Predicting Long-Term Prognoses and Grading Platinum Sensitivity Using a Novel Progression-Free Interval Criterion in Ovarian Clear Cell Carcinoma: A Multi-Institutional Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
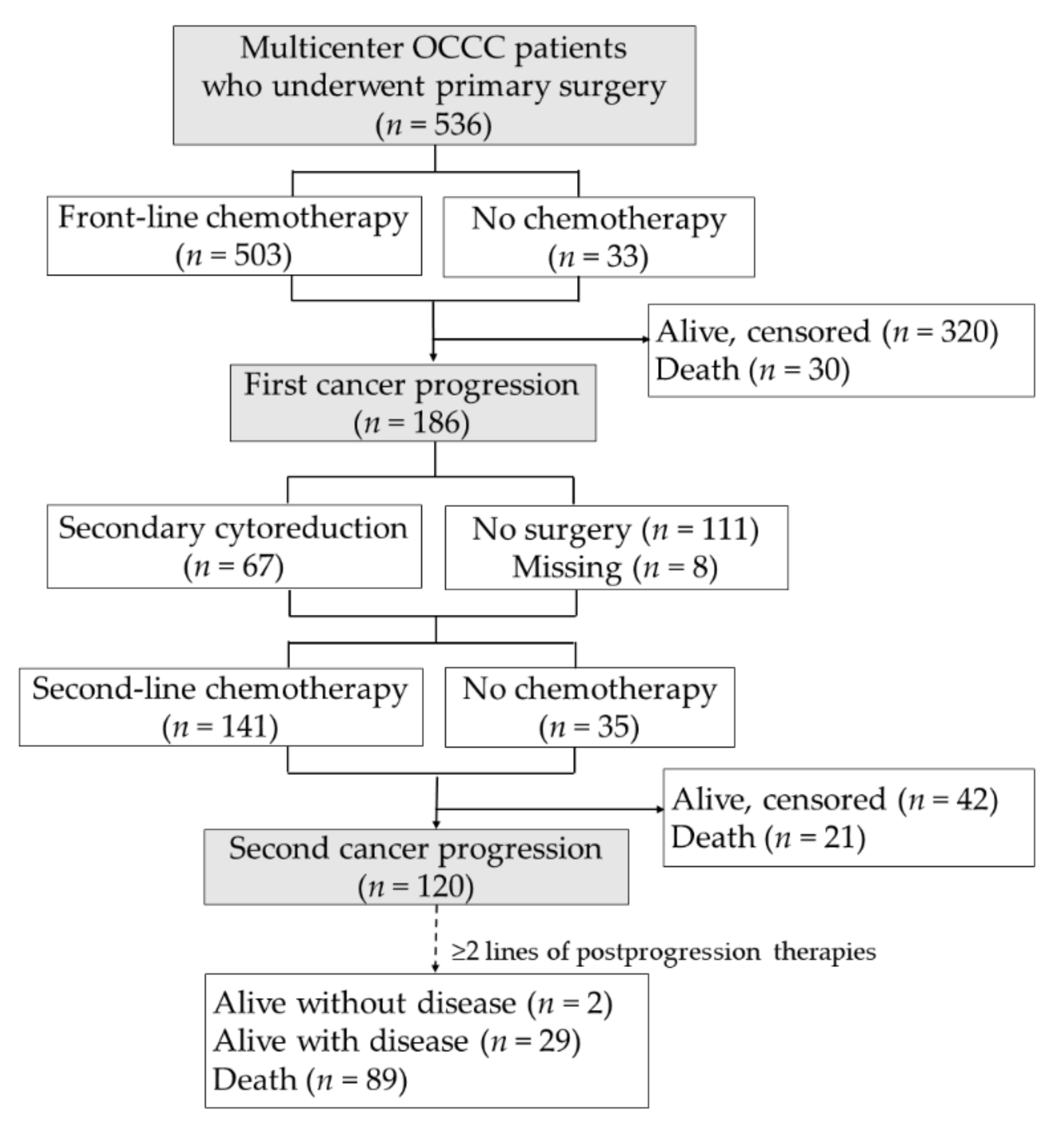
2.1. Participants
2.2. Data Collection and Outcome Assessments
2.3. Statistical Analyses
3. Results
3.1. Patient Demographics
3.1.1. Complete OCCC Cohort
3.1.2. Relapse Cohort
3.2. Grading Platinum Sensitivity by PFI
3.3. PFI-Associated Factors
3.4. Stage-Associated Factors
3.5. Clinical Outcomes
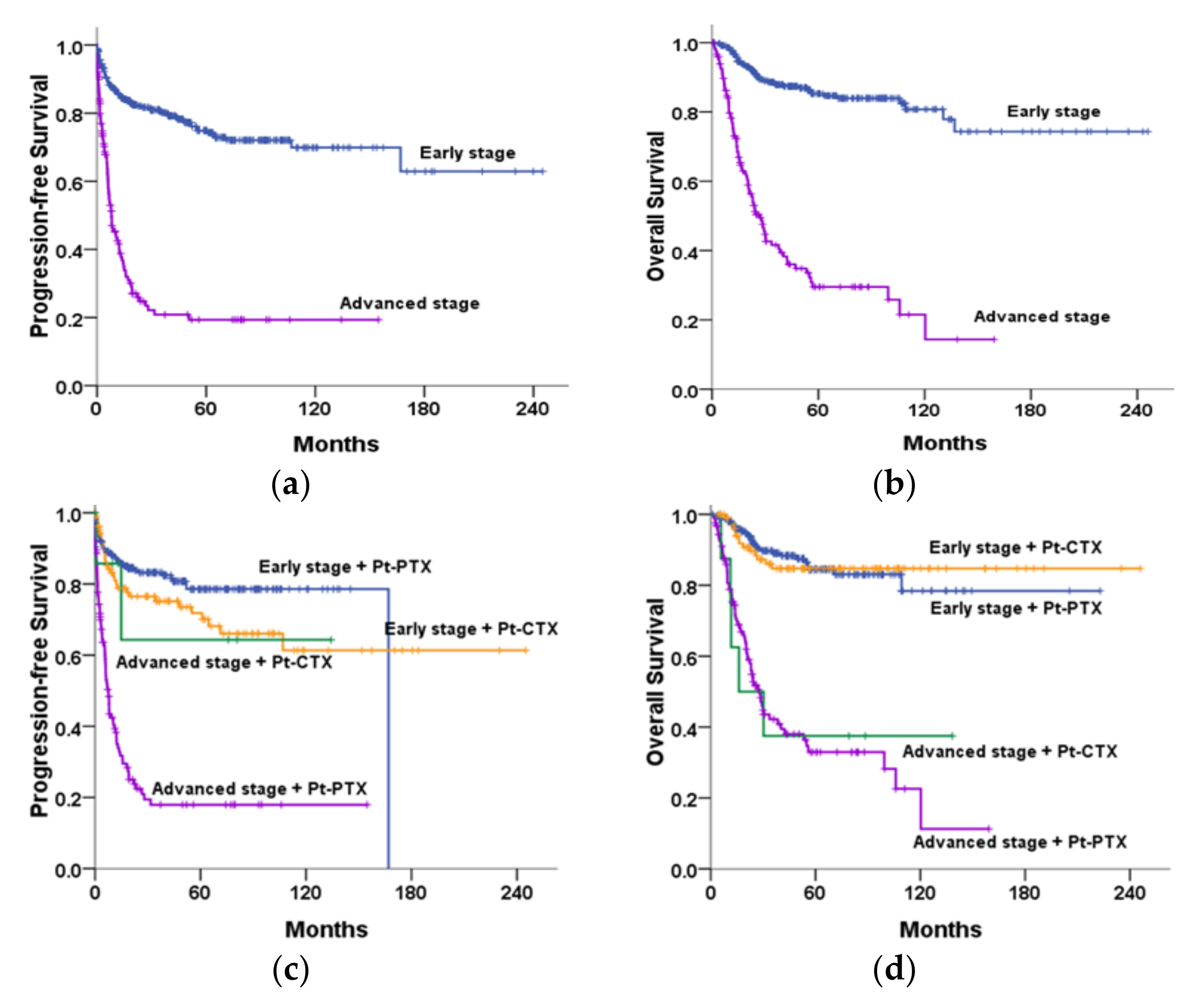
3.5.1. Stage-Associated and Treatment-Associated Survival
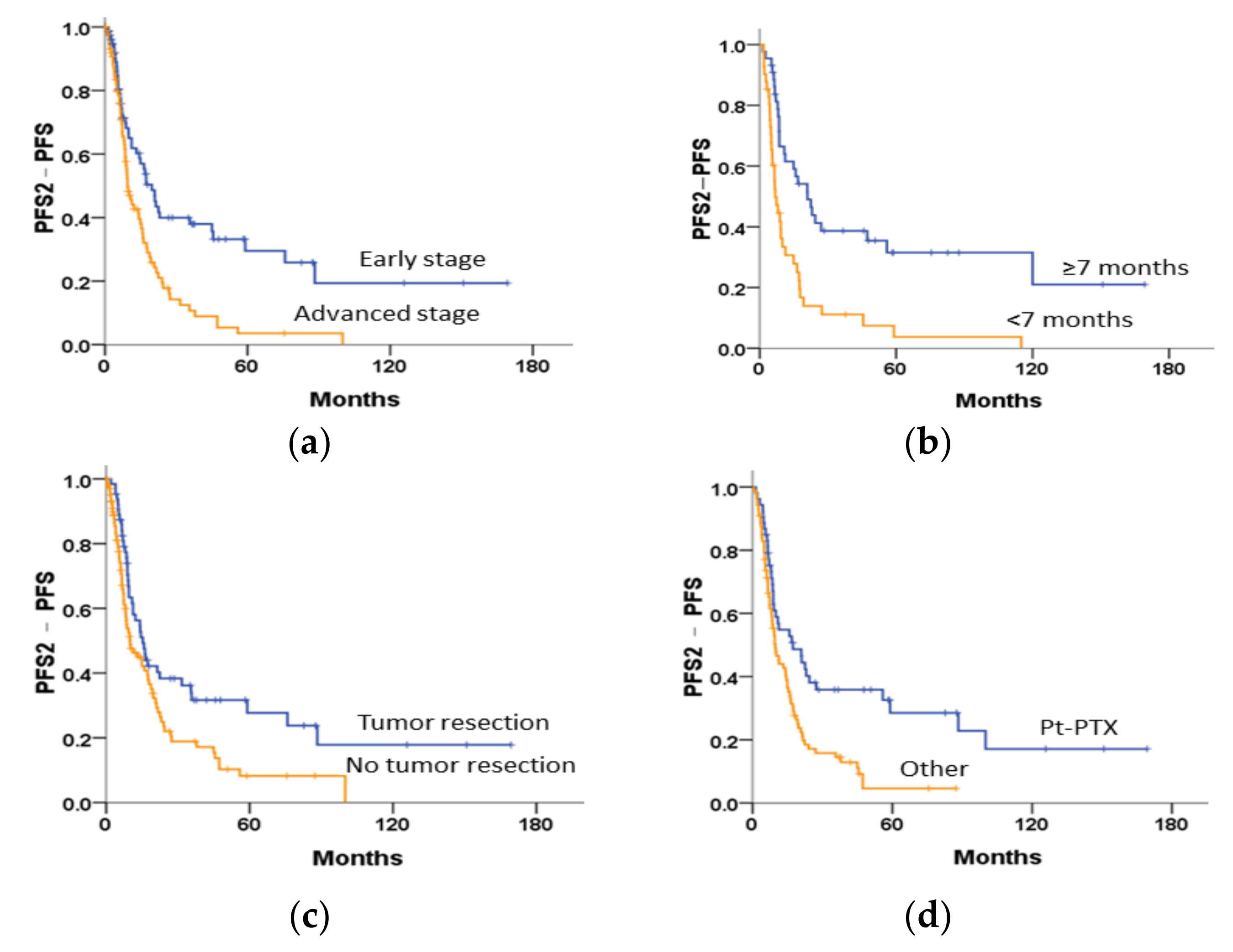
3.5.2. Survival Curves following the First Relapse
3.6. Univariate and Multivariate Analyses
3.6.1. Complete OCCC Cohort
3.6.2. Early-Stage OCCC Cohort
3.6.3. Relapsed OCCC Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Bishop, K.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2014; National Cancer Institute: Bethesda, MD, USA, 2017; Data Posted to the SEER Web Site. [Google Scholar]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Provincial Health Service Authority. Histological Classification of Ovarian Carcinoma, BC Cancer. Available online: http://www.bccancer.bc.ca/books/ovary-epithelial-carcinoma/histological-classification-of-ovarian-carcinoma (accessed on 15 January 2021).
- Okamoto, A.; Glasspool, R.M.; Mabuchi, S.; Matsumura, N.; Nomura, H.; Itamochi, H.; Takano, M.; Takano, T.; Susumu, N.; Aoki, D.; et al. Gynecologic Cancer InterGroup (GCIG) consensus review for clear cell carcinoma of the ovary. Int. J. Gynecol. Cancer 2014, 24, S20–S25, Erratum in Int. J. Gynecol. Cancer 2015, 25, 721. [Google Scholar] [CrossRef] [PubMed]
- Köbel, M.; Kang, E.Y. The evolution of ovarian carcinoma subclassification. Cancers 2022, 14, 416. [Google Scholar] [CrossRef]
- Jones, S.; Wang, T.L.; Shih, I.; Mao, T.L.; Nakayama, K.; Roden, R.; Glas, R.; Slamon, D.; Diaz, L.A., Jr.; Vogelstein, B.; et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science 2010, 330, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Chandler, R.L.; Damrauer, J.S.; Raab, J.R.; Schisler, J.C.; Wilkerson, M.D.; Didion, J.P.; Starmer, J.; Serber, D.; Yee, D.; Xiong, J.; et al. Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat. Commun. 2015, 6, 6118. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, H.; Jimbo, H.; Okada, S.; Matsumoto, K.; Onda, T.; Yasugi, T.; Taketani, Y. Prevalence of endometriosis in ovarian cancer. Gynecol. Obstet. Investig. 2000, 50, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Okamoto, A.; Enomoto, T.; Hamano, T.; Aotani, E.; Terao, Y.; Suzuki, N.; Mikami, M.; Yaegashi, N.; Kato, K.; et al. Randomized phase III trial of irinotecan plus cisplatin compared with paclitaxel plus carboplatin as first-line chemotherapy for ovarian clear cell carcinoma: JGOG3017/GCIG trial. J. Clin. Oncol. 2016, 34, 2881–2887. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Chen, C.A.; Chiang, C.J.; Hsu, T.H.; Lin, M.C.; You, S.L.; Cheng, W.F.; Lai, M.S. Trends in incidence and survival outcome of epithelial ovarian cancer: 30-year national population-based registry in Taiwan. J. Gynecol. Oncol. 2013, 24, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Aoki, D. Annual report of Gynecologic Oncology Committee, Japan Society of Obstetrics and Gynecology, 2013. J. Obstet. Gynaecol. Res. 2014, 40, 338–348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crozier, M.A.; Copeland, L.J.; Silva, E.G.; Gershenson, D.M.; Stringer, C.A. Clear cell carcinoma of the ovary: A study of 59 cases. Gynecol. Oncol. 1989, 35, 199–203. [Google Scholar] [CrossRef]
- Scarfone, G.; Bergamini, A.; Noli, S.; Villa, A.; Cipriani, S.; Taccagni, G.; Vigano, P.; Candiani, M.; Parazzini, F.; Mangili, G. Characteristics of clear cell ovarian cancer arising from endometriosis: A two center cohort study. Gynecol. Oncol. 2014, 133, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, S.; Aoki, D.; Tominaga, E.; Susumu, N.; Udagawa, Y.; Nozawa, S. Prognosis of Japanese patients with ovarian clear cell carcinoma associated with pelvic endometriosis: Clinicopathologic evaluation. Gynecol. Oncol. 1999, 72, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Kamura, T.; Kigawa, J.; Terakawa, N.; Kikuchi, Y.; Kita, T.; Suzuki, M.; Sato, I.; Taguchi, K. Clinical characteristics of clear cell carcinoma of the ovary: A distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer 2000, 88, 2584–2589. [Google Scholar] [CrossRef]
- Winter, W.E., 3rd; Maxwell, G.L.; Tian, C.; Carlson, J.W.; Ozols, R.F.; Rose, P.G.; Markman, M.; Armstrong, D.K.; Muggia, F.; McGuire, W.P.; et al. Prognostic factors for stage III epithelial ovarian cancer: A gynecologic oncology group study. J. Clin. Oncol. 2007, 25, 3621–3627. [Google Scholar] [CrossRef] [PubMed]
- Del Carmen, M.G.; Birrer, M.; Schorge, J.O. Clear cell carcinoma of the ovary: A review of the literature. Gynecol. Oncol. 2012, 126, 481–490. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Ovarian Cancer—Version 3. 2020. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1453 (accessed on 15 January 2021).
- Harries, M.; Gore, M. Part II: Chemotherapy for epithelial ovarian cancer-treatment of recurrent disease. Lancet Oncol. 2002, 3, 537–545. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Futagami, M.; Watanabe, J.; Sato, N.; Terada, Y.; Miura, F.; Sugiyama, T.; Takano, T.; Yaegashi, N.; Kojimahara, T.; et al. Redistribution of resistance and sensitivity to platinum during the observation period following treatment of epithelial ovarian cancer. Mol. Clin. Oncol. 2014, 2, 212–218. [Google Scholar] [CrossRef][Green Version]
- Kondo, E.; Tabata, T.; Suzuki, N.; Aoki, D.; Yahata, H.; Kotera, Y.; Tokuyama, O.; Fujiwara, K.; Kimura, E.; Terauchi, F.; et al. The post-progression survival of patients with recurrent or persistent ovarian clear cell carcinoma: Results from a randomized phase III study in JGOG3017/GCIG. J. Gynecol. Oncol. 2020, 31, e94. [Google Scholar] [CrossRef] [PubMed]
- IBM Corp. IBM SPSS Statistics for Windows; Version 21.0; IBM Corp.: Armonk, NY, USA, 2012. [Google Scholar]
- Ye, S.; Yang, J.; You, Y.; Cao, D.; Huang, H.; Wu, M.; Chen, J.; Lang, J.; Shen, K. Comparison of clinical characteristic and prognosis between ovarian clear cell carcinoma and serous carcinoma: A 10-year cohort study of chinese patients. PLoS ONE 2015, 10, e0133498. [Google Scholar] [CrossRef] [PubMed]
- Sahin, H.; Sari, M.E.; Cuylan, Z.F.; Haberal, A.N.; Sirvan, L.; Coban, G.; Yalcin, I.; Güngör, T.; Celik, H.; Meydanli, M.M.; et al. Is the presence of endometriosis associated with a survival benefit in pure ovarian clear cell carcinoma? Arch. Gynecol. Obstet. 2018, 297, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.A.; Dong, F.; Young, R.H.; Oliva, E. Clear cell carcinoma of the ovary: Evaluation of prognostic parameters based on a clinicopathological analysis of 100 cases. Histopathology 2015, 66, 808–815. [Google Scholar] [CrossRef]
- Ku, F.C.; Wu, R.C.; Yang, L.Y.; Tang, Y.H.; Chang, W.Y.; Yang, J.E.; Wang, C.C.; Jung, S.M.; Lin, C.T.; Chang, T.C.; et al. Clear cell carcinomas of the ovary have poorer outcomes compared with serous carcinomas: Results from a single-center Taiwanese study. J. Formos. Med. Assoc. 2018, 117, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Hatae, M.; Watanabe, Y.; Yaegashi, N.; Ishiko, O.; Kodama, S.; Yamaguchi, S.; Ochiai, K.; Takano, M.; Yokota, H.; et al. Outcomes of fertility-sparing surgery for stage I epithelial ovarian cancer: A proposal for patient selection. J. Clin. Oncol. 2010, 28, 1727–1732, Erratum in J. Clin. Oncol. 2011, 29, 4725. [Google Scholar] [CrossRef] [PubMed]
- Prodromidou, A.; Theofanakis, C.; Thomakos, N.; Haidopoulos, D.; Rodolakis, A. Fertility sparing surgery for early-stage clear cell carcinoma of the ovary; A systematic review and analysis of obstetric outcomes. Eur. J. Surg. Oncol. 2021, 47, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; Cecere, S.C.; Magazzino, F.; Katsaros, D.; Ottaiano, A.; Gadducci, A.; Pisano, C.; Scalone, S.; Rabaiotti, E.; Salutari, V.; et al. Second-line chemotherapy in recurrent clear cell ovarian cancer: Results from the multicenter Italian trials in ovarian cancer (MITO-9). Oncology 2014, 86, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Tomao, F.; D’Incalci, M.; Biagioli, E.; Peccatori, F.A.; Colombo, N. Restoring platinum sensitivity in recurrent ovarian cancer by extending the platinum-free interval: Myth or reality? Cancer 2017, 123, 3450–3459. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M.; Trimble, E.; Tinker, A.; Alberts, D.; Avall-Lundqvist, E.; Brady, M.; Harter, P.; Pignata, S.; Pujade-Lauraine, E.; Sehouli, J.; et al. Clinical trials in recurrent ovarian cancer. Int. J. Gynecol. Cancer 2011, 21, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, K.; Gao, B.; Mapagu, C.; Fereday, S.; Emmanuel, C.; Alsop, K.; Traficante, N.; Harnett, P.R.; Bowtell, D.D.; de Fazio, A.; et al. Response rates to second-line platinum-based therapy in ovarian cancer patients challenge the clinical definition of platinum resistance. Gynecol. Oncol. 2018, 150, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.D.; Matulonis, U.A.; Herzog, T.J.; Coleman, R.L.; Monk, B.J.; Markman, M. Moving beyond the platinum sensitive/resistant paradigm for patients with recurrent ovarian cancer. Gynecol. Oncol. 2016, 141, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Aebi, S.; Castiglione, M. Epithelial ovarian carcinoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann. Oncol. 2008, 19, ii14–ii16. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, H.; Mikami, M.; Nagase, S.; Kobayashi, Y.; Tabata, T.; Kaneuchi, M.; Satoh, T.; Hirashima, Y.; Matsumura, N.; Yokoyama, Y.; et al. The 2020 Japan Society of Gynecologic Oncology guidelines for the treatment of ovarian cancer, fallopian tube cancer, and primary peritoneal cancer. J. Gynecol. Oncol. 2021, 32, e49. [Google Scholar] [CrossRef] [PubMed]
- Stuart, G.C.; Kitchener, H.; Bacon, M.; du Bois, A.; Friedlander, M.; Ledermann, J.; Marth, C.; Thigpen, T.; Trimble, E. Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: Report from the Fourth Ovarian Cancer Consensus Conference. Int. J. Gynecol. Cancer 2011, 21, 750–755. [Google Scholar] [CrossRef]
- Husseinzadeh, N.; Husseinzadeh, H.D. mTOR inhibitors and their clinical application in cervical, endometrial and ovarian cancers: A critical review. Gynecol. Oncol. 2014, 133, 375–381. [Google Scholar] [CrossRef]
- Sasano, T.; Mabuchi, S.; Kuroda, H.; Kawano, M.; Matsumoto, Y.; Takahashi, R.; Hisamatsu, T.; Sawada, K.; Hashimoto, K.; Isobe, A.; et al. Preclinical efficacy for AKT targeting in clear cell carcinoma of the ovary. Mol. Cancer Res. 2015, 13, 795–806. [Google Scholar] [CrossRef]
- Chan, J.K.; Brady, W.; Monk, B.J.; Brown, J.; Shahin, M.S.; Rose, P.G.; Kim, J.H.; Secord, A.A.; Walker, J.L.; Gershenson, D.M. A phase II evaluation of sunitinib in the treatment of persistent or recurrent clear cell ovarian carcinoma: An NRG oncology/gynecologic oncology group study (GOG-254). Gynecol. Oncol. 2018, 150, 247–252. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Sill, M.W.; Makker, V.; Mutch, D.G.; Carlson, J.W.; Darus, C.J.; Mannel, R.S.; Bender, D.P.; Crane, E.K.; Aghajanian, C. A randomized phase II study of cabozantinib versus weekly paclitaxel in the treatment of persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer: An NRG oncology/gynecologic oncology group study. Gynecol. Oncol. 2019, 152, 548–553. [Google Scholar] [CrossRef]
- Colombo, I.; Genta, S.; Martorana, F.; Guidi, M.; Frattini, M.; Samartzis, E.P.; Brandt, S.; Gaggetta, S.; Moser, L.; Pascale, M.; et al. Phase I dose-escalation study of the dual PI3K-mTORC1/2 inhibitor gedatolisib in combination with paclitaxel and carboplatin in patients with advanced solid tumors. Clin. Cancer Res. 2021, 27, 5012–5019. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Ikeda, T.; Minami, M.; Kawaguchi, A.; Murayama, T.; Kanai, M.; Mori, Y.; Matsumoto, S.; Chikuma, S.; et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J. Clin. Oncol. 2015, 33, 4015–4022. [Google Scholar] [CrossRef]
- Maiorano, B.A.; Maiorano, M.; Lorusso, D.; Maiello, E. Ovarian cancer in the era of immune checkpoint inhibitors: State of the art and future perspectives. Cancers 2021, 13, 4438. [Google Scholar] [CrossRef] [PubMed]
- Hamanishi, J.; Takeshima, N.; Katsumata, N.; Ushijima, K.; Kimura, T.; Takeuchi, S.; Matsumoto, K.; Ito, K.; Mandai, M.; Nakai, H.; et al. Nivolumab versus gemcitabine or pegylated liposomal doxorubicin for patients with platinum-resistant ovarian cancer: Open-label, randomized trial in Japan (NINJA). J. Clin. Oncol. 2021, 39, 3671–3681. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Z.; Chen, L.; Pang, J.; Wu, H.; Liang, Z. Next-generation sequencing reveals a very low prevalence of deleterious mutations of homologous recombination repair genes and homologous recombination deficiency in ovarian clear cell carcinoma. Front. Oncol. 2022, 11, 798173. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | OCCC Patients (n = 536) |
|---|---|
| Age, years, mean ± SD | 50.0 ± 9.9 |
| Menarche age, years, median ± IQR (range) | 13.0 ± 1.0 (10.0–18.0) |
| CA125 level at diagnosis, U/mL, median ± IQR (range) | 101.5 ± 324 (6.0–16,659.0) |
| Nulliparity | 141 (30.4) |
| Parity, mean ± SD | 1.5 ± 1.3 |
| Menopause | 279 (52.1) |
| Most common symptoms, n (%) | |
| Asymptomatic | 43 (8.0) |
| Abdominal pain | 112 (20.9) |
| Abdominal bloating | 132 (24.6) |
| Abnormal uterine bleeding | 17 (3.2) |
| Other symptoms | 101 (18.8) |
| Endometriosis present pathologically | 209 (39.0) |
| FIGO stage, n (%) | |
| I | 308 (57.5) |
| II | 63 (12.3) |
| III | 116 (21.1) |
| IV | 34 (6.2) |
| Unknown | 15 (2.8) |
| Primary staging/cytoreduction, n (%) | |
| RD < 1 cm | 429 (80.0) |
| RD ≥ 1 cm | 61 (11.4) |
| Unknown | 46 (8.5) |
| Front-line chemotherapy, n (%) | |
| Pt-PTX | 354 (66.0) |
| Pt-CTX | 120 (22.4) |
| Other platinum-based regimens, n (%) | 29 (5.4) |
| None | 33 (6.2) |
| Cycles of front-line chemotherapy, median ± IQR (range) | 6.0 ± 1.0 (1–11) |
| <6, n (%) | 134 (26.6) |
| ≥6, n (%) | 369 (73.4) |
| PFI after primary chemotherapy, n (%) | |
| ≥12 months | 320 (63.6) |
| 6–12 months | 56 (11.1) |
| <6 months | 127 (25.2) |
| Surgical resection after the first relapse, n (%) | 67 (36.0) |
| Chemotherapy after the first relapse, n (%) | |
| None | 35 (18.8) |
| Pt-PTX | 55 (29.6) |
| Pt-CTX | 2 (1.1) |
| Other | 87 (46.8) |
| PFI after Front-Line Chemotherapy to the First Relapse (Months) | No. of Patients with Pt-Based Chemotherapy | No. of Patients with the Second Relapse | Median PFS2-PFS (Months) | HR | 95% CI |
|---|---|---|---|---|---|
| <6 | 38 | 34 | 6.8 | 1.00 | – |
| ≥6 | 47 | 32 | 20.9 | 0.41 | 0.25–0.68 |
| 6–12 | 16 | 15 | 9.2 | 0.76 | 0.41–1.40 |
| ≥12 | 31 | 16 | 24.4 | 0.27 | 0.15–0.50 |
| <7 | 41 | 37 | 6.8 | 1.00 | – |
| ≥7 | 44 | 28 | 21.0 | 0.37 | 0.22–0.61 |
| 7–12 | 13 | 12 | 11.2 | 0.68 | 0.35–1.31 |
| ≥12 | 31 | 16 | 24.4 | 0.27 | 0.14–0.49 |
| 7–14 | 18 | 16 | 14.9 | 0.53 | 0.29–0.97 |
| ≥14 | 26 | 12 | 24.4 | 0.26 | 0.13–0.50 |
| 7–16 | 20 | 18 | 11.2 | 0.57 | 0.32–1.01 |
| ≥16 | 24 | 10 | NR | 0.22 | 0.11–0.45 |
| 7–18 | 23 | 21 | 11.2 | 0.60 | 0.35–1.03 |
| ≥18 | 21 | 7 | NR | 0.17 | 0.07–0.38 |
| <8 | 46 | 41 | 8.5 | 1.00 | – |
| ≥8 | 39 | 24 | 23.0 | 0.38 | 0.23–0.65 |
| 8–16 | 15 | 14 | 8.6 | 0.65 | 0.35–1.21 |
| ≥16 | 24 | 10 | NR | 0.24 | 0.12–0.49 |
| <10 | 49 | 44 | 8.5 | 1.00 | – |
| ≥10 | 36 | 21 | 24.4 | 0.35 | 0.20–0.60 |
| 10–18 | 15 | 14 | 8.6 | 0.64 | 0.35–1.19 |
| ≥18 | 21 | 7 | NR | 0.18 | 0.08–0.41 |
| <12 | 54 | 49 | 8.6 | 1.00 | – |
| ≥12 | 31 | 16 | 24.4 | 0.30 | 0.16–0.54 |
| 12–18 | 10 | 9 | 8.5 | 0.57 | 0.27–1.18 |
| >18 | 21 | 7 | NR | 0.18 | 0.08–0.41 |
| Variables | PFI ≥7 Months n = 366 | PFI <7 Months n = 137 | p | PFI ≥12 Months n = 318 | PFI <12 Months n = 185 | p |
|---|---|---|---|---|---|---|
| Age >50 years | 179 (48.9) | 60 (43.8) | 0.271 | 154 (48.4) | 85 (45.9) | 0.584 |
| Menopause | 192 (52.5) | 68 (49.6) | 0.516 | 162 (50.9) | 98 (53.0) | 0.667 |
| CA125 level ≥114.5 U/mL | 126 (34.4) | 88 (64.2) | <0.001 | 103 (32.4) | 111 (60.0) | <0.001 |
| Endometriosis present | 147 (40.1) | 52 (38.0) | 0.628 | 132 (41.5) | 67 (36.2) | 0.424 |
| FIGO stage | <0.001 | <0.001 | ||||
| I | 248 (67.8) | 41 (29.9) | 225 (70.8) | 64 (34.6) | ||
| II | 43 (11.7) | 17 (12.4) | 39 (12.3) | 21 (11.4) | ||
| III | 46 (12.6) | 64 (46.7) | 31 (9.7) | 79 (42.7) | ||
| IV | 18 (4.9) | 13 (9.5) | 12 (3.8) | 19 (10.3) | ||
| Primary cytoreduction | <0.001 | <0.001 | ||||
| Optimal (RD <1 cm) | 316 (86.3) | 94 (68.6) | 278 (87.4) | 132 (71.3) | ||
| Suboptimal (RD ≥1 cm) | 17 (4.6) | 35 (25.5) | 11 (3.5) | 41 (22.2) | ||
| Front-line chemotherapy | 0.192 | 0.121 | ||||
| Pt-PTX | 250 (68.3) | 104 (75.9) | 214 (67.3) | 140 (75.7) | ||
| Pt-CTX | 95 (26.0) | 25 (18.2) | 85 (26.7) | 35 (18.9) | ||
| Other | 21 (5.7) | 8 (5.8) | 19 (6.0) | 10 (5.4) | ||
| Cycles of front-line chemotherapy | 0.005 | 0.068 | ||||
| <6 | 85 (23.2) | 49 (35.8) | 76 (23.9) | 58 (31.4) | ||
| ≥6 | 281 (76.8) | 88 (64.2) | 242 (76.1) | 127 (68.6) | ||
| Response to primary chemotherapy | <0.001 | <0.001 | ||||
| CR/PR | 335 (91.5) | 68 (49.6) | 298 (93.7) | 105 (56.8) | ||
| SD/PD | 22 (6.0) | 64 (46.7) | 11 (3.5) | 75 (40.5) | ||
| Tumour resection after the first relapse | 0.076 | 0.034 | ||||
| No | 114 (79.2) | 67 (69.1) | 98 (81.0) | 83 (69.2) | ||
| Yes | 30 (20.8) | 30 (30.9) | 23 (19.0) | 37 (30.8) | ||
| Chemotherapy after the first relapse | <0.001 | <0.001 | ||||
| Pt-PTX | 33 (54.1) | 16 (21.6) | 29 (70.7) | 20 (21.3) | ||
| Other | 28 (45.9) | 58 (78.4) | 12 (29.3) | 74 (78.7) |
| Variables | HR for the First Progression (95% CI) | HR for Death (95% CI) |
|---|---|---|
| Age (≥50 vs. <50 years) | 0.89 (0.62–1.27) | 2.98 (1.60–5.57) |
| CA125 level at diagnosis (≥ 114.5 versus <114.5 U/mL) | 1.57 (1.03–2.40) | 0.71 (0.34–1.47) |
| Endometriosis present (yes versus no) | 1.00 (0.69–1.44) | 0.93 (0.52–1.64) |
| FIGO stage (advanced versus early) | 3.21 (1.98–5.22) | 1.41 (0.67–2.95) |
| Primary staging/cytoreduction (RD ≥ 1 cm versus <1 cm) | 1.30 (0.81–2.08) | 1.12 (0.59–2.34) |
| Front-line chemotherapy (Pt-PTX versus Pt-CTX) | 0.69 (0.41–1.16) | 0.71 (0.25–2.03) |
| Cycles of chemotherapy (≥6 versus <6) | 0.88 (0.59–1.33) | 1.04 (0.51–2.10) |
| Response to chemotherapy (SD/PD versus CR/PR) | 4.56 (2.88–7.23) | 1.47 (0.79–2.73) |
| Progression-free interval (<7 months versus ≥7 months) | - | 7.85 (3.63–16.94) |
| Surgical resection after the first relapse (yes versus no) | - | 0.60 (0.29–1.23) |
| Chemotherapy after the first relapse (others versus Pt-PTX) | - | 1.23 (0.52–2.89) |
| Variables | HR for the First Progression (95% CI) | HR for Death (95% CI) |
|---|---|---|
| Age (≥50 versus <50 years) | 0.72 (0.39–1.31) | 2.39 (0.67–8.50) |
| CA125 level at diagnosis (≥ 114.5 versus <114.5 U/mL) | 2.63 (1.48–4.67) | 0.61 (0.17–2.19) |
| Endometriosis present (yes versus no) | 0.74 (0.41–1.33) | 0.44 (0.14–1.35) |
| Primary staging/cytoreduction (RD ≥ 1 cm versus <1 cm) | 1.38 (0.39–4.92) | 0.81 (0.09–7.81) |
| Front-line chemotherapy (Pt-PTX versus Pt-CTX) | 0.61 (0.34–1.09) | 0.48 (0.12–1.99) |
| Cycles of chemotherapy (≥6 versus <6) | 0.76 (0.41–1.41) | 2.22 (0.57–8.61) |
| Response to chemotherapy (SD/PD versus CR/PR) | 22.77 (11.64–44.56) | 1.83 (0.44–7.57) |
| PFI after primary chemotherapy (<7 versus ≥7 months) | - | 4.82 (1.32–17.60) |
| Surgical resection after the first relapse (yes versus no) | - | 0.23 (0.06–0.91) |
| Chemotherapy after the first relapse (other versus Pt-PTX) | - | 1.64 (0.41–6.62) |
| Variables | HR for the Next Progression (95% CI) | HR for Post- Progression Death (95% CI) |
|---|---|---|
| Age (≥50 versus <50) | 1.49 (0.97–2.30) | 2.26 (1.37–3.72) |
| FIGO stage (advanced versus early) | 1.50 (0.95–2.37) | 1.73 (1.01–2.96) |
| Response to chemotherapy (SD/PD versus CR/PR) | 1.33 (0.85–2.08) | 1.26 (0.76–2.10) |
| Progression-free interval (<7 versus ≥7 months) | 2.46 (1.54–3.93) | 3.86 (2.15–6.96) |
| Surgical resection after the first relapse (yes versus no) | 0.83 (0.52–1.32) | 0.83 (0.50–1.41) |
| Chemotherapy after the first relapse (other versus Pt-PTX) | 1.04 (0.62–1.75) | 0.95 (0.51–1.78) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, C.-Y.; Cheng, W.-F.; Chen, M.-Y.; Lin, H.; Ho, C.-M.; Hung, Y.-C.; Huang, L.-W.; Wang, P.-H.; Yu, M.-H.; Huang, Y.-F. Predicting Long-Term Prognoses and Grading Platinum Sensitivity Using a Novel Progression-Free Interval Criterion in Ovarian Clear Cell Carcinoma: A Multi-Institutional Cohort Study. Cancers 2022, 14, 1746. https://doi.org/10.3390/cancers14071746
Chou C-Y, Cheng W-F, Chen M-Y, Lin H, Ho C-M, Hung Y-C, Huang L-W, Wang P-H, Yu M-H, Huang Y-F. Predicting Long-Term Prognoses and Grading Platinum Sensitivity Using a Novel Progression-Free Interval Criterion in Ovarian Clear Cell Carcinoma: A Multi-Institutional Cohort Study. Cancers. 2022; 14(7):1746. https://doi.org/10.3390/cancers14071746
Chicago/Turabian StyleChou, Cheng-Yang, Wen-Fang Cheng, Min-Yu Chen, Hao Lin, Chih-Ming Ho, Yao-Ching Hung, Lee-Wen Huang, Po-Hui Wang, Mu-Hsien Yu, and Yu-Fang Huang. 2022. "Predicting Long-Term Prognoses and Grading Platinum Sensitivity Using a Novel Progression-Free Interval Criterion in Ovarian Clear Cell Carcinoma: A Multi-Institutional Cohort Study" Cancers 14, no. 7: 1746. https://doi.org/10.3390/cancers14071746
APA StyleChou, C.-Y., Cheng, W.-F., Chen, M.-Y., Lin, H., Ho, C.-M., Hung, Y.-C., Huang, L.-W., Wang, P.-H., Yu, M.-H., & Huang, Y.-F. (2022). Predicting Long-Term Prognoses and Grading Platinum Sensitivity Using a Novel Progression-Free Interval Criterion in Ovarian Clear Cell Carcinoma: A Multi-Institutional Cohort Study. Cancers, 14(7), 1746. https://doi.org/10.3390/cancers14071746






