Prostate Cancer and Sleep Disorders: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Definition, Diagnosis and Underlying Mechanisms of Sleep Disturbances in Cancer Patients and, in Particular, Prostate Cancer Patients
1.2. Methodologies for Investigating Sleep Disturbances in Prostate Cancer Patients
1.3. Rationale and Objectives of the Systematic Review
2. Materials and Methods
2.1. Literature Search Method and Evidence Acquisition
2.2. Quality Assessment
3. Results
3.1. Results of the Literature Search
Characteristics of the Studies Included
3.2. Evidence Synthesis of Prostate Cancer Treatments and Sleep Disorders
3.2.1. Androgen Deprivation Therapy (ADT)
3.2.2. Radiotherapy for Localized PCa (Primary Curative or Adjuvant)
3.2.3. ADT Combined with Radiotherapy (Primary Curative or Adjuvant)
3.2.4. Prostatectomy
3.2.5. Novel Hormonal Agents
4. Discussion
4.1. Limitations and Strengths
4.2. Suggestions for Future Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korfage, I.J.; Essink-Bot, M.L.; Borsboom, G.J.; Madalinska, J.B.; Kirkels, W.J.; Habbema, J.D.; Schroder, F.H.; de Koning, H.J. Five-year follow-up of health-related quality of life after primary treatment of localized prostate cancer. Int. J. Cancer 2005, 116, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Tobaldini, E.; Costantino, G.; Solbiati, M.; Cogliati, C.; Kara, T.; Nobili, L.; Montano, N. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci. Biobehav. Rev. 2017, 74, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Roth, T. Insomnia: Definition, prevalence, etiology, and consequences. J. Clin. Sleep Med. 2007, 3, S7–S10. [Google Scholar] [CrossRef] [Green Version]
- Chee, M.W.; Chuah, L.Y.; Venkatraman, V.; Chan, W.Y.; Philip, P.; Dinges, D.F. Functional imaging of working memory following normal sleep and after 24 and 35 h of sleep deprivation: Correlations of fronto-parietal activation with performance. NeuroImage 2006, 31, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Killgore, W.D. Effects of sleep deprivation on cognition. Prog. Brain Res. 2010, 185, 105–129. [Google Scholar] [CrossRef]
- Savard, J.; Morin, C.M. Insomnia in the context of cancer: A review of a neglected problem. J. Clin. Oncol. 2001, 19, 895–908. [Google Scholar] [CrossRef]
- Matthews, E.E.; Tanner, J.M.; Dumont, N.A. Sleep Disturbances in Acutely Ill Patients with Cancer. Crit. Care Nurs. Clin. N. Am. 2016, 28, 253–268. [Google Scholar] [CrossRef]
- Doghramji, K. The epidemiology and diagnosis of insomnia. Am. J. Manag. Care 2006, 12, S214–S220. [Google Scholar]
- Savard, J.; Ivers, H.; Villa, J.; Caplette-Gingras, A.; Morin, C.M. Natural course of insomnia comorbid with cancer: An 18-month longitudinal study. J. Clin. Oncol. 2011, 29, 3580–3586. [Google Scholar] [CrossRef]
- Morin, C.M.; LeBlanc, M.; Daley, M.; Gregoire, J.P.; Mérette, C. Epidemiology of insomnia: Prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006, 7, 123–130. [Google Scholar] [CrossRef]
- Ohayon, M.M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med. Rev. 2002, 6, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carioli, G.; Bertuccio, P.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E.; Malvezzi, M. European cancer mortality predictions for the year 2020 with a focus on prostate cancer. Ann. Oncol. 2020, 31, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Pineros, M.; Znaor, A.; Soerjomataram, I. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- Savard, J.; Simard, S.; Hervouet, S.; Ivers, H.; Lacombe, L.; Fradet, Y. Insomnia in men treated with radical prostatectomy for prostate cancer. Psychooncology 2005, 14, 147–156. [Google Scholar] [CrossRef]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J. Clin. 1972, 22, 232–240. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [Green Version]
- de Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.R.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N. Engl. J. Med. 2018, 378, 1408–1418. [Google Scholar] [CrossRef]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef] [Green Version]
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 2974–2986. [Google Scholar] [CrossRef] [PubMed]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): Final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019, 20, 686–700. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.E.; Chen, Y.H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J. Clin. Oncol. 2018, 36, 1080–1087. [Google Scholar] [CrossRef] [Green Version]
- Lardas, M.; Liew, M.; van den Bergh, R.C.; De Santis, M.; Bellmunt, J.; Van den Broeck, T.; Cornford, P.; Cumberbatch, M.G.; Fossati, N.; Gross, T.; et al. Quality of Life Outcomes after Primary Treatment for Clinically Localised Prostate Cancer: A Systematic Review. Eur. Urol. 2017, 72, 869–885. [Google Scholar] [CrossRef]
- Esper, P.; Mo, F.; Chodak, G.; Sinner, M.; Cella, D.; Pienta, K.J. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology 1997, 50, 920–928. [Google Scholar] [CrossRef]
- van Andel, G.; Bottomley, A.; Fossa, S.D.; Efficace, F.; Coens, C.; Guerif, S.; Kynaston, H.; Gontero, P.; Thalmann, G.; Akdas, A.; et al. An international field study of the EORTC QLQ-PR25: A questionnaire for assessing the health-related quality of life of patients with prostate cancer. Eur. J. Cancer 2008, 44, 2418–2424. [Google Scholar] [CrossRef]
- Rico-Rosillo, M.; Vega-Robledo, G. Sleep and immune system. Rev. Alerg. Mex. 2018, 65, 160–170. [Google Scholar] [PubMed] [Green Version]
- Reutrakul, S.; van Cauter, E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism 2018, 84, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Koren, D.; Taveras, E.M. Association of sleep disturbances with obesity, insulin resistance and the metabolic syndrome. Metabolism 2018, 84, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Micić, D.D.; Šumarac-Dumanović, M.; Šušić, V.; Pejković, D.; Polovina, S. Sleep and metabolic disorders. Glas. Srp. Akad. Nauka Med. 2011, 51, 5–25. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef]
- Graci, G. Pathogenesis and management of cancer-related insomnia. J. Support. Oncol. 2005, 3, 349–359. [Google Scholar]
- Sharpley, C.F.; Christie, D.R.H.; Bitsika, V.; Miller, B.J. Trajectories of total depression and depressive symptoms in prostate cancer patients receiving six months of hormone therapy. Psycho-Oncology 2017, 26, 60–66. [Google Scholar] [CrossRef]
- Savard, J.; Hervouet, S.; Ivers, H. Prostate cancer treatments and their side effects are associated with increased insomnia. Psycho-Oncology 2013, 22, 1381–1388. [Google Scholar] [CrossRef]
- Dosani, M.; Morris, W.J.; Tyldesley, S.; Pickles, T. The Relationship between Hot Flashes and Testosterone Recovery after 12 Months of Androgen Suppression for Men with Localised Prostate Cancer in the ASCENDE-RT Trial. Clin. Oncol. 2017, 29, 696–701. [Google Scholar] [CrossRef]
- Spielman, A.J.; Glovisky, P.B. The Varied Nature of Insomnia. In Case Studies in Insomnia; Hauri, P.J., Ed.; Plenum Press: New York, NY, USA, 1991; pp. 1–15. [Google Scholar]
- Howell, D.; Oliver, T.K.; Keller-Olaman, S.; Davidson, J.R.; Garland, S.; Samuels, C.; Savard, J.; Harris, C.; Aubin, M.; Olson, K.; et al. Sleep disturbance in adults with cancer: A systematic review of evidence for best practices in assessment and management for clinical practice. Ann. Oncol. 2014, 25, 791–800. [Google Scholar] [CrossRef]
- Santoso, A.M.M.; Jansen, F.; de Vries, R.; Leemans, C.R.; van Straten, A.; Verdonck-de Leeuw, I.M. Prevalence of sleep disturbances among head and neck cancer patients: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 47, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, S.D.; Song, J.; Gatus, L.; Lu, K.H.; Basen-Engquist, K.M. Endometrial cancer survivors’ sleep patterns before and after a physical activity intervention: A retrospective cohort analysis. Gynecol. Oncol. 2018, 149, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, A.; Acebo, C. The role of actigraphy in sleep medicine. Sleep Med. Rev. 2002, 6, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, S.; Conley, S.; Redeker, N.S. Discrepancy between wrist-actigraph and polysomnographic measures of sleep in patients with stable heart failure and a novel approach to evaluating discrepancy. J. Sleep Res. 2019, 28, e12717. [Google Scholar] [CrossRef]
- Costa, A.R.; Fontes, F.; Pereira, S.; Gonçalves, M.; Azevedo, A.; Lunet, N. Impact of breast cancer treatments on sleep disturbances—A systematic review. Breast 2014, 23, 697–709. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Savard, J.; Ivers, H.; Savard, M.-H.; Morin, C.M. Cancer treatments and their side effects are associated with aggravation of insomnia: Results of a longitudinal study. Cancer 2015, 121, 1703–1711. [Google Scholar] [CrossRef]
- Gonzalez, B.D.; Small, B.J.; Cases, M.G.; Williams, N.L.; Fishman, M.N.; Jacobsen, P.B.; Jim, H.S.L. Sleep disturbance in men receiving androgen deprivation therapy for prostate cancer: The role of hot flashes and nocturia. Cancer 2018, 124, 499–506. [Google Scholar] [CrossRef]
- Hanisch, L.J.; Gehrman, P.R. Circadian rhythm of hot flashes and activity levels among prostate cancer patients on androgen deprivation therapy. Aging Male 2011, 14, 243–248. [Google Scholar] [CrossRef]
- Koskderelioglu, A.; Gedizlioglu, M.; Ceylan, Y.; Gunlusoy, B.; Kahyaoglu, N. Quality of sleep in patients receiving androgen deprivation therapy for prostate cancer. Neurol. Sci. 2017, 38, 1445–1451. [Google Scholar] [CrossRef]
- Saini, A.; Berruti, A.; Cracco, C.; Sguazzotti, E.; Porpiglia, F.; Russo, L.; Bertaglia, V.; Picci, R.L.; Negro, M.; Tosco, A.; et al. Psychological distress in men with prostate cancer receiving adjuvant androgen-deprivation therapy. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Challapalli, A.; Edwards, S.M.; Abel, P.; Mangar, S.A. Evaluating the prevalence and predictive factors of vasomotor and psychological symptoms in prostate cancer patients receiving hormonal therapy: Results from a single institution experience. Urol. Oncol. Semin. Orig. Investig. 2018, 10, 29–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miaskowski, C.; Paul, S.M.; Cooper, B.A.; Lee, K.; Dodd, M.; West, C.; Aouizerat, B.E.; Dunn, L.; Swift, P.S.; Wara, W. Predictors of the Trajectories of Self-Reported Sleep Disturbance in Men with Prostate Cancer During and Following Radiation Therapy. Sleep 2011, 34, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Thomas, K.S.; Motivala, S.; Olmstead, R.; Irwin, M.R. Sleep depth and fatigue: Role of cellular inflammatory activation. Brain Behav. Immun. 2011, 25, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Garrett, K.; Dhruva, A.; Koetters, T.; West, C.; Paul, S.M.; Dunn, L.B.; Aouizerat, B.E.; Cooper, B.A.; Dodd, M.; Lee, K.; et al. Differences in sleep disturbance and fatigue between patients with breast and prostate cancer at the initiation of radiation therapy. J. Pain Symptom Manag. 2011, 42, 239–250. [Google Scholar] [CrossRef]
- Holliday, E.B.; Dieckmann, N.F.; McDonald, T.L.; Hung, A.Y.; Thomas, C.R., Jr.; Wood, L.J. Relationship between fatigue, sleep quality and inflammatory cytokines during external beam radiation therapy for prostate cancer: A prospective study. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2016, 118, 105–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maguire, R.; Drummond, F.J.; Hanly, P.; Gavin, A.; Sharp, L. Problems sleeping with prostate cancer: Exploring possible risk factors for sleep disturbance in a population-based sample of survivors. Support. Care Cancer 2019, 27, 3365–3373. [Google Scholar] [CrossRef] [Green Version]
- Hervouet, S.; Savard, J.; Simard, S.; Ivers, H.; Laverdière, J.; Vigneault, E.; Fradet, Y.; Lacombe, L. Psychological functioning associated with prostate cancer: Cross-sectional comparison of patients treated with radiotherapy, brachytherapy, or surgery. J. Pain Symptom Manag. 2005, 30, 474–484. [Google Scholar] [CrossRef]
- Tulk, J.; Rash, J.A.; Thoms, J.; Wassersug, R.; Gonzalez, B.; Garland, S.N. Androgen deprivation therapy and radiation for prostate cancer—Cognitive impairment, sleep, symptom burden: A prospective study. BMJ Support. Palliat. Care 2021. online ahead of print. [Google Scholar] [CrossRef]
- Sánchez-Martínez, V.; Buigues, C.; Navarro-Martínez, R.; García-Villodre, L.; Jeghalef, N.; Serrano-Carrascosa, M.; Rubio-Briones, J.; Cauli, O. Analysis of Brain Functions in Men with Prostate Cancer under Androgen Deprivation Therapy: A One-Year Longitudinal Study. Life 2021, 11, 227. [Google Scholar] [CrossRef]
- Hanisch, L.J.; Gooneratne, N.S.; Soin, K.; Gehrman, P.R.; Vaughn, D.J.; Coyne, J.C. Sleep and daily functioning during androgen deprivation therapy for prostate cancer. Eur. J. Cancer Care 2011, 20, 549–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, K.S.; Bower, J.; Hoyt, M.A.; Sepah, S. Disrupted sleep in breast and prostate cancer patients undergoing radiation therapy: The role of coping processes. Psycho-Oncology 2010, 19, 767–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horwitz, E.M.; Bae, K.; Hanks, G.E.; Porter, A.; Grignon, D.J.; Brereton, H.D.; Venkatesan, V.; Lawton, C.A.; Rosenthal, S.A.; Sandler, H.M.; et al. Ten-Year Follow-Up of Radiation Therapy Oncology Group Protocol 92-02: A Phase III Trial of the Duration of Elective Androgen Deprivation in Locally Advanced Prostate Cancer. J. Clin. Oncol. 2008, 26, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.U.; Hunt, D.; McGowan, D.G.; Amin, M.B.; Chetner, M.P.; Bruner, D.W.; Leibenhaut, M.H.; Husain, S.M.; Rotman, M.; Souhami, L.; et al. Radiotherapy and Short-Term Androgen Deprivation for Localized Prostate Cancer. N. Engl. J. Med. 2011, 365, 107–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amico, A.V.; Chen, M.-H.; Renshaw, A.; Loffredo, M.; Kantoff, P.W. Long-term Follow-up of a Randomized Trial of Radiation With or Without Androgen Deprivation Therapy for Localized Prostate Cancer. JAMA 2015, 314, 1291–1293. [Google Scholar] [CrossRef] [PubMed]
- Killock, D. Benefits of adding ADT to RT confirmed. Nat. Rev. Clin. Oncol. 2020, 17, 7. [Google Scholar] [CrossRef]
- Mottet, N.; Peneau, M.; Mazeron, J.J.; Molinie, V.; Richaud, P. Addition of radiotherapy to long-term androgen deprivation in locally advanced prostate cancer: An open randomised phase 3 trial. Eur. Urol. 2012, 62, 213–219. [Google Scholar] [CrossRef]
- Warde, P.; Mason, M.; Ding, K.; Kirkbride, P.; Brundage, M.; Cowan, R.; Gospodarowicz, M.; Sanders, K.; Kostashuk, E.; Swanson, G.; et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: A randomised, phase 3 trial. Lancet 2011, 378, 2104–2111. [Google Scholar] [CrossRef] [Green Version]
- Widmark, A.; Klepp, O.; Solberg, A.; Damber, J.-E.; Angelsen, A.; Fransson, P.; Lund, J.-Å.; Tasdemir, I.; Hoyer, M.; Wiklund, F.; et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): An open randomised phase III trial. Lancet 2009, 373, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Labrize, F.; Cany, L.; Massard, C.; Loriot, Y.; Sargos, P.; Gross-Goupil, M.; Roubaud, G. Enzalutamide and sleep apnea: An emerging central nervous system side-effect? Ann. Oncol. 2016, 27, 206. [Google Scholar] [CrossRef]
- Tombal, B.; Saad, F.; Penson, D.; Hussain, M.; Sternberg, C.N.; Morlock, R.; Ramaswamy, K.; Ivanescu, C.; Attard, G. Patient-reported outcomes following enzalutamide or placebo in men with non-metastatic, castration-resistant prostate cancer (PROSPER): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2019, 20, 556–569. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Evans, C.P.; Kim, C.S.; Kimura, G.; et al. Enzalutamide in Men with Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur. Urol. 2017, 71, 151–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2019, 380, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez Soto, Á.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef]
- Fizazi, K.; Scher, H.I.; Molina, A.; Logothetis, C.J.; Chi, K.N.; Jones, R.J.; Staffurth, J.N.; North, S.; Vogelzang, N.J.; Saad, F.; et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012, 13, 983–992. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; Fizazi, K.; Saad, F.; Mulders, P.F.; Sternberg, C.N.; Miller, K.; Logothetis, C.J.; Shore, N.D.; Small, E.J.; et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015, 16, 152–160. [Google Scholar] [CrossRef]
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.S.; Attard, G.; Chowdhury, S.; Cross, W.; et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomised controlled phase 3 trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef] [Green Version]
- Hershman, D.L.; Unger, J.M.; Wright, J.D.; Ramsey, S.; Till, C.; Tangen, C.M.; Barlow, W.E.; Blanke, C.; Thompson, I.M.; Hussain, M. Adverse Health Events Following Intermittent and Continuous Androgen Deprivation in Patients With Metastatic Prostate Cancer. JAMA Oncol. 2016, 2, 453–461. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep 2010, 33, 585–592. [Google Scholar] [CrossRef]
- Jike, M.; Itani, O.; Watanabe, N.; Buysse, D.J.; Kaneita, Y. Long sleep duration and health outcomes: A systematic review, meta-analysis and meta-regression. Sleep Med. Rev. 2018, 39, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Espie, C.A.; Fleming, L.; Cassidy, J.; Samuel, L.; Taylor, L.M.; White, C.A.; Douglas, N.J.; Engleman, H.M.; Kelly, H.L.; Paul, J. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J. Clin. Oncol. 2008, 26, 4651–4658. [Google Scholar] [CrossRef] [PubMed]
- Savard, J.; Villa, J.; Ivers, H.; Simard, S.; Morin, C.M. Prevalence, natural course, and risk factors of insomnia comorbid with cancer over a 2-month period. J. Clin. Oncol. 2009, 27, 5233–5239. [Google Scholar] [CrossRef] [PubMed]
- Sprod, L.K.; Palesh, O.G.; Janelsins, M.C.; Peppone, L.J.; Heckler, C.E.; Adams, M.J.; Morrow, G.R.; Mustian, K.M. Exercise, sleep quality, and mediators of sleep in breast and prostate cancer patients receiving radiation therapy. Community Oncol. 2010, 7, 463–471. [Google Scholar] [CrossRef] [Green Version]
- Kumari, M.; Badrick, E.; Ferrie, J.; Perski, A.; Marmot, M.; Chandola, T. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J. Clin. Endocrinol. Metab. 2009, 94, 4801–4809. [Google Scholar] [CrossRef] [Green Version]
- Walker, H.W.; Borniger, C.J. Molecular Mechanisms of Cancer-Induced Sleep Disruption. Int. J. Mol. Sci. 2019, 20, 2780. [Google Scholar] [CrossRef] [Green Version]
- Kiss, Z.; Ghosh, P.M. Women In Cancer Thematic Review: Circadian rhythmicity and the influence of ‘clock’ genes on prostate cancer. Endocr.-Relat. Cancer 2016, 23, T123–T134. [Google Scholar] [CrossRef] [Green Version]
- Gillessen, S.; Attard, G.; Beer, T.M.; Beltran, H.; Bjartell, A.; Bossi, A.; Briganti, A.; Bristow, R.G.; Chi, K.N.; Clarke, N.; et al. Management of Patients with Advanced Prostate Cancer: Report of the Advanced Prostate Cancer Consensus Conference 2019. Eur. Urol. 2020, 77, 508–547. [Google Scholar] [CrossRef]
- Pávó, I.; Varga, C.; Szücs, M.; László, F.; SzÉcsi, M.; Gardi, J.; László, F.A. Effects of testosterone on the rat renal medullary vasopressin receptor concentration and the antidiuretic response. Life Sci. 1995, 56, 1215–1222. [Google Scholar] [CrossRef]
- Deka, R.; Rose, B.S.; Bryant, A.K.; Sarkar, R.R.; Nalawade, V.; McKay, R.; Murphy, J.D.; Simpson, D.R. Androgen deprivation therapy and depression in men with prostate cancer treated with definitive radiation therapy. Cancer 2019, 125, 1070–1080. [Google Scholar] [CrossRef]
- van de Water, A.T.M.; Holmes, A.; Hurley, D.A. Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography—A systematic review. J. Sleep Res. 2011, 20, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Erwin, C.W.; Marsh, G.R. Ambulatory polysomnography in the study of patients with disorders of initiating and maintaining sleep. Semin. Neurol. 1990, 10, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Nixon, G.M.; Brouillette, R.T. Diagnostic techniques for obstructive sleep apnoea: Is polysomnography necessary? Paediatr. Respir. Rev. 2002, 3, 18–24. [Google Scholar] [CrossRef] [PubMed]
- George, C.F. Standards for polysomnography in Canada. The Standards Committees of the Canadian Sleep Society and the Canadian Thoracic Society. Can. Med. Assoc. J. 1996, 155, 1673–1678. [Google Scholar]
- Rundo, J.V.; Downey, R., 3rd. Polysomnography. Handb. Clin. Neurol. 2019, 160, 381–392. [Google Scholar] [CrossRef]
- Zhou, J.; Jolly, S. Obstructive sleep apnea and fatigue in head and neck cancer patients. Am. J. Clin. Oncol. 2015, 38, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Faiz, S.A.; Balachandran, D.; Hessel, A.C.; Lei, X.; Beadle, B.M.; William, W.N., Jr.; Bashoura, L. Sleep-related breathing disorders in patients with tumors in the head and neck region. Oncologist 2014, 19, 1200–1206. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, T.S.; Shade, M.Y.; Breton, G.; Gilbert, M.R.; Mahajan, A.; Scheurer, M.E.; Vera, E.; Berger, A.M. Sleep-wake disturbance in patients with brain tumors. Neuro Oncol. 2017, 19, 323–335. [Google Scholar] [CrossRef]
- Parker, K.P.; Bliwise, D.L.; Ribeiro, M.; Jain, S.R.; Vena, C.I.; Kohles-Baker, M.K.; Rogatko, A.; Xu, Z.; Harris, W.B. Sleep/Wake patterns of individuals with advanced cancer measured by ambulatory polysomnography. J. Clin. Oncol. 2008, 26, 2464–2472. [Google Scholar] [CrossRef]
- Reinsel, R.A.; Starr, T.D.; O’Sullivan, B.; Passik, S.D.; Kavey, N.B. Polysomnographic Study of Sleep in Survivors of Breast Cancer. J. Clin. Sleep Med. 2015, 11, 1361–1370. [Google Scholar] [CrossRef]

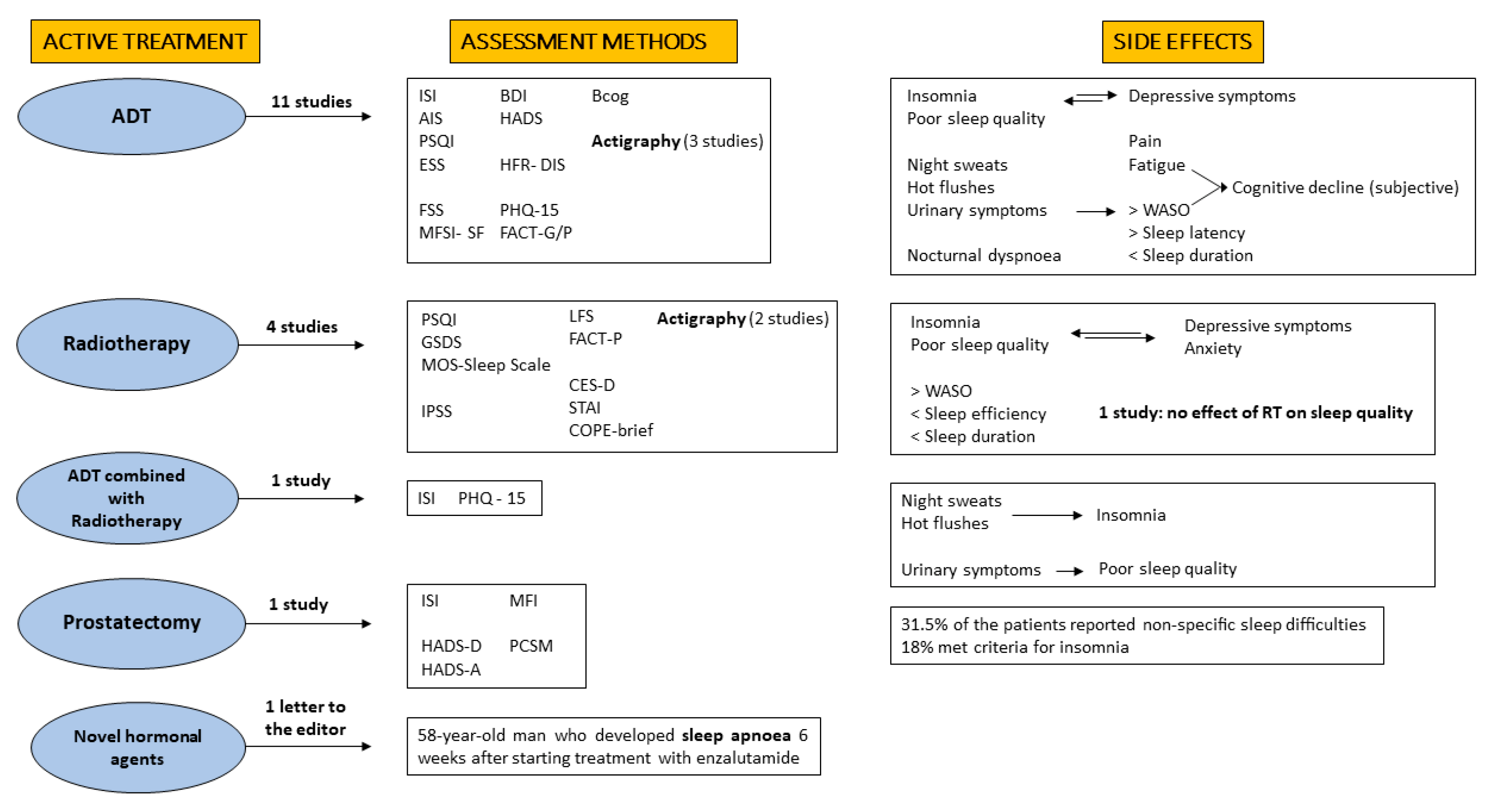
| Authors | Study Design | Number of Participants | Inclusion/Exclusion Criteria | Methods | Results | Quality Assessment |
|---|---|---|---|---|---|---|
| Savard, J. et al., 2015 [50] | Prospective, cohort | Tot. n = 728 BC n = 465 PC n = 263 treatment during study (RT 4.2%, CHT 0.8%, hormone therapy 0.4% (76% LHRH, 76% bicalutamide)) | Inclusion PCa: non-metastatic, after prostatectomy Exclusion: neoadjuvant cancer treatment; brachytherapy, severe cognitive impairment or severe psychiatric disorder; sleep disorder | ISI PHQ-15 | PCa was consistently associated with insomnia, and this association was strongly mediated by night sweats. Significantly higher ISI scores at 14, 16 and 18 months when exposed to hormone therapy. | good |
| * Gonzalez, B.D. et al., 2018 [51] | Prospective cohort study | Tot. PCa 177 PC n = 99 (receiving ADT n = 78) Control group no cancer n = 108 | Inclusion: ADT for non-metastatic or asymptomatic metastatic PCa, ADT for ≥6 months. Patients not treated with ADT with non-metastatic prostate cancer treated only by prostatectomy for prostate cancer, and not receiving testosterone supplementation | ISI HFR-DIS Actigraphy (3 days at one time point at 6 months) | ADT recipients reported worse subjective sleep disturbances over time. Nocturia mediated the association between ADT and objective sleep disturbances. Hot flash interference mediated the association between ADT and subjective sleep disturbances. | good |
| * Hanisch, L.J. et al., 2011 [52] | Cross-sectional | n = 60 ADT only | Inclusion: ongoing ADT, exclusion: recent surgery, radiation, chemotherapy or myelosuppressive medication 49% metastatic, 30% BCR, 21% localized disease at time of enrolment | Actigraphy (7 days, one time point) Daily Diary ESS FACT-G | ADT associated with sleep disturbances. Patients receiving ADT had lower sleep quality with difficulty in falling asleep, sleep fragmentation and daily napping. They presented a reduced TST (6 h), but no interference with the activities of daily life. Nocturia and hot flashes were common causes of sleep disruption. | good |
| Koskderelioglu, A. et al., 2017 [53] | Cross-sectional | Tot. 106 prostatectomy adj. ADT > 6 months n = 48 no adj. ADT n = 58 | Inclusion: prostatectomy Adj. ADT or follow-up only Exclusion: patients with major stroke, sleep disorders, dementia, Parkinson’s disease, traumatic brain injury, epilepsy and psychiatric condition | PSQI BDI ESS FSS | ADT patients reported higher levels of depression, worse quality of sleep and more severe fatigue (p < 0.001). PSQI scores showed a positive correlation with BDI and FSS scores. ADT was strongly associated with PSQI and FSS at multivariate analysis. | good |
| Saini, A. et al., 2013 [54] | Cross-sectional | Tot. 103 ADT n = 49 no ADT n = 54 | Inclusion: prostatectomy or 3D-RT, no metastatic disease; absence of major comorbidities; PS 0–1, testosterone < 0.5 ng/mL. Exclusion: history of neuropsychiatric disease or drugs, progressive disease at the study entry | FACT-P HADS BIS PSQI | No difference was found between the 2 groups for total PSQI and the other relevant items, except for daytime dysfunction (p = 0.03). | good |
| Challapalli, A. et al., 2018 [55] | Prospective, single-cohort | Tot. 250 (54% > 6 months ADT, 89% LHRH-agonists) | Inclusion: prostate cancer patients on ADT | specific questionnaire on vasomotor symptoms | 80% of ADT-treated patients had sleep problems, which were more prevalent in younger patients with higher BMI. | good |
| Miaskowski, C. et al., 2011 [56] | Prospective, single-cohort | Tot. n = 82 RT (primary or adj) | Inclusion: primary or adjuvant RT, KPS > 60 Exclusion: metastatic disease, had more than one cancer diagnosis or had a diagnosed sleep disorder | PSQI GSDS CES-D STAI NRS LFS | Sleep disturbances increased during RT and decreased after the completion of RT. Younger men with co-occurring depression and anxiety had the greatest risk for sleep disturbances during RT. ADT before RT (51% of patients) and fatigue are not predictors of sleep disturbances. | good |
| Thomas, K.S. et al., 2011 [57] | Prospective, cohort | Tot. n = 56 BC n = 33 PC n = 23 (primary RT) | Inclusion criteria PCa: radiation therapy for early stage Exclusion: recurrent cancer; prior or planned treatment with chemotherapy; immunosuppressive medication or tobacco. | MOS-Sleep Scale COPE-brief FACT-P | PCa: RT was associated with a decrease in TST. Sleep latency increased at the beginning of RT and during treatment, but decreased at follow-up. There was no significant change in sleep quality over the course of treatment. | fair |
| * Garrett, K. et al., 2011 [58] | Cross-sectional | Tot. 160 BC n = 78 PC n= 82 (RT primary or adj.) | Inclusion criteria PCa: primary or adjuvant RT; KPS > 60 exclusion: metastatic disease; more than one cancer diagnosis; sleep disorder | PSQI GSDS LFS Actigraphy (48 h one time point) | Results PCa: Sleep disturbances and fatigue are significant burdens. Significantly lower TST, lower sleep efficiency and higher percentage of WASO compared to patients with BC. | good |
| * Holliday, E.B. et al., 2016 [59] | Prospective, single-cohort | Tot. 28 all RT | Inclusion: EBRT for T1-T2 PCa. Exclusion criteria: concurrent ADT; brachytherapy; psychiatric disorders treatment for any cancer | IPSS Actigraphy | Sleep efficiency improved during radiotherapy, fatigue increased and was associated with reduced QoL. | good |
| Savard, J. et al., 2013 [40] | Prospective, cohort | Tot. 60 RT + ADT n = 28 RT n = 32 | Inclusion: non-metastatic prostate cancer, scheduled to receive curative RT only or RT plus ADT; Exclusion: prior history of cancer; score <24 on the Mini-Mental State Examination, any treatment for cancer | ISI PHQ | A significant interaction effect was found indicating an increase in insomnia scores in ADT + RT patients at 2, 4 and 6 months, as compared with baseline, and stable scores in RT only patients. A significant mediating role of hot flashes and night sweats was found in the relationship between ADT and insomnia, while nocturia mediated the association between RT and poor sleep quality. | good |
| Savard, J. et al., 2005 [15] | Cross-sectional | Tot. 327 all RP | Inclusion: radical prostatectomy for prostate cancer within the past 10 years. | ISI HADS-D HADS-A MFI PCSM | 31.5% of the patients reported non-specific sleep difficulties and 18% of them met criteria for insomnia. In 95% of the cases, insomnia was chronic. In 50% of patients with insomnia, the onset of sleep difficulties followed the cancer diagnosis. Risk factors for insomnia were younger age, worse prognosis, intestinal pain, depression and ADT-related symptoms (for patients undergoing ADT). | good |
| Maguire, R. et al., 2018 [60] | Cross-sectional design | n = 3348 | Inclusion: being at least 2 years post diagnosis | EORTC QLQC30 QLQPR25 EQ5D-5L | Sleep disturbances have a positive association with side effects such as urinary symptoms, hormone treatment-related symptoms, intestinal symptoms and depression/anxiety. | good |
| Hervouet, S. et al., 2005 [61] | Cross-sectional | Tot. 861 RT n = 392 BR n = 188 RP n = 28 Current hormone therapy (10.2%; 4.8%; 20.6%) Lifetime hormone therapy (93.6%; 77.1%; 54.5%) | Inclusion: RT, BR, RP as an initial treatment for PC within the past 7 years; age < 80 at study entry Exclusion: any other type of cancer; orchiectomy; chemotherapy; severe cognitive impairment | HADS-D HADS-A ISI MFI PCSM EORTC QLQC30 | Sexual difficulties were the most frequently reported (70.5%), followed by insomnia (31.9%), anxiety (23.7%), fatigue (18.5%) and depression (17.0%). Patients treated with RT had higher levels of clinically significant insomnia (n = 137; 35%) compared to men receiving RP (n = 84; 30%), scores of fatigue motivation were higher in ongoing hormone therapy group. | good |
| * Tulk, J. et al., 2021 [62] | Prospective, single-cohort | n = 24 | Inclusion: ADT after RT; age > 18 at study entry Exclusion: prior history of cancer diagnosis and treatment. | FACT-Cog ISI PSQI Sleep Diary HADS MFSI-SF Actigraphy HFR-DIS | The worsening of subjectively estimated wake after sleep onset (sleep diary) was a predictor of subjective cognitive decline in the first 12 months of ADT. | good |
| Sánchez-Martínez, V. et al., 2021 [63] | Prospective, single-cohort | n = 33 | Inclusion: ADT with or without previous prostatectomy Exclusion: history of other chemotherapy treatment for prostate or any other cancer, cognitive deterioration, relevant change in the health status that could influence sleep quality, mood or cognitive performance. | AIS BCog GDS | Lower subjective sleep quality and more depressive symptoms after one year of follow-up (first assessment in the six months to one year treatment with ADT). | fair |
| Adverse Events | PROSPER [74] n = 1401 nmCRPC n (%) | ARCHES [25] n = 1150 mHSPC n (%) | ENZAMET [26] n = 1125 mHSPC n (%) | PREVAIL [75] n = 1717 mCRPC Chemo Naive n (%) | AFFIRM [76] n = 1199 mCRPC after Docetaxel Failure n (%) | ARAMIS [77] n = 1509 nmCRPC n (%) | SPARTAN [21] n = 1207 nmCRPC n (%) | TITAN [78] n = 1052 mHSPC n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enza Plus ADT | Placebo Plus ADT | Enza Plus ADT | Placebo Plus ADT | Enza Plus ADT | SOC Plus ADT | Enza Plus ADT | Placebo Plus ADT | Enza Plus ADT | Placebo Plus ADT | Daro Plus ADT | Placebo Plus ADT | Apa Plus ADT | Placebo Plus ADT | Apa Plus ADT | Placebo Plus ADT | |
| -sleep disorder | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr |
| -fatigue | ||||||||||||||||
| all grades | 303 (33) | 64 (14) | 138 (24) | 112 (19.5) | nr | nr | 310 (36) | 218 (36) | 260 (34) | 116 (29) | 115 | 48 (8.7) | 244 (30.4) | 84 (21.1) | 103 | 86 (16.3) |
| G3 | 3 (3) | 3 (1) | 10 (1.7) | 9 (1.6) | 31 (6) | 4 (1) | 16 (2) | 16 (2) | 50 (6) | 29 (7) | (12.1) | 5 (0.9) | 7 (0.9) | 1 (0.3) | (19.7) | 0 |
| -dizziness | ||||||||||||||||
| all grades | 91 (10) | 20 (4) | 29 (5.1) | 20 (3.5) | nr | nr | nr | nr | nr | nr | 4 (80.4) | 22 (4.0) | 75 (9.3) | 25 (6.3) | 8 (1.5) | nr |
| G3 | 4 (<1) | 0 | 0 | 0 | nr | nr | nr | nr | nr | nr | 43 (4.5) | 1 (0.2) | 5 (0.6) | 0 | nr | nr |
| -cognitive/memory impairment | ||||||||||||||||
| all grades | 48 (5) | 9 (2) | 26 (4.5) | 12 (2.1) | nr | nr | nr | nr | nr | nr | 2 (0.2) | 8 (1.5) | 41 (5.1) | 12 (3) | nr | nr |
| G3 | 1 (<1) | 0 | 4 (0.7) | 0 | nr | nr | nr | nr | nr | nr | 9 (0.9) | 0 | 0 | 0 | nr | nr |
| -syncope | ||||||||||||||||
| all grades | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | 0 | nr | nr | nr | nr | nr |
| G3 | nr | nr | nr | nr | 20 (4) | 6 (1) | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr |
| -delirium | ||||||||||||||||
| all grades | nr | nr | nr | nr | 0 | 1 (<1) | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr |
| -headache | ||||||||||||||||
| all grades | 85 (9) | 21 (5) | nr | nr | nr | nr | 91 (10) | 59 (7) | 93 (12) | 22 (6) | nr | nr | nr | nr | nr | nr |
| G3 | 2 (<1) | 0 | nr | nr | nr | nr | 2 (<1) | 3 (<1) | 6(<1) | 0 | nr | nr | nr | nr | nr | nr |
| -seizures | ||||||||||||||||
| all grades | 3 (1) | 0 | nr | nr | nr | nr | 1 (<1) | 1 (<1) | 5 (<1) | 0 | 2 (0.2) | 1 (0.2) | 2 (0.2) | 0 | 3 (0.6) | 2 (0.4) |
| G3 | 2 (1) | 0 | nr | nr | nr | nr | 1 (<1) | 0 | 5 (<1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Adverse Events | COU-AA-301 [79] n = 1195 mCRPC after Docetaxel Failure n (%) | COU-AA-302 [80] n = 1088 mCRPC Chemo Naive n (%) | LATITUDE [24] n = 1199 mHSPC n (%) | STAMPEDE [81] n = 1917 PC Not Previously Treated with Hormone Therapy n (%) | SWOG S9346 [82] n= 1134 mHSPC n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Abiraterone Plus Prednisone Plus ADT | Placebo Plus Prednisone Plus ADT | Abiraterone Plus Prednisone Plus ADT | Placebo Plus Prednisone Plus ADT | Abiraterone Plus Prednisone Plus ADT | Double Placebo Plus ADT | Abiraterone Plus Prednisone plus ADT +/− Radiotherapy | Double Placebo Plus ADT +/− Radiotherapy | Continuous ADT | Intermittent ADT | |
| -sleep disorder | ||||||||||
| all grades | nr | nr | nr | nr | nr | nr | 222 (23) | 180 (19) | nr | nr |
| G3 | nr | nr | nr | nr | nr | nr | 14 (1) | 6 (1) | nr | nr |
| -fatigue | ||||||||||
| all grades | 346 (44) | 169 (43) | 212 (39) | 185 (34) | 77 (13) | 86 (14) | 424 (45) | 400 (42) | nr | nr |
| G3 | 64 (8) | 36 (9) | nr | nr | 10 (2) | 14 (2) | 15 (2) | 21 (2) | nr | nr |
| -fluid retention and edema | ||||||||||
| all grades | 241 (31) | 88 (22) | nr | nr | nr | nr | 176 (19) | 134 (14) | nr | nr |
| G3 | 16 (2) | 4 (1) | nr | nr | nr | nr | 5 (1) | 0 (0) | nr | nr |
| -back pain | ||||||||||
| all grades | 233 (30) | 129 (33) | 173 (32) | 173 (32) | 110 (18) | 123 (20) | 0 (0) | 0 (0) | nr | nr |
| G3 | 44 (6) | 37 (9) | nr | nr | 14 (2) | nr | 0 (0) | 0 (0) | nr | nr |
| -nausea * | ||||||||||
| all grades | 233 (30) | 124 (32) | 120 (22) | 118 (22) | nr | nr | 132 (14) | 81 (8) | nr | nr |
| G3 | 12 (2) | 10 (3) | nr | nr | nr | nr | 1 (0) | 1 (0) | nr | nr |
| -arthralgia | ||||||||||
| all grades | 215 (27) | 89 (23) | 154 (28) | 129 (24) | nr | nr | nr | nr | nr | nr |
| G3 | 33 (4) | 16 (4) | nr | nr | nr | nr | nr | nr | nr | nr |
| -constipation | ||||||||||
| all grades | 206 (13) | 89 (23) | 125 (23) | 103 (19) | 103 (19) | nr | 866 (90) | 660 (70) | nr | nr |
| G3 | 8 (1) | 16 (4) | nr | nr | nr | nr | 1 (0) | 5 (1) | nr | nr |
| -bone pain | ||||||||||
| all grades | 194 (25) | 110 (28) | 106 (20) | 103 (19) | 74 (12) | 88 (15) | nr | nr | nr | nr |
| G3 | 42 (5) | 25 (6) | nr | nr | 20 (3) | 17 (3) | nr | nr | 26 (3.6) | 30 (4) |
| -vomiting | ||||||||||
| all grades | 168 (21) | 97 (25) | nr | nr | nr | nr | 63 (7) | 34 (4) | nr | nr |
| G3 | 13 (2) | 11 (3) | nr | nr | nr | nr | 4 (0) | 1 (0) | nr | nr |
| -diarrhea | ||||||||||
| all grades | 139 (18) | 53 (14) | 117 (22) | 96 (18) | nr | nr | 229 (24) | 194 (20) | nr | nr |
| G3 | 5 (1) | 5 (1) | nr | nr | nr | nr | 13 (1) | 8 (1) | nr | nr |
| -muscle spasm | ||||||||||
| all grades | nr | nr | 75 (14) | 110 (20) | nr | nr | nr | nr | nr | nr |
| G3 | nr | nr | nr | nr | nr | nr | nr | nr | 1 (0) | 2 (<1) |
| -hot flashes | ||||||||||
| all grades | nr | nr | 121 (22) | 98 (88) | nr | nr | 496 (52) | 510 (53) | nr | nr |
| G3 | nr | nr | nr | nr | nr | nr | 41 (4) | 39 (4) | 20 (6) | 16 (5) |
| -spinal-cord compression | ||||||||||
| all grades | nr | nr | nr | nr | 14 (2) | 12 (2) | nr | nr | nr | nr |
| G3 | nr | nr | nr | nr | 12 (12) | 7 (1) | nr | nr | nr | nr |
| neurologic disorders ** | 4 | |||||||||
| all grades | nr | nr | nr | nr | nr | nr | nr | nr | 43 (14) | 6 (14) |
| G3 | nr | nr | nr | nr | nr | nr | nr | nr | 15 (2) | 15 (2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sparasci, D.; Napoli, I.; Rossi, L.; Pereira-Mestre, R.; Manconi, M.; Treglia, G.; Marandino, L.; Ottaviano, M.; Turco, F.; Mangan, D.; et al. Prostate Cancer and Sleep Disorders: A Systematic Review. Cancers 2022, 14, 1784. https://doi.org/10.3390/cancers14071784
Sparasci D, Napoli I, Rossi L, Pereira-Mestre R, Manconi M, Treglia G, Marandino L, Ottaviano M, Turco F, Mangan D, et al. Prostate Cancer and Sleep Disorders: A Systematic Review. Cancers. 2022; 14(7):1784. https://doi.org/10.3390/cancers14071784
Chicago/Turabian StyleSparasci, Davide, Ilenia Napoli, Lorenzo Rossi, Ricardo Pereira-Mestre, Mauro Manconi, Giorgio Treglia, Laura Marandino, Margaret Ottaviano, Fabio Turco, Dylan Mangan, and et al. 2022. "Prostate Cancer and Sleep Disorders: A Systematic Review" Cancers 14, no. 7: 1784. https://doi.org/10.3390/cancers14071784
APA StyleSparasci, D., Napoli, I., Rossi, L., Pereira-Mestre, R., Manconi, M., Treglia, G., Marandino, L., Ottaviano, M., Turco, F., Mangan, D., Gillessen, S., & Vogl, U. M. (2022). Prostate Cancer and Sleep Disorders: A Systematic Review. Cancers, 14(7), 1784. https://doi.org/10.3390/cancers14071784







