Interspatial Distribution of Tumor and Immune Cells in Correlation with PD-L1 in Molecular Subtypes of Gastric Cancers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; Van Grieken, N.C.T.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Akyala, A.I.; Verhaar, A.P.; Peppelenbosch, M.P. Immune checkpoint inhibition in gastric cancer: A systematic review. J. Cell. Immunother. 2018, 4, 49–55. [Google Scholar] [CrossRef]
- Chenard-Poirier, M.; Smyth, E.C. Immune Checkpoint Inhibitors in the Treatment of Gastroesophageal Cancer. Drugs 2019, 79, 1–10. [Google Scholar] [CrossRef]
- Smyth, E.C.; Wotherspoon, A.; Peckitt, C.; Gonzalez, D.; Hulkki-Wilson, S.; Eltahir, Z.; Fassan, M.; Rugge, M.; Valeri, N.; Okines, A.; et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol. 2017, 3, 1197–1203. [Google Scholar] [CrossRef] [Green Version]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, F.; Zhou, N.; Gu, Y.M.; Zhang, Y.T.; He, Y.D.; Wang, L.; Yang, L.X.; Zhao, Y.; Li, Y.M. Efficacy and safety of immune checkpoint inhibitors in advanced gastric or gastroesophageal junction cancer: A systematic review and meta-analysis. Oncoimmunology 2019, 8, e1581547. [Google Scholar] [CrossRef] [Green Version]
- Davis, A.A.; Patel, V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef]
- Kono, K.; Nakajima, S.; Mimura, K. Current status of immune checkpoint inhibitors for gastric cancer. Gastric Cancer 2020, 23, 565–578. [Google Scholar] [CrossRef]
- Kim, S.T.; Cristescu, R.; Bass, A.J.; Kim, K.M.; Odegaard, J.I.; Kim, K.; Liu, X.Q.; Sher, X.; Jung, H.; Lee, M.; et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 2018, 24, 1449–1458. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.P.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wei, X.L.; Wang, F.H.; Xu, N.; Shen, L.; Dai, G.H.; Yuan, X.L.; Chen, Y.; Yang, S.J.; Shi, J.H.; et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann. Oncol. 2019, 30, 1479–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.; Chang, Y.H.; Heo, Y.J.; Kim, S.; Kim, N.K.; Park, J.O.; Kang, W.K.; Lee, J.; Kim, K.M. Four distinct immune microenvironment subtypes in gastric adenocarcinoma with special reference to microsatellite instability. ESMO Open 2018, 3, e000326. [Google Scholar] [CrossRef] [Green Version]
- Dislich, B.; Stein, A.; Seiler, C.A.; Kroll, D.; Berezowska, S.; Zlobec, I.; Galvan, J.; Slotta-Huspenina, J.; Walch, A.; Langer, R. Expression patterns of programmed death-ligand 1 in esophageal adenocarcinomas: Comparison between primary tumors and metastases. Cancer Immunol. Immunother. 2017, 66, 777–786. [Google Scholar] [CrossRef]
- Teng, M.W.; Ngiow, S.F.; Ribas, A.; Smyth, M.J. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015, 75, 2139–2145. [Google Scholar] [CrossRef] [Green Version]
- Dislich, B.; Blaser, N.; Berger, M.D.; Gloor, B.; Langer, R. Preservation of Epstein-Barr virus status and mismatch repair protein status along the metastatic course of gastric cancer. Histopathology 2020, 76, 740–747. [Google Scholar] [CrossRef]
- Zlobec, I.; Koelzer, V.H.; Dawson, H.; Perren, A.; Lugli, A. Next-generation tissue microarray (ngTMA) increases the quality of biomarker studies: An example using CD3, CD8, and CD45RO in the tumor microenvironment of six different solid tumor types. J. Transl. Med. 2013, 11, 104. [Google Scholar] [CrossRef] [Green Version]
- Xie, T.; Zhang, Z.; Zhang, X.; Qi, C.; Shen, L.; Peng, Z. Appropriate PD-L1 Cutoff Value for Gastric Cancer Immunotherapy: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 646355. [Google Scholar] [CrossRef]
- Valentini, A.M.; Di Pinto, F.; Coletta, S.; Guerra, V.; Armentano, R.; Caruso, M.L. Tumor microenvironment immune types in gastric cancer are associated with mismatch repair however, not HER2 status. Oncol. Lett. 2019, 18, 1775–1785. [Google Scholar] [CrossRef]
- Derks, S.; Liao, X.; Chiaravalli, A.M.; Xu, X.; Camargo, M.C.; Solcia, E.; Sessa, F.; Fleitas, T.; Freeman, G.J.; Rodig, S.J.; et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget 2016, 7, 32925–32932. [Google Scholar] [CrossRef] [PubMed]
- Gullo, I.; Oliveira, P.; Athelogou, M.; Goncalves, G.; Pinto, M.L.; Carvalho, J.; Valente, A.; Pinheiro, H.; Andrade, S.; Almeida, G.M.; et al. New insights into the inflamed tumor immune microenvironment of gastric cancer with lymphoid stroma: From morphology and digital analysis to gene expression. Gastric Cancer 2019, 22, 77–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böger, C.; Behrens, H.-M.; Mathiak, M.; Krüger, S.; Kalthoff, H.; Röcken, C. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget 2016, 7, 24269–24283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Choi, M.G.; Kim, K.; Kim, K.M.; Kim, S.T.; Park, S.H.; Cristescu, R.; Peter, S.; Lee, J. High PD-L1 expression in gastric cancer (GC) patients and correlation with molecular features. Pathol. Res. Pract. 2020, 216, 152881. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, M.; An, M.; Klempner, S.J.; Lee, H.; Kim, K.M.; Sa, J.K.; Cho, H.J.; Hong, J.Y.; Lee, T.; Min, Y.W.; et al. Determinants of Response and Intrinsic Resistance to PD-1 Blockade in Microsatellite Instability-High Gastric Cancer. Cancer Discov. 2021, 11, 2168–2185. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.D.; Zahurak, M.; Murphy, A.; Cornish, T.; Cuka, N.; Abdelfatah, E.; Yang, S.; Duncan, M.; Ahuja, N.; Taube, J.M.; et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut 2017, 66, 794–801. [Google Scholar] [CrossRef]
- Xing, X.; Guo, J.; Ding, G.; Li, B.; Dong, B.; Feng, Q.; Li, S.; Zhang, J.; Ying, X.; Cheng, X.; et al. Analysis of PD1, PDL1, PDL2 expression and T cells infiltration in 1014 gastric cancer patients. Oncoimmunology 2018, 7, e1356144. [Google Scholar] [CrossRef] [Green Version]
- Rudolf, J.; Buttner-Herold, M.; Erlenbach-Wunsch, K.; Posselt, R.; Jessberger, J.; Haderlein, M.; Hecht, M.; Hartmann, A.; Fietkau, R.; Distel, L. Regulatory T cells and cytotoxic T cells close to the epithelial-stromal interface are associated with a favorable prognosis. Oncoimmunology 2020, 9, 1746149. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Huang, Y.K.; Kong, J.C.; Sun, Y.; Tantalo, D.G.; Yeang, H.X.A.; Ying, L.; Yan, F.; Xu, D.; Halse, H.; et al. High-dimensional analyses reveal a distinct role of T-cell subsets in the immune microenvironment of gastric cancer. Clin. Transl. Immunol. 2020, 9, e1127. [Google Scholar] [CrossRef]
- Kim, K.-J.; Yang, H.K.; Kim, W.H.; Kang, G.H. Combined prognostic effect of PD-L1 expression and immunoscore in microsatellite-unstable advanced gastric cancers. Oncotarget 2017, 8, 58887. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, J.; Hao, Y.; Nie, Y.; Li, Z.; Qian, M.; Liang, Q.; Yu, J.; Zeng, M.; Wu, K. Differentiated tumor immune microenvironment of Epstein-Barr virus-associated and negative gastric cancer: Implication in prognosis and immunotherapy. Oncotarget 2017, 8, 67094–67103. [Google Scholar] [CrossRef] [PubMed]
- Ock, C.Y.; Keam, B.; Kim, S.; Lee, J.S.; Kim, M.; Kim, T.M.; Jeon, Y.K.; Kim, D.W.; Chung, D.H.; Heo, D.S. Pan-Cancer Immunogenomic Perspective on the Tumor Microenvironment Based on PD-L1 and CD8 T-Cell Infiltration. Clin. Cancer Res. 2016, 22, 2261–2270. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.; Kim, K.M. PD-L1 expression in gastric cancer: Interchangeability of 22C3 and 28-8 pharmDx assays for responses to immunotherapy. Mod. Pathol. 2021, 34, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Koh, J.; Na, H.Y.; Kwak, Y.; Lee, K.W.; Ahn, S.H.; Park, D.J.; Kim, H.H.; Lee, H.S. PD-L1 Testing in Gastric Cancer by the Combined Positive Score of the 22C3 PharmDx and SP263 Assay with Clinically Relevant Cut-offs. Cancer Res. Treat. 2020, 52, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van De Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [Green Version]
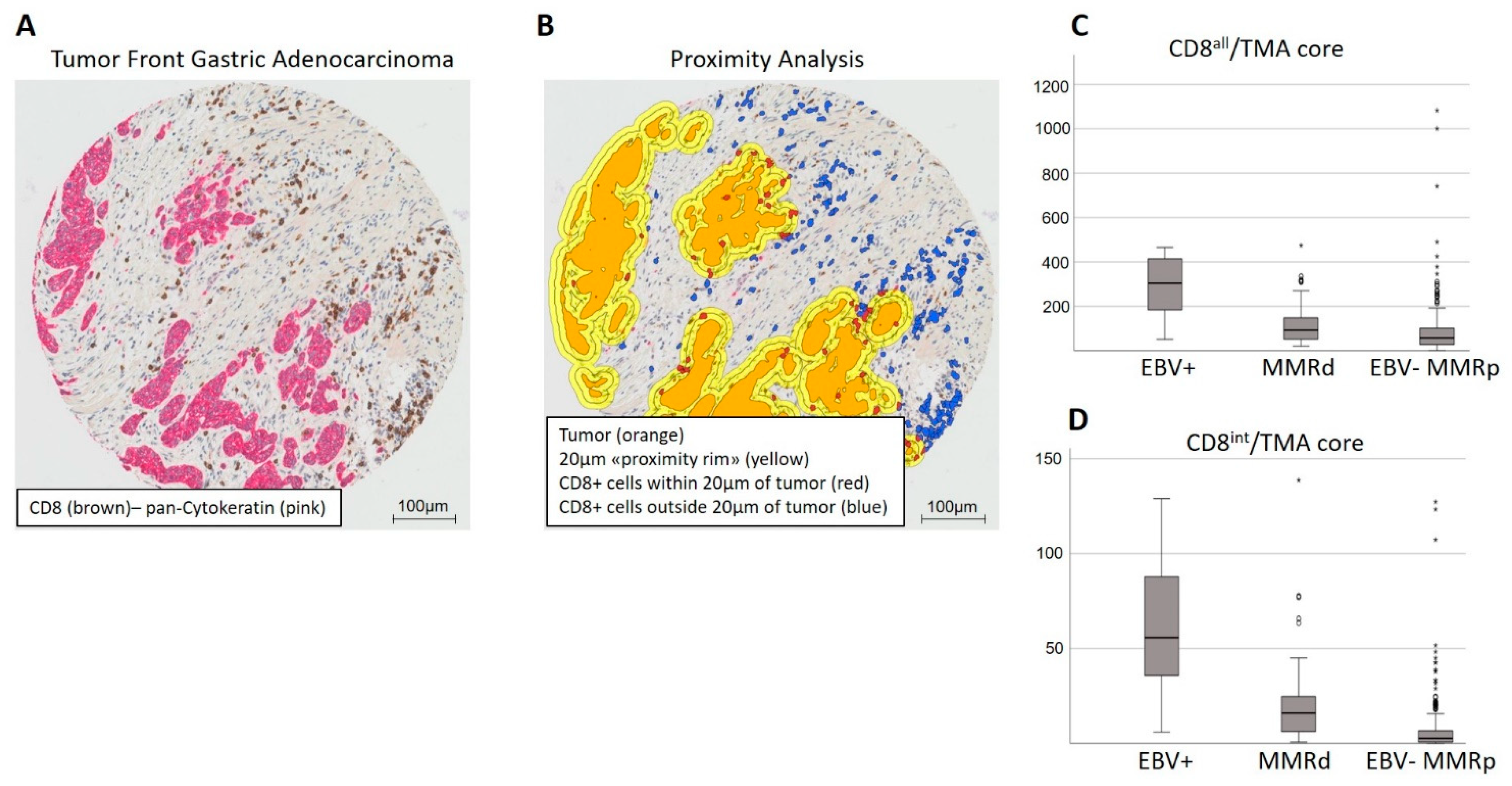
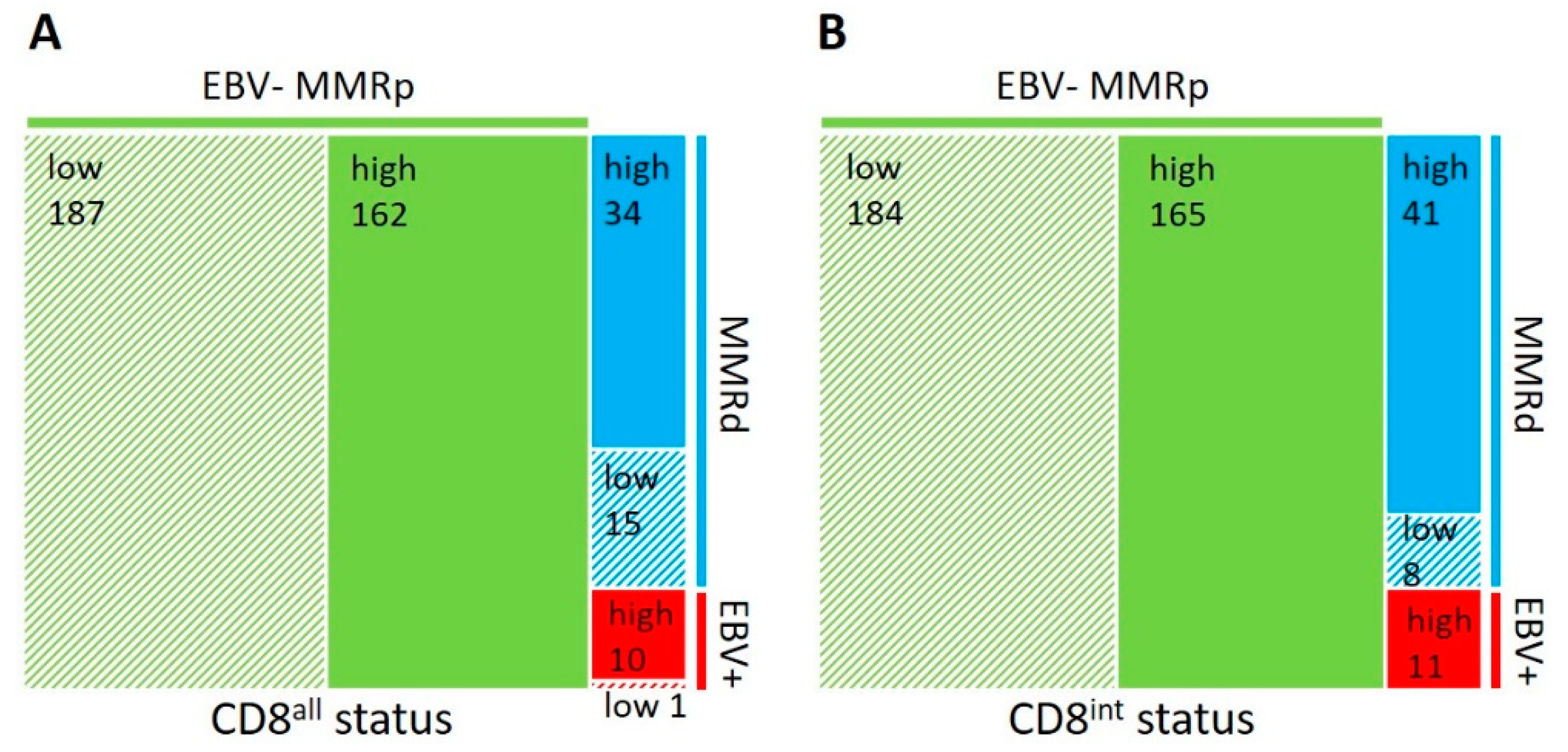

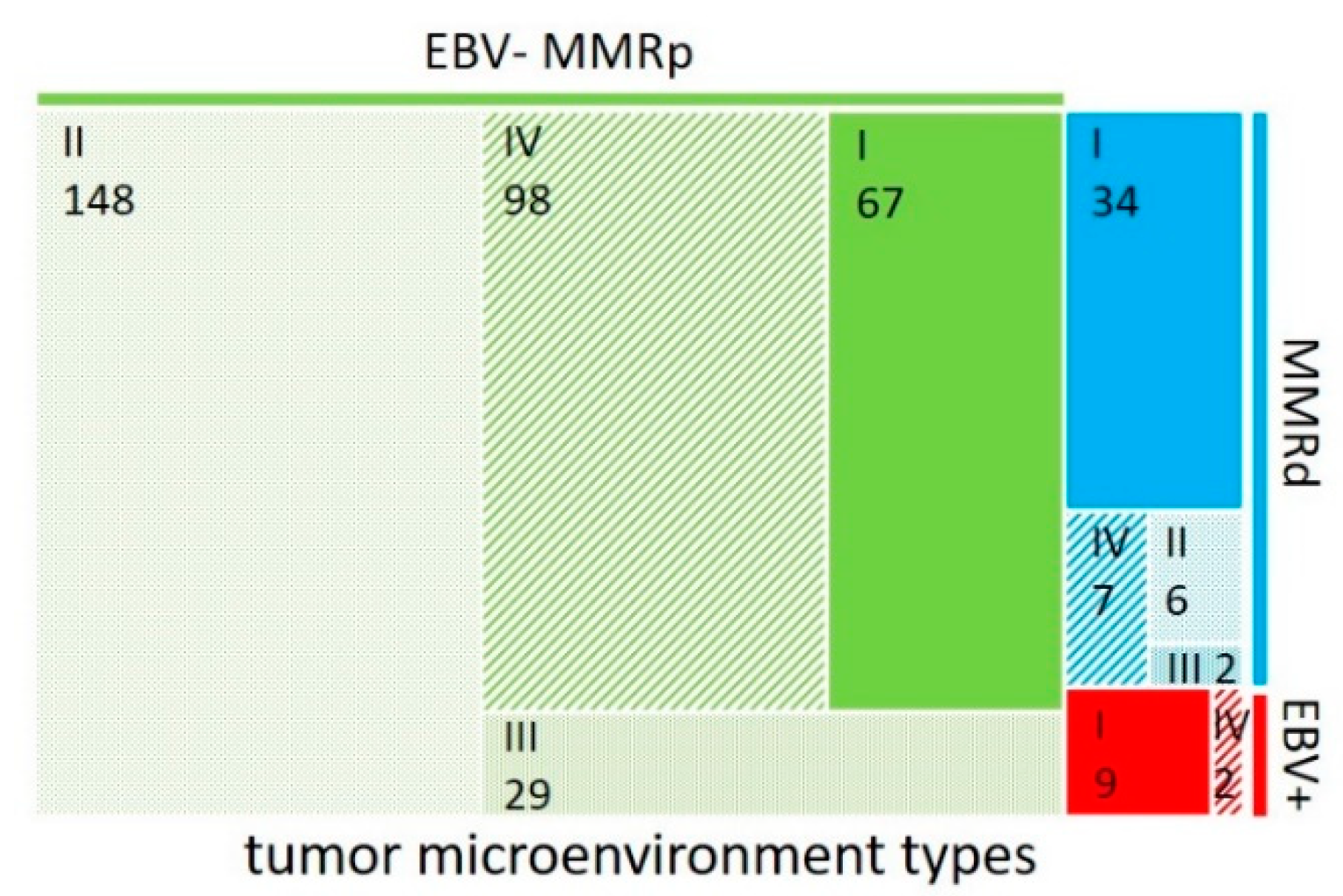
| Factors | n | CD8all High | CD8all Low | p-Value | CD8int High | CD8int Low | p-Value | n | CPS ≥ 1 | CPS < 1 | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pT | 0.033 | 0.001 | 0.015 | ||||||||
| T1 | 45 (11%) | 20 (44.4%) | 25 (55.5%) | 29 (64.4%) | 16 (35.6%) | 48 (11.7%) | 16 (33.3%) | 32 (66.7%) | |||
| T2 | 54 (13.3%) | 36 (66.7%) | 18 (33.3%) | 37 (68.5%) | 17 (31.5%) | 55 (13.5%) | 28 (50.9%) | 27 (49.1%) | |||
| T3 | 146 (35.9%) | 80 (54.8%) | 66 (45.2%) | 87 (59.6%) | 59 (40.4%) | 144 (35.3%) | 54 (37.5%) | 90 (62.5%) | |||
| T4 | 162 (39.8%) | 71 (43.8%) | 91 (56.2%) | 65 (40.1%) | 97 (59.9%) | 161 (39.5%) | 44 (27.3%) | 117 (72.7%) | |||
| pN | 0.37 | 0.045 | 0.112 | ||||||||
| N0 | 112 (27.5%) | 61 (54.5%) | 51 (45.5%) | 69 (61.6%) | 43 (38.4%) | 112 (27.8%) | 46 (41.1%) | 66 (58.9%) | |||
| N1-3 | 295 (72.5%) | 146 (49.5%) | 149 (50.5%) | 149 (50.5%) | 146 (49.5%) | 291 (72.2%) | 95 (32.6%) | 196 (67.4%) | |||
| Grading | 0.278 | 0.107 | 0.041 | ||||||||
| G1 | 21 (5.2%) | 8 (38.1%) | 13 (61.9%) | 14 (66.6%) | 7 (33.3%) | 23 (5.6%) | 8 (34.8%) | 15 (65.2%) | |||
| G2 | 96 (23.6%) | 45 (46.9%) | 51 (53.1%) | 58 (60.4%) | 38 (39.6%) | 97 (23.8%) | 44 (45.4%) | 53 (54.6%) | |||
| G3 | 290 (71.2%) | 154 (53.1%) | 136 (46.9%) | 146 (50.3%) | 144 (49.7%) | 288 (70.6%) | 90 (31.3%) | 198 (68.7%) | |||
| Laurén | 0.933 | 0.022 | <0.001 | ||||||||
| Intestinal | 207 (50.9%) | 102 (49.3%) | 105 (50.7%) | 125 (60.4%) | 82 (39.6%) | 211 (51.7%) | 101 (47.9%) | 110 (52.1%) | |||
| Diffuse | 131 (32.2%) | 69 (52.7%) | 62 (47.3%) | 58 (44.3%) | 73 (55.7%) | 127 (31.1%) | 22 (17.3%) | 105 (82.7%) | |||
| Mixed | 65 (16%) | 34 (52.3%) | 31 (47.7%) | 32 (49.2%) | 33 (50.8%) | 66 (16.2%) | 16 (24.2%) | 50 (75.8%) | |||
| Indeterm. | 4 (0.9%) | 2 (50%) | 2 (50%) | 3 (75%) | 1 (25%) | 4 (1%) | 3 (75%) | 1 (25%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dislich, B.; Mertz, K.D.; Gloor, B.; Langer, R. Interspatial Distribution of Tumor and Immune Cells in Correlation with PD-L1 in Molecular Subtypes of Gastric Cancers. Cancers 2022, 14, 1736. https://doi.org/10.3390/cancers14071736
Dislich B, Mertz KD, Gloor B, Langer R. Interspatial Distribution of Tumor and Immune Cells in Correlation with PD-L1 in Molecular Subtypes of Gastric Cancers. Cancers. 2022; 14(7):1736. https://doi.org/10.3390/cancers14071736
Chicago/Turabian StyleDislich, Bastian, Kirsten D. Mertz, Beat Gloor, and Rupert Langer. 2022. "Interspatial Distribution of Tumor and Immune Cells in Correlation with PD-L1 in Molecular Subtypes of Gastric Cancers" Cancers 14, no. 7: 1736. https://doi.org/10.3390/cancers14071736
APA StyleDislich, B., Mertz, K. D., Gloor, B., & Langer, R. (2022). Interspatial Distribution of Tumor and Immune Cells in Correlation with PD-L1 in Molecular Subtypes of Gastric Cancers. Cancers, 14(7), 1736. https://doi.org/10.3390/cancers14071736







