKenna, W.; et al. Targeting ATR in vivo using the novel inhibitor VE-822 results in selective sensitization of pancreatic tumors to radiation. Cell Death Dis. 2012, 3, e441. [Google Scholar] [CrossRef]
- Talele, S.; Mohammad, A.; Kim, M.; Burgenske, D.; Tuma, A.C.M.; Sarkaria, J.N.; Elmquist, W.F. Abstract 4858: CNS delivery of VX-970: A selective ATR inhibitor for radiosensitization in GBM. Cancer Res. 2019, 79, 4858. [Google Scholar] [CrossRef]
- Chen, E.M.; Quijano, A.R.; Seo, Y.E.; Jackson, C.; Josowitz, A.D.; Noorbakhsh, S.; Merlettini, A.; Sundaram, R.K.; Focarete, M.L.; Jiang, Z.; et al. Biodegradable PEG-poly(ω-pentadecalactone-co-p-dioxanone) nanoparticles for enhanced and sustained drug delivery to treat brain tumors. Biomaterials 2018, 178, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Wakimoto, H.; Martuza, R.L.; Rabkin, S.D. Abstract 1122: ATR inhibitors synergize with PARP inhibitors in killing glioblastoma stem cells and treating glioblastoma. Cancer Res. 2017, 77, 1122. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, H.; Diplas, B.H.; Liu, S.; Liu, J.; Wang, D.; Lu, Y.; Zhu, Q.; Wu, J.; Wang, W.; et al. Genome-Wide CRISPR-Cas9 Screen Reveals Selective Vulnerability of ATRX-Mutant Cancers to WEE1 Inhibition. Cancer Res. 2020, 80, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, A.B.; Lewis, C.W.; Pearce, J.J.; Luong, D.; Chan, G.K.; Gamper, A.M. Inhibiting Wee1 and ATR kinases produces tumor-selective synthetic lethality and suppresses metastasis. J. Clin. Investig. 2019, 129, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Matheson, C.J.; Backos, D.S.; Reigan, P. Targeting WEE1 Kinase in Cancer. Trends. Pharmacol. Sci. 2016, 37, 872–881. [Google Scholar] [CrossRef]
- Vendetti, F.P.; Karukonda, P.; Clump, D.A.; Teo, T.; Lalonde, R.; Nugent, K.; Ballew, M.; Kiesel, B.F.; Beumer, J.H.; Sarkar, S.N.; et al. ATR kinase inhibitor AZD6738 potentiates CD8+ T cell-dependent antitumor activity following radiation. J. Clin. Investig. 2018, 128, 3926–3940. [Google Scholar] [CrossRef] [PubMed]
- Foote, K.M.; Nissink, J.W.M.; McGuire, T.; Turner, P.; Guichard, S.; Yates, J.W.T.; Lau, A.; Blades, K.; Heathcote, D.; Odedra, R.; et al. Discovery and Characterization of AZD6738, a Potent Inhibitor of Ataxia Telangiectasia Mutated and Rad3 Related (ATR) Kinase with Application as an Anticancer Agent. J. Med. Chem. 2018, 61, 9889–9907. [Google Scholar] [CrossRef] [PubMed]
- Fròsina, G.; Profumo, A.; Marubbi, D.; Marcello, D.; Ravetti, J.L.; Daga, A. ATR kinase inhibitors NVP-BEZ235 and AZD6738 effectively penetrate the brain after systemic administration. Radiat. Oncol. 2018, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Toledo, L.I.; Murga, M.; Zur, R.; Soria, R.; Rodriguez, A.; Martinez, S.; Oyarzabal, J.; Pastor, J.; Bischoff, J.R.; Fernandez-Capetillo, O. A cell-based screen identifies ATR inhibitors with synthetic lethal properties for cancer-associated mutations. Nat. Struct. Mol. Biol. 2011, 18, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Xie, G.; Zhou, G.; Cheng, Y.; Zhang, G.; Yao, G.; Chen, Y.; Li, Y.; Zhao, G. NVP-BEZ235, a novel dual PI3K-mTOR inhibitor displays anti-glioma activity and reduces chemoresistance to temozolomide in human glioma cells. Cancer Lett. 2015, 367, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Gil del Alcazar, C.R.; Hardebeck, M.C.; Mukherjee, B.; Tomimatsu, N.; Gao, X.; Yan, J.; Xie, X.J.; Bachoo, R.; Li, L.; Habib, A.A.; et al. Inhibition of DNA double-strand break repair by the dual PI3K/mTOR inhibitor NVP-BEZ235 as a strategy for radiosensitization of glioblastoma. Clin. Cancer Res. 2014, 20, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Long, L.M.; Yang, N.; Zhang, Q.Q.; Ji, W.J.; Zhao, J.H.; Qin, Z.H.; Wang, Z.; Chen, G.; Liang, Z.Q. NVP-BEZ235, a novel dual PI3K/mTOR inhibitor, enhances the radiosensitivity of human glioma stem cells in vitro. Acta Pharmacol. Sin. 2013, 34, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Cerniglia, G.J.; Karar, J.; Tyagi, S.; Christofidou-Solomidou, M.; Rengan, R.; Koumenis, C.; Maity, A. Inhibition of autophagy as a strategy to augment radiosensitization by the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235. Mol. Pharmacol. 2012, 82, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Kuger, S.; Graus, D.; Brendtke, R.; Günther, N.; Katzer, A.; Lutyj, P.; Polat, B.; Chatterjee, M.; Sukhorukov, V.L.; Flentje, M.; et al. Radiosensitization of Glioblastoma Cell Lines by the Dual PI3K and mTOR Inhibitor NVP-BEZ235 Depends on Drug-Irradiation Schedule. Transl. Oncol. 2013, 6, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Netland, I.A.; Førde, H.E.; Sleire, L.; Leiss, L.; Rahman, M.A.; Skeie, B.S.; Gjerde, C.H.; Enger, P.; Goplen, D. Dactolisib (NVP-BEZ235) toxicity in murine brain tumour models. BMC Cancer 2016, 16, 657. [Google Scholar] [CrossRef]
- Mukherjee, B.; Tomimatsu, N.; Amancherla, K.; Camacho, C.V.; Pichamoorthy, N.; Burma, S. The dual PI3K/mTOR inhibitor NVP-BEZ235 is a potent inhibitor of ATM- and DNA-PKCs-mediated DNA damage responses. Neoplasia 2012, 14, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Wise-Draper, T.M.; Moorthy, G.; Salkeni, M.A.; Karim, N.A.; Thomas, H.E.; Mercer, C.A.; Beg, M.S.; O’Gara, S.; Olowokure, O.; Fathallah, H.; et al. A Phase Ib Study of the Dual PI3K/mTOR Inhibitor Dactolisib (BEZ235) Combined with Everolimus in Patients with Advanced Solid Malignancies. Target Oncol. 2017, 12, 323–332. [Google Scholar] [CrossRef]
- Fazio, N.; Buzzoni, R.; Baudin, E.; Antonuzzo, L.; Hubner, R.A.; Lahner, H.; De Herder, W.W.; Raderer, M.; Teulé, A.; Capdevila, J.; et al. A Phase II Study of BEZ235 in Patients with Everolimus-resistant, Advanced Pancreatic Neuroendocrine Tumours. Anticancer Res. 2016, 36, 713–719. [Google Scholar]
- Topatana, W.; Juengpanich, S.; Li, S.; Cao, J.; Hu, J.; Lee, J.; Suliyanto, K.; Ma, D.; Zhang, B.; Chen, M.; et al. Advances in synthetic lethality for cancer therapy: Cellular mechanism and clinical translation. J. Hematol. Oncol. 2020, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Bartek, J.; Lukas, J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 2003, 3, 421–429. [Google Scholar] [CrossRef]
- Busino, L.; Chiesa, M.; Draetta, G.F.; Donzelli, M. Cdc25A phosphatase: Combinatorial phosphorylation, ubiquitylation and proteolysis. Oncogene 2004, 23, 2050–2056. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Xie, T.; Luo, L.; Xu, C.; Gao, Q.; Shen, L.; Wan, F.; Lei, T.; Ye, F. Radiation-induced G2/M arrest rarely occurred in glioblastoma stem-like cells. Int. J. Radiat. Biol. 2018, 94, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Pilié, P.G.; Tang, C.; Mills, G.B.; Yap, T.A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 2019, 16, 81–104. [Google Scholar] [CrossRef] [PubMed]
- Matthews, T.P.; Jones, A.M.; Collins, I. Structure-based design, discovery and development of checkpoint kinase inhibitors as potential anticancer therapies. Expert Opin. Drug Discov. 2013, 8, 621–640. [Google Scholar] [CrossRef] [PubMed]
- Daud, A.I.; Ashworth, M.T.; Strosberg, J.; Goldman, J.W.; Mendelson, D.; Springett, G.; Venook, A.P.; Loechner, S.; Rosen, L.S.; Shanahan, F.; et al. Phase I dose-escalation trial of checkpoint kinase 1 inhibitor MK-8776 as monotherapy and in combination with gemcitabine in patients with advanced solid tumors. J. Clin. Oncol. 2015, 33, 1060–1066. [Google Scholar] [CrossRef]
- Sausville, E.A.; Arbuck, S.G.; Messmann, R.; Headlee, D.; Bauer, K.S.; Lush, R.M.; Murgo, A.; Figg, W.D.; Lahusen, T.; Jaken, S.; et al. Phase I trial of 72-h continuous infusion UCN-01 in patients with refractory neoplasms. J. Clin. Oncol. 2001, 19, 2319–2333. [Google Scholar] [CrossRef]
- Laquente, B.; Lopez-Martin, J.; Richards, D.; Illerhaus, G.; Chang, D.Z.; Kim, G.; Stella, P.; Richel, D.; Szcylik, C.; Cascinu, S.; et al. A phase II study to evaluate LY2603618 in combination with gemcitabine in pancreatic cancer patients. BMC Cancer 2017, 17, 137. [Google Scholar] [CrossRef] [PubMed]
- Scagliotti, G.; Kang, J.H.; Smith, D.; Rosenberg, R.; Park, K.; Kim, S.W.; Su, W.C.; Boyd, T.E.; Richards, D.A.; Novello, S.; et al. Phase II evaluation of LY2603618, a first-generation CHK1 inhibitor, in combination with pemetrexed in patients with advanced or metastatic non-small cell lung cancer. Investig. New Drugs 2016, 34, 625–635. [Google Scholar] [CrossRef]
- Wehler, T.; Thomas, M.; Schumann, C.; Bosch-Barrera, J.; Viñolas Segarra, N.; Dickgreber, N.J.; Dalhoff, K.; Sebastian, M.; Corral Jaime, J.; Alonso, M.; et al. A randomized, phase 2 evaluation of the CHK1 inhibitor, LY2603618, administered in combination with pemetrexed and cisplatin in patients with advanced nonsquamous non-small cell lung cancer. Lung Cancer 2017, 108, 212–216. [Google Scholar] [CrossRef]
- Sausville, E.; Lorusso, P.; Carducci, M.; Carter, J.; Quinn, M.F.; Malburg, L.; Azad, N.; Cosgrove, D.; Knight, R.; Barker, P.; et al. Phase I dose-escalation study of AZD7762, a checkpoint kinase inhibitor, in combination with gemcitabine in US patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2014, 73, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Moore, K.; Patel, M.; Grant, S.C.; Burris, H.A., 3rd; William, W.N., Jr.; Jones, S.; Meric-Bernstam, F.; Infante, J.; Golden, L.; et al. Evaluation of Prexasertib, a Checkpoint Kinase 1 Inhibitor, in a Phase Ib Study of Patients with Squamous Cell Carcinoma. Clin. Cancer Res. 2018, 24, 3263–3272. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Infante, J.; Janku, F.; Jones, S.; Nguyen, L.M.; Burris, H.; Naing, A.; Bauer, T.M.; Piha-Paul, S.; Johnson, F.M.; et al. Phase I Study of LY2606368, a Checkpoint Kinase 1 Inhibitor, in Patients With Advanced Cancer. J. Clin. Oncol. 2016, 34, 1764–1771. [Google Scholar] [CrossRef]
- Lee, J.M.; Nair, J.; Zimmer, A.; Lipkowitz, S.; Annunziata, C.M.; Merino, M.J.; Swisher, E.M.; Harrell, M.I.; Trepel, J.B.; Lee, M.J.; et al. Prexasertib, a cell cycle checkpoint kinase 1 and 2 inhibitor, in BRCA wild-type recurrent high-grade serous ovarian cancer: A first-in-class proof-of-concept phase 2 study. Lancet. Oncol. 2018, 19, 207–215. [Google Scholar] [CrossRef]
- Gatti-Mays, M.E.; Karzai, F.H.; Soltani, S.N.; Zimmer, A.; Green, J.E.; Lee, M.J.; Trepel, J.B.; Yuno, A.; Lipkowitz, S.; Nair, J.; et al. A Phase II Single Arm Pilot Study of the CHK1 Inhibitor Prexasertib (LY2606368) in BRCA Wild-Type, Advanced Triple-Negative Breast Cancer. Oncologist 2020, 25, 1013. [Google Scholar] [CrossRef]
- Do, K.; Kochupurakkal, B.S.; Kelland, S.; de Jonge, A.; Hedglin, J.; Powers, A.; Quinn, N.; Gannon, C.; Vuong, L.; Parmar, K.; et al. Phase 1 Combination Study of the CHK1 inhibitor prexasertib, and the PARP inhibitor olaparib, in high-grade serous ovarian cancer and other solid tumors. Clin. Cancer Res. 2021, 27, 4710–4716. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.R.; Hollebecque, A.; Postel-Vinay, S.; Bauer, T.M.; Blackwood, E.M.; Evangelista, M.; Mahrus, S.; Peale, F.V.; Lu, X.; Sahasranaman, S.; et al. Phase I Study of GDC-0425, a Checkpoint Kinase 1 Inhibitor, in Combination with Gemcitabine in Patients with Refractory Solid Tumors. Clin. Cancer Res. 2017, 23, 2423–2432. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Infante, J.R.; Shapiro, G.I.; Moore, K.N.; LoRusso, P.M.; Hamilton, E.; Cousin, S.; Toulmonde, M.; Postel-Vinay, S.; Tolaney, S.; et al. Phase I study of the checkpoint kinase 1 inhibitor GDC-0575 in combination with gemcitabine in patients with refractory solid tumors. Ann. Oncol. 2018, 29, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Krishnamurthy Nemani, V.; Du, G.; Montano, R.; Song, R.; Gimi, B.; Swartz, H.M.; Eastman, A.; Khan, N. Monitoring oxygen levels in orthotopic human glioma xenograft following carbogen inhalation and chemotherapy by implantable resonator-based oximetry. Int. J. Cancer 2015, 136, 1688–1696. [Google Scholar] [CrossRef]
- Hirose, Y.; Berger, M.S.; Pieper, R.O. Abrogation of the Chk1-mediated G(2) checkpoint pathway potentiates temozolomide-induced toxicity in a p53-independent manner in human glioblastoma cells. Cancer Res. 2001, 61, 5843–5849. [Google Scholar] [PubMed]
- Signore, M.; Pelacchi, F.; di Martino, S.; Runci, D.; Biffoni, M.; Giannetti, S.; Morgante, L.; De Majo, M.; Petricoin, E.F.; Stancato, L.; et al. Combined PDK1 and CHK1 inhibition is required to kill glioblastoma stem-like cells in vitro and in vivo. Cell Death Dis. 2014, 5, e1223. [Google Scholar] [CrossRef]
- Tang, Y.; Dai, Y.; Grant, S.; Dent, P. Enhancing CHK1 inhibitor lethality in glioblastoma. Cancer Biol. Ther. 2012, 13, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.M.; Galbán, S.; Li, F.; Heist, K.A.; Galbán, C.J.; Lawrence, T.S.; Holland, E.C.; Thomae, T.L.; Chenevert, T.L.; Rehemtulla, A.; et al. DW-MRI as a Predictive Biomarker of Radiosensitization of GBM through Targeted Inhibition of Checkpoint Kinases. Transl. Oncol. 2013, 6, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Patties, I.; Kallendrusch, S.; Böhme, L.; Kendzia, E.; Oppermann, H.; Gaunitz, F.; Kortmann, R.D.; Glasow, A. The Chk1 inhibitor SAR-020106 sensitizes human glioblastoma cells to irradiation, to temozolomide, and to decitabine treatment. J. Exp. Clin. Cancer Res. 2019, 38, 420. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Thoms, J.A.I.; Stringer, B.W.; Chung, S.A.; Ensbey, K.S.; Jue, T.R.; Jahan, Z.; Subramanian, S.; Anande, G.; Shen, H.; et al. Constitutive CHK1 Expression Drives a pSTAT3-CIP2A Circuit that Promotes Glioblastoma Cell Survival and Growth. Mol. Cancer Res. 2020, 18, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lai, G.; Wan, F.; Xiao, Z.; Zeng, L.; Wang, X.; Ye, F.; Lei, T. Knockdown of checkpoint kinase 1 is associated with the increased radiosensitivity of glioblastoma stem-like cells. Tohoku J. Exp. Med. 2012, 226, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Jobson, A.G.; Lountos, G.T.; Lorenzi, P.L.; Llamas, J.; Connelly, J.; Cerna, D.; Tropea, J.E.; Onda, A.; Zoppoli, G.; Kondapaka, S.; et al. Cellular inhibition of checkpoint kinase 2 (Chk2) and potentiation of camptothecins and radiation by the novel Chk2 inhibitor PV1019 [7-nitro-1H-indole-2-carboxylic acid {4-[1-(guanidinohydrazone)-ethyl]-phenyl}-amide]. J. Pharmacol. Exp. Ther. 2009, 331, 816–826. [Google Scholar] [CrossRef]
- Vanan, I.; Dong, Z.; Tosti, E.; Warshaw, G.; Symons, M.; Ruggieri, R. Role of a DNA damage checkpoint pathway in ionizing radiation-induced glioblastoma cell migration and invasion. Cell Mol. Neurobiol. 2012, 32, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, Z.; Painter, C.L.; Zhang, Q.; Chen, E.; Arango, M.E.; Kuszpit, K.; Zasadny, K.; Hallin, M.; Hallin, J.; et al. PF-00477736 mediates checkpoint kinase 1 signaling pathway and potentiates docetaxel-induced efficacy in xenografts. Clin. Cancer Res. 2009, 15, 4630–4640. [Google Scholar] [CrossRef]
- Chan, C.Y.; Tan, K.V.; Cornelissen, B. PARP Inhibitors in Cancer Diagnosis and Therapy. Clin. Cancer Res. 2021, 27, 1585–1594. [Google Scholar] [CrossRef]
- Gupta, S.K.; Smith, E.J.; Mladek, A.C.; Tian, S.; Decker, P.A.; Kizilbash, S.H.; Kitange, G.J.; Sarkaria, J.N. PARP Inhibitors for Sensitization of Alkylation Chemotherapy in Glioblastoma: Impact of Blood-Brain Barrier and Molecular Heterogeneity. Front Oncol. 2018, 8, 670. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.F.; Stanciu, M.; Humphrey, M.R.; Gorham, J.; Wakimoto, H.; Nishihara, R.; Lees, J.; Zou, L.; Martuza, R.L.; Rabkin, S.D. Myc targeted CDK18 promotes ATR and homologous recombination to mediate PARP inhibitor resistance in glioblastoma. Nat. Commun. 2019, 10, 2910. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, F.; Nagashima, H.; Ning, J.; Koerner, M.V.A.; Wakimoto, H.; Cahill, D.P. Restoration of Temozolomide Sensitivity by PARP Inhibitors in Mismatch Repair Deficient Glioblastoma is Independent of Base Excision Repair. Clin. Cancer Res. 2020, 26, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Sandhu, S.K.; Carden, C.P.; de Bono, J.S. Poly(ADP-ribose) polymerase (PARP) inhibitors: Exploiting a synthetic lethal strategy in the clinic. CA Cancer J. Clin. 2011, 61, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jette, N.; Moussienko, D.; Bebb, D.G.; Lees-Miller, S.P. ATM-Deficient Colorectal Cancer Cells Are Sensitive to the PARP Inhibitor Olaparib. Transl. Oncol. 2017, 10, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.T.; Muzik, H.; Turhan, A.G.; Zamò, A.; O’Connor, M.J.; Bebb, D.G.; Lees-Miller, S.P. ATM deficiency sensitizes mantle cell lymphoma cells to poly(ADP-ribose) polymerase-1 inhibitors. Mol. Cancer Ther. 2010, 9, 347–357. [Google Scholar] [CrossRef]
- Majuelos-Melguizo, J.; Rodríguez, M.I.; López-Jiménez, L.; Rodríguez-Vargas, J.M.; Martí Martín-Consuegra, J.M.; Serrano-Sáenz, S.; Gavard, J.; de Almodóvar, J.M.; Oliver, F.J. PARP targeting counteracts gliomagenesis through induction of mitotic catastrophe and aggravation of deficiency in homologous recombination in PTEN-mutant glioma. Oncotarget 2015, 6, 4790–4803. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; de Gooijer, M.C.; Roig, E.M.; Buil, L.C.; Christner, S.M.; Beumer, J.H.; Würdinger, T.; Beijnen, J.H.; van Tellingen, O. ABCB1, ABCG2, and PTEN determine the response of glioblastoma to temozolomide and ABT-888 therapy. Clin. Cancer Res. 2014, 20, 2703–2713. [Google Scholar] [CrossRef]
- Xiong, Y.; Guo, Y.; Liu, Y.; Wang, H.; Gong, W.; Wang, X.; Gao, Y.; Yu, F.; Su, D.; Wang, F.; et al. Pamiparib is a potent and selective PARP inhibitor with unique potential for the treatment of brain tumor. Neoplasia 2020, 22, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Lester, A.; Rapkins, R.; Nixdorf, S.; Khasraw, M.; McDonald, K. Combining PARP inhibitors with radiation therapy for the treatment of glioblastoma: Is PTEN predictive of response? Clin. Transl. Oncol. 2017, 19, 273–278. [Google Scholar] [CrossRef]
- Brown, J.S.; O’Carrigan, B.; Jackson, S.P.; Yap, T.A. Targeting DNA Repair in Cancer: Beyond PARP Inhibitors. Cancer Discov. 2017, 7, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, R.; Chalmers, A.J. The potential of PARP inhibitors in neuro-oncology. CNS Oncol. 2012, 1, 85–97. [Google Scholar] [CrossRef]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, X.; Gao, F.; de Groot, J.F.; Koul, D.; Yung, W.K.A. PARP-mediated PARylation of MGMT is critical to promote repair of temozolomide-induced O6-methylguanine DNA damage in glioblastoma. Neuro Oncol. 2021, 23, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Murai, J.; Huang, S.Y.; Das, B.B.; Renaud, A.; Zhang, Y.; Doroshow, J.H.; Ji, J.; Takeda, S.; Pommier, Y. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res. 2012, 72, 5588–5599. [Google Scholar] [CrossRef] [PubMed]
- Min, A.; Im, S.A. PARP Inhibitors as Therapeutics: Beyond Modulation of PARylation. Cancers 2020, 12, 394. [Google Scholar] [CrossRef] [PubMed]
- Parmar, K.; Kochupurakkal, B.S.; Lazaro, J.B.; Wang, Z.C.; Palakurthi, S.; Kirschmeier, P.T.; Yang, C.; Sambel, L.A.; Färkkilä, A.; Reznichenko, E.; et al. The CHK1 Inhibitor Prexasertib Exhibits Monotherapy Activity in High-Grade Serous Ovarian Cancer Models and Sensitizes to PARP Inhibition. Clin. Cancer Res. 2019, 25, 6127–6140. [Google Scholar] [CrossRef]
- Anderson, V.E.; Walton, M.I.; Eve, P.D.; Boxall, K.J.; Antoni, L.; Caldwell, J.J.; Aherne, W.; Pearl, L.H.; Oliver, A.W.; Collins, I.; et al. CCT241533 is a potent and selective inhibitor of CHK2 that potentiates the cytotoxicity of PARP inhibitors. Cancer Res. 2011, 71, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Vandenberg, C.J.; Lieschke, E.; Di Rago, L.; Scott, C.L.; Majewski, I.J. CHK2 Inhibition Provides a Strategy to Suppress Hematologic Toxicity from PARP Inhibitors. Mol. Cancer Res. 2021, 19, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Wang, J.; Mishail, D.; Wang, C.Y. Recent advancements in PARP inhibitors-based targeted cancer therapy. Precis. Clin. Med. 2020, 3, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Ghorai, A.; Mahaddalkar, T.; Thorat, R.; Dutt, S. Sustained inhibition of PARP-1 activity delays glioblastoma recurrence by enhancing radiation-induced senescence. Cancer Lett. 2020, 490, 44–53. [Google Scholar] [CrossRef]
- Venere, M.; Hamerlik, P.; Wu, Q.; Rasmussen, R.D.; Song, L.A.; Vasanji, A.; Tenley, N.; Flavahan, W.A.; Hjelmeland, A.B.; Bartek, J.; et al. Therapeutic targeting of constitutive PARP activation compromises stem cell phenotype and survival of glioblastoma-initiating cells. Cell Death Differ. 2014, 21, 258–269. [Google Scholar] [CrossRef]
- Lesueur, P.; Lequesne, J.; Grellard, J.M.; Dugué, A.; Coquan, E.; Brachet, P.E.; Geffrelot, J.; Kao, W.; Emery, E.; Berro, D.H.; et al. Phase I/IIa study of concomitant radiotherapy with olaparib and temozolomide in unresectable or partially resectable glioblastoma: OLA-TMZ-RTE-01 trial protocol. BMC Cancer 2019, 19, 198. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, A.J.; Gutierrez-Quintana, R.; Walker, D.J.; Williams, K.; Forster, D.; Jackson, M.R.; Derby, S.; Stobo, J.; Sweeting, L.; Kelly, C.; et al. Abstract IA-006: Enhancing the therapeutic ratio for glioblastoma by combining radiation therapy with PARP inhibitors. Clin. Cancer Res. 2021, 27, IA-006. [Google Scholar] [CrossRef]
- Barazzuol, L.; Jena, R.; Burnet, N.G.; Meira, L.B.; Jeynes, J.C.; Kirkby, K.J.; Kirkby, N.F. Evaluation of poly (ADP-ribose) polymerase inhibitor ABT-888 combined with radiotherapy and temozolomide in glioblastoma. Radiat. Oncol. 2013, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Dungey, F.A.; Löser, D.A.; Chalmers, A.J. Replication-dependent radiosensitization of human glioma cells by inhibition of poly(ADP-Ribose) polymerase: Mechanisms and therapeutic potential. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.L.; Kwon, H.C.; Burgan, W.E.; Carter, D.; Beam, K.; Weizheng, X.; Zhang, J.; Slusher, B.S.; Chakravarti, A.; Tofilon, P.J.; et al. In vitro and in vivo radiosensitization of glioblastoma cells by the poly (ADP-ribose) polymerase inhibitor E7016. Clin. Cancer Res. 2009, 15, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.J.; Mulligan, E.A.; Grogan, P.T.; Mladek, A.C.; Carlson, B.L.; Schroeder, M.A.; Curtin, N.J.; Lou, Z.; Decker, P.A.; Wu, W.; et al. Effective sensitization of temozolomide by ABT-888 is lost with development of temozolomide resistance in glioblastoma xenograft lines. Mol. Cancer Ther. 2009, 8, 407–414. [Google Scholar] [CrossRef]
- Lemasson, B.; Wang, H.; Galbán, S.; Li, Y.; Zhu, Y.; Heist, K.A.; Tsein, C.; Chenevert, T.L.; Rehemtulla, A.; Galbán, C.J.; et al. Evaluation of Concurrent Radiation, Temozolomide and ABT-888 Treatment Followed by Maintenance Therapy with Temozolomide and ABT-888 in a Genetically Engineered Glioblastoma Mouse Model. Neoplasia 2016, 18, 82–89. [Google Scholar] [CrossRef]
- Jue, T.R.; Nozue, K.; Lester, A.J.; Joshi, S.; Schroder, L.B.; Whittaker, S.P.; Nixdorf, S.; Rapkins, R.W.; Khasraw, M.; McDonald, K.L. Veliparib in combination with radiotherapy for the treatment of MGMT unmethylated glioblastoma. J. Transl. Med. 2017, 15, 61. [Google Scholar] [CrossRef]
- Albert, J.M.; Cao, C.; Kim, K.W.; Willey, C.D.; Geng, L.; Xiao, D.; Wang, H.; Sandler, A.; Johnson, D.H.; Colevas, A.D.; et al. Inhibition of poly(ADP-ribose) polymerase enhances cell death and improves tumor growth delay in irradiated lung cancer models. Clin. Cancer Res. 2007, 13, 3033–3042. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, M.; Mukhopadhyay, P.; Godlewski, G.; Bátkai, S.; Haskó, G.; Liaudet, L.; Pacher, P. Poly(ADP-ribose)polymerase inhibition decreases angiogenesis. Biochem. Biophys Res. Commun. 2006, 350, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.L.; Ricks, C.B.; Bohm, A.K.; Lun, X.; Maxwell, L.; Safdar, S.; Bukhari, S.; Gerber, A.; Sayeed, W.; Bering, E.A.; et al. ABT-888 restores sensitivity in temozolomide resistant glioma cells and xenografts. PLoS ONE 2018, 13, e0202860. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.L.; Meode, M.; Tan, M.; Maxwell, L.; Bering, E.A.; Pedersen, H.; Willms, J.; Liao, J.; Black, S.; Cairncross, J.G.; et al. PARP inhibition suppresses the emergence of temozolomide resistance in a model system. J. Neurooncol. 2020, 148, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Mladek, A.C.; Carlson, B.L.; Boakye-Agyeman, F.; Bakken, K.K.; Kizilbash, S.H.; Schroeder, M.A.; Reid, J.; Sarkaria, J.N. Discordant in vitro and in vivo chemopotentiating effects of the PARP inhibitor veliparib in temozolomide-sensitive versus -resistant glioblastoma multiforme xenografts. Clin. Cancer Res. 2014, 20, 3730–3741. [Google Scholar] [CrossRef] [PubMed]
- Kleinberg, L.; Supko, J.G.; Mikkelsen, T.; Blakeley, J.O.N.; Stevens, G.; Ye, X.; Desideri, S.; Ryu, S.; Desai, B.; Giranda, V.L.; et al. Phase I adult brain tumor consortium (ABTC) trial of ABT-888 (veliparib), temozolomide (TMZ), and radiotherapy (RT) for newly diagnosed glioblastoma multiforme (GBM) including pharmacokinetic (PK) data. J. Clin. Oncol. 2013, 31, 2065. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, X.; Li, X.; Liu, H.; Zhao, B.; Elkabets, M.; Liu, Y.; Wang, W.; Wang, R.; Zhong, Y.; et al. BKM120 sensitizes glioblastoma to the PARP inhibitor rucaparib by suppressing homologous recombination repair. Cell Death Dis. 2021, 12, 546. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.J.; Cooper, E.; Schweder, P.; Mee, E.; Turner, C.; Faull, R.; Denny, W.A.; Dragunow, M.; Park, T.I.; Jose, J. PARP inhibitor cyanine dye conjugate with enhanced cytotoxic and antiproliferative activity in patient derived glioblastoma cell lines. Bioorg. Med. Chem. Lett. 2020, 30, 127252. [Google Scholar] [CrossRef] [PubMed]
- Parrish, K.E.; Cen, L.; Murray, J.; Calligaris, D.; Kizilbash, S.; Mittapalli, R.K.; Carlson, B.L.; Schroeder, M.A.; Sludden, J.; Boddy, A.V.; et al. Efficacy of PARP Inhibitor Rucaparib in Orthotopic Glioblastoma Xenografts Is Limited by Ineffective Drug Penetration into the Central Nervous System. Mol. Cancer Ther. 2015, 14, 2735–2743. [Google Scholar] [CrossRef] [PubMed]
- Plummer, R.; Jones, C.; Middleton, M.; Wilson, R.; Evans, J.; Olsen, A.; Curtin, N.; Boddy, A.; McHugh, P.; Newell, D.; et al. Phase I study of the poly(ADP-ribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced solid tumors. Clin. Cancer Res. 2008, 14, 7917–7923. [Google Scholar] [CrossRef] [PubMed]
- Lesueur, P.; Chevalier, F.; El-Habr, E.A.; Junier, M.P.; Chneiweiss, H.; Castera, L.; Müller, E.; Stefan, D.; Saintigny, Y. Radiosensitization Effect of Talazoparib, a Parp Inhibitor, on Glioblastoma Stem Cells Exposed to Low and High Linear Energy Transfer Radiation. Sci. Rep. 2018, 8, 3664. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Yuan, B.; Chen, H.D.; Xu, L.; Tian, Y.N.; Zhang, A.; He, J.X.; Miao, Z.H. Acquired resistance of phosphatase and tensin homolog-deficient cells to poly(ADP-ribose) polymerase inhibitor and Ara-C mediated by 53BP1 loss and SAMHD1 overexpression. Cancer Sci. 2018, 109, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Gao, F.; Zheng, S.; Zhang, C.; Martinez-Ledesma, E.; Ezhilarasan, R.; Ding, J.; Li, X.; Feng, N.; Multani, A.; et al. EGFR Amplification Induces Increased DNA Damage Response and Renders Selective Sensitivity to Talazoparib (PARP Inhibitor) in Glioblastoma. Clin. Cancer Res. 2020, 26, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Kizilbash, S.H.; Gupta, S.K.; Chang, K.; Kawashima, R.; Parrish, K.E.; Carlson, B.L.; Bakken, K.K.; Mladek, A.C.; Schroeder, M.A.; Decker, P.A.; et al. Restricted Delivery of Talazoparib Across the Blood-Brain Barrier Limits the Sensitizing Effects of PARP Inhibition on Temozolomide Therapy in Glioblastoma. Mol. Cancer Ther. 2017, 16, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Sambade, M.J.; Van Swearingen, A.E.D.; McClure, M.B.; Deal, A.M.; Santos, C.; Sun, K.; Wang, J.; Mikule, K.; Anders, C.K. Efficacy and pharmacodynamics of niraparib in BRCA-mutant and wild-type intracranial triple-negative breast cancer murine models. Neuro-Oncol. Adv. 2019, 1, vdz005. [Google Scholar] [CrossRef] [PubMed]
- Miknyoczki, S.; Chang, H.; Grobelny, J.; Pritchard, S.; Worrell, C.; McGann, N.; Ator, M.; Husten, J.; Deibold, J.; Hudkins, R.; et al. The selective poly(ADP-ribose) polymerase-1(2) inhibitor, CEP-8983, increases the sensitivity of chemoresistant tumor cells to temozolomide and irinotecan but does not potentiate myelotoxicity. Mol. Cancer Ther. 2007, 6, 2290–2302. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Pamiparib: First Approval. Drugs 2021, 81, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Plummer, R.; Stephens, P.; Aissat-Daudigny, L.; Cambois, A.; Moachon, G.; Brown, P.D.; Campone, M. Phase 1 dose-escalation study of the PARP inhibitor CEP-9722 as monotherapy or in combination with temozolomide in patients with solid tumors. Cancer Chemothe.r Pharmacol. 2014, 74, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.A.; Kossatz, S.; Weber, W.; Beheshti, M.; Morgenroth, A.; Mottaghy, F.M. Advancements in PARP1 Targeted Nuclear Imaging and Theranostic Probes. J. Clin. Med. 2020, 9, 2130. [Google Scholar] [CrossRef] [PubMed]
- Puentes, L.N.; Makvandi, M.; Mach, R.H. Molecular Imaging: PARP-1 and Beyond. J. Nucl. Med. 2021, 62, 765–770. [Google Scholar] [CrossRef]
- Thisgaard, H.; Halle, B.; Aaberg-Jessen, C.; Olsen, B.B.; Therkelsen, A.S.; Dam, J.H.; Langkjær, N.; Munthe, S.; Någren, K.; Høilund-Carlsen, P.F.; et al. Highly Effective Auger-Electron Therapy in an Orthotopic Glioblastoma Xenograft Model using Convection-Enhanced Delivery. Theranostics 2016, 6, 2278–2291. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wilson, T.C.; Jannetti, S.A.; Guru, N.; Pillarsetty, N.; Reiner, T.; Pirovano, G. Improved radiosynthesis of (123)I-MAPi, an auger theranostic agent. Int. J. Radiat. Biol. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Damia, G. Targeting DNA-PK in cancer. Mutat. Res. 2020, 821, 111692. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Huang, Z.; Zhai, K.; Huang, Q.; Tao, W.; Kim, L.; Wu, Q.; Almasan, A.; Yu, J.S.; Li, X.; et al. Inhibiting DNA-PK induces glioma stem cell differentiation and sensitizes glioblastoma to radiation in mice. Sci. Transl. Med. 2021, 13, eabc7275. [Google Scholar] [CrossRef] [PubMed]
- Kase, M.; Vardja, M.; Lipping, A.; Asser, T.; Jaal, J. Impact of PARP-1 and DNA-PK expression on survival in patients with glioblastoma multiforme. Radiother. Oncol. 2011, 101, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, H.; Liu, T.; Huang, M.; Butter, P.P.; Li, C.; Zhang, L.; Kao, G.D.; Gong, Y.; Maity, A.; et al. Temporal DNA-PK activation drives genomic instability and therapy resistance in glioma stem cells. JCI Insight 2018, 3, e98096. [Google Scholar] [CrossRef]
- Sun, Q.; Guo, Y.; Liu, X.; Czauderna, F.; Carr, M.I.; Zenke, F.T.; Blaukat, A.; Vassilev, L.T. Therapeutic Implications of p53 Status on Cancer Cell Fate Following Exposure to Ionizing Radiation and the DNA-PK Inhibitor M3814. Mol. Cancer Res. 2019, 17, 2457–2468. [Google Scholar] [CrossRef]
- Pospisilova, M.; Seifrtova, M.; Rezacova, M. Small molecule inhibitors of DNA-PK for tumor sensitization to anticancer therapy. J. Physiol. Pharmacol. 2017, 68, 337–344. [Google Scholar] [PubMed]
- Medová, M.; Medo, M.; Hovhannisyan, L.; Muñoz-Maldonado, C.; Aebersold, D.M.; Zimmer, Y. DNA-PK in human malignant disorders: Mechanisms and implications for pharmacological interventions. Pharmacol. Ther. 2020, 215, 107617. [Google Scholar] [CrossRef] [PubMed]
- Zenke, F.T.; Zimmermann, A.; Sirrenberg, C.; Dahmen, H.; Kirkin, V.; Pehl, U.; Grombacher, T.; Wilm, C.; Fuchss, T.; Amendt, C.; et al. Pharmacologic Inhibitor of DNA-PK, M3814, Potentiates Radiotherapy and Regresses Human Tumors in Mouse Models. Mol. Cancer Ther. 2020, 19, 1091–1101. [Google Scholar] [CrossRef]
- van Bussel, M.T.J.; Awada, A.; de Jonge, M.J.A.; Mau-Sørensen, M.; Nielsen, D.; Schöffski, P.; Verheul, H.M.W.; Sarholz, B.; Berghoff, K.; El Bawab, S.; et al. A first-in-man phase 1 study of the DNA-dependent protein kinase inhibitor peposertib (formerly M3814) in patients with advanced solid tumours. Br. J. Cancer 2021, 124, 728–735. [Google Scholar] [CrossRef]
- Timme, C.R.; Rath, B.H.; O’Neill, J.W.; Camphausen, K.; Tofilon, P.J. The DNA-PK Inhibitor VX-984 Enhances the Radiosensitivity of Glioblastoma Cells Grown In Vitro and as Orthotopic Xenografts. Mol. Cancer Ther. 2018, 17, 1207–1216. [Google Scholar] [CrossRef]
- Khan, A.J.; Misenko, S.M.; Thandoni, A.; Schiff, D.; Jhawar, S.R.; Bunting, S.F.; Haffty, B.G. VX-984 is a selective inhibitor of non-homologous end joining, with possible preferential activity in transformed cells. Oncotarget 2018, 9, 25833–25841. [Google Scholar] [CrossRef]
- Fok, J.H.L.; Ramos-Montoya, A.; Vazquez-Chantada, M.; Wijnhoven, P.W.G.; Follia, V.; James, N.; Farrington, P.M.; Karmokar, A.; Willis, S.E.; Cairns, J.; et al. AZD7648 is a potent and selective DNA-PK inhibitor that enhances radiation, chemotherapy and olaparib activity. Nat. Commun. 2019, 10, 5065. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Karmokar, A.; Farrington, P.M.; James, N.H.; Ramos-Montoya, A.; Bickerton, S.J.; Hughes, G.D.; Illidge, T.M.; Cadogan, E.B.; Davies, B.R.; et al. Inhibition of DNA-PK with AZD7648 Sensitizes Tumor Cells to Radiotherapy and Induces Type I IFN-Dependent Durable Tumor Control. Clin. Cancer Res. 2021, 27, 4353–4366. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Sapinoso, L.M.; Tran, T.; Gaffney, B.; Wong, L.; Sankar, S.; Raymon, H.K.; Mortensen, D.S.; Xu, S. CC-115, a dual inhibitor of mTOR kinase and DNA-PK, blocks DNA damage repair pathways and selectively inhibits ATM-deficient cell growth in vitro. Oncotarget 2017, 8, 74688–74702. [Google Scholar] [CrossRef] [PubMed]
- Munster, P.; Mita, M.; Mahipal, A.; Nemunaitis, J.; Massard, C.; Mikkelsen, T.; Cruz, C.; Paz-Ares, L.; Hidalgo, M.; Rathkopf, D.; et al. First-In-Human Phase I Study Of A Dual mTOR Kinase And DNA-PK Inhibitor (CC-115) In Advanced Malignancy. Cancer Manag. Res. 2019, 11, 10463–10476. [Google Scholar] [CrossRef]
- Alexander, B.M.; Trippa, L.; Gaffey, S.; Arrillaga-Romany, I.C.; Lee, E.Q.; Rinne, M.L.; Ahluwalia, M.S.; Colman, H.; Fell, G.; Galanis, E.; et al. Individualized Screening Trial of Innovative Glioblastoma Therapy (INSIGhT): A Bayesian Adaptive Platform Trial to Develop Precision Medicines for Patients With Glioblastoma. JCO Precis. Oncol. 2019, 3, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Rasco, D.W.; Papadopoulos, K.P.; Pourdehnad, M.; Gandhi, A.K.; Hagner, P.R.; Li, Y.; Wei, X.; Chopra, R.; Hege, K.; DiMartino, J.; et al. A First-in-Human Study of Novel Cereblon Modulator Avadomide (CC-122) in Advanced Malignancies. Clin. Cancer Res. 2019, 25, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Bendell, J.C.; Varghese, A.M.; Hyman, D.M.; Bauer, T.M.; Pant, S.; Callies, S.; Lin, J.; Martinez, R.; Wickremsinhe, E.; Fink, A.; et al. A First-in-Human Phase 1 Study of LY3023414, an Oral PI3K/mTOR Dual Inhibitor, in Patients with Advanced Cancer. Clin. Cancer Res. 2018, 24, 3253–3262. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.M.; Hyman, D.M.; Caird, I.; Won, H.; Soldan, K.; Seier, K.; Iasonos, A.; Tew, W.P.; O’Cearbhaill, R.E.; Grisham, R.N.; et al. Phase 2 study of LY3023414 in patients with advanced endometrial cancer harboring activating mutations in the PI3K pathway. Cancer 2020, 126, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.P.; Hung, C.Y.; Lusvarghi, S.; Huang, Y.H.; Tseng, P.J.; Hung, T.H.; Yu, J.S.; Ambudkar, S.V. Overexpression of ABCB1 and ABCG2 contributes to reduced efficacy of the PI3K/mTOR inhibitor samotolisib (LY3023414) in cancer cell lines. Biochem. Pharmacol. 2020, 180, 114137. [Google Scholar] [CrossRef] [PubMed]
- Kopa, P.; Macieja, A.; Gulbas, I.; Pastwa, E.; Poplawski, T. Inhibition of DNA-PK potentiates the synergistic effect of NK314 and etoposide combination on human glioblastoma cells. Mol. Biol. Rep. 2020, 47, 67–76. [Google Scholar] [CrossRef]
- Ismail, I.H.; Mårtensson, S.; Moshinsky, D.; Rice, A.; Tang, C.; Howlett, A.; McMahon, G.; Hammarsten, O. SU11752 inhibits the DNA-dependent protein kinase and DNA double-strand break repair resulting in ionizing radiation sensitization. Oncogene 2004, 23, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Zhao, Z.; Qu, Y.; Zhang, M.; Wang, H.; Zhang, Z.; Zhou, W.; Fan, X.; Yu, C.; Zhan, Q.; et al. Targeting hyperactivated DNA-PKcs by KU0060648 inhibits glioma progression and enhances temozolomide therapy via suppression of AKT signaling. Oncotarget 2016, 7, 55555–55571. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Sun, C.; Zhang, H.; Miao, G.; Di, C.X.; Zhou, X.; Zhou, R.; Wang, Z. DNA-PKcs deficiency inhibits glioblastoma cell-derived angiogenesis after ionizing radiation. J. Cell Physiol. 2015, 230, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Quiros, S.; Roos, W.P.; Kaina, B. Rad51 and BRCA2--New molecular targets for sensitizing glioma cells to alkylating anticancer drugs. PLoS ONE 2011, 6, e27183. [Google Scholar] [CrossRef] [PubMed]
- Poty, S.; Mandleywala, K.; O’Neill, E.; Knight, J.C.; Cornelissen, B.; Lewis, J.S. (89)Zr-PET imaging of DNA double-strand breaks for the early monitoring of response following α- and β-particle radioimmunotherapy in a mouse model of pancreatic ductal adenocarcinoma. Theranostics 2020, 10, 5802–5814. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.; Kersemans, V.; Allen, P.D.; Terry, S.Y.A.; Torres, J.B.; Mosley, M.; Smart, S.; Lee, B.Q.; Falzone, N.; Vallis, K.A.; et al. Imaging DNA Damage Repair In Vivo After (177)Lu-DOTATATE Therapy. J. Nucl. Med. 2020, 61, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.J.; Bajrami, I.; Lord, C.J. Synthetic Lethality and Cancer-Penetrance as the Major Barrier. Trends Cancer 2018, 4, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Galia, A.; Calogero, A.E.; Condorelli, R.; Fraggetta, F.; La Corte, A.; Ridolfo, F.; Bosco, P.; Castiglione, R.; Salemi, M. PARP-1 protein expression in glioblastoma multiforme. Eur. J. Histochem. 2012, 56, e9. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Carter, C.A.; Kelly, R.J.; Gutierrez, M.; Kummar, S.; Szabo, E.; Yancey, M.A.; Ji, J.; Mannargudi, B.; Woo, S.; et al. A phase I combination study of olaparib with cisplatin and gemcitabine in adults with solid tumors. Clin. Cancer Res. 2012, 18, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, M.S.; Bartelink, I.H.; Aggarwal, R.R.; Leng, J.; Zhang, J.Z.; Pawlowska, N.; Terranova-Barberio, M.; Grabowsky, J.A.; Gewitz, A.; Chien, A.J.; et al. Differential Toxicity in Patients with and without DNA Repair Mutations: Phase I Study of Carboplatin and Talazoparib in Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 6400–6410. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Das, T.; Suman, S.K.; Kumar, C.; Sarma, H.D.; Dash, A. Targeted Tumor Therapy with Radiolabeled DNA Intercalator: A Possibility? Preclinical Investigations with (177)Lu-Acridine. Biomed. Res. Int. 2020, 2020, 9514357. [Google Scholar] [CrossRef] [PubMed]
- Kubalanza, K.; Konecny, G.E. Mechanisms of PARP inhibitor resistance in ovarian cancer. Curr. Opin. Obstet. Gynecol. 2020, 32, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.K.; Harrell, M.I.; Oza, A.M.; Oaknin, A.; Ray-Coquard, I.; Tinker, A.V.; Helman, E.; Radke, M.R.; Say, C.; Vo, L.T.; et al. BRCA Reversion Mutations in Circulating Tumor DNA Predict Primary and Acquired Resistance to the PARP Inhibitor Rucaparib in High-Grade Ovarian Carcinoma. Cancer Discov. 2019, 9, 210–219. [Google Scholar] [CrossRef]
- Ruiz, S.; Mayor-Ruiz, C.; Lafarga, V.; Murga, M.; Vega-Sendino, M.; Ortega, S.; Fernandez-Capetillo, O. A Genome-wide CRISPR Screen Identifies CDC25A as a Determinant of Sensitivity to ATR Inhibitors. Mol. Cell 2016, 62, 307–313. [Google Scholar] [CrossRef]
- Henssen, A.G.; Reed, C.; Jiang, E.; Garcia, H.D.; von Stebut, J.; MacArthur, I.C.; Hundsdoerfer, P.; Kim, J.H.; de Stanchina, E.; Kuwahara, Y.; et al. Therapeutic targeting of PGBD5-induced DNA repair dependency in pediatric solid tumors. Sci. Transl. Med. 2017, 9, aam9078. [Google Scholar] [CrossRef] [PubMed]
- Hinrichsen, I.; Ackermann, A.; Düding, T.; Graband, A.; Filmann, N.; Plotz, G.; Zeuzem, S.; Brieger, A. Loss of MLH1 sensitizes colon cancer cells to DNA-PKcs inhibitor KU60648. Mol. Carcinog. 2017, 56, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Beebe, J.; Zhang, J.T. CC-115, a Dual Mammalian Target of Rapamycin/DNA-Dependent Protein Kinase Inhibitor in Clinical Trial, Is a Substrate of ATP-Binding Cassette G2, a Risk Factor for CC-115 Resistance. J. Pharmacol. Exp. Ther. 2019, 371, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Shirakami, Y.; Watabe, T.; Obata, H.; Kaneda, K.; Ooe, K.; Liu, Y.; Teramoto, T.; Toyoshima, A.; Shinohara, A.; Shimosegawa, E.; et al. Synthesis of [211At]4-astato-L-phenylalanine by dihydroxyboryl-astatine substitution reaction in aqueous solution. Sci. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef]
- Pozzi, O.R.; Zalutsky, M.R. Radiopharmaceutical chemistry of targeted radiotherapeutics, part 4: Strategies for (211)At labeling at high activities and radiation doses of (211)At α-particles. Nucl. Med. Biol. 2017, 46, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Filippone, N.R.; Reiner, T.; Roberts, S. Sensors and Inhibitors for the Detection of Ataxia Telangiectasia Mutated (ATM) Protein Kinase. Mol. Pharm. 2021, 18, 2470–2481. [Google Scholar] [CrossRef] [PubMed]
- Degorce, S.L.; Barlaam, B.; Cadogan, E.; Dishington, A.; Ducray, R.; Glossop, S.C.; Hassall, L.A.; Lach, F.; Lau, A.; McGuire, T.M.; et al. Discovery of Novel 3-Quinoline Carboxamides as Potent, Selective, and Orally Bioavailable Inhibitors of Ataxia Telangiectasia Mutated (ATM) Kinase. J. Med. Chem. 2016, 59, 6281–6292. [Google Scholar] [CrossRef] [PubMed]
- Pike, K.G.; Barlaam, B.; Cadogan, E.; Campbell, A.; Chen, Y.; Colclough, N.; Davies, N.L.; de-Almeida, C.; Degorce, S.L.; Didelot, M.; et al. The Identification of Potent, Selective, and Orally Available Inhibitors of Ataxia Telangiectasia Mutated (ATM) Kinase: The Discovery of AZD0156 (8-{6-[3-(Dimethylamino)propoxy]pyridin-3-yl}-3-methyl-1-(tetrahydro-2H-pyran-4-yl)-1,3-dihydro-2H-imidazo[4,5-c]quinolin-2-one). J. Med. Chem. 2018, 61, 3823–3841. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Thomas, S.E.; Chaplin, A.K.; Hardwick, S.W.; Chirgadze, D.Y.; Blundell, T.L. Structural insights into inhibitor regulation of the DNA repair protein DNA-PKcs. Nature 2022, 601, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.I.; Eve, P.D.; Hayes, A.; Valenti, M.; De Haven Brandon, A.; Box, G.; Boxall, K.J.; Aherne, G.W.; Eccles, S.A.; Raynaud, F.I.; et al. The preclinical pharmacology and therapeutic activity of the novel CHK1 inhibitor SAR-020106. Mol. Cancer Ther. 2010, 9, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Yamamori, T.; Bo, T.; Sakai, Y.; Inanami, O. MK-8776, a novel Chk1 inhibitor, exhibits an improved radiosensitizing effect compared to UCN-01 by exacerbating radiation-induced aberrant mitosis. Transl. Oncol. 2017, 10, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Guzi, T.J.; Paruch, K.; Dwyer, M.P.; Labroli, M.; Shanahan, F.; Davis, N.; Taricani, L.; Wiswell, D.; Seghezzi, W.; Penaflor, E.; et al. Targeting the replication checkpoint using SCH 900776, a potent and functionally selective CHK1 inhibitor identified via high content screening. Mol. Cancer Ther. 2011, 10, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.R.; Yang, Z.Z.; Wang, S.J.; Zhang, L.; Luo, J.R.; Feng, Y.; Yu, X.L.; Chen, X.X.; Guo, X.M. The Chk1 inhibitor MK-8776 increases the radiosensitivity of human triple-negative breast cancer by inhibiting autophagy. Acta Pharmacol. Sin. 2017, 38, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Labroli, M.A.; Dwyer, M.P.; Poker, C.; Keertikar, K.M.; Rossman, R.; Guzi, T.J. A convergent preparation of the CHK1 inhibitor MK-8776 (SCH 900776). Tetrahedron. Lett. 2016, 57, 2601–2603. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).