Exosomes on Endometrial Cancer: A Biomarkers Treasure Trove?
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Endometrial Cancer
1.2. Liquid Biopsy
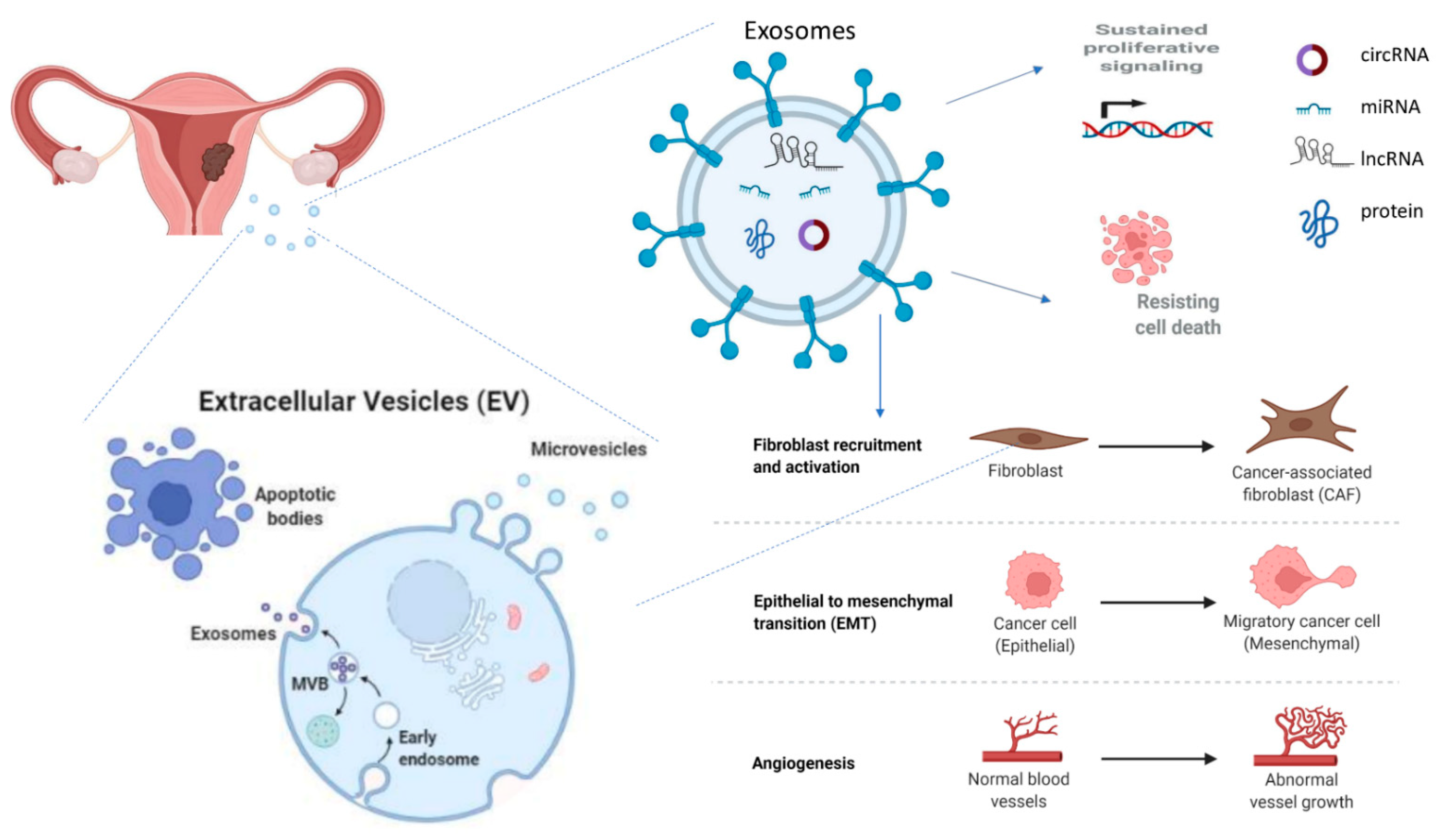
1.3. Exosomes
2. Exosomes in the Pathophysiology of Endometrial Cancer
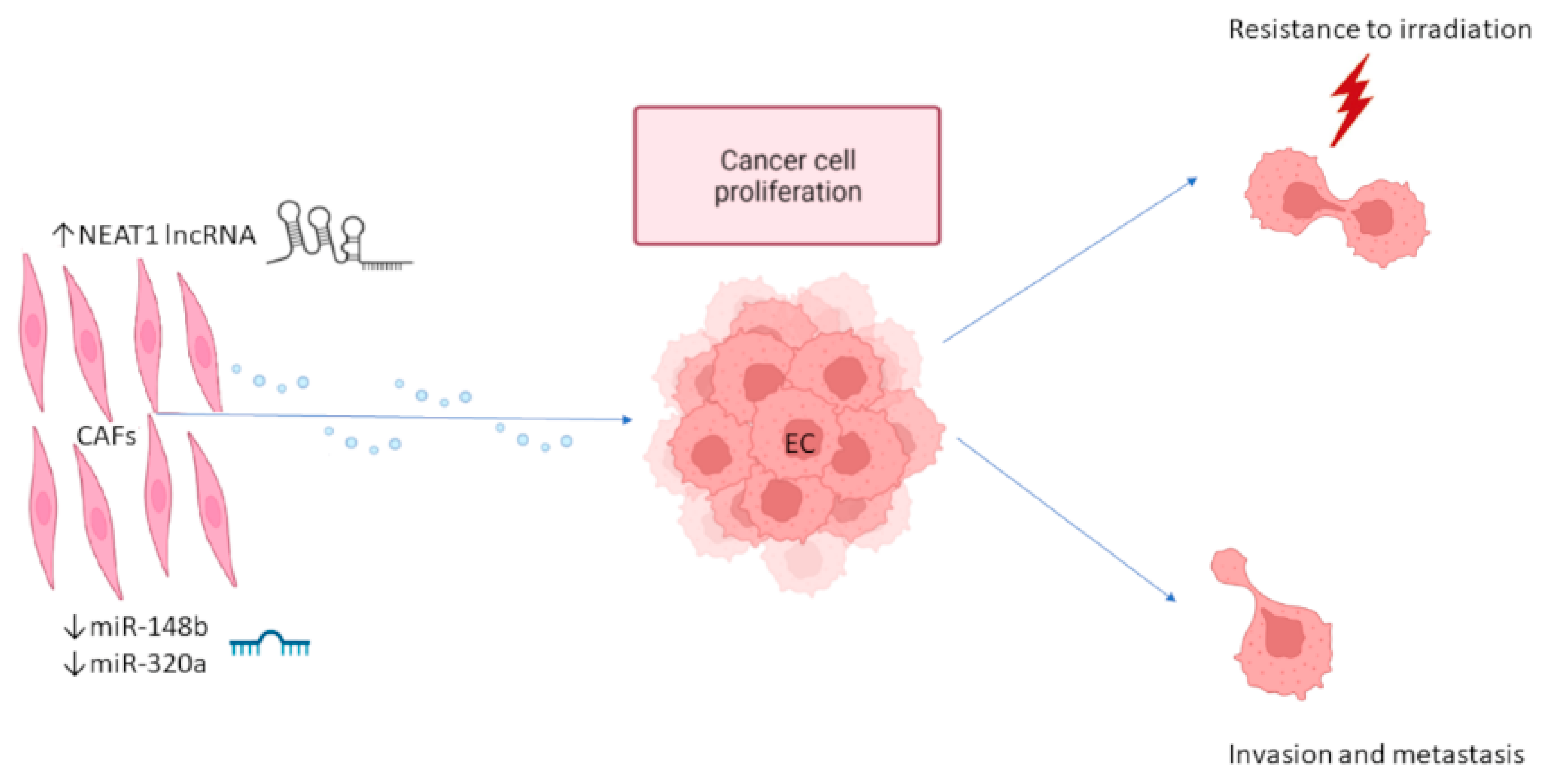
2.1. Exosome-Mediated Interaction between EC Cells and CAFs
2.2. Exosome-Mediated Communication between EC Cells and Tumor Infiltrating Immune Cells
2.3. EC-Derived Exosomes Involved in Cancer Progression
3. Exosomal Biomarkers in Biological Fluids of EC Patients
3.1. Plasma-Serum Biomarkers
3.1.1. Proteins
3.1.2. miRNAs
3.1.3. circRNAs
3.2. Peritoneal Lavage Exosomal Biomarkers
3.3. Urine Exosomal Biomarkers
3.4. Uterine Blood–Uterine Aspirate Biomarkers
| Origin | Biomarker | Biomarker Type | Expression | Function/Significance | Potential Application | Ref |
|---|---|---|---|---|---|---|
| EC CAFs | NEAT1 | lncRNA | ↑ | EC growth | Therapy | [44] |
| EC CAFs | miR-148b | miRNA | ↓ | EC cell invasion, EMT | Therapy | [45] |
| EC CAFs | miR-320a | miRNA | ↓ | EC cell proliferation | Therapy | [46] |
| EC TAMs | hsa_circ_0001610 | circRNA | ↑ | EC cell proliferation Resistance to irradiation | Therapy | [59] |
| EC TAMs | miR-192-5p | miRNA | ↓ | EC cell proliferation EMT | Therapy | [60] |
| CD45RO-CD8+ TILs | miR-765 | miRNA | ↑ | EC growth inhibition | Therapy | [62] |
| EC cells | miR-133a | miRNA | ↑ | Communication between EC and stromal cells | Therapy | [50] |
| EC cells | DLEU1 | lncRNA | ↑ | EC cell proliferation, migration and invasion | Therapy | [63] |
| EC cells (hypoxia) | miR-21 | miRNA | ↑ | EC cell proliferation and migration, ↓OS ** | Prognosis Therapy | [53,56,57,58] |
| hUMSCs | miR-503-3p | miRNA | ↑ | EC growth inhibition | Therapy | [80] |
| Serum from stage III adenocarcinoma patients | hsa_circ_0109046 hsa_circ_0002577 | circRNAs | ↑ | EC migration and invasion | Diagnosis | [70] |
| Serum * | miR-93 | miRNA | ↑ | Correlation with smoking, tumor grade, advanced stage, metastasis, ↓OS | Diagnosis Prognosis | [69] |
| Serum * | miR-205 | miRNA | ↓ | Correlation with smoking, lymph node spread, advanced stage, ↓OS | Diagnosis Prognosis | [69] |
| Plasma | LGALS3BP | Protein | ↑ | EC growth, angiogenesis | Diagnosis Prognosis | [65] |
| Plasma | miR-15a-5p | miRNAs | ↑ | Correlation with tumor size and depth of invasion, similar expression in EEC and non-EEC patients | Diagnosis of early EC | [67] |
| Plasma | miR-106b-5p miR-107 | miRNAs | ↑ | Unknown, similar expression in EEC and non-EEC patients | Diagnosis | [67] |
| Plasma * | miR-151a-5p | miRNA | ↑ | Unknown | Diagnosis | [68] |
| Plasma from EEC (n = 19) and non-EEC (n = 3) patients. | miR-10b-5p, miR- 34b-3p, miR-34c- 5p, miR-34c-3p, miR-449b-5p, miR-200b-3p, miR-383-5p, miR-2110 | miRNAs | ↓ | EC progression ↓ miR-10b-5p correlates with ↓OS miR-200b-3p inhibits EMT | Diagnosis | [71] |
| Plasma from EEC (n = 26), non-EEC (n = 12) and other EC subtypes (n = 3) patients. | ANXA2 | Protein | ↑ | Correlates with high grade, non-endometrioid subtype, advanced stage, and increased risk of recurrence | Diagnosis Prognosis | [66] |
| Urine | hsa-miR-200c-3p | miRNA | ↑ | EC growth inhibition EMT block | Diagnosis | [74] |
| Serum from PCOS patients | miR-590-3p miR-27a-5p | miRNAs | ↑ | miR-27a-5p promotes EC cell migration-invasion | Diagnosis | [64] |
| Serum from PCOS patients | miR-375a-3p miR-19b-3p | miRNAs | ↓ | Unknown | Diagnosis | [64] |
4. Exosomes as Therapeutic Agents
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gayther, S.A.; Pharoah, P.D. The inherited genetics of ovarian and endometrial cancer. Curr. Opin. Genet. Dev. 2010, 20, 231–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, K.; Brewer, M.A. Endometrial cancer: Is this a new disease? Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Lortet-Tieulent, J.; Ferlay, J.; Bray, F.; Jemal, A. International patterns and trends in endometrial cancer incidence, 1978–2013. JNCI J. Natl. Cancer Inst. 2018, 110, 354–361. [Google Scholar] [CrossRef]
- Raglan, O.; Kalliala, I.; Markozannes, G.; Cividini, S.; Gunter, M.J.; Nautiyal, J.; Gabra, H.; Paraskevaidis, E.; Martin-Hirsch, P.; Tsilidis, K.K. Risk factors for endometrial cancer: An umbrella review of the literature. Int. J. Cancer 2019, 145, 1719–1730. [Google Scholar] [CrossRef] [Green Version]
- National Cancer Institute. SEER Cancer Statistics Review, 1975–2017; Howlader, N., Noone, A.M., Krapcho, M., Miller, D., Brest, A., Yu, M., Ruhl, J., Tatalovich, Z., Mariotto, A., Lewis, D.R., et al., Eds.; National Cancer Institute: Bethesda, MD, USA, 2020; Based on November 2019 SEER Data Submission, Posted to the SEER Web Site, April 2020. Available online: https://seer.cancer.gov/csr/1975_2017/ (accessed on 1 March 2022).
- Cree, I.A.; White, V.A.; Indave, B.I.; Lokuhetty, D. Revising the WHO classification: Female genital tract tumours. Histopathology 2020, 76, 151–156. [Google Scholar] [CrossRef]
- Suarez, A.A.; Felix, A.S.; Cohn, D.E. Bokhman Redux: Endometrial cancer “types” in the 21st century. Gynecol. Oncol. 2017, 144, 243–249. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board (Ed.) World Health Organization Classification of Tumours, 5th ed.; Female Genital Tumours; IARC Press: Lyon, France, 2020. [Google Scholar]
- Levine, D.A. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef] [Green Version]
- Talhouk, A.; McConechy, M.; Leung, S.; Li-Chang, H.; Kwon, J.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef] [Green Version]
- Kommoss, S.; McConechy, M.; Kommoss, F.; Leung, S.; Bunz, A.; Magrill, J.; Britton, H.; Grevenkamp, F.; Karnezis, A.; Yang, W. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann. Oncol. 2018, 29, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Vermij, L.; Smit, V.; Nout, R.; Bosse, T. Incorporation of molecular characteristics into endometrial cancer management. Histopathology 2020, 76, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Angelico, G.; Travaglino, A.; Inzani, F.; Arciuolo, D.; Valente, M.; D’Alessandris, N.; Scaglione, G.; Fiorentino, V.; Raffone, A.; et al. New Pathological and Clinical Insights in Endometrial Cancer in View of the Updated ESGO/ESTRO/ESP Guidelines. Cancers 2021, 13, 2623. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.R.; Cooper, K.; Croce, S.; Djordevic, B.; Herrington, S.; Howitt, B.; Hui, P.; Ip, P.; Koebel, M.; Lax, S. International Society of Gynecological Pathologists (ISGyP) endometrial cancer project: Guidelines from the special techniques and ancillary studies group. Int. J. Gynecol. Pathol. 2019, 38, S114. [Google Scholar] [CrossRef] [PubMed]
- Kesterson, J.P.; Fanning, J. Fertility-sparing treatment of endometrial cancer: Options, outcomes and pitfalls. J. Gynecol. Oncol. 2012, 23, 120. [Google Scholar] [CrossRef] [Green Version]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Van Weelden, W.J.; Massuger, L.F.; Pijnenborg, J.; Romano, A. Anti-estrogen treatment in endometrial cancer: A systematic review. Front. Oncol. 2019, 9, 359. [Google Scholar] [CrossRef] [Green Version]
- Muinelo-Romay, L.; Casas-Arozamena, C.; Abal, M. Liquid biopsy in endometrial cancer: New opportunities for personalized oncology. Int. J. Mol. Sci. 2018, 19, 2311. [Google Scholar] [CrossRef] [Green Version]
- Alix-Panabières, C.; Pantel, K. Liquid biopsy: From discovery to clinical application. Cancer Discov. 2021, 11, 858–873. [Google Scholar] [CrossRef]
- Mader, S.; Pantel, K. Liquid biopsy: Current status and future perspectives. Oncol. Res. Treat. 2017, 40, 404–408. [Google Scholar] [CrossRef]
- Hadjimichael, A.C.; Pergaris, A.; Kaspiris, A.; Foukas, A.F.; Theocharis, S.E. Liquid Biopsy: A New Translational Diagnostic and Monitoring Tool for Musculoskeletal Tumors. Int. J. Mol. Sci. 2021, 22, 11526. [Google Scholar] [CrossRef] [PubMed]
- Ura, B.; Monasta, L.; Arrigoni, G.; Franchin, C.; Radillo, O.; Peterlunger, I.; Ricci, G.; Scrimin, F. A proteomic approach for the identification of biomarkers in endometrial cancer uterine aspirate. Oncotarget 2017, 8, 109536. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, E.; Lesur, A.; Devis, L.; Cabrera, S.; Matias-Guiu, X.; Hirschfeld, M.; Asberger, J.; Van Oostrum, J.; Gómez-Tato, A.; Reventos, J. Targeted proteomics identifies proteomic signatures in liquid biopsies of the endometrium to diagnose endometrial cancer and assist in the prediction of the optimal surgical treatment. Clin. Cancer Res. 2017, 23, 6458–6467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mota, A.; Colás, E.; García-Sanz, P.; Campoy, I.; Rojo-Sebastián, A.; Gatius, S.; García, Á.; Chiva, L.; Alonso, S.; Gil-Moreno, A. Genetic analysis of uterine aspirates improves the diagnostic value and captures the intra-tumor heterogeneity of endometrial cancers. Mod. Pathol. 2017, 30, 134–145. [Google Scholar] [CrossRef] [Green Version]
- Herrero, C.; Abal, M.; Muinelo-Romay, L. Circulating extracellular vesicles in gynecological tumors: Realities and challenges. Front. Oncol. 2020, 10, 565666. [Google Scholar] [CrossRef]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [Green Version]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Georgantzoglou, N.; Pergaris, A.; Masaoutis, C.; Theocharis, S. Extracellular vesicles as biomarkers carriers in bladder cancer: Diagnosis, surveillance, and treatment. Int. J. Mol. Sci. 2021, 22, 2744. [Google Scholar] [CrossRef]
- Masaoutis, C.; Al Besher, S.; Koutroulis, I.; Theocharis, S. Exosomes in nephropathies: A rich source of novel biomarkers. Dis. Mark. 2020, 2020, 8897833. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Ratajczak, J. Extracellular microvesicles/exosomes: Discovery, disbelief, acceptance, and the future? Leukemia 2020, 34, 3126–3135. [Google Scholar] [CrossRef]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Agrahari, V.; Burnouf, P.-A.; Chew, C.H.; Burnouf, T. Extracellular microvesicles as new industrial therapeutic frontiers. Trends Biotechnol. 2019, 37, 707–729. [Google Scholar] [CrossRef] [PubMed]
- Klyachko, N.L.; Arzt, C.J.; Li, S.M.; Gololobova, O.A.; Batrakova, E.V. Extracellular vesicle-based therapeutics: Preclinical and clinical investigations. Pharmaceutics 2020, 12, 1171. [Google Scholar] [CrossRef]
- Esfandyari, S.; Elkafas, H.; Chugh, R.M.; Park, H.-S.; Navarro, A.; Al-Hendy, A. Exosomes as Biomarkers for Female Reproductive Diseases Diagnosis and Therapy. Int. J. Mol. Sci. 2021, 22, 2165. [Google Scholar] [CrossRef]
- Gilabert-Estelles, J.; Braza-Boils, A.; Ramon, L.A.; Zorio, E.; Medina, P.; Espana, F.; Estelles, A. Role of microRNAs in gynecological pathology. Curr. Med. Chem. 2012, 19, 2406–2413. [Google Scholar] [CrossRef]
- Chen, S.; Sun, K.-X.; Liu, B.-L.; Zong, Z.-H.; Zhao, Y. MicroRNA-505 functions as a tumor suppressor in endometrial cancer by targeting TGF-α. Mol. Cancer 2016, 15, 11. [Google Scholar] [CrossRef] [Green Version]
- Roy, M.; Yang, Y.-P.; Bosquet, O.; Deo, S.K.; Daunert, S. Understanding the Role and Clinical Applications of Exosomes in Gynecologic Malignancies: A Review of the Current Literature. Cancers 2021, 14, 158. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of extracellular vesicles: General methodologies and latest trends. BioMed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Niu, Z.; Pang, R.T.; Liu, W.; Li, Q.; Cheng, R.; Yeung, W.S. Polymer-based precipitation preserves biological activities of extracellular vesicles from an endometrial cell line. PLoS ONE 2017, 12, e0186534. [Google Scholar] [CrossRef] [Green Version]
- Maida, Y.; Takakura, M.; Nishiuchi, T.; Yoshimoto, T.; Kyo, S. Exosomal transfer of functional small RNA s mediates cancer-stroma communication in human endometrium. Cancer Med. 2016, 5, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-T.; Zhou, Z.-Y.; Luo, Y.-L.; Luo, Q.; Chen, S.-B.; Zhao, J.-C.; Chen, Q.-R. Exosomal lncRNA NEAT1 from cancer-associated fibroblasts facilitates endometrial cancer progression via miR-26a/b-5p-mediated STAT3/YKL-40 signaling pathway. Neoplasia 2021, 23, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Li, B.L.; Lu, W.; Qu, J.J.; Ye, L.; Du, G.Q.; Wan, X.P. Loss of exosomal miR-148b from cancer-associated fibroblasts promotes endometrial cancer cell invasion and cancer metastasis. J. Cell. Physiol. 2019, 234, 2943–2953. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Y.; Liu, H.; Shen, W. Extracellular vesicle encapsulated microRNA-320a inhibits endometrial cancer by suppression of the HIF1α/VEGFA axis. Exp. Cell Res. 2020, 394, 112113. [Google Scholar] [CrossRef]
- Berg, A.; Fasmer, K.E.; Mauland, K.K.; Ytre-Hauge, S.; Hoivik, E.A.; Husby, J.A.; Tangen, I.L.; Trovik, J.; Halle, M.K.; Woie, K. Tissue and imaging biomarkers for hypoxia predict poor outcome in endometrial cancer. Oncotarget 2016, 7, 69844. [Google Scholar] [CrossRef]
- Dziobek, K.; Oplawski, M.; Grabarek, B.O.; Zmarzły, N.; Tomala, B.; Halski, T.; Leśniak, E.; Januszyk, K.; Brus, R.; Kiełbasiński, R. Changes in the expression profile of VEGF-A, VEGF-B, VEGFR-1, VEGFR-2 in different grades of endometrial cancer. Curr. Pharm. Biotechnol. 2019, 20, 955. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, A.; Oda, K.; Ikeda, Y.; Sone, K.; Fukuda, T.; Inaba, K.; Makii, C.; Enomoto, A.; Hosoya, N.; Tanikawa, M. PI3K/mTOR pathway inhibition overcomes radioresistance via suppression of the HIF1-α/VEGF pathway in endometrial cancer. Gynecol. Oncol. 2015, 138, 174–180. [Google Scholar] [CrossRef]
- Shi, S.; Tan, Q.; Feng, F.; Huang, H.; Liang, J.; Cao, D.; Wang, Z. Identification of core genes in the progression of endometrial cancer and cancer cell-derived exosomes by an integrative analysis. Sci. Rep. 2020, 10, 9862. [Google Scholar] [CrossRef]
- Gu, S.; Ni, T.; Wang, J.; Liu, Y.; Fan, Q.; Wang, Y.; Huang, T.; Chu, Y.; Sun, X.; Wang, Y. CD47 blockade inhibits tumor progression through promoting phagocytosis of tumor cells by M2 polarized macrophages in endometrial cancer. J. Immunol. Res. 2018, 2018, 6156757. [Google Scholar] [CrossRef]
- Cassetta, L.; Fragkogianni, S.; Sims, A.H.; Swierczak, A.; Forrester, L.M.; Zhang, H.; Soong, D.Y.; Cotechini, T.; Anur, P.; Lin, E.Y. Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell 2019, 35, 588–602.e10. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; He, Y.; Peng, F.; Yang, J.; Yuan, C. Endometrial cancer cells promote M2-like macrophage polarization by delivering exosomal miRNA-21 under hypoxia condition. J. Immunol. Res. 2020, 2020, 9731049. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Torres, K.; Paszkowski, T.; Radej, S.; Staśkiewicz, G.J.; Ceccaroni, M.; Pesci, A.; Maciejewski, R. Highly increased maspin expression corresponds with up-regulation of miR-21 in endometrial cancer: A preliminary report. Int. J. Gynecol. Cancer 2011, 21, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Eismann, J.; Hirschfeld, M.; Erbes, T.; Rücker, G.; Jäger, M.; Ritter, A.; Weiss, D.; Gitsch, G.; Mayer, S. Hypoxia-and acidosis-driven aberrations of secreted microRNAs in endometrial cancer in vitro. Oncol. Rep. 2017, 38, 993–1004. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Yan, L.; Zhao, X.; Li, C.; Fu, Y. microRNA-21 overexpression contributes to cell proliferation by targeting PTEN in endometrioid endometrial cancer. Oncol. Lett. 2012, 4, 1290–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.; Geng, P.; Li, Y.; Wei, X.; Cheng, J. MicroRNA-21 promotes endometrial carcinoma proliferation and invasion by targeting PTEN. Int. J. Clin. Exp. Pathol. 2017, 10, 11489. [Google Scholar] [PubMed]
- Doberstein, K.; Bretz, N.P.; Schirmer, U.; Fiegl, H.; Blaheta, R.; Breunig, C.; Müller-Holzner, E.; Reimer, D.; Zeimet, A.G.; Altevogt, P. miR-21-3p is a positive regulator of L1CAM in several human carcinomas. Cancer Lett. 2014, 354, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Shi, Y.; Dong, M.; Jiang, L.; Yang, J.; Liu, Z. Exosomal transfer of tumor-associated macrophage-derived hsa_circ_0001610 reduces radiosensitivity in endometrial cancer. Cell Death Dis. 2021, 12, 818. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, H.; Li, Y.; Su, R. MiR-192-5p-Modified Tumor-Associated Macrophages-Derived Exosome Suppressed Endometrial Cancer Progression Through Targeting IRAK1/NF-κB Signaling. Reprod. Sci. 2022, 29, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Xie, B.; Zhang, L.; Li, C.; Shan, W.; Yang, B.; Luo, X.; Gu, C.; He, Q.; Jin, H. Infiltrating macrophages induce ERα expression through an IL17A-mediated epigenetic mechanism to sensitize endometrial cancer cells to estrogen. Cancer Res. 2016, 76, 1354–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.-J.; Zhang, J.; Xie, F.; Wu, J.-N.; Ye, J.-F.; Wang, J.; Wu, K.; Li, M.-Q. CD45RO-CD8+ T cell-derived exosomes restrict estrogen-driven endometrial cancer development via the ERβ/miR-765/PLP2/Notch axis. Theranostics 2021, 11, 5330. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Guo, S.; Zhang, D.; Tian, X.; Xie, X. Exosomal-lncRNA DLEU1 accelerates the proliferation, migration, and invasion of endometrial carcinoma cells by regulating microRNA-E2F3. OncoTargets Ther. 2020, 13, 8651. [Google Scholar] [CrossRef]
- Che, X.; Jian, F.; Chen, C.; Liu, C.; Liu, G.; Feng, W. PCOS serum-derived exosomal miR-27a-5p stimulates endometrial cancer cells migration and invasion. J. Mol. Endocrinol. 2020, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, M.; Tong, H.; Tan, Y.; Hu, X.; Wang, K.; Wan, X. Plasma exosomes from endometrial cancer patients contain LGALS3BP to promote endometrial cancer progression. Oncogene 2021, 40, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Herrero, C.; de la Fuente, A.; Casas-Arozamena, C.; Sebastian, V.; Prieto, M.; Arruebo, M.; Abalo, A.; Colás, E.; Moreno-Bueno, G.; Gil-Moreno, A. Extracellular vesicles-based biomarkers represent a promising liquid biopsy in endometrial cancer. Cancers 2019, 11, 2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Wang, W.; Wang, F.; Yang, S.; Hu, J.; Lu, B.; Pan, Z.; Ma, Y.; Zheng, M.; Zhou, L.; et al. Plasma-derived exosomal miR-15a-5p as a promising diagnostic biomarker for early detection of endometrial carcinoma. Mol. Cancer 2021, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Cao, M.; Liu, C.; Zhang, C.; Li, C.; Cheng, W.; Zhang, S.; Zhang, H.; Zhu, W. Three plasma-based microRNAs as potent diagnostic biomarkers for endometrial cancer. Cancer Biomark. 2021, 31, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Yang, J.; Wang, Y.; Liu, X. Exosomal miRNA-93 and miRNA-205 expression in endometrial cancer. J. King Saud Univ.-Sci. 2020, 32, 1111–1115. [Google Scholar] [CrossRef]
- Xu, H.; Gong, Z.; Shen, Y.; Fang, Y.; Zhong, S. Circular RNA expression in extracellular vesicles isolated from serum of patients with endometrial cancer. Epigenomics 2018, 10, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Roman-Canal, B.; Moiola, C.P.; Gatius, S.; Bonnin, S.; Ruiz-Miró, M.; González, E.; González-Tallada, X.; Llordella, I.; Hernández, I.; Porcel, J.M. EV-associated miRNAs from peritoneal lavage are a source of biomarkers in endometrial cancer. Cancers 2019, 11, 839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiroki, E.; Akahira, J.I.; Suzuki, F.; Nagase, S.; Ito, K.; Suzuki, T.; Sasano, H.; Yaegashi, N. Changes in microRNA expression levels correlate with clinicopathological features and prognoses in endometrial serous adenocarcinomas. Cancer Sci. 2010, 101, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Korpal, M.; Kang, Y. The emerging role of miR-200 family of microRNAs in epithelial-mesenchymal transition and cancer metastasis. RNA Biol. 2008, 5, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.; Moxley, K.; Ruskin, R.; Dhanasekaran, D.N.; Zhao, Y.D.; Ramesh, R. A non-invasive liquid biopsy screening of urine-derived exosomes for miRNAs as biomarkers in endometrial cancer patients. AAPS J. 2018, 20, 82. [Google Scholar] [CrossRef]
- Hur, K.; Toiyama, Y.; Takahashi, M.; Balaguer, F.; Nagasaka, T.; Koike, J.; Hemmi, H.; Koi, M.; Boland, C.R.; Goel, A. MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut 2013, 62, 1315–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Záveský, L.; Jandáková, E.; Turyna, R.; Langmeierová, L.; Weinberger, V.; Drábková, L.Z.; Hůlková, M.; Hořínek, A.; Dušková, D.; Feyereisl, J. Evaluation of cell-free urine microRNAs expression for the use in diagnosis of ovarian and endometrial cancers. A pilot study. Pathol. Oncol. Res. 2015, 21, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Zavesky, L.; Jandakova, E.; Turyna, R.; Langmeierova, L.; Weinberger, V.; Minar, L. Supernatant versus exosomal urinary microRNAs. Two fractions with different outcomes in gynaecological cancers. Neoplasma 2016, 63, 121–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Garcia, E.; Lesur, A.; Devis, L.; Campos, A.; Cabrera, S.; van Oostrum, J.; Matias-Guiu, X.; Gil-Moreno, A.; Reventos, J.; Colas, E. Development of a sequential workflow based on LC-PRM for the verification of endometrial cancer protein biomarkers in uterine aspirate samples. Oncotarget 2016, 7, 53102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziechciowski, M.; Zapala, B.; Skotniczny, K.; Gawlik, K.; Pawlica-Gosiewska, D.; Piwowar, M.; Balajewicz-Nowak, M.; Basta, P.; Solnica, B.; Pitynski, K. Diagnostic and prognostic relevance of microparticles in peripheral and uterine blood of patients with endometrial cancer. Ginekol. Pol. 2018, 89, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, X.; Li, Y.; Yan, P.; Zhang, H. Human umbilical cord blood mesenchymal stem cells-derived exosomal microRNA-503-3p inhibits progression of human endometrial cancer cells through downregulating MEST. Cancer Gene Ther. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Hua, X.; Du Yuanna, Z.R.; Junjun, M. Exosomal miR-499a-5p Inhibits Endometrial Cancer Growth and Metastasis via Targeting VAV3. Cancer Manag. Res. 2020, 12, 13541. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, K.; Ai, H. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles inhibit endometrial cancer cell proliferation and migration through delivery of exogenous miR-302a. Stem Cells Int. 2019, 2019, 8108576. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sykaras, A.G.; Christofidis, K.; Politi, E.; Theocharis, S. Exosomes on Endometrial Cancer: A Biomarkers Treasure Trove? Cancers 2022, 14, 1733. https://doi.org/10.3390/cancers14071733
Sykaras AG, Christofidis K, Politi E, Theocharis S. Exosomes on Endometrial Cancer: A Biomarkers Treasure Trove? Cancers. 2022; 14(7):1733. https://doi.org/10.3390/cancers14071733
Chicago/Turabian StyleSykaras, Alexandros G., Konstantinos Christofidis, Ekaterini Politi, and Stamatios Theocharis. 2022. "Exosomes on Endometrial Cancer: A Biomarkers Treasure Trove?" Cancers 14, no. 7: 1733. https://doi.org/10.3390/cancers14071733
APA StyleSykaras, A. G., Christofidis, K., Politi, E., & Theocharis, S. (2022). Exosomes on Endometrial Cancer: A Biomarkers Treasure Trove? Cancers, 14(7), 1733. https://doi.org/10.3390/cancers14071733







