Minimally Invasive Preclinical Monitoring of the Peritoneal Cavity Tumor Microenvironment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Cells
2.2. Mouse Tumor Models
2.3. In Vivo Intraperitoneal Lavage (IVIPL)
2.4. Antibodies and Flow Cytometry
2.5. Statistical Analysis
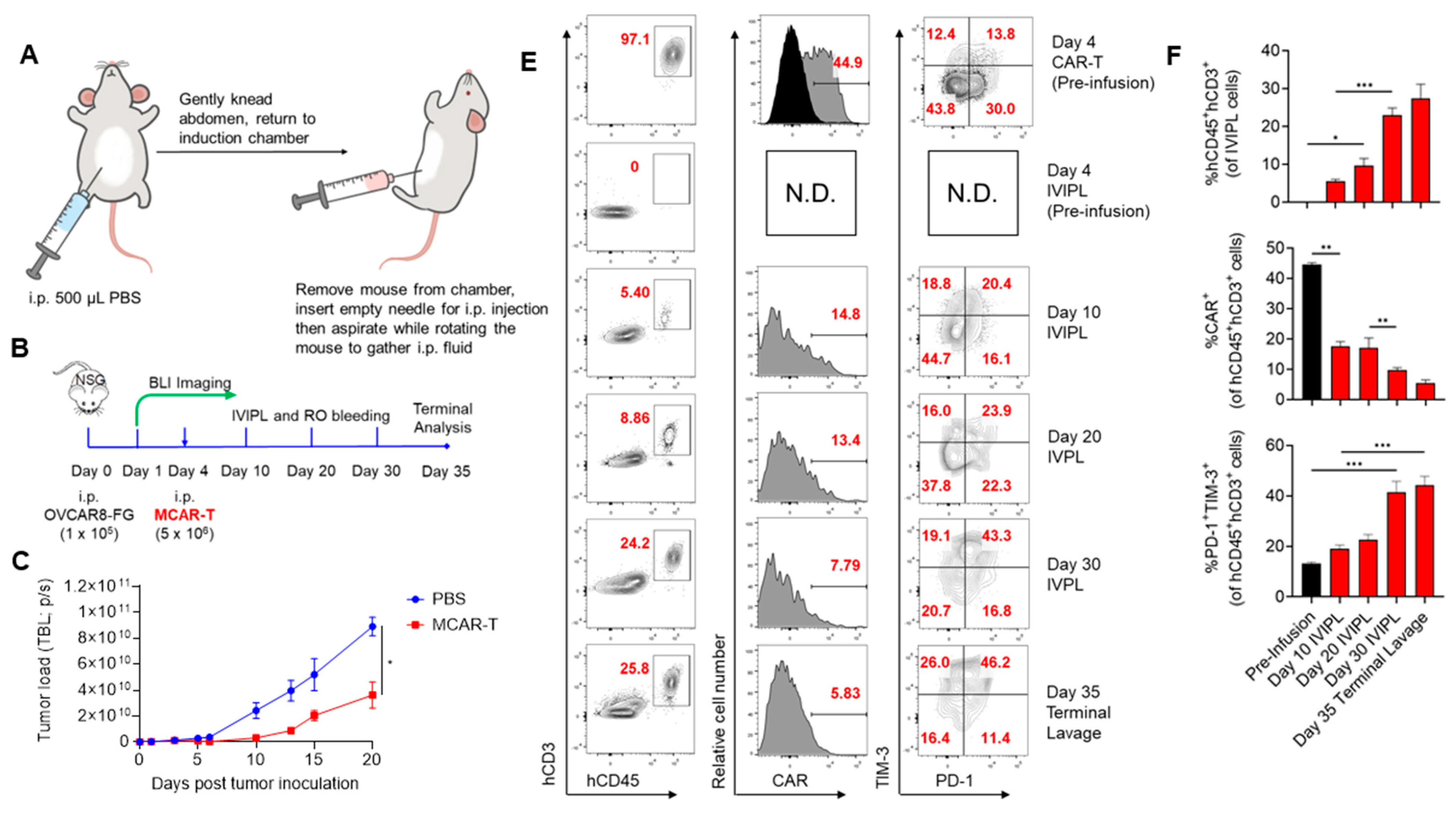
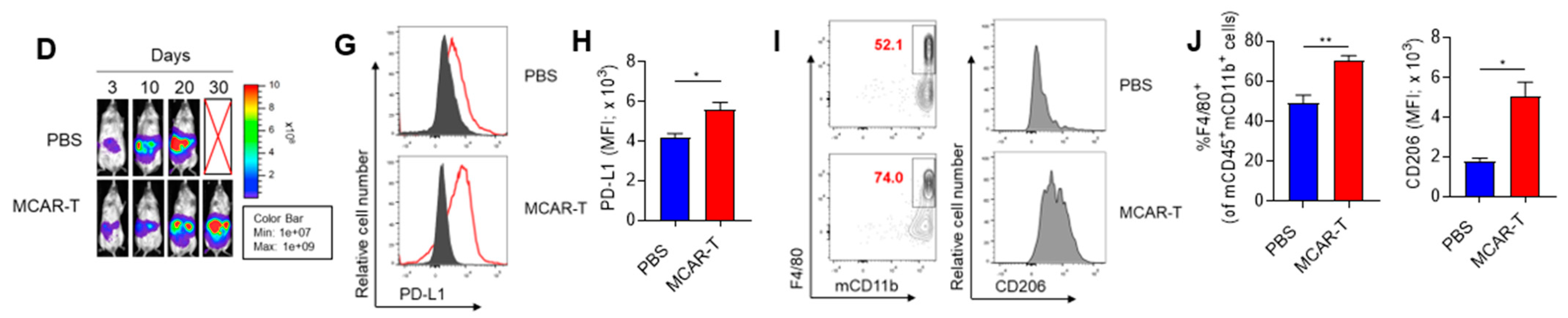
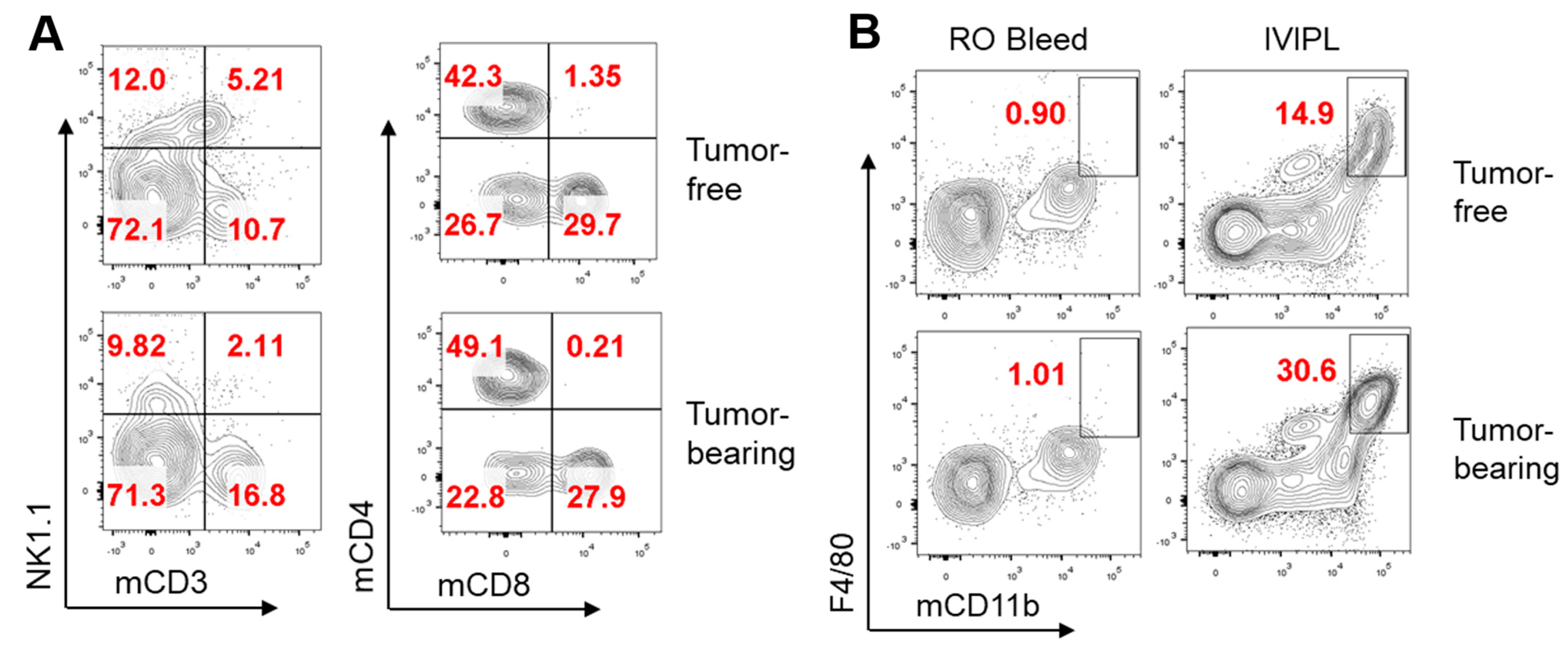
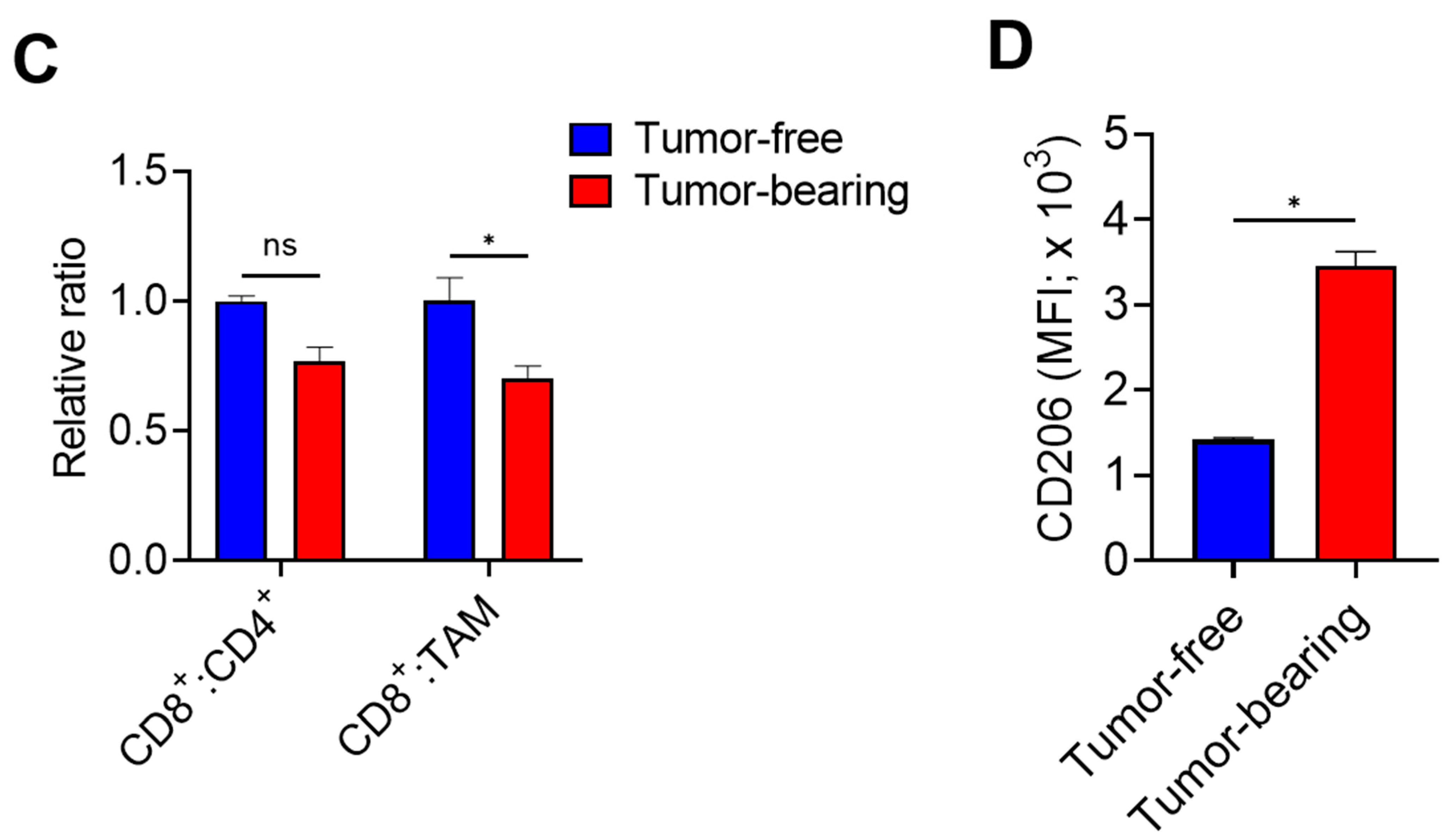
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Isaza-Restrepo, A.; Martin-Saavedra, J.S.; Velez-Leal, J.L.; Vargas-Barato, F.; Riveros-Dueñas, R. The Peritoneum: Beyond the Tissue - A Review. Front. Physiol. 2018, 9, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Baal, J.O.A.M.; Van de Vijver, K.K.; Nieuwland, R.; van Noorden, C.J.F.; van Driel, W.J.; Sturk, A.; Kenter, G.G.; Rikkert, L.G.; Lok, C.A.R. The Histophysiology and Pathophysiology of the Peritoneum. Tissue Cell 2017, 49, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Runyon, B.A. Care of Patients with Ascites. N. Engl. J. Med. 1994, 330, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S. New Therapies and Insights into the Changing Landscape of Colorectal Cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 79–80. [Google Scholar] [CrossRef]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian Cancer: An Integrated Review. Semin. Oncol. Nurs. 2019, 35, 151–156. [Google Scholar] [CrossRef]
- Coccolini, F.; Gheza, F.; Lotti, M.; Virzì, S.; Iusco, D.; Ghermandi, C.; Melotti, R.; Baiocchi, G.; Giulini, S.M.; Ansaloni, L.; et al. Peritoneal Carcinomatosis. World J. Gastroenterol. 2013, 19, 6979–6994. [Google Scholar] [CrossRef]
- Ahmed, N.; Stenvers, K.L. Getting to Know Ovarian Cancer Ascites: Opportunities for Targeted Therapy-Based Translational Research. Front. Oncol. 2013, 3, 256. [Google Scholar] [CrossRef] [Green Version]
- Kipps, E.; Tan, D.S.P.; Kaye, S.B. Meeting the Challenge of Ascites in Ovarian Cancer: New Avenues for Therapy and Research. Nat. Rev. Cancer 2013, 13, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Rohaan, M.W.; Van Den Berg, J.H.; Kvistborg, P.; Haanen, J.B.A.G. Adoptive Transfer of Tumor-Infiltrating Lymphocytes in Melanoma: A Viable Treatment Option 11 Medical and Health Sciences 1107 Immunology 11 Medical and Health Sciences 1112 Oncology and Carcinogenesis. J. Immunother. Cancer 2018, 6, 1–16. [Google Scholar] [CrossRef]
- Haslauer, T.; Greil, R.; Zaborsky, N.; Geisberger, R. Car T-Cell Therapy in Hematological Malignancies. Int. J. Mol. Sci. 2021, 22, 8996. [Google Scholar] [CrossRef]
- Sharma, P.; Kanapuru, B.; George, B.; Lin, X.; Xu, Z.; Bryan, W.W.; Pazdur, R.; Theoret, M.R. FDA Approval Summary: Idecabtagene Vicleucel for Relapsed or Refractory Multiple Myeloma. Clin. Cancer Res. 2022, OF1–OF6. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Tang, Y.; Li, W.; Zeng, Q.; Chang, D. Efficiency of CAR-T Therapy for Treatment of Solid Tumor in Clinical Trials: A Meta-Analysis. Dis. Markers 2019, 2019, 3425291. [Google Scholar] [CrossRef] [Green Version]
- Rafiq, S.; Hackett, C.S.; Brentjens, R.J. Engineering Strategies to Overcome the Current Roadblocks in CAR T Cell Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, B.; Song, Y.S. Ascites Modulates Cancer Cell Behavior, Contributing to Tumor Heterogeneity in Ovarian Cancer. Cancer Sci. 2016, 107, 1173–1178. [Google Scholar] [CrossRef] [Green Version]
- Heath, O.; Berlato, C.; Maniati, E.; Lakhani, A.; Pegrum, C.; Kotantaki, P.; Barry, S.T.; Annibaldi, A.; Barton, D.P.; Elorbany, S.; et al. Chemotherapy Induces Tumor-Associated Macrophages That Aid Adaptive Immune Responses in Ovarian Cancer. Cancer Immunol. Res. 2021, 9, 665–681. [Google Scholar] [CrossRef]
- Antonio, M.; Giulia, G.; Cristina, M.; Luciana, T.; Mel, L. Role of M1-Polarized Tumor- Associated Macrophages in the Prognosis of Advanced Ovarian Cancer Patients. Sci. Rep. 2020, 10, 6096. [Google Scholar] [CrossRef] [Green Version]
- Chyuan, I.; Chu, C. Targeting the Tumor Microenvironment for Improving Therapeutic Effectiveness in Cancer Immunotherapy: Focusing on Immune Checkpoint Inhibitors and Combination Therapies. Cancers 2021, 13, 1188. [Google Scholar] [CrossRef]
- Fang, H.; Declerck, Y.A. Targeting the Tumor Microenvironment: From Understanding Pathways to Effective Clinical Trials. Cancer Res. 2013, 73, 4965–4977. [Google Scholar] [CrossRef] [Green Version]
- Ray, A.; Dittel, B.N. Isolation of Mouse Peritoneal Cavity Cells. J. Vis. Exp. 2010, 9–11. [Google Scholar] [CrossRef]
- Zhu, Y.; Smith, D.J.; Zhou, Y.; Li, Y.R.; Yu, J.; Lee, D.; Wang, Y.C.; Di Biase, S.; Wang, X.; Hardoy, C.; et al. Development of Hematopoietic Stem Cell-Engineered Invariant Natural Killer T Cell Therapy for Cancer. Cell Stem Cell 2019, 25, 542–557.e9. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, E.A. Sampling and Preparation of Mouse and Rat Serum. Cold Spring Harb. Protoc. 2017, 2017, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Motulsky, H. Prism, 8.0.1; Graphpad Software: San Diego, CA, USA, 2018. [Google Scholar]
- Ford, R.B.; Mazzaferro, E. Kirk & Bistner’s Handbook of Veterinary Procedures and Emergency Treatment, 9th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Klampatsa, A.; Dimou, V.; Albelda, S.M. Mesothelin-Targeted CAR-T Cell Therapy for Solid Tumors. Expert Opin. Biol. Ther. 2021, 21, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Haanen, J.B.A.G.; Robert, C. Immune Checkpoint Inhibitors. Prog. Tumor Res. 2015, 42, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Roby, K.F.; Taylor, C.C.; Sweetwood, J.P.; Cheng, Y.; Pace, J.L.; Tawfik, O.; Persons, D.L.; Smith, P.G.; Terranova, P.F. Development of a Syngeneic Mouse Model for Events Related to Ovarian Cancer. Carcinogenesis 2000, 21, 585–591. [Google Scholar] [CrossRef]
- Miller, J.E.; Ahn, S.H.; Marks, R.M.; Monsanto, S.P.; Fazleabas, A.T.; Koti, M.; Tayade, C. IL-17A Modulates Peritoneal Macrophage Recruitment and M2 Polarization in Endometriosis. Front. Immunol. 2020, 11, 108. [Google Scholar] [CrossRef]
- Romero, C.R.; Herzig, D.S.; Etogo, A.; Nunez, J.; Mahmoudizad, R.; Fang, G.; Murphey, E.D.; Toliver-Kinsky, T.; Sherwood, E.R. The Role of Interferon-γ in the Pathogenesis of Acute Intra-Abdominal Sepsis. J. Leukoc. Biol. 2010, 88, 725–735. [Google Scholar] [CrossRef] [Green Version]
- Gautam, A.; Park, B.K.; Kim, T.H.; Akauliya, M.; Kim, D.; Maharjan, S.; Park, J.; Kim, J.; Lee, H.; Park, M.S.; et al. Peritoneal Cells Mediate Immune Responses and Cross-Protection against Influenza A Virus. Front. Immunol. 2019, 10, 1160. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.Y.; Lee, S.N.; Chang, S.Y.; Ko, H.J.; Ryu, S.; Kweon, M.N. A Mouse Model of Shigellosis by Intraperitoneal Infection. J. Infect. Dis. 2014, 209, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Bella, Á.; Di Trani, C.A.; Fernández-Sendin, M.; Arrizabalaga, L.; Cirella, A.; Teijeira, Á.; Medina-Echeverz, J.; Melero, I.; Berraondo, P.; Aranda, F. Mouse Models of Peritoneal Carcinomatosis to Develop Clinical Applications. Cancers 2021, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.D.; Shaw, J.C.; Sobin, L.H. From the Archives of the AFIP: Secondary Tumors and Tumorlike Lesions of the Peritoneal Cavity: Imaging Features with Pathologic Correlation. Radiographics 2009, 29, 347–373. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA. Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Boland, P.M.; Ma, W.W. Immunotherapy for Colorectal Cancer. Cancers 2017, 9, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odunsi, K. Immunotherapy in Ovarian Cancer. Ann. Oncol. 2017, 28, viii1–viii7. [Google Scholar] [CrossRef]
- DeRenzo, C.; Gottschalk, S. Genetic Modification Strategies to Enhance CAR T Cell Persistence for Patients with Solid Tumors. Front. Immunol. 2019, 10, 218. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T Cell Therapy: Current Limitations and Potential Strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Kircher, M.F.; Gambhir, S.S.; Grimm, J. Noninvasive Cell-Tracking Methods. Nat. Rev. Clin. Oncol. 2011, 8, 677–688. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2020, 56, 15. [Google Scholar] [CrossRef] [Green Version]
- Milone, M.C.; Fish, J.D.; Carpenito, C.; Carroll, R.G.; Binder, G.K.; Teachey, D.; Samanta, M.; Lakhal, M.; Gloss, B.; Danet-Desnoyers, G.; et al. Chimeric Receptors Containing CD137 Signal Transduction Domains Mediate Enhanced Survival of T Cells and Increased Antileukemic Efficacy in Vivo. Mol. Ther. 2009, 17, 1453–1464. [Google Scholar] [CrossRef]
- Zhao, Z.; Condomines, M.; van der Stegen, S.J.C.; Perna, F.; Kloss, C.C.; Gunset, G.; Plotkin, J.; Sadelain, M. Structural Design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T Cells. Cancer Cell 2015, 28, 415–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Moon, E.; Carpenito, C.; Paulos, C.M.; Liu, X.; Brennan, A.L.; Chew, A.; Carroll, R.G.; Scholler, J.; Levine, B.L.; et al. Multiple Injections of Electroporated Autologous T Cells Expressing a Chimeric Antigen Receptor Mediate Regression of Human Disseminated Tumor. Cancer Res. 2010, 70, 9053–9061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanitis, E.; Poussin, M.; Hagemann, I.S.; Coukos, G.; Sandaltzopoulos, R.; Scholler, N.; Powell, D.J. Redirected Antitumor Activity of Primary Human Lymphocytes Transduced with a Fully Human Anti-Mesothelin Chimeric Receptor. Mol. Ther. 2012, 20, 633–643. [Google Scholar] [CrossRef] [Green Version]
- Ang, W.X.; Li, Z.; Chi, Z.; Du, S.H.; Chen, C.; Tay, J.C.K.; Toh, H.C.; Connolly, J.E.; Xu, X.H.; Wang, S. Intraperitoneal Immunotherapy with T Cells Stably and Transiently Expressing Anti-EpCAM CAR in Xenograft Models of Peritoneal Carcinomatosis. Oncotarget 2017, 8, 13545–13559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murad, J.P.; Kozlowska, A.K.; Lee, H.J.; Ramamurthy, M.; Chang, W.C.; Yazaki, P.; Colcher, D.; Shively, J.; Cristea, M.; Forman, S.J.; et al. Effective Targeting of TAG72+peritoneal Ovarian Tumors via Regional Delivery of CAR-Engineered T Cells. Front. Immunol. 2018, 9, 2268. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.F.; Xu, X.; Li, L.; Ma, Y.; Jin, Q.; Viley, A.; Allen, C.; Natarajan, P.; Shivakumar, R.; Peshwa, M.V.; et al. Development of Anti-Human Mesothelin-Targeted Chimeric Antigen Receptor Messenger RNA-Transfected Peripheral Blood Lymphocytes for Ovarian Cancer Therapy. Hum. Gene Ther. 2018, 29, 614–625. [Google Scholar] [CrossRef]
- Owens, G.L.; Sheard, V.E.; Kalaitsidou, M.; Blount, D.; Lad, Y.; Cheadle, E.J.; Edmondson, R.J.; Kooner, G.; Gilham, D.E.; Harrop, R. Preclinical Assessment of CAR T-Cell Therapy Targeting the Tumor Antigen 5T4 in Ovarian Cancer. J. Immunother. 2018, 41, 130–140. [Google Scholar] [CrossRef] [Green Version]
- Giavridis, T.; Van Der Stegen, S.J.C.; Eyquem, J.; Hamieh, M.; Piersigilli, A.; Sadelain, M. CAR T Cell-Induced Cytokine Release Syndrome Is Mediated by Macrophages and Abated by IL-1 Blockade Letter. Nat. Med. 2018, 24, 731–738. [Google Scholar] [CrossRef]
- Guedan, S.; Madar, A.; Casado-Medrano, V.; Shaw, C.; Wing, A.; Liu, F.; Young, R.M.; June, C.H.; Posey, A.D. Single Residue in CD28-Costimulated CAR-T Cells Limits Long-Term Persistence and Antitumor Durability. J. Clin. Investig. 2020, 130, 3087–3097. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Li, C.; Bian, H.; Qian, W.; Jin, K.; Xu, T.; Guo, X.; Lu, X.; Su, F. Targeting CDK7 Suppresses Super Enhancer-Linked Inflammatory Genes and Alleviates CAR T Cell-Induced Cytokine Release Syndrome. Mol. Cancer 2021, 20, 5. [Google Scholar] [CrossRef]
- Pang, N.; Shi, J.; Qin, L.; Chen, A.; Tang, Y.; Yang, H.; Huang, Y.; Wu, Q.; Li, X.; He, B.; et al. IL-7 and CCL19-Secreting CAR-T Cell Therapy for Tumors with Positive Glypican-3 or Mesothelin. J. Hematol. Oncol. 2021, 14, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, H.; Meng, F.; Li, J.; Zhang, S. Combined Trabectedin and Anti-PD1 Antibody Produces a Synergistic Antitumor Effect in a Murine Model of Ovarian Cancer. J. Transl. Med. 2015, 13, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, L.; Bachelder, R.E.; Kennedy, M.; Chen, P.H.; Chi, J.T.; Berchuck, A.; Cianciolo, G.; Pizzo, S.V. Syngeneic Murine Ovarian Cancer Model Reveals That Ascites Enriches for Ovarian Cancer Stem-like Cells Expressing Membrane GRP78. Mol. Cancer Ther. 2015, 14, 747–756. [Google Scholar] [CrossRef] [Green Version]
- Au, K.K.; Peterson, N.; Truesdell, P.; Reid-Schachter, G.; Khalaj, K.; Ren, R.; Francis, J.A.; Graham, C.H.; Craig, A.W.; Koti, M. CXCL10 Alters the Tumour Immune Microenvironment and Disease Progression in a Syngeneic Murine Model of High-Grade Serous Ovarian Cancer. Gynecol. Oncol. 2017, 145, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; Merlino, G.; Van Dyke, T. Preclinical Mouse Cancer Models: A Maze of Opportunities and Challenges. Cell 2015, 163, 39–53. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Dunn, Z.S.; Chen, X.; MacMullan, M.; Cinay, G.; Wang, H.; Liu, J.; Hu, F.; Wang, P. Adenosine Deaminase 1 Overexpression Enhances the Antitumor Efficacy of Chimeric Antigen Receptor-Engineered T Cells. Hum. Gene Ther. 2021, 33, 223–236. [Google Scholar] [CrossRef]
- Li, Y.R.; Zhou, Y.; Kim, Y.J.; Zhu, Y.; Ma, F.; Yu, J.; Wang, Y.C.; Chen, X.; Li, Z.; Zeng, S.; et al. Development of Allogeneic HSC-Engineered INKT Cells for off-the-Shelf Cancer Immunotherapy. Cell Rep. Med. 2021, 2, 100449. [Google Scholar] [CrossRef]
- Ipseiz, N.; Czubala, M.A.; Bart, V.M.T.; Davies, L.C.; Jenkins, R.H.; Brennan, P.; Taylor, P.R. Effective In Vivo Gene Modification in Mouse Tissue-Resident Peritoneal Macrophages by Intraperitoneal Delivery of Lentiviral Vectors. Mol. Ther. Methods Clin. Dev. 2020, 16, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Hogg, C.; Panir, K.; Dhami, P.; Rosser, M.; Mack, M.; Soong, D.; Pollard, J.W.; Jenkins, S.J.; Horne, A.W.; Greaves, E. Macrophages Inhibit and Enhance Endometriosis Depending on Their Origin. Proc. Natl. Acad. Sci. USA 2021, 118, e2013776118. [Google Scholar] [CrossRef]
- Blacher, E.; Tsai, C.; Litichevskiy, L.; Shipony, Z.; Iweka, C.A.; Schneider, K.M.; Chuluun, B.; Heller, H.C.; Menon, V.; Thaiss, C.A.; et al. Aging Disrupts Circadian Gene Regulation and Function in Macrophages. Nat. Immunol. 2022, 23, 229–236. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunn, Z.S.; Li, Y.-R.; Yu, Y.; Lee, D.; Gibbons, A.; Kim, J.J.; Zhou, T.Y.; Li, M.; Nguyen, M.; Cen, X.; et al. Minimally Invasive Preclinical Monitoring of the Peritoneal Cavity Tumor Microenvironment. Cancers 2022, 14, 1775. https://doi.org/10.3390/cancers14071775
Dunn ZS, Li Y-R, Yu Y, Lee D, Gibbons A, Kim JJ, Zhou TY, Li M, Nguyen M, Cen X, et al. Minimally Invasive Preclinical Monitoring of the Peritoneal Cavity Tumor Microenvironment. Cancers. 2022; 14(7):1775. https://doi.org/10.3390/cancers14071775
Chicago/Turabian StyleDunn, Zachary Spencer, Yan-Ruide Li, Yanqi Yu, Derek Lee, Alicia Gibbons, James Joon Kim, Tian Yang Zhou, Mulin Li, Mya Nguyen, Xinjian Cen, and et al. 2022. "Minimally Invasive Preclinical Monitoring of the Peritoneal Cavity Tumor Microenvironment" Cancers 14, no. 7: 1775. https://doi.org/10.3390/cancers14071775
APA StyleDunn, Z. S., Li, Y.-R., Yu, Y., Lee, D., Gibbons, A., Kim, J. J., Zhou, T. Y., Li, M., Nguyen, M., Cen, X., Zhou, Y., Wang, P., & Yang, L. (2022). Minimally Invasive Preclinical Monitoring of the Peritoneal Cavity Tumor Microenvironment. Cancers, 14(7), 1775. https://doi.org/10.3390/cancers14071775









