Pentafecta for Radical Nephroureterectomy in Patients with High-Risk Upper Tract Urothelial Carcinoma: A Proposal for Standardization of Quality Care Metrics
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Cohort Description
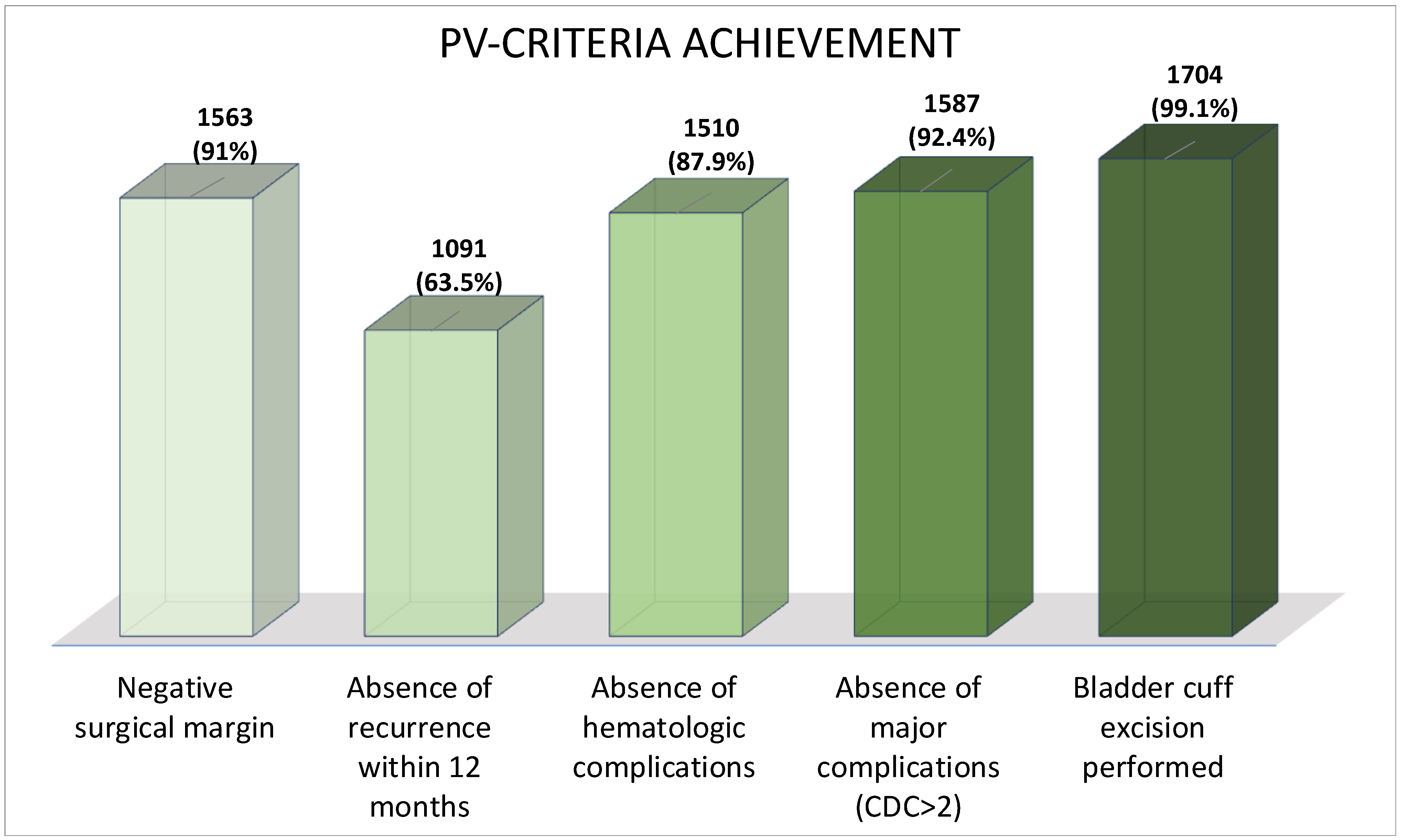
2.2. Pentafecta Criteria
2.3. Treatment-Specific Features and Follow-Up
2.4. Study Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Clinicopathological Characteristics
3.2. Perioperative Outcomes
3.3. Pentafecta Validation
3.4. Pathological Characteristics
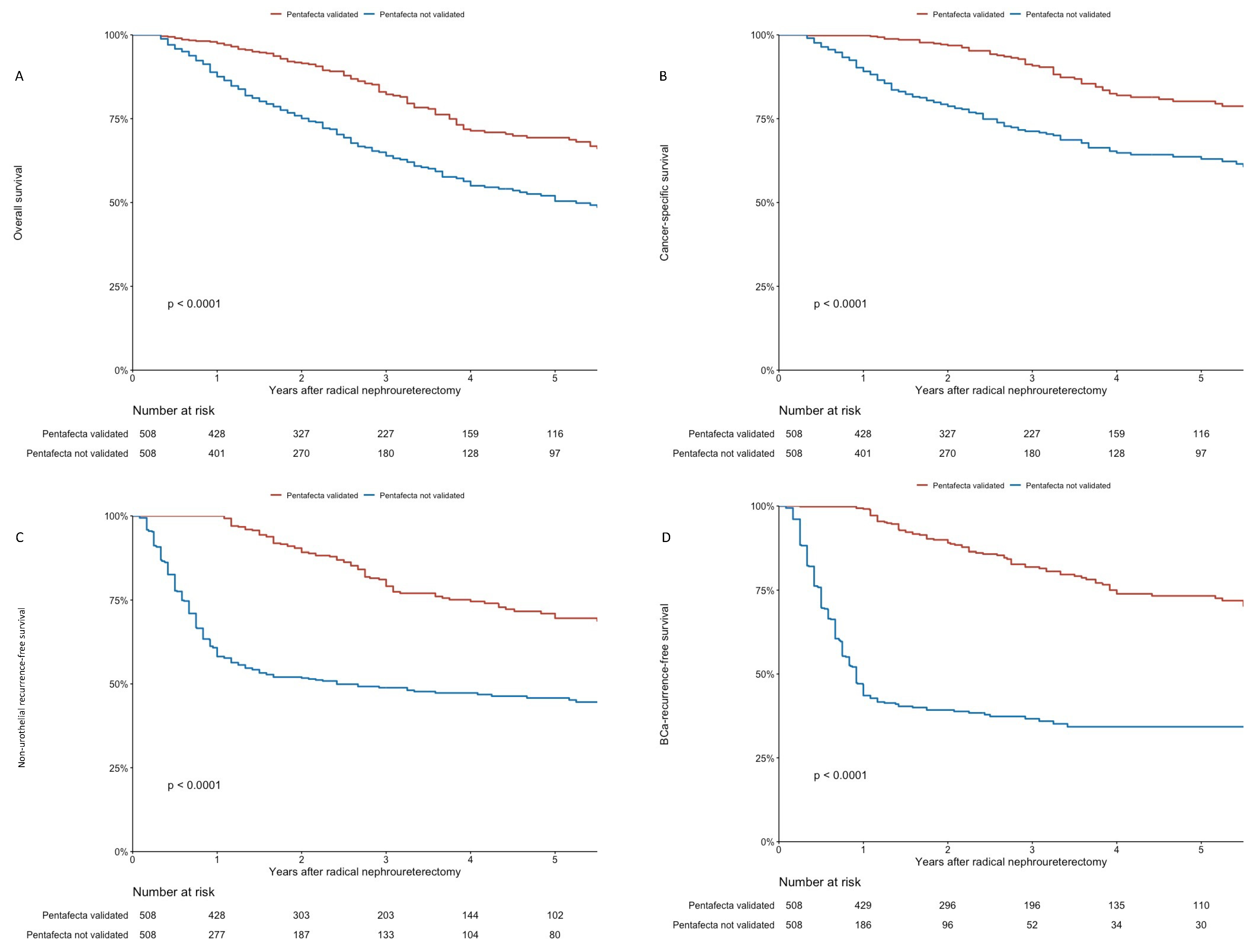
3.5. Oncological Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soria, F.; Shariat, S.F.; Lerner, S.P.; Fritsche, H.M.; Rink, M.; Kassouf, W.; Spiess, P.E.; Lotan, Y.; Ye, D.; Fernandez, M.I.; et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J. Urol. 2017, 35, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Roupret, M.; Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Comperat, E.M.; Cowan, N.C.; Dominguez-Escrig, J.L.; Gontero, P.; Hugh Mostafid, A.; et al. EAU Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma. 2020. Available online: https://uroweb.org/guidelines (accessed on 1 December 2021).
- Favaretto, R.L.; Shariat, S.F.; Savage, C.; Godoy, G.; Chade, D.C.; Kaag, M.; Bochner, B.H.; Coleman, J.; Dalbagni, G. Combining imaging and ureteroscopy variables in a preoperative multivariable model for prediction of muscle-invasive and non-organ confined disease in patients with upper tract urothelial carcinoma. BJU Int. 2012, 109, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Chromecki, T.F.; Cha, E.K.; Fajkovic, H.; Margulis, V.; Novara, G.; Scherr, D.S.; Lotan, Y.; Raman, J.D.; Kassouf, W.; Bensalah, K.; et al. The impact of tumor multifocality on outcomes in patients treated with radical nephroureterectomy. Eur. Urol. 2012, 61, 245–253. [Google Scholar] [CrossRef]
- Remzi, M.; Haitel, A.; Margulis, V.; Karakiewicz, P.; Montorsi, F.; Kikuchi, E.; Zigeuner, R.; Weizer, A.; Bolenz, C.; Bensalah, K.; et al. Tumour architecture is an independent predictor of outcomes after nephroureterectomy: A multi-institutional analysis of 1363 patients. BJU Int. 2009, 103, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Zigeuner, R.; Shariat, S.F.; Margulis, V.; Karakiewicz, P.I.; Roscigno, M.; Weizer, A.; Kikuchi, E.; Remzi, M.; Raman, J.D.; Bolenz, C.; et al. Tumour necrosis is an indicator of aggressive biology in patients with urothelial carcinoma of the upper urinary tract. Eur. Urol. 2010, 57, 575–581. [Google Scholar] [CrossRef]
- Seisen, T.; Granger, B.; Colin, P.; Leon, P.; Utard, G.; Renard-Penna, R.; Comperat, E.; Mozer, P.; Cussenot, O.; Shariat, S.F.; et al. A Systematic Review and Meta-analysis of Clinicopathologic Factors Linked to Intravesical Recurrence After Radical Nephroureterectomy to Treat Upper Tract Urothelial Carcinoma. Eur. Urol. 2015, 67, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Roupret, M.; Hupertan, V.; Seisen, T.; Colin, P.; Xylinas, E.; Yates, D.R.; Fajkovic, H.; Lotan, Y.; Raman, J.D.; Zigeuner, R.; et al. Prediction of cancer specific survival after radical nephroureterectomy for upper tract urothelial carcinoma: Development of an optimized postoperative nomogram using decision curve analysis. J. Urol. 2013, 189, 1662–1669. [Google Scholar] [CrossRef]
- Khalifeh, A.; Autorino, R.; Hillyer, S.P.; Laydner, H.; Eyraud, R.; Panumatrassamee, K.; Long, J.A.; Kaouk, J.H. Comparative outcomes and assessment of trifecta in 500 robotic and laparoscopic partial nephrectomy cases: A single surgeon experience. J. Urol. 2013, 189, 1236–1242. [Google Scholar] [CrossRef]
- Salomon, L.; Saint, F.; Anastasiadis, A.G.; Sebe, P.; Chopin, D.; Abbou, C.C. Combined reporting of cancer control and functional results of radical prostatectomy. Eur. Urol. 2003, 44, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Gierth, M.; Rink, M.; Schmid, M.; Chun, F.K.; Dahlem, R.; Roghmann, F.; Palisaar, R.J.; Noldus, J.; Ellinger, J.; et al. Optimizing outcome reporting after radical cystectomy for organ-confined urothelial carcinoma of the bladder using oncological trifecta and pentafecta. World J. Urol. 2015, 33, 1945–1950. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, G.E.; Winter, M.; Medina, L.G.; Ashrafi, A.N.; Miranda, G.; Tafuri, A.; Landsberger, H.; Lin-Brande, M.; Rajarubendra, N.; De Castro Abreu, A.; et al. Radical cystectomy pentafecta: A proposal for standardisation of outcomes reporting following robot-assisted radical cystectomy. BJU Int. 2020, 125, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, V.R.; Sivaraman, A.; Coelho, R.F.; Chauhan, S.; Palmer, K.J.; Orvieto, M.A.; Camacho, I.; Coughlin, G.; Rocco, B. Pentafecta: A new concept for reporting outcomes of robot-assisted laparoscopic radical prostatectomy. Eur. Urol. 2011, 59, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Freifeld, Y.; Ghandour, R.; Singla, N.; Woldu, S.; Clinton, T.; Kulangara, R.; Bagrodia, A.; Matin, S.F.; Petros, F.G.; Raman, J.D.; et al. Preoperative predictive model and nomogram for disease recurrence following radical nephroureterectomy for high grade upper tract urothelial carcinoma. Urol. Oncol. 2019, 37, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Krabbe, L.M.; Eminaga, O.; Shariat, S.F.; Hutchinson, R.C.; Lotan, Y.; Sagalowsky, A.I.; Raman, J.D.; Wood, C.G.; Weizer, A.Z.; Roscigno, M.; et al. Postoperative Nomogram for Relapse-Free Survival in Patients with High Grade Upper Tract Urothelial Carcinoma. J. Urol. 2017, 197, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Raman, J.D.; Lin, Y.K.; Shariat, S.F.; Krabbe, L.M.; Margulis, V.; Arnouk, A.; Lallas, C.D.; Trabulsi, E.J.; Drouin, S.J.; Roupret, M.; et al. Preoperative nomogram to predict the likelihood of complications after radical nephroureterectomy. BJU Int. 2017, 119, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Margulis, V.; Youssef, R.F.; Karakiewicz, P.I.; Lotan, Y.; Wood, C.G.; Zigeuner, R.; Kikuchi, E.; Weizer, A.; Raman, J.D.; Remzi, M.; et al. Preoperative multivariable prognostic model for prediction of nonorgan confined urothelial carcinoma of the upper urinary tract. J. Urol. 2010, 184, 453–458. [Google Scholar] [CrossRef]
- Roupret, M.; Colin, P.; Yates, D.R. A new proposal to risk stratify urothelial carcinomas of the upper urinary tract (UTUCs) in a predefinitive treatment setting: Low-risk versus high-risk UTUCs. Eur. Urol. 2014, 66, 181–183. [Google Scholar] [CrossRef]
- O’Brien, T.; Ray, E.; Singh, R.; Coker, B.; Beard, R.; British Association of Urological Surgeons Section of Oncology. Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: A prospective, multicentre, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C Trial). Eur. Urol. 2011, 60, 703–710. [Google Scholar] [CrossRef]
- Ito, A.; Shintaku, I.; Satoh, M.; Ioritani, N.; Aizawa, M.; Tochigi, T.; Kawamura, S.; Aoki, H.; Numata, I.; Takeda, A.; et al. Prospective randomized phase II trial of a single early intravesical instillation of pirarubicin (THP) in the prevention of bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma: The THP Monotherapy Study Group Trial. J. Clin. Oncol. 2013, 31, 1422–1427. [Google Scholar] [CrossRef]
- Hwang, E.C.; Sathianathen, N.J.; Jung, J.H.; Kim, M.H.; Dahm, P.; Risk, M.C. Single-dose intravesical chemotherapy after nephroureterectomy for upper tract urothelial carcinoma. Cochrane Database Syst. Rev. 2019, 5, CD013160. [Google Scholar] [CrossRef] [PubMed]
- Favaretto, R.L.; Shariat, S.F.; Chade, D.C.; Godoy, G.; Adamy, A.; Kaag, M.; Bochner, B.H.; Coleman, J.; Dalbagni, G. The effect of tumor location on prognosis in patients treated with radical nephroureterectomy at Memorial Sloan-Kettering Cancer Center. Eur. Urol. 2010, 58, 574–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, T.; Hashimoto, Y.; Kobayashi, H.; Iizuka, J.; Nakazawa, H.; Ito, F.; Tanabe, K. Template-based lymphadenectomy in urothelial carcinoma of the upper urinary tract: Impact on patient survival. Int. J. Urol. 2010, 17, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Matin, S.F.; Sfakianos, J.P.; Espiritu, P.N.; Coleman, J.A.; Spiess, P.E. Patterns of Lymphatic Metastases in Upper Tract Urothelial Carcinoma and Proposed Dissection Templates. J. Urol. 2015, 194, 1567–1574. [Google Scholar] [CrossRef] [Green Version]
- Lughezzani, G.; Jeldres, C.; Isbarn, H.; Shariat, S.F.; Sun, M.; Pharand, D.; Widmer, H.; Arjane, P.; Graefen, M.; Montorsi, F.; et al. A critical appraisal of the value of lymph node dissection at nephroureterectomy for upper tract urothelial carcinoma. Urology 2010, 75, 118–124. [Google Scholar] [CrossRef]
- Roscigno, M.; Brausi, M.; Heidenreich, A.; Lotan, Y.; Margulis, V.; Shariat, S.F.; Van Poppel, H.; Zigeuner, R. Lymphadenectomy at the time of nephroureterectomy for upper tract urothelial cancer. Eur. Urol. 2011, 60, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Comperat, E.M.; Burger, M.; Gontero, P.; Mostafid, A.H.; Palou, J.; Roupret, M.; van Rhijn, B.W.G.; Shariat, S.F.; Sylvester, R.J.; Zigeuner, R.; et al. Grading of Urothelial Carcinoma and The New “World Health Organisation Classification of Tumours of the Urinary System and Male Genital Organs 2016”. Eur. Urol. Focus 2019, 5, 457–466. [Google Scholar] [CrossRef]
- Brierley, J.D.; Wittekind, C.; International Union against Cancer (UICC). TNM Classification of Malignant Tumours, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Flaig, T.W.; Spiess, P.E.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chang, S.; Downs, T.M.; Efstathiou, J.A.; Friedlander, T.; et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2020, 18, 329–354. [Google Scholar] [CrossRef] [Green Version]
- White, I.R.; Royston, P.; Wood, A.M. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 2011, 30, 377–399. [Google Scholar] [CrossRef]
- Kenigsberg, A.P.; Smith, W.; Meng, X.; Ghandour, R.; Rapoport, L.; Bagrodia, A.; Lotan, Y.; Woldu, S.L.; Margulis, V. Robotic Nephroureterectomy vs Laparoscopic Nephroureterectomy: Increased Utilization, Rates of Lymphadenectomy, Decreased Morbidity Robotically. J. Endourol. 2021, 35, 312–318. [Google Scholar] [CrossRef]
- Ikeda, M.; Matsumoto, K.; Hirayama, T.; Koguchi, D.; Murakami, Y.; Matsuda, D.; Okuno, N.; Utsunomiya, T.; Taoka, Y.; Irie, A.; et al. Selected High-Risk Patients with Upper Tract Urothelial Carcinoma Treated with Radical Nephroureterectomy for Adjuvant Chemotherapy: A Multi-Institutional Retrospective Study. Clin. Genitourin. Cancer 2018, 16, e669–e675. [Google Scholar] [CrossRef] [PubMed]
- Peyronnet, B.; Seisen, T.; Dominguez-Escrig, J.L.; Bruins, H.M.; Yuan, C.Y.; Lam, T.; Maclennan, S.; N’Dow, J.; Babjuk, M.; Comperat, E.; et al. Oncological Outcomes of Laparoscopic Nephroureterectomy Versus Open Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: An European Association of Urology Guidelines Systematic Review. Eur. Urol. Focus 2019, 5, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Xylinas, E.; Rink, M.; Cha, E.K.; Clozel, T.; Lee, R.K.; Fajkovic, H.; Comploj, E.; Novara, G.; Margulis, V.; Raman, J.D.; et al. Impact of distal ureter management on oncologic outcomes following radical nephroureterectomy for upper tract urothelial carcinoma. Eur. Urol. 2014, 65, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Xylinas, E.; Kluth, L.; Passoni, N.; Trinh, Q.D.; Rieken, M.; Lee, R.K.; Fajkovic, H.; Novara, G.; Margulis, V.; Raman, J.D.; et al. Prediction of intravesical recurrence after radical nephroureterectomy: Development of a clinical decision-making tool. Eur. Urol. 2014, 65, 650–658. [Google Scholar] [CrossRef] [PubMed]
- König, F.; Shariat, S.F.; Karakiewicz, P.I.; Mun, D.H.; Rink, M.; Pradere, B. Quality indicators for the management of high-risk upper tract urothelial carcinoma requiring radical nephroureterectomy. Curr. Opin. Urol. 2021, 31, 291–296. [Google Scholar] [CrossRef]
- Hung, A.J.; Cai, J.; Simmons, M.N.; Gill, I.S. “Trifecta” in partial nephrectomy. J. Urol. 2013, 189, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Raman, J.D.; Jafri, S.M. Complications Following Radical Nephroureterectomy. Curr. Urol. Rep. 2016, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Kocher, N.J.; Canes, D.; Bensalah, K.; Roupret, M.; Lallas, C.; Margulis, V.; Shariat, S.; Colin, P.; Matin, S.; Tracy, C.; et al. Incidence and preoperative predictors for major complications following radical nephroureterectomy. Transl. Androl. Urol. 2020, 9, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Tikkinen, K.A.O.C.R.; Gould, M.K.; Naspro, R.; Novara, G.; Sandset, P.M.; Violette, P.D.; Guyatt, G.H. EAU Guidelines on Thromboprophylaxis. In Proceedings of the EAU Annual Congress, Amsterdam, The Netherlands, 17–21 July 2020; Available online: http://uroweb.org/guidelines/compilations-of-all-guidelines/ (accessed on 1 December 2021).
- Tikkinen, K.A.; Agarwal, A.; Craigie, S.; Cartwright, R.; Gould, M.K.; Haukka, J.; Naspro, R.; Novara, G.; Sandset, P.M.; Siemieniuk, R.A.; et al. Systematic reviews of observational studies of risk of thrombosis and bleeding in urological surgery (ROTBUS): Introduction and methodology. Syst. Rev. 2014, 3, 150. [Google Scholar] [CrossRef]
- Tikkinen, K.A.O.; Craigie, S.; Agarwal, A.; Violette, P.D.; Novara, G.; Cartwright, R.; Naspro, R.; Siemieniuk, R.A.C.; Ali, B.; Eryuzlu, L.; et al. Procedure-specific Risks of Thrombosis and Bleeding in Urological Cancer Surgery: Systematic Review and Meta-analysis. Eur. Urol. 2018, 73, 242–251. [Google Scholar] [CrossRef] [Green Version]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristic | Initial Cohort | PSM-Adjusted Cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| Total Cohort | Pentafecta Validation | Total Cohort | Pentafecta Validation | |||||
| n = 1718 | Pentafecta Validated, n = 844 (49%) | Pentafecta Not Validated, n = 874 (51%) | p-Value | n = 1016 | Pentafecta Validated, n = 508 (50%) | Pentafecta Not Validated, n = 508 (50%) | p-Value | |
| Age | 71 (64, 78) | 71 (64, 77) | 72 (64, 78) | 0.12 | 71 (64, 78) | 71 (65, 78) | 72 (64, 78) | 0.8 |
| Sex | 0.14 | 0.8 | ||||||
| male | 1153 (67%) | 552 (65%) | 601 (69%) | 681 (67%) | 339 (67%) | 342 (67%) | ||
| female | 565 (33%) | 292 (35%) | 273 (31%) | 335 (33%) | 169 (33%) | 166 (33%) | ||
| BMI | 26.0 (23.0, 29.0) | 26.0 (23.4, 29.0) | 25.4 (23.0, 28.7) | 0.027 | 25.8 (23.0, 28.7) | 26.0 (23.1, 28.5) | 25.7 (22.9, 28.7) | 0.6 |
| Unknown | 267 | 106 | 161 | - | - | - | ||
| ECOG | 0.047 | 0.8 | ||||||
| 0 | 844 (62%) | 451 (65%) | 393 (58%) | 630 (62%) | 318 (63%) | 312 (61%) | ||
| 1 | 401 (29%) | 188 (27%) | 213 (32%) | 293 (29%) | 146 (29%) | 147 (29%) | ||
| 2 | 108 (7.9%) | 46 (6.6%) | 62 (9.2%) | 83 (8.2%) | 38 (7.5%) | 45 (8.9%) | ||
| 3 | 14 (1.0%) | 8 (1.2%) | 6 (0.9%) | 10 (1.0%) | 6 (1.2%) | 4 (0.8%) | ||
| Unknown | 351 | 151 | 200 | - | - | - | ||
| Diabetes mellitus | 289 (22%) | 152 (21%) | 137 (22%) | 0.7 | 202 (20%) | 103 (20%) | 99 (19%) | 0.8 |
| Unknown | 378 | 128 | 250 | - | - | - | ||
| ASA | 0.2 | 0.4 | ||||||
| 1 | 101 (6.8%) | 57 (7.6%) | 44 (6.0%) | 78 (7.7%) | 43 (8.5%) | 35 (6.9%) | ||
| 2 | 676 (46%) | 343 (46%) | 333 (46%) | 494 (49%) | 247 (49%) | 247 (49%) | ||
| 3 | 669 (45%) | 338 (45%) | 331 (45%) | 426 (42%) | 212 (42%) | 214 (42%) | ||
| 4 | 35 (2.4%) | 12 (1.6%) | 23 (3.1%) | 18 (1.8%) | 6 (1.2%) | 12 (2.4%) | ||
| Unknown | 237 | 94 | 143 | - | - | - | ||
| Hypertension | 682 (56%) | 354 (54%) | 328 (58%) | 0.14 | 575 (56.6%) | 271 (26.7%) | 304 (29.9%) | 0.9 |
| Unknown | 505 | 192 | 313 | - | - | - | ||
| NAC | 115 (6.7%) | 46 (5.5%) | 69 (7.9%) | 0.043 | 94 (9.3%) | 41 (8.1%) | 53 (10%) | 0.2 |
| CT tumor stage | 0.043 | |||||||
| cT0 | 83 (4.8%) | 41 (4.9%) | 42 (4.8%) | 48 (4.7%) | 25 (4.9%) | 23 (4.5%) | 0.9 | |
| cTa/cT1 | 559 (33%) | 297 (35%) | 262 (30%) | 318 (31%) | 162 (32%) | 156 (31%) | ||
| cT2 | 248 (14%) | 108 (13%) | 140 (16%) | 150 (15%) | 74 (15%) | 76 (15%) | ||
| cT ≥ 3 | 267 (15.6%) | 124 (14.9%) | 143 (16.3%) | 161 (15.6%) | 86 (16.6%) | 75 (14.6%) | ||
| cTx (infiltration unclear) | 561 (33%) | 274 (32%) | 287 (33%) | 339 (33%) | 161 (32%) | 178 (35%) | ||
| CT lymph nodes | 0.005 | 0.5 | ||||||
| cN0 | 1176 (79%) | 604 (82%) | 572 (77%) | 782 (77%) | 397 (78%) | 385 (76%) | ||
| lymph nodes < 1 cm | 182 (12%) | 86 (12%) | 96 (13%) | 139 (14%) | 69 (14%) | 70 (14%) | ||
| lymph nodes > 1 cm | 122 (8.2%) | 44 (6.0%) | 78 (10%) | 95 (9.4%) | 42 (8.3%) | 53 (10%) | ||
| Previous bladder cancer | 510 (31%) | 209 (25%) | 301 (36%) | <0.001 | 320 (31%) | 158 (31%) | 162 (32%) | 0.8 |
| Unknown | 57 | 18 | 39 | |||||
| Smoking status | 0.9 | 0.9 | ||||||
| currently smoking | 388 (25%) | 200 (25%) | 188 (24%) | 380 (37%) | 191 (38%) | 189 (37%) | ||
| former smoking | 618 (39%) | 308 (39%) | 310 (40%) | 406 (40%) | 199 (39%) | 207 (41%) | ||
| never smoked | 573 (36%) | 289 (36%) | 284 (36%) | 230 (23%) | 118 (23%) | 112 (22%) | ||
| Unknown | 139 | 47 | 92 | - | - | - | ||
| Follow-up | 28 (13, 52) | 33 (15, 59) | 24 (12, 46) | <0.001 | 29 (14, 52) | 32 (15, 59) | 26 (13, 47) | <0.001 |
| Characteristic | Initial Cohort | PSM-Adjusted Cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| Total Cohort | Pentafecta Validation | Total Cohort | Pentafecta Validation | |||||
| n = 1718 | Pentafecta Validated, n = 844 (49%) | Pentafecta Not Validated, n = 874 (51%) | p-Value | n = 1016 | Pentafecta Validated, n = 508 (50%) | Pentafecta Not Validated, n = 508 (50%) | p-Value | |
| Surgical approach | <0.001 | 0.9 | ||||||
| open | 689 (40%) | 290 (34%) | 399 (46%) | 430 (42%) | 214 (42%) | 216 (43%) | ||
| laparoscopic or robotic | 1029 (60%) | 554 (66%) | 475 (54%) | 586 (58%) | 294 (58%) | 292 (57%) | ||
| Side | 0.2 | 0.5 | ||||||
| left | 838 (50%) | 403 (48%) | 435 (52%) | 496 (49%) | 242 (48%) | 254 (50%) | ||
| right | 840 (50%) | 433 (52%) | 407 (48%) | 520 (51%) | 266 (52%) | 254 (50%) | ||
| bilateral | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Unknown | 40 | 8 | 32 | - | - | - | ||
| Transfusion | 153 (9.7%) | 0 (0%) | 153 (20%) | <0.001 | 119 (12%) | 1 (0.2%) | 118 (23%) | <0.001 |
| Unknown | 146 | 26 | 120 | - | - | - | ||
| Surgery duration | 251 (195, 325) | 247 (190, 317) | 258 (195, 338) | 0.049 | 247 (190, 320) | 245 (192, 316) | 250 (187, 328) | 0.5 |
| Unknown | 677 | 298 | 379 | - | - | - | ||
| Lymph node dissection (LND) | 722 (42%) | 340 (40%) | 382 (44%) | 0.2 | 456 (45%) | 222 (44%) | 234 (46%) | 0.4 |
| Postoperative instillation | 197 (14%) | 98 (14%) | 99 (15%) | 0.5 | 176 (17%) | 88 (17%) | 88 (17%) | 0.9 |
| Unknown | 358 | 137 | 221 | - | - | - | ||
| Hospital length of stay | 7.0 (4.0, 10.0) | 5.0 (3.0, 9.0) | 8.0 (5.0, 13.0) | <0.001 | 7.0 (4.0, 10.0) | 6.0 (4.0, 9.0) | 8.0 (4.0, 12.0) | <0.001 |
| Unknown | 309 | 83 | 226 | |||||
| Complications | <0.001 | <0.001 | ||||||
| No complication | 1142 (72%) | 694 (82%) | 448 (60%) | 721 (71%) | 416 (82%) | 305 (60%) | ||
| CDC I | 100 (6.3%) | 57 (6.8%) | 43 (5.8%) | 65 (6.4%) | 36 (7%) | 29 (6%) | ||
| CDC II | 216 (14%) | 92 (11%) | 124 (17%) | 142 (14%) | 56 (11%) | 86 (17%) | ||
| CDC III | 82 (5.2%) | 0 (0%) | 82 (11%) | 56 (5.5%) | 0 (0%) | 56 (11%) | ||
| CDC IV | 33 (2.1%) | 0 (0%) | 33 (4.4%) | 22 (2%) | 0 (0%) | 22 (4%) | ||
| CDC V | 15 (0.9%) | 0 (0%) | 15 (2.0%) | 10 (0.9%) | 0 (0%) | 10 (2%) | ||
| Characteristic | Initial Cohort | PSM-Adjusted Cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| Total Cohort | Pentafecta Validation | Total Cohort | Pentafecta Validation | |||||
| n = 1718 | Pentafecta Validated, n = 844 (49%) | Pentafecta Not Validated, n = 874 (51%) | p-Value | n = 1016 | Pentafecta Validated, n = 508 (50%) | Pentafecta Not Validated, n = 508 (50%) | p-Value | |
| Pathological tumor stage | <0.001 | 0.6 | ||||||
| pT0 | 48 (2.8%) | 26 (3.1%) | 22 (2.5%) | 24 (2.4%) | 15 (3.0%) | 9 (1.8%) | ||
| pTa | 340 (20%) | 215 (25%) | 125 (14%) | 181 (18%) | 92 (18%) | 89 (18%) | ||
| pTis | 89 (5.2%) | 48 (5.7%) | 41 (4.7%) | 41 (4.0%) | 20 (3.9%) | 21 (4.1%) | ||
| pT1 | 322 (19%) | 168 (20%) | 154 (18%) | 184 (18%) | 95 (19%) | 89 (18%) | ||
| pT2 | 271 (16%) | 125 (15%) | 146 (17%) | 173 (17%) | 83 (16%) | 90 (18%) | ||
| pT3 | 581 (34%) | 248 (29%) | 333 (38%) | 375 (37%) | 189 (37%) | 186 (37%) | ||
| pT4 | 67 (3.9%) | 14 (1.7%) | 53 (6.1%) | 38 (3.7%) | 14 (2.8%) | 24 (4.7%) | ||
| Pathological tumor grade | 0.074 | 0.4 | ||||||
| no tumor | 24 (1.4%) | 13 (1.5%) | 11 (1.3%) | 15 (1.5%) | 8 (1.6%) | 7 (1.4%) | ||
| Low-grade | 326 (19%) | 178 (21%) | 148 (17%) | 191 (19%) | 101 (20%) | 90 (18%) | ||
| High-grade | 1368 (80%) | 653 (77%) | 715 (82%) | 810 (81%) | 399 (80%) | 411 (82%) | ||
| Multifocal | 704 (41%) | 299 (36%) | 405 (47%) | <0.001 | 396 (39%) | 199 (39%) | 197 (39%) | 0.9 |
| Unknown | 9 | 4 | 5 | - | - | - | ||
| Lymph node involvement | <0.001 | 0.3 | ||||||
| no | 561 (33%) | 288 (34%) | 273 (31%) | 319 (31%) | 162 (32%) | 157 (31%) | ||
| yes | 214 (12%) | 73 (8.6%) | 141 (16%) | 148 (15%) | 65 (13%) | 83 (16%) | ||
| Nx (No LND) | 943 (55%) | 483 (57%) | 460 (53%) | 549 (54%) | 281 (55%) | 268 (53%) | ||
| Lymphovascular invasion | 330 (19%) | 121 (14%) | 209 (24%) | <0.001 | 214 (21%) | 101 (20%) | 113 (22%) | 0.4 |
| Concomitant carcinoma in situ | 313 (18%) | 131 (16%) | 182 (21%) | 0.004 | 173 (17%) | 84 (17%) | 89 (18%) | 0.7 |
| Characteristic | Overall Survival | Cancer-Specific Survival | Non-Urothelial Recurrence-Free Survival | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable | |||||||||||||
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Pentafecta validation (Reference: PNV) | ||||||||||||||||||
| Pentafecta validated | 0.54 | 0.43, 0.68 | <0.001 | 0.52 | 0.41, 0.66 | <0.001 | 0.37 | 0.27, 0.50 | <0.001 | 0.34 | 0.25, 0.47 | <0.001 | 0.28 | 0.22, 0.35 | <0.001 | 0.24 | 0.19, 0.30 | <0.001 |
| Age | 1.03 | 1.01, 1.04 | <0.001 | 1.02 | 1.00, 1.03 | 0.016 | 1.00 | 0.99, 1.02 | 0.604 | 1.00 | 0.99, 1.02 | 0.7 | 1.01 | 1.00, 102 | 0.295 | 1.00 | 0.99, 1.02 | 0.4 |
| Sex (Reference: Male) | ||||||||||||||||||
| female | 0.88 | 0.69, 1.12 | 0.308 | 0.85 | 0.66, 1.10 | 0.2 | 1.05 | 0.79, 1.41 | 0.716 | 1.07 | 0.78, 1.46 | 0.7 | 1.08 | 0.87, 1.34 | 0.484 | 0.96 | 0.76, 1.21 | 0.7 |
| ASA | 1.43 | 1.20, 1.72 | <0.001 | 1.31 | 1.04, 1.66 | 0.023 | 1.18 | 0.95, 1.47 | 0.135 | 1.13 | 0.85, 1.51 | 0.4 | 1.16 | 0.98, 1.37 | 0.076 | 1.15 | 0.93, 1.41 | 0.2 |
| ECOG | 1.40 | 1.21, 1.63 | <0.001 | 1.09 | 0.92, 1.28 | 0.3 | 1.51 | 1.27, 1.80 | <0.001 | 1.20 | 0.98, 1.47 | 0.083 | 1.23 | 1.07, 1.42 | 0.004 | 1.09 | 0.94, 1.28 | 0.3 |
| BMI | 0.99 | 0.97, 1.01 | 0.427 | 0.97 | 0.95, 1.00 | 0.036 | 0.99 | 0.96, 1.01 | 0.331 | 0.98 | 0.95, 1.01 | 0.2 | 1.00 | 0.98, 1.03 | 0.661 | 1.01 | 0.99, 1.03 | 0.4 |
| Preoperative creatinine | 1.13 | 1.03, 1.23 | 0.008 | 1.03 | 0.89, 1.19 | 0.7 | 1.11 | 0.99, 1.24 | 0.076 | 1.00 | 0.84, 1.20 | 0.9 | 0.97 | 0.86, 1.10 | 0.657 | 0.78 | 0.64, 0.94 | 0.010 |
| NAC (Reference: No) | ||||||||||||||||||
| yes | 1.25 | 0.86, 1.83 | 0.243 | 0.87 | 0.56, 1.35 | 0.5 | 1.83 | 1.23, 2.73 | 0.003 | 1.10 | 0.68, 1.78 | 0.7 | 1.75 | 1.28, 2.38 | <0.001 | 1.02 | 0.70, 1.48 | 0.9 |
| CT stage (Reference: cT0) | ||||||||||||||||||
| cTa/cT1 | 0.74 | 0.56, 0.97 | 0.027 | 0.85 | 0.63, 1.15 | 0.3 | 0.71 | 0.50, 1.00 | 0.049 | 0.73 | 0.50, 1.08 | 0.12 | 1.01 | 0.78, 1.30 | 0.945 | 1.05 | 0.79, 1.39 | 0.7 |
| cT2 | 0.59 | 0.39, 0.87 | 0.008 | 0.56 | 0.36, 0.86 | 0.008 | 0.74 | 0.47, 1.16 | 0.188 | 0.53 | 0.32, 0.90 | 0.018 | 0.99 | 0.71, 1.37 | 0.945 | 0.88 | 0.61, 1.27 | 0.5 |
| ≥cT3 | 1.13 | 0.83, 1.54 | 0.441 | 0.99 | 0.70, 1.40 | 0.9 | 1.24 | 0.85, 1.81 | 0.259 | 0.94 | 0.62, 1.42 | 0.8 | 1.46 | 1.08, 1.95 | 0.013 | 1.30 | 0.95, 1.80 | 0.10 |
| CT lymph nodes (Reference: cN0) | ||||||||||||||||||
| Lymphnodes < 1 cm | 1.19 | 0.87, 1.63 | 0.277 | 1.03 | 0.72, 1.47 | 0.9 | 1.45 | 1.00, 2.10 | 0.052 | 1.13 | 0.73, 1.74 | 0.6 | 1.41 | 1.06, 1.87 | 0.017 | 1.14 | 0.82, 1.57 | 0.4 |
| Lymphnodes > 1 cm | 2.17 | 1.59, 2.95 | <0.001 | 1.55 | 1.06, 2.28 | 0.024 | 2.80 | 1.96, 3.99 | <0.001 | 1.57 | 1.00, 2.48 | 0.052 | 2.59 | 1.94, 3.45 | <0.001 | 2.24 | 1.56, 3.23 | <0.001 |
| CT hydronephrosis | 0.93 | 0.74, 1.16 | 0.501 | 1.06 | 0.83, 1.35 | 0.6 | 1.04 | 0.79, 1.37 | 0.783 | 1.25 | 0.93, 1.68 | 0.15 | 0.93 | 0.75, 1.14 | 0.476 | 0.96 | 0.77, 1.19 | 0.7 |
| Pathological tumor stage (Reference: pT0) | ||||||||||||||||||
| pTa | 1.20 | 0.29, 4.97 | 0.802 | 0.65 | 0.08, 5.03 | 0.7 | 0.57 | 0.13, 2.47 | 0.456 | 0.66 | 0.08, 5.62 | 0.7 | 0.42 | 0.18, 1.01 | 0.053 | 0.80 | 0.18, 3.57 | 0.8 |
| pTis | 1.21 | 0.26, 5.52 | 0.808 | 0.60 | 0.07, 5.05 | 0.6 | 0.77 | 0.16, 3.83 | 0.752 | 0.93 | 0.09, 9.08 | 0.9 | 0.78 | 0.30, 2.03 | 0.605 | 1.15 | 0.24, 5.55 | 0.9 |
| pT1 | 1.42 | 0.34, 5.83 | 0.631 | 0.78 | 0.10, 5.97 | 0.8 | 0.95 | 0.23, 3.99 | 0.948 | 1.17 | 0.14, 9.74 | 0.9 | 0.71 | 0.31, 1.66 | 0.431 | 1.28 | 0.29, 5.65 | 0.7 |
| pT2 | 1.62 | 0.39, 6.70 | 0.502 | 1.14 | 0.15, 8.70 | 0.9 | 1.06 | 0.25, 4.47 | 0.932 | 1.35 | 0.16, 11.1 | 0.8 | 0.94 | 0.40, 2.17 | 0.876 | 1.41 | 0.32, 6.18 | 0.6 |
| pT3 | 2.88 | 0.71, 11.63 | 0.138 | 1.58 | 0.21, 11.9 | 0.7 | 2.08 | 0.51, 8.42 | 0.307 | 2.06 | 0.26, 16.6 | 0.5 | 1.46 | 0.65, 3.30 | 0.363 | 2.07 | 0.48, 8.94 | 0.3 |
| pT4 | 5.94 | 1.39, 25.42 | 0.0163 | 3.36 | 0.42, 26.6 | 0.3 | 4.80 | 1.10, 20.89 | 0.037 | 4.41 | 0.52, 37.3 | 0.2 | 3.30 | 1.35, 8.07 | 0.009 | 4.08 | 0.90, 18.6 | 0.069 |
| Pathological tumor grade (Reference: Low-grade) | ||||||||||||||||||
| High-grade | 1.84 | 1.33, 2.53 | <0.001 | 1.31 | 0.90, 1.92 | 0.2 | 2.71 | 1.71, 4.30 | <0.001 | 1.70 | 0.98, 2.93 | 0.058 | 2.04 | 1.48, 2.81 | <0.001 | 1.56 | 1.08, 2.26 | 0.019 |
| Concomitant carcinoma in situ (Reference: No) | ||||||||||||||||||
| yes | 1.02 | 0.77, 1.37 | 0.881 | 0.91 | 0.65, 1.27 | 0.6 | 1.11 | 0.78, 1.57 | 0.569 | 0.82 | 0.54, 1.24 | 0.4 | 1.28 | 0.99, 1.66 | 0.055 | 0.99 | 0.74, 1.34 | 0.9 |
| Multifocal tumor (Reference: No) | ||||||||||||||||||
| yes | 1.29 | 1.03, 1.62 | 0.024 | 1.48 | 1.14, 1.91 | 0.003 | 1.38 | 1.05, 1.82 | 0.022 | 1.59 | 1.15, 2.19 | 0.005 | 1.09 | 0.88, 1.35 | 0.42 | 1.23 | 0.98, 1.56 | 0.080 |
| Surgical approach (Reference: Open) | ||||||||||||||||||
| Laparoscopic or robotic | 0.77 | 0.62, 0.97 | 0.024 | 0.81 | 0.64, 1.04 | 0.093 | 0.64 | 0.49, 0.85 | 0.002 | 0.75 | 0.55, 1.02 | 0.067 | 0.89 | 0.72, 1.10 | 0.267 | 0.96 | 0.77, 1.21 | 0.7 |
| LND (Reference: No) | ||||||||||||||||||
| Yes | 1.05 | 0.84, 1.32 | 0.651 | 0.68 | 0.37, 1.27 | 0.2 | 1.52 | 1.15, 2.00 | 0.003 | 1.03 | 0.45, 2.37 | 0.9 | 1.33 | 1.08, 1.63 | 0.008 | 0.73 | 0.42, 1.28 | 0.3 |
| Lymph node involvement (Reference: No) | ||||||||||||||||||
| yes | 2.86 | 2.03, 4.01 | <0.001 | 1.97 | 1.33, 2.93 | <0.001 | 3.65 | 2.48, 5.37 | <0.001 | 2.18 | 1.39, 3.41 | <0.001 | 3.17 | 2.35, 4.27 | <0.001 | 1.70 | 1.19, 2.43 | 0.004 |
| Nx | 1.32 | 1.00, 1.73 | 0.047 | 0.96 | 0.52, 1.77 | 0.9 | 1.11 | 0.79, 1.56 | 0.549 | 1.23 | 0.53, 2.82 | 0.6 | 1.13 | 0.88, 1.46 | 0.335 | 0.92 | 0.53, 1.59 | 0.8 |
| Lymphovascular invasion (Reference: No) | ||||||||||||||||||
| yes | 1.44 | 1.10, 1.89 | 0.007 | 0.96 | 0.71, 1.29 | 0.8 | 1.86 | 1.37, 2.54 | <0.001 | 1.15 | 0.82, 1.62 | 0.4 | 2.21 | 1.76, 2.78 | <0.001 | 1.50 | 1.16, 1.94 | 0.002 |
| Previous bladder cancer (Reference: No) | ||||||||||||||||||
| yes | 1.17 | 0.92, 1.47 | 0.198 | 1.20 | 0.93, 1.54 | 0.2 | 1.22 | 0.91, 1.62 | 0.181 | 1.59 | 1.16, 2.18 | 0.004 | 0.99 | 0.79, 1.24 | 0.939 | 1.10 | 0.86, 1.40 | 0.5 |
| Postoperative instillation (Reference: No) | ||||||||||||||||||
| Yes | 0.79 | 0.57, 1.09 | 0.154 | 0.66 | 0.46, 0.97 | 0.032 | 0.61 | 0.39, 0.96 | 0.031 | 0.48 | 0.28, 0.83 | 0.009 | 0.98 | 0.74, 1.29 | 0.887 | 0.80 | 0.58, 1.09 | 0.15 |
| Characteristic | OR | 95% CI | p-Value |
|---|---|---|---|
| Age | 1.00 | 0.99, 1.01 | 0.9 |
| Gender (Ref.: Male) | |||
| female | 0.97 | 0.75, 1.26 | 0.8 |
| ASA | 1.11 | 0.92, 1.34 | 0.3 |
| ECOG | 1.04 | 0.87, 1.24 | 0.7 |
| BMI | 1.00 | 0.98, 1.03 | 0.9 |
| Smoking status (Ref.: never) | |||
| former smoking | 1.05 | 0.79, 1.39 | 0.7 |
| currently smoking | 0.96 | 0.69, 1.33 | 0.8 |
| Multifocal (Ref.: No) | |||
| yes | 0.98 | 0.76, 1.27 | 0.9 |
| Diabetes mellitus (Ref.: No) | |||
| yes | 0.95 | 0.70, 1.30 | 0.8 |
| Preoperative creatinine | 1.09 | 0.96, 1.26 | 0.2 |
| CT stage (Ref.: cT0) | |||
| cT1 | 0.89 | 0.66, 1.20 | 0.4 |
| cT2 | 0.95 | 0.65, 1.39 | 0.8 |
| ≥cT3 | 0.81 | 0.56, 1.17 | 0.3 |
| CT lymph nodes (Ref.: cT0) | |||
| Lymphnodes < 1 cm | 1.06 | 0.74, 1.53 | 0.7 |
| Lymphnodes > 1 cm | 1.45 | 0.96, 2.20 | 0.08 |
| CT hydronephrosis (Ref.: No) | |||
| Yes | 1.10 | 0.86, 1.41 | 0.5 |
| Surgical approach (Ref.: Open) | |||
| laparoscopic or robotic | 0.98 | 0.77, 1.26 | 0.9 |
| Tumor side (Ref.: left) | |||
| right | 0.91 | 0.71, 1.16 | 0.5 |
| LAD (Ref.: No) | |||
| Yes | 1.08 | 0.85, 1.39 | 0.5 |
| Postoperative instillation (Ref.: No) | |||
| Yes | 0.97 | 0.70, 1.35 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
König, F.; Grossmann, N.C.; Soria, F.; D’Andrea, D.; Juvet, T.; Potretzke, A.; Djaladat, H.; Ghoreifi, A.; Kikuchi, E.; Hayakawa, N.; et al. Pentafecta for Radical Nephroureterectomy in Patients with High-Risk Upper Tract Urothelial Carcinoma: A Proposal for Standardization of Quality Care Metrics. Cancers 2022, 14, 1781. https://doi.org/10.3390/cancers14071781
König F, Grossmann NC, Soria F, D’Andrea D, Juvet T, Potretzke A, Djaladat H, Ghoreifi A, Kikuchi E, Hayakawa N, et al. Pentafecta for Radical Nephroureterectomy in Patients with High-Risk Upper Tract Urothelial Carcinoma: A Proposal for Standardization of Quality Care Metrics. Cancers. 2022; 14(7):1781. https://doi.org/10.3390/cancers14071781
Chicago/Turabian StyleKönig, Frederik, Nico C. Grossmann, Francesco Soria, David D’Andrea, Tristan Juvet, Aaron Potretzke, Hooman Djaladat, Alireza Ghoreifi, Eiji Kikuchi, Nozomi Hayakawa, and et al. 2022. "Pentafecta for Radical Nephroureterectomy in Patients with High-Risk Upper Tract Urothelial Carcinoma: A Proposal for Standardization of Quality Care Metrics" Cancers 14, no. 7: 1781. https://doi.org/10.3390/cancers14071781
APA StyleKönig, F., Grossmann, N. C., Soria, F., D’Andrea, D., Juvet, T., Potretzke, A., Djaladat, H., Ghoreifi, A., Kikuchi, E., Hayakawa, N., Mari, A., Khene, Z.-E., Fujita, K., Raman, J. D., Breda, A., Fontana, M., Sfakianos, J. P., Pfail, J. L., Laukhtina, E., ... Pradere, B. (2022). Pentafecta for Radical Nephroureterectomy in Patients with High-Risk Upper Tract Urothelial Carcinoma: A Proposal for Standardization of Quality Care Metrics. Cancers, 14(7), 1781. https://doi.org/10.3390/cancers14071781














