Simple Summary
Physical activity is known to reduce breast cancer risk and improve patient prognosis. However, the association between pre-diagnostic physical activity and the aggressiveness of breast cancer is unclear. Here, we assessed the effects of pre-diagnostic physical activity on breast cancer aggressiveness among breast cancer patients. Despite not improving overall survival, higher levels of pre-diagnostic physical activity contributed to less aggressive forms of breast tumours. These results underline the importance of physical activity in improving patient prognosis.
Abstract
Physical activity (PA) is known to reduce breast cancer (BC) risk and improve patient prognosis. However, the association between pre-diagnostic PA and the aggressiveness of BC is unclear. We investigated the associations between PA, BC tumour characteristics, and survival. This retrospective observational study included 7688 BC patients from the Singapore Breast Cancer Cohort (2010–2016). PA information from the questionnaire included intensity (light/moderate/vigorous) and duration (<1 h/1–2 h/>2 h per week). A PA score (1–5) incorporating intensity and duration was calculated. Associations between PA score and tumour characteristics such as stage, histological grade, nodal and hormone receptor status were examined using multinomial regression. Moreover, 10-year overall survival was estimated using Cox regression analysis in 6572 patients after excluding patients with invalid survival data and stage IV disease. Breast tumours associated with higher PA score were more likely to be non-invasive (ORinvasive vs. non-invasive(reference) [95% CI]: 0.71 [0.58–0.87], p-trend = 0.001), of lower grade (ORpoorly vs. well differentiated(reference): 0.69 [0.52–0.93], p = 0.014), ER-positive (ORER-negative vs. ER-positive(reference): 0.94 [0.89–1.00], p-trend = 0.049), PR-positive (ORPR-negative vs. PR-positive(reference): 0.82 [0.67–0.99], p = 0.041), HER2-negative (ORHER2-negative vs. HER2-positive(reference): 1.29 [1.02–1.62], p-trend = 0.002), and less likely to be of HER2-overexpressed subtype (ORHER2-overexpressed vs. Luminal A(reference): 0.89 [0.81–0.98], p-trend = 0.018). These associations (odds ratios) were more pronounced among post-menopausal patients. A higher PA score did not improve survival. Higher levels of pre-diagnostic PA were associated with less aggressive tumours in BC patients. This illustrated another benefit of PA in addition to its known role in BC risk reduction.
1. Introduction
Breast cancer is the most common cancer among women [1]. Despite the continuous medical advancements to improve detection and intervention methods, it remains important to highlight the major lifestyle factors that can be easily modified and engaged by people in order to reduce breast cancer occurrence [2,3]. One of these factors is physical activity (PA), which has been reported in several epidemiological studies to be a predominant modifiable risk factor for the development of breast cancer [2,4].
Previous studies have demonstrated an inverse association between both pre-diagnostic and post-diagnostic PA with breast cancer mortality [5,6,7,8]. Additionally, the updated World Cancer Research Foundation comprehensive review judged the evidence of physical activity’s preventive role against breast cancer as strong [9]. Furthermore, a dose-dependent relationship between PA and cancer risk and mortality is well established and reported by several meta-analyses and systematic reviews involving various cancers [10,11]. Notably, PA confers an average relative breast cancer risk reduction of 12–25% when comparing individuals with the highest to the lowest category of PA [4,11,12]. To adequately enjoy health benefits, it is recommended that individuals engage in 150 to 300 min of moderate-intensity PA or 75 to 100 min of vigorous intensity aerobic PA per week [13].
Regular PA is associated with many positive impacts on health, well-being and survival in both healthy women and breast cancer patients. However, the relationship between PA and breast cancer severity and aggressiveness such as the stage at diagnosis and grade is not well-known. In this study, we examine the associations between pre-diagnostic PA and breast cancer characteristics at diagnosis and overall survival in a large population of Asian breast cancer patients.
2. Methods
2.1. Study Population
The Singapore Breast Cancer Cohort (SGBCC) is a multicenter cohort study of breast cancer patients in Singapore. Established in 2010, it was established with the purpose of investigating the associations between various genetic and non-genetic factors and breast cancer risk (cohort profile described in [14]). Patients were recruited across six recruitment sites, between 2010 to 2016, namely: National University Hospital (NUH, Singapore, Singapore), KK Women’s and Children’s Hospital (KKH, Singapore, Singapore), Tan Tock Seng Hospital (TTSH, Singapore, Singapore), National Cancer Centre Singapore (NCCS, Singapore, Singapore), Singapore General Hospital (SGH, Singapore, Singapore), and Changi General Hospital (CGH, Singapore, Singapore). The recruiting hospitals collectively treat ~76% of the breast cancer patients in Singapore [14].
Eligible patients have to be (1) diagnosed with breast carcinoma in situ or invasive breast cancer; (2) citizens or permanent residents of Singapore; (3) aged 21 years and above. As part of the recruitment process, patients completed a structured questionnaire which included questions relating to breast cancer risk factors (i.e., pre-diagnostic PA level, reproductive factors, and family history of breast cancer), with assistance as required from a trained study coordinator. The SGBCC questionnaire was adapted from the KARolinska MAmmography Project for Risk Prediction of Breast Cancer (KARMA) study’s questionnaire [15].
2.2. Assessment of PA
Information on PA was obtained from the questionnaire administered at recruitment. Questions included “How much PA/sports do you practice in your free time?” Examples of each PA level were given in the questionnaire (light: walking, driving, housework; moderate: brisk walking, cycling, easy swimming; vigorous: jogging, vigorous swimming, aerobic exercises). Respondents were asked to select the highest level of PA (light, moderate, or vigorous) and to report how much time was engaged in exercise (never, less than one hour per week, one to two hours per week, or more than two hours per week). Information on the highest PA level engaged between the age 18 to 30 (adulthood) was collected.
Based on intensity and duration of PA reported, a PA score ranging from 1 to 5 was calculated for each time point (Figure 1). Patients who engaged in vigorous activities were combined into one group regardless of PA duration due to the small number of patients. As there were very few who chose “never”, these people were classified under Score 1.
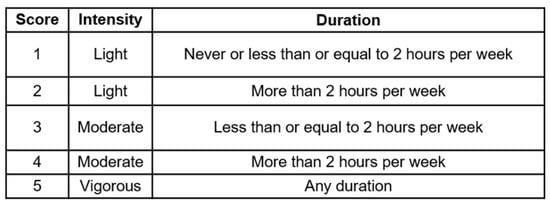
Figure 1.
Physical activity score. Scores were calculated based on the highest intensity and duration of physical activity engaged by patient per week.
2.3. Demographics and Breast Cancer Risk Factor Data
Baseline information on sociodemographics and breast cancer risk factors were obtained at the time of recruitment via the structured questionnaire. The variables included ethnicity, smoking (yes, no, or missing) and alcohol consumption (yes, no, or missing), previous benign lump or gynaecological surgery (yes, no, or missing), family history of breast and ovarian cancer (yes, no, or missing), reproductive factors and body size one year prior to diagnosis etc. Body size one year prior to diagnosis was based on the nine-level Stunkard Figure Rating scale of body size, a somatotype pictogram which is validated for the estimation of a person’s body mass index [16,17]. Details on how menopausal status was coded may be found in Supplementary Figure S1. Medical history, specifically, previous diagnoses of heart attack, asthma, renal disease, stroke, diabetes, and previous cancer, was also collected. Comorbidities were combined and scored according to the Charlson Comorbidity Index (CCI) [18].
2.4. Clinical Data
Clinical data on tumour characteristics and treatment modalities were obtained through medical records. The variables included disease stage (stage 0, I, II, III, IV), nodal involvement (yes or no), tumour size (≤2 cm, >2–5 cm, and >5 cm, other/missing), histological grade (well-, moderately-, poorly differentiated), estrogen receptor (ER) status (positive or negative), progesterone receptor (PR) status (positive or negative), human epidermal growth factor receptor 2 (HER2) status (positive or negative), surgery (yes/no), radiotherapy (yes/no), any chemotherapy (neoadjuvant or adjuvant, yes/no), endocrine therapy (yes/no), and targeted therapy (yes/no). Tumour behaviour was derived from stage at diagnosis, where stage 0 indicated non-invasive behaviour, and stages I to IV indicated invasive behaviour. Intrinsic-like subtypes were defined using immunohistochemical markers for ER, PR and HER2 in conjunction with histologic grade; luminal A [ER+/PR+, HER2-, well or moderately differentiated], luminal B [HER2-] (ER+/PR+, HER2-, and poorly differentiated), luminal B [HER2+](ER+/PR+, HER2+, and poorly differentiated), HER2-enriched [HER2+], triple-negative [ER-, PR-, and HER2-] [19].
2.5. Passive Follow-Up
Information on vital status and cause of death were obtained via a linkage with the Registry of Births and Deaths [14], using each individual’s unique National Registration Identity Card (NRIC) number. Hospitals had differing schedules in updating their in-house breast cancer registry; in turn, the collection of variables ended at different points (NUH: 30 April 2017; KKH: 30 June 2017; CGH: 16 April 2018; TTSH: 30 April 2018). For SGH and NCCS, not all NRICs are sent to the registry at the same time, the date of follow-up was obtained from the electronic medical records; all recorded deaths are verified with the Registry of Births and Deaths.
2.6. Exclusions
Figure 2 summarizes the exclusions performed for this study. We excluded 2 patients without age at diagnosis, 7 male patients, 8 patients without information on PA, and 63 patients diagnosed at below 30 years old. The analytical cohort comprised 7688 breast cancer patients. Further exclusion was performed for survival analysis (refer to Figure 2 for more details), where 6572 patients were retained.
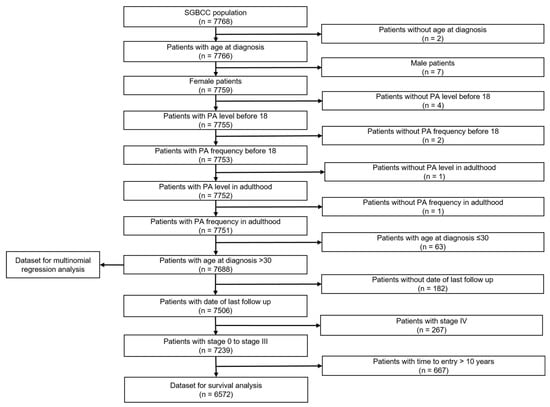
Figure 2.
Flow chart of study population. Population comprised of breast cancer patients in the Singapore Breast Cancer Cohort (SGBCC), recruited between 2011 and 2016.
2.7. Statistical Analysis
Characteristics of the study population were described by frequency and percentage for categorical variables, and by mean and standard deviation (SD) for continuous variables. The associations between PA and patient characteristics were studied using the Chi-square test and Kruskal-Wallis test, for categorical and continuous variables, respectively.
To assess associations between pre-diagnostic PA score and tumour characteristics, multinomial logistic regression models (multinom function in R package “nnet”) were performed, adjusting for age at diagnosis, body size one year prior to cancer diagnosis, ethnicity, and recruitment site. We ran a sensitivity analysis excluding patients who passed away within 1 year of study entry and another separate sensitivity analysis excluding patients who engaged in less than one hour per week of vigorous activity (PA Score 5). We also examined the association of pre-diagnostic PA score and tumour characteristics in subgroups defined by menopausal status at diagnosis (pre- or post-menopausal). Additionally, we investigated the individual effects of pre-diagnostic PA intensity and duration on tumour characteristics. To account for the effect of PA intensity on PA duration and vice-versa, further adjustments were also conducted. Missing data in covariates were replaced by adding a ’missing’ indicator category.
Overall survival was studied using Cox proportional hazard models (survival package in R, where the Surv (time at entry, follow-up time, event)) command was used to estimate hazard ratios (HR) and corresponding 95% confidence intervals (CI). Time at entry was defined as the time between the date of recruitment and the date of diagnosis. Follow-up time was defined as the time between the date of death/last follow-up date and the diagnosis/recruitment date, censored at ten years post-diagnosis. Breast cancer patients who were recruited ten years after diagnosis were left truncated in the model. In the multivariable Cox regression model, effect of pre-diagnostic PA on survival was adjusted for age at diagnosis and recruitment site. Further adjustments were made for variables found to be significant with survival in univariate models, such as ethnicity, smoking status, CCI, age at menarche, age at first full term pregnancy, hormone receptor use, stage, grade, nodal status, tumour size, estrogen receptor status at diagnosis, and breast cancer treatment history (surgery, chemotherapy, and adjuvant endocrine therapy). Various subgroup analyses were performed to further explore associations between pre-diagnostic PA and survival. These subgroups include hormone receptor and HER2-status, stage at diagnosis, and menopausal status at diagnosis. Further sensitivity analysis excluding patients who engaged in less than 1 hour per week of vigorous activity (PA Score 5) was also conducted.
3. Results
Demographics and breast cancer risk factor characteristics of the analytical cohort are described in Table 1a. In these 7688 patients, the mean age at diagnosis was 53.5 years (interquartile range: 46.5–61.2). Table 1b describes disease characteristics and treatment. At diagnosis, 6236 (81.1%) had invasive cancers, 4714 (61.3%) had no nodal involvement; 4957 (64.5%), 4368 (56.8%) and 1490 (19.4%) had ER-, PR- and HER2-positive tumours respectively. Other sociodemographic, medical history, breast cancer risk factors, disease and treatment characteristics that were explored can be found in Supplementary Table S1.

Table 1.
Characteristics of SGBCC study population by pre-diagnostic physical activity (PA) score (n = 7688). p-value (p) for categorical variables are based on Chi-square test and p-value for continuous variables are based on Kruskal-Wallis test.
3.1. Pre-Diagnostic PA and Disease Characteristics
Table 2 shows the associations between pre-diagnostic PA score and disease characteristics, adjusted for age at diagnosis, ethnicity, body size one year prior to diagnosis, and recruitment site. Compared to those with a PA score 2 (reference category for all comparisons), those with highest PA score (score 5) were significantly less likely to be diagnosed with invasive cancer (OR invasive vs. non-invasive (reference) [95%CI]: 0.71 [0.58–0.87], p = 0.001, p-trend = 0.001). This means that if a patient were to increase her PA score from 2 (reference category) to 5, her risk of developing invasive breast cancer is 0.71 times that of a patient with PA score 2, given the other variables in the model are held constant. With regards to stage, among those with invasive cancer, those who engaged in the highest level of PA were significantly less likely to be diagnosed with stage II breast cancer (OR stage II vs. stage I (reference): 0.80 [0.65–0.98], p = 0.028). This inverse association, was, however, not significant when comparing stage I to stages III or IV. Breast cancer patients with higher PA scores were also less likely to be diagnosed with high-grade tumours, especially for those with score 3 (OR poorly vs. well differentiated (reference): 0.69 [0.52–0.93], p = 0.014). Other higher PA scores also showed lower risk of developing high-grade tumours but the association was not significant (score 4 OR poorly vs. well differentiated (reference): 0.84 [0.66–1.08], p = 0.176; score 5 OR poorly vs. well differentiated (reference): 0.81 [0.62–1.05], p = 0.112).

Table 2.
Associations between pre-diagnostic physical activity (PA) score and disease characteristics adjusted for age at diagnosis, body size one year prior to diagnosis, ethnicity, and recruitment site (n = 7688). Odds ratios (OR) and 95% confidence intervals (CI) were estimated using multinomial regression. p indicates p-value obtained from the Wald test. Results in bold indicate p ≤ 0.05.
In terms of hormone receptor and HER2 status, higher PA scores were associated with a decreased risk of being diagnosed with ER-negative, PR-negative, and HER2-positive cancers. More specifically, a higher PA score reduced the risk of ER-negative tumours (OR ER-negative vs. ER-positive (reference): 0.94 [0.89–1.00], p-trend = 0.049). In addition, compared to those with PA score 2, those with score 4 were less likely to be diagnosed with PR-negative cancers (OR PR-negative vs. PR-positive (reference): 0.82 [0.67–0.99], p = 0.041), even though this trend was no longer significant when considering PA score as a continuous variable (p-trend = 0.051). Those with the highest PA score had a higher odds of being diagnosed with HER2-negative cancers (OR HER2-negative vs. HER2-positive (reference): 1.29 [1.02–1.62], p = 0.033, p-trend = 0.002). Notably, an increase in PA score was associated with lower odds of HER2-overexpressed cancer (OR HER2-overexpressed vs. Luminal A (reference): 0.89 [0.81–0.98], p-trend = 0.018).
The results did not significantly change in sensitivity analyses excluding patients who passed away within one year of study entry (Supplementary Table S2). Similarly, in a separate analysis excluding patients who reported less than 1 hour of vigorous PA (PA Score 5), the results remained largely unchanged, with the exception of ER status (Supplementary Table S3). In contrast, PA was not significantly associated with disease aggressiveness among patients diagnosed with non-invasive breast cancer (Supplementary Table S4).
3.2. Pre-Diagnostic PA and Survival
Among the 6572 patients included for survival analysis, a total of 394 deaths occurred within 10 years after diagnosis. After adjusting for age at diagnosis and recruitment site, higher PA score did not confer any significant survival benefit (HR: 0.98 [0.89–1.08], p = 0.647) (Table 3a). Further adjustments for variables that were significant in the univariate Cox regression (Supplementary Table S5) did not show any appreciable change (data not shown). Subset analyses by stage, hormone receptor status, and HER2 subsets, adjusted for age at diagnosis and recruitment site, showed similar results (Supplementary Table S6a,b). The trends persisted when the outcome considered was deaths due to breast cancer (n = 234) (Table 3b). The results remained largely unchanged in sensitivity analysis excluding patients who reported less than 1 hour of vigorous PA (PA Score 5) (Supplementary Table S7).

Table 3.
Cox regression model showing association between pre-diagnostic physical activity (PA) score and ten-year survival, adjusted for age at diagnosis and recruitment site (n = 6572). Hazard ratios (HR) and 95% confidence intervals (CI) were estimated using Cox regression models.
3.3. Subset Analysis by Menopausal Status
We conducted further analysis by sub-setting the population according to menopausal status at diagnosis (pre-, n = 3617; post-, n = 4017) (Table 4). Trends observed among patients with pre-menopausal breast cancer (Table 4a) were less significant than the results observed in Table 2. Even though breast cancer patients with the highest PA score were less likely to develop invasive cancer (OR invasive vs. non-invasive (reference): 0.73 [0.56–0.94], p = 0.016, p-trend = 0.11), the effects of high PA score on lower stage and grade at diagnosis were no longer significant in this group. Effect of PA score on hormone receptor status (OR ER-negative vs. ER-positive (reference): 0.97 [0.88–1.04], p-trend = 0.387; OR PR-negative vs. PR-positive (reference): 0.95 [0.88–1.02], p = 0.137) were also no longer significant in this group, even though similar trends were observed. HER2 status remained significantly associated with PA score, where higher PA score reduced the risk of HER2-positive cancers (OR HER2-negative vs. HER2-positive (reference): 1.11 [1.02–1.20], p-trend = 0.01).

Table 4.
Associations between pre-diagnostic physical activity (PA) score and disease characteristics adjusted for age at diagnosis, body size one year prior to diagnosis, ethnicity, and recruitment site for (a) pre-menopausal (n = 3671) and (b) post-menopausal breast cancer (n = 4017). Odds ratios (OR) and 95% confidence intervals (CI) were estimated using multinomial regression. P indicates p-value obtained from the Wald test. Results in bold indicate p ≤ 0.05.
In contrast, the trends observed between PA score and tumour characteristics among post-menopausal patients (Table 4b) were similar to the results observed in Table 2. Mainly, higher PA score reduced risk of invasive cancer (OR invasive vs. non-invasive (reference): 0.71 [0.50–1.00], p = 0.05, p-trend = 0.003). Among those with invasive cancer, it was also observed that higher PA score was significantly associated with reduced risk of stage II (OR stage II vs. stage I (reference): 0.71 [0.50–1.00], p = 0.048) and high-grade (OR poorly vs. well differentiated (reference): 0.88 [0.79–0.98], p-trend = 0.018) cancers. Similar to the pre-menopausal subgroup, effect of PA score on hormone receptor status (OR ER-negative vs. ER-positive (reference): 0.93 [0.85–1.05], p-trend = 0.099; OR PR-negative vs. PR-positive (reference): 0.97 [0.89–1.05], p = 0.391) was not significant in this subset. Furthermore, the effect of PA score on HER2 status (OR HER2-negative vs. HER2-positive (reference): 1.07 [0.98–1.17], p-trend = 0.128) was no longer statistically significant.
Survival benefit was not observed in survival analysis performed with pre- (HR: 1.04 [0.91–1.18], p = 0.611) and post-menopausal (HR: 0.91 [0.79–1.05], p = 0.213) subgroups (Supplementary Table S6c).
3.4. Subset Analysis by PA Intensity and Duration
In order to study individual effects of PA intensity and duration on disease characteristics, multinomial regression for each variable was conducted separately (Supplementary Table S8). Interestingly, increasing intensity and duration of PA have contrasting effects on tumour characteristics. Compared to light levels (reference category), those who engaged in vigorous levels of PA were less likely to develop invasive cancer (OR invasive vs. non-invasive (reference): 0.70 [0.57–0.86], p ≤ 0.001). Among those with invasive cancer, those who engaged in higher levels of PA were less likely to develop high-grade (moderate OR poorly vs. well differentiated (reference): 0.80 [0.65–0.97], p = 0.024) and HER2-positive (moderate OR HER2-negative vs. HER2-positive (reference): 1.18 [1.00–1.40], p = 0.048; vigorous OR HER2-negative vs. HER2-positive (reference): 1.33 [1.06–1.67], p = 0.015) tumour. On the contrary, compared to those who engaged in more than 2 h of PA (reference category), those who engaged in only 1 to 2 h of PA per week had a statistically significant decreased risk of high grade (OR poorly vs. well differentiated (reference): 0.73 [0.57–0.94], p = 0.014) and large (OR >5 cm vs. ≤2 cm (reference): 0.68 [0.46–1.00], p = 0.05) tumour.
When pre-diagnostic PA intensity was further adjusted for pre-diagnostic PA duration, engaging in vigorous levels of pre-diagnostic PA remained significantly associated with reduced risk of invasive cancer (OR invasive vs. non-invasive (reference): 0.71 [0.58–0.88], p = 0.001) and HER2-positive tumour (OR HER2-negative vs. HER2-positive (reference): 1.31 [1.04–1.65], p = 0.015) (data not shown). However, the effect of pre-diagnostic PA duration on tumour characteristics was no longer statistically significant after adjusting for intensity (data not shown).
4. Discussion
In this large, retrospective, multicenter cohort study of 7688 breast cancer patients, a high pre-diagnostic PA score in adulthood (18–30 years) was associated with lower breast cancer stage and grade at diagnosis. High PA score was also statistically associated with ER-positive, PR-positive, and HER2-negative subtypes. We did not observe a significant impact of a high PA score on overall survival. To our knowledge, this is the largest study exploring pre-diagnostic PA and breast cancer characteristics in Asian breast cancer patients.
The influence of PA on breast cancer risk and outcomes has been extensively studied. In these studies, pre-diagnostic PA has been shown to be correlated with a lower incidence of breast cancer [4,11,12]. Conversely, a sedentary lifestyle elevated the risk for cancer development in general [20]. The impact of PA on subgroups by ethnicity and menopausal status has been studied. Compared to Caucasian women, Asians are associated with an appreciable larger reduction in breast cancer risk with PA [12]. While we could not look at breast cancer risk reduction in our study cohort of Asian breast cancer patients, our results suggest that pre-diagnostic PA may affect the aggressiveness of the disease developed. Characteristics that are more favorable were observed in tumours associated with high pre-diagnostic PA scores.
There are inconsistencies regarding the relationship between PA and hormone-receptor status of breast cancers. Our findings in this large Asian breast cancer cohort were in agreement with the majority of previous work. A Spanish case-control study (1389 histologically confirmed invasive BC cases and 1712 controls) found that high PA predisposed to ER-positive, PR-positive and HER2-negative breast cancers [21]. In addition, the Canadian Breast Cancer Study (692 women with incident breast cancer and 644 controls) reported that total lifetime moderate-to-vigorous PA during leisure time exhibited a reduced risk of hormone-receptor (ER or PR) negative breast cancers [22]. The observed effect was confined to HER2-negative tumours [22]. In contrast, a conflicting study found that PA was associated with a reduced risk of ER-positive, PR-positive breast cancers [23]. It should be noted that these studies specified the effects of the type of PA, such as leisure, occupational or household, on hormone receptor and HER2 status in breast cancer [21,22,23], which can explain the difference in findings. However, PA information collected for our study did not allow us to specify types of PA. With regard to the exact relationship between PA and hormone receptors and HER2 status, further investigation will be required.
Stage and grade at diagnosis are important factors determining the aggressiveness of breast cancer. These tumour characteristics impact treatment options, with chemotherapy more likely being offered if the tumour is of a higher stage or grade. The associations between PA and tumour characteristics other than hormone receptors and HER2 status are, however, not commonly reported. Our results regarding invasiveness from stage to hormone receptor statuses highlights the additional roles that pre-diagnostic PA may play, in addition to primary cancer prevention.
Compared to women who screened, non-screeners have been associated with higher rates of advanced and deadly breast cancers [24,25]. In our study, screen-detection is mainly associated with stage 0 and I cancers. Women engaging in physical activity may participate more in screening than physically inactive women. Hence, stage 0 and I breast cancers may be more prevalent among physically active than among physically inactive women. It cannot be discounted that physical activity may be associated with less advanced stage cancer simply because of a difference in screening attendance between physically active and inactive women.
Comparing the highest to the lowest scores of PA, pre-menopausal and post-menopausal women have been reported to be associated with an estimated breast cancer risk reduction of 27%, and 31%, respectively [12]. As menopausal status at breast cancer diagnosis may be associated with distinct risk factors or tumour characteristic profiles, we carried out subset analyses by menopausal status. Our results showed that high PA was associated with more favorable disease at presentation in both subsets, with the association being stronger in the post-menopausal subsets. This aligns with previous publications, where post-menopausal patients were observed to experience greater risk reduction and survival benefit from PA engagement as compared to pre-menopausal patients [26,27]. Chollet-Hinto et al. reported no large differences between menopausal status in a study looking at biologic and etiologic heterogeneity of breast cancer, and concluded that age, rather than menopausal status, may be the key player in determining tumour characteristics [28]. Nonetheless, our results suggest that pre-diagnostic PA is of advantage to both pre- and post-menopausal breast cancer patients.
The mechanisms by which PA reduces breast cancer risk are unclear. Several hypotheses were proposed to explain the effect of PA on cancer development and progression. Particularly for breast cancer, these mechanisms include the lowering of hormones such as estrogen [29] and the avoidance of high insulin levels [30] with PA. Other protective factors include reducing inflammation [31], improving immune system function and decreasing BMI since obesity is linked to many cancers, including breast cancer [32]. As the effects of obesity and PA could be interrelated, the analysis was adjusted for the patients’ body size prior to diagnosis, to exclude obesity as a confounding factor.
Despite the correlations between higher pre-diagnostic PA and more favorable tumour characteristics, there was no significant improvement in overall survival. The potential protective effects of physical activity on survival suggested by other studies could be mediated by physiological changes after diagnosis, such as changes in body size and metabolism [33,34], which was not accounted for in this study. In addition, this study did not account for changes in levels of PA post-diagnosis, which impacts survival [35,36].
Results observed from the separately analyzed effects of PA intensity and duration on disease characteristics were further supported by current guidelines for physical activity, where adults are recommended to engage in either longer duration (150 to 300 min) of moderate or shorter duration (75 to 150 min) of vigorous activity per week [37]. More specifically, it is recommended that individuals perform 500 to 1000 Metabolic Equivalent Tasks (MET) minutes per week to gain potential health benefits or risk reductions. Importantly, our results showed that intensity, compared to duration of PA engagement, is a more important indicator of possible disease outcomes. The exact relationship between PA duration, intensity, and cancer outcome remains unclear. However, the existing literature has shown that compared to moderate-intensity continuous training, engaging in high intensity interval exercise provided greater health improvements such as improved resting blood pressure [38] and cardiorespiratory fitness [39], which might point to the possible effect of PA intensity on improved disease outcomes.
Strengths of this paper included a large study cohort from multiple recruitment sites in Singapore that treat a majority of the breast cancer patients in the country. Participation rate was high (86%) [14] and each questionnaire was completed with little unavailable data. In most cases, there was a dedicated research coordinator to assist the patient in the interpretation of the questionnaire, if needed. The use of a unique National Registration Identity Card (NRIC) for every Singaporean citizen or permanent resident enables healthcare utilisation at all levels to be linked [40]. The clinical data of breast cancer characteristics and outcomes were well kept and retrieved from well-maintained electronic databases, accounting for little missing data. Loss to follow-up due to emigration is expected to be minimal for the duration of the study.
This study is not without limitations. The PA score examined in our study was not a validated tool. Furthermore, patients were limited to the options of light, moderate or vigorous levels with specific durations. Additionally, patients were also asked to report the highest level of PA rather than the habitual pattern of PA. These factors could have resulted in response bias, though there were specific explanations on the questionnaire to guide the patients to make the most appropriate choice. However, accurate measurement of PA is known to be challenging [41]. Nonetheless, our findings can serve as a reference for other studies looking at the associations between PA, breast cancer aggressiveness and fatality. In addition, being a retrospective study, recall bias cannot be eliminated. Moreover, there could be residual confounding and effect modification that could have been missed with our study design. As with all the other epidemiological studies, a causal relationship cannot be conclusively drawn because of various potential confounders. For example, the possibility that the lower cancer stage associated with high PA level may attributed to other factors, such as their engagement in other healthy lifestyle habits, cannot be excluded. To establish a causal effect of PA on breast cancer, large, randomized trials should be planned.
5. Conclusions
The relationship between high PA levels and reduced breast cancer risk is recognized. Our study reveals that high pre-diagnostic PA levels may also lower breast cancer aggressiveness in patients who develop the disease. Whether PA levels before breast cancer diagnosis improves survival remains unclear. The results of this study may add value in improving public health recommendations regarding PA for breast cancer risk reduction. Further investigation taking into account more potential confounders, clinical and recommended treatments for breast cancer are needed to clarify the relationship between PA levels and survival.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14071756/s1, Figure S1: Details on deriving menopausal status at diagnosis; Table S1: Other characteristics of SGBCC study population by pre-diagnostic physical activity (PA) score (n = 7688); Table S2: Associations between pre-diagnostic physical activity (PA) score and disease characteristics for patients, excluding those that pass away within 1 year of study entry (n = 7563); Table S3: Associations between pre-diagnostic physical activity (PA) score and disease characteristics for patients, excluding those who engaged in less than an hour of vigorous exercise (n = 7621); Table S4: Associations between pre-diagnostic physical activity (PA) score and disease characteristics for patients with non-invasive cancer (n = 1097); Table S5: Cox regression model showing association between various factors and overall survival, unadjusted; Table S6: Cox regression model showing association between pre-diagnostic physical activity (PA) score and breast cancer overall survival, adjusted for age at diagnosis and recruitment site, for (a) stage subsets, (b) estrogen receptor, progesterone receptor, and HER2 subsets, and (c) menopausal status at diagnosis subsets; Table S7: Cox regression model showing association between pre-diagnostic physical activity (PA) score and ten-year survival, excluding those who engaged in less than an hour of vigorous exercise (n = 6507); Table S8: Associations between pre-diagnostic physical activity (PA) (a) intensity, and (b) duration and disease characteristics.
Author Contributions
Conceptualization, J.L. and G.H.L.; Formal analysis, Z.L.L., G.H.L. and J.L.; Funding acquisition, J.L. and M.H.; Project administration, J.L.; Writing—original draft, Z.L.L., G.H.L. and J.L.; Writing—review & editing, Z.L.L., G.H.L., P.J.H., A.J.K., Y.S.Y., A.T.W.O., B.K.T.T., E.Y.T., S.-M.T., V.K.-M.T., J.L. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
SGBCC was supported by the National Research Foundation Singapore (NRF-NRFF2017-02, awarded to J Li), BMRC Central Research Fund (Applied Translational Research, awarded to J Li), NUS Start-up Grant, National University Cancer Institute Singapore (NCIS) Centre Grant [NMRC/CG/NCIS/2010, NMRC/CG/012/2013, CGAug16M005], Saw Swee Hock School of Public Health Research Programme of Research Seed Funding (Breast Cancer Prevention Program), Yong Loo Lin School of Medicine Breast Cancer Screening Prevention Programme, Asian Breast Cancer Research Fund, and the NMRC Clinician Scientist Award (Senior Investigator Category) [NMRC/CSA-SI/0015/2017] awarded to M.H.
Institutional Review Board Statement
SGBCC was approved by the National Healthcare Group Domain Specific Review Board (reference number: 2009/00501) and the SingHealth Centralised Institutional Review Board (CIRB Ref: 2019/2246 [2010/632/B]).
Informed Consent Statement
Informed consent was obtained from all patients. They were informed that they can withdraw their consent and stop participation at any time without disclosing the reasons and without negative consequences for their future medical care. All patients have consented to publishing their data anonymously.
Data Availability Statement
Due to ethical reasons and institutional guidelines, the data presented in the study cannot be shared publicly. For ethical issues, please contact the National Healthcare Group Domain Specific Review Board (Email: OHRPP@nhg.com.sg) and the SingHealth Centralised Institutional Review Board (Email: irb@singhealth.com.sg). Data are available to interested researchers with some access restrictions applied upon request. All requests can be directed to the Singapore Breast Cancer Cohort (SGBCC) scientific steering committee. Interested researchers may contact the Principal Investigator, Mikael Hartman at mikael_hartman@nuhs.edu.sg for more details. List of available data can be found in https://blog.nus.edu.sg/sgbcc/for-researchers/, accessed on 7 February 2022.
Acknowledgments
We are very grateful for all our patients’ generous support and cooperation. We also want to thank Jenny Liu and Siew Li Tan, Siok Hoon Yeo, Kimberley Chua, Ting Ting Koh, Michelle Jia Qi Mo, Ying Jia Chew, Jing Jing Hong, Jin Yin Lee, Charlotte Ong, Kah Aik Tan, Ganga Devi D/O Chandrasegran, Nur Khaliesah Binte Mohamed Risa, and Nayli Nur Hannah Bte Mazlan for their contributions.
Conflicts of Interest
The authors declare no potential conflicts of interest.
Abbreviations
| BC | breast cancer |
| BMI | body mass index, kg/m2 |
| CI | confidence interval |
| ER | estrogen receptor |
| HER2 | human epidermal growth factor receptor 2 |
| HR | hazard ratio |
| OR | odds ratio |
| PA | physical activity |
| PR | progesterone receptor |
| SD | standard deviation |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, S.S.; Thygesen, L.C.; Tolstrup, J.S.; Gronbaek, M. Modifiable risk factors and survival in women diagnosed with primary breast cancer: Results from a prospective cohort study. Eur. J. Cancer Prev. 2010, 19, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Killelea, B.K.; Gallagher, E.J.; Feldman, S.M.; Port, E.; King, T.; Boolbol, S.K.; Franco, R.; Fei, K.; Le Roith, D.; Bickell, N.A. The effect of modifiable risk factors on breast cancer aggressiveness among black and white women. Am. J. Surg. 2019, 218, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Pizot, C.; Boniol, M.; Mullie, P.; Koechlin, A.; Boniol, M.; Boyle, P.; Autier, P. Physical activity, hormone replacement therapy and breast cancer risk: A meta-analysis of prospective studies. Eur. J. Cancer 2016, 52, 138–154. [Google Scholar] [CrossRef] [Green Version]
- Spei, M.E.; Samoli, E.; Bravi, F.; La Vecchia, C.; Bamia, C.; Benetou, V. Physical activity in breast cancer survivors: A systematic review and meta-analysis on overall and breast cancer survival. Breast 2019, 44, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.D.; Chen, W.Y.; Feskanich, D.; Kroenke, C.H.; Colditz, G.A. Physical activity and survival after breast cancer diagnosis. JAMA 2005, 293, 2479–2486. [Google Scholar] [CrossRef] [Green Version]
- Carmichael, A.R.; Daley, A.J.; Rea, D.W.; Bowden, S.J. Physical activity and breast cancer outcome: A brief review of evidence, current practice and future direction. Eur. J. Surg. Oncol. 2010, 36, 1139–1148. [Google Scholar] [CrossRef] [Green Version]
- Cleveland, R.J.; Eng, S.M.; Stevens, J.; Bradshaw, P.T.; Teitelbaum, S.L.; Neugut, A.I.; Gammon, M.D. Influence of prediagnostic recreational physical activity on survival from breast cancer. Eur. J. Cancer Prev. 2012, 21, 46–54. [Google Scholar] [CrossRef] [Green Version]
- World Cancer Research Foundation/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective; World Cancer Research Fund and American Institute for Cancer Research: Washington, DC, USA, 2018; Available online: Dietandcancerreport.org (accessed on 7 February 2022).
- Li, T.; Wei, S.; Shi, Y.; Pang, S.; Qin, Q.; Yin, J.; Deng, Y.; Chen, Q.; Wei, S.; Nie, S.; et al. The dose-response effect of physical activity on cancer mortality: Findings from 71 prospective cohort studies. Br. J. Sports Med. 2016, 50, 339–345. [Google Scholar] [CrossRef]
- McTiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef]
- Friedenreich, C.M. The role of physical activity in breast cancer etiology. Semin. Oncol. 2010, 37, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.J.; Yeoh, Y.S.; Miao, H.; Lim, S.H.; Tan, E.Y.; Tan, B.K.T.; Tan, V.K.M.; Tan, S.M.; Yong, W.S.; Wong, F.Y.; et al. Cohort profile: The Singapore Breast Cancer Cohort (SGBCC), a multi-center breast cancer cohort for evaluation of phenotypic risk factors and genetic markers. PLoS ONE 2021, 16, e0250102. [Google Scholar] [CrossRef] [PubMed]
- Gabrielson, M.; Eriksson, M.; Hammarstrom, M.; Borgquist, S.; Leifland, K.; Czene, K.; Hall, P. Cohort Profile: The Karolinska Mammography Project for Risk Prediction of Breast Cancer (KARMA). Int. J. Epidemiol. 2017, 46, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, A.A.; Semnani, S.; Pourshams, A.; Khademi, H.; Roshandel, G.; Boffetta, P.; Malekzadeh, R. Pictogram use was validated for estimating individual’s body mass index. J. Clin. Epidemiol. 2010, 63, 655–659. [Google Scholar] [CrossRef]
- Stunkard, A.J.; Sorensen, T.; Schulsinger, F. Use of the Danish Adoption Register for the study of obesity and thinness. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1983, 60, 115–120. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thurlimann, B.; Senn, H.J. Strategies for subtypes—Dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef]
- Patel, A.V.; Hildebrand, J.S.; Campbell, P.T.; Teras, L.R.; Craft, L.L.; McCullough, M.L.; Gapstur, S.M. Leisure-Time Spent Sitting and Site-Specific Cancer Incidence in a Large U.S. Cohort. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1350–1359. [Google Scholar] [CrossRef] [Green Version]
- Huerta, J.M.; Molina, A.J.; Chirlaque, M.D.; Yepes, P.; Moratalla-Navarro, F.; Moreno, V.; Amiano, P.; Guevara, M.; Moreno-Iribas, C.; Llorca, J.; et al. Domain-specific patterns of physical activity and risk of breast cancer sub-types in the MCC-Spain study. Breast Cancer Res. Treat. 2019, 177, 749–760. [Google Scholar] [CrossRef]
- Shi, J.; Kobayashi, L.C.; Grundy, A.; Richardson, H.; SenGupta, S.K.; Lohrisch, C.A.; Spinelli, J.J.; Aronson, K.J. Lifetime moderate-to-vigorous physical activity and ER/PR/HER-defined post-menopausal breast cancer risk. Breast Cancer Res. Treat. 2017, 165, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.E.; Steindorf, K.; Mutschelknauss, E.; Slanger, T.; Kropp, S.; Obi, N.; Flesch-Janys, D.; Chang-Claude, J. Physical activity and postmenopausal breast cancer: Effect modification by breast cancer subtypes and effective periods in life. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3402–3410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, S.W.; Tabar, L.; Yen, A.M.; Dean, P.B.; Smith, R.A.; Jonsson, H.; Tornberg, S.; Chen, S.L.; Chiu, S.Y.; Fann, J.C.; et al. Mammography screening reduces rates of advanced and fatal breast cancers: Results in 549,091 women. Cancer 2020, 126, 2971–2979. [Google Scholar] [CrossRef] [PubMed]
- Webb, M.L.; Cady, B.; Michaelson, J.S.; Bush, D.M.; Calvillo, K.Z.; Kopans, D.B.; Smith, B.L. A failure analysis of invasive breast cancer: Most deaths from disease occur in women not regularly screened. Cancer 2014, 120, 2839–2846. [Google Scholar] [CrossRef] [PubMed]
- Lahart, I.M.; Metsios, G.S.; Nevill, A.M.; Carmichael, A.R. Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015, 54, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Boyle, T.; Heyworth, J.; Glass, D.C.; Saunders, C.; Fritschi, L. Lifetime physical activity and risk of breast cancer in pre-and post-menopausal women. Breast Cancer Res. Treat. 2015, 152, 449–462. [Google Scholar] [CrossRef] [Green Version]
- Chollet-Hinton, L.; Anders, C.K.; Tse, C.K.; Bell, M.B.; Yang, Y.C.; Carey, L.A.; Olshan, A.F.; Troester, M.A. Breast cancer biologic and etiologic heterogeneity by young age and menopausal status in the Carolina Breast Cancer Study: A case-control study. Breast Cancer Res. 2016, 18, 1–10. [Google Scholar] [CrossRef] [Green Version]
- de Boer, M.C.; Worner, E.A.; Verlaan, D.; van Leeuwen, P.A.M. The Mechanisms and Effects of Physical Activity on Breast Cancer. Clin. Breast Cancer 2017, 17, 272–278. [Google Scholar] [CrossRef]
- Winzer, B.M.; Whiteman, D.C.; Reeves, M.M.; Paratz, J.D. Physical activity and cancer prevention: A systematic review of clinical trials. Cancer Causes Control 2011, 22, 811–826. [Google Scholar] [CrossRef]
- Orlandella, F.M.; De Stefano, A.E.; Iervolino, P.L.C.; Buono, P.; Soricelli, A.; Salvatore, G. Dissecting the molecular pathways involved in the effects of physical activity on breast cancers cells: A narrative review. Life Sci. 2021, 265, 118790. [Google Scholar] [CrossRef]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 2017, 67, 378–397. [Google Scholar] [CrossRef] [PubMed]
- Friedenreich, C.M.; Shaw, E.; Neilson, H.K.; Brenner, D.R. Epidemiology and biology of physical activity and cancer recurrence. J. Mol. Med. 2017, 95, 1029–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannioto, R.A.; Dighe, S.; Mahoney, M.C.; Moysich, K.B.; Sen, A.; Hulme, K.; McCann, S.E.; Ambrosone, C.B. Habitual recreational physical activity is associated with significantly improved survival in cancer patients: Evidence from the Roswell Park Data Bank and BioRepository. Cancer Causes Control 2019, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.M.; Al-Homaidh, A. Physical activity and survival after breast cancer diagnosis: Meta-analysis of published studies. Med. Oncol. 2011, 28, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Beasley, J.M.; Kwan, M.L.; Chen, W.Y.; Weltzien, E.K.; Kroenke, C.H.; Lu, W.; Nechuta, S.J.; Cadmus-Bertram, L.; Patterson, R.E.; Sternfeld, B.; et al. Meeting the physical activity guidelines and survival after breast cancer: Findings from the after breast cancer pooling project. Breast Cancer Res. Treat. 2012, 131, 637–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Physical Activity Guidelines Advisory Committee 2018. 2018 Physical Activity Guidelines Advisory Committee Scientific Report; Department of Health & Human Services: Washington, DC, USA, 2018. Available online: https://health.gov/sites/default/files/2019-09/PAG_Advisory_Committee_Report.pdf (accessed on 7 February 2022).
- Costa, E.C.; Hay, J.L.; Kehler, D.S.; Boreskie, K.F.; Arora, R.C.; Umpierre, D.; Szwajcer, A.; Duhamel, T.A. Effects of High-Intensity Interval Training Versus Moderate-Intensity Continuous Training On Blood Pressure in Adults with Pre- to Established Hypertension: A Systematic Review and Meta-Analysis of Randomized Trials. Sports Med. 2018, 48, 2127–2142. [Google Scholar] [CrossRef]
- Sultana, R.N.; Sabag, A.; Keating, S.E.; Johnson, N.A. The Effect of Low-Volume High-Intensity Interval Training on Body Composition and Cardiorespiratory Fitness: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 1687–1721. [Google Scholar] [CrossRef]
- Emmanuel, S. Quality assurance in medicine: Research and evaluation activities towards quality control in Singapore. Ann. Acad. Med. Singap. 1993, 22, 129–133. [Google Scholar]
- Helmerhorst, H.J.; Brage, S.; Warren, J.; Besson, H.; Ekelund, U. A systematic review of reliability and objective criterion-related validity of physical activity questionnaires. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 1–55. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).