Health-Related Quality of Life in Metastatic Colorectal Cancer Patients Treated with Curative Resection and/or Local Ablative Therapy or Systemic Therapy in the Finnish RAXO-Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Curative and Non-Curative Treatment Phases
2.3. Finnish Reference Population for 15D and EQ-5D-3L
2.4. HRQoL Instruments
2.5. Statistical Analysis
3. Results
3.1. Study Population and Questionnaires
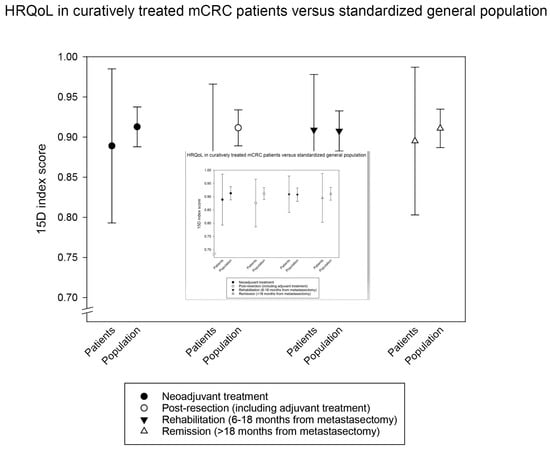
3.2. Multi-Cross-Sectional HRQoL Index Measures in Curative and Non-Curative Treatment Phases
3.3. Longitudinal HRQoL Index Measures in Curative and Non-Curative Treatment Phases
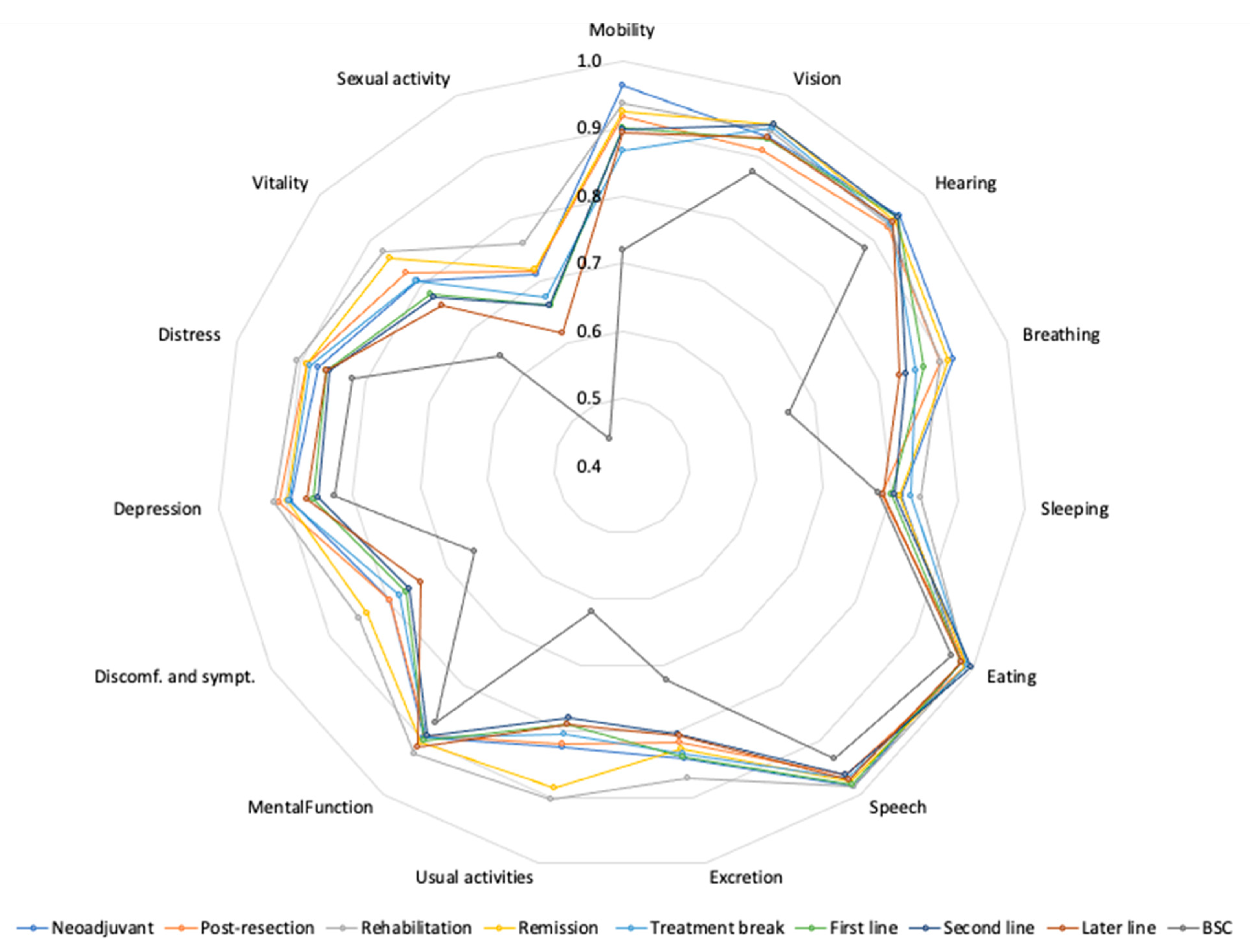
3.4. 15D Profile Measures
3.5. QLQ-C30 and QLQ-CR29 Functioning Scales
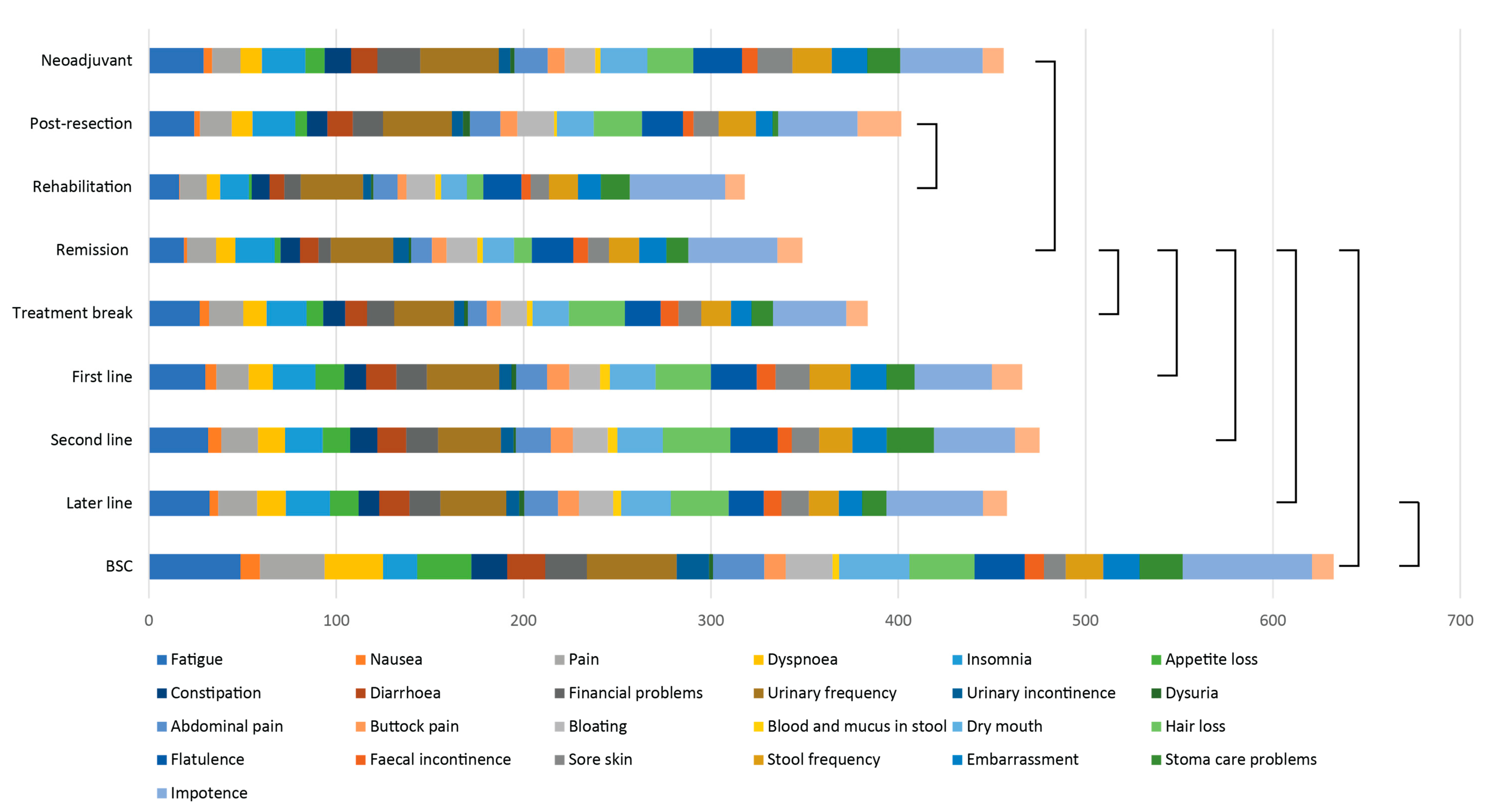
3.6. QLQ-C30 and QLQ-CR29 Symptom Scales
3.7. First 15D Index Score and QLQ-C30 Physical Functioning as Prognostic Markers
3.8. Association of Remission Phase Index Scores with Demographic Factors, Metastatic Sites and Metastasectomies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Osterlund, P.; Salminen, T.; Soveri, L.-M.; Kallio, R.; Kellokumpu, I.; Lamminm, A.; Halonen, A.; Ristam, R.; Lantto, E.; Uutela, A.; et al. Repeated centralized multidisciplinary team assessment of resectability, clinical behavior, and outcomes in 1086 Finnish metastatic colorectal cancer patients (RAXO): A nationwide prospective intervention study. Lancet Reg. Health Eur. 2021, 3, 100049. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Langenhoff, B.S.; Krabbe, P.F.; Wobbes, T.; Ruers, T.J. Quality of life as an outcome measure in surgical oncology. Br. J. Surg. 2001, 88, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Griffin, A.; Blazeby, J.; Conroy, T.; Efficace, F. Health-related quality of life as a valid outcome in the treatment of advanced colorectal cancer. Eur. J. Surg. Oncol. 2007, 33 (Suppl. 2), S95–S104. [Google Scholar] [CrossRef] [PubMed]
- Bonnetain, F.; Borg, C.; Adams, R.R.; Ajani, J.A.; Benson, A.; Bleiberg, H.; Chibaudel, B.; Diaz-Rubio, E.; Douillard, J.Y.; Fuchs, C.S.; et al. How health-related quality of life assessment should be used in advanced colorectal cancer clinical trials. Ann. Oncol. 2017, 28, 2077–2085. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, P.; Marandino, L.; De Luca, E.; Zichi, C.; Reale, M.L.; Pignataro, D.; Di Stefano, R.F.; Ghisoni, E.; Mariniello, A.; Trevisi, E.; et al. Quality of life assessment and reporting in colorectal cancer: A systematic review of phase III trials published between 2012 and 2018. Crit. Rev. Oncol. Hematol. 2020, 146, 102877. [Google Scholar] [CrossRef]
- Wiering, B.; Oyen, W.J.G.; Adang, E.M.M.; van der Sijp, J.R.M.; Roumen, R.M.; de Jong, K.P.; Ruers, T.J.M.; Krabbe, P.F.M. Long-term global quality of life in patients treated for colorectal liver metastases. Br. J. Surg. 2011, 98, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Langenhoff, B.S.; Krabbe, P.F.M.; Peerenboom, L.; Wobbes, T.; Ruers, T.J.M. Quality of life after surgical treatment of colorectal liver metastases. Br. J. Surg. 2006, 93, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.R.; Blazeby, J.M.; Brookes, S.T.; John, T.; Welsh, F.K.; Rees, M. Patient-reported outcomes in long-term survivors of metastatic colorectal cancer needing liver resection. Br. J. Surg. 2014, 101, 1468–1474. [Google Scholar] [CrossRef]
- Studer, P.; Horn, T.; Haynes, A.; Candinas, D.; Banz, V.M. Quality of life after hepatic resection. Br. J. Surg. 2018, 105, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Cashin, P.; Mahteme, H.; Syk, I.; Frödin, J.; Glimelius, B.; Graf, W. Quality of life and cost effectiveness in a randomized trial of patients with colorectal cancer and peritoneal metastases. Eur. J. Surg. Oncol. 2018, 44, 983–990. [Google Scholar] [CrossRef]
- Wei, A.C.; Coburn, N.G.; Devitt, K.S.; Serrano, P.E.; Moulton, C.-A.; Cleary, S.P.; Law, C.; Moore, M.J.; Gallinger, S. Survival Following Resection of Intra- and Extra-Hepatic Metastases from Colorectal Cancer: A Phase II Trial. Ann. Surg. Oncol. 2016, 23, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- Sintonen, H. The 15D instrument of health-related quality of life: Properties and applications. Ann. Med. 2001, 33, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Dolan, P. Modeling valuations for EuroQol health states. Med. Care 1997, 35, 1095–1108. [Google Scholar] [CrossRef]
- Pickard, A.S.; Neary, M.P.; Cella, D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual. Life Outcomes 2007, 5, 70. [Google Scholar] [CrossRef] [Green Version]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. JNCI J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Gujral, S.; Conroy, T.; Fleissner, C.; Sezer, O.; King, P.M.; Avery, K.N.L.; Sylvester, P.; Koller, M.; Sprangers, M.A.G.; Blazeby, J.M. Assessing quality of life in patients with colorectal cancer: An update of the EORTC quality of life questionnaire. Eur. J. Cancer 2007, 43, 1564–1573. [Google Scholar] [CrossRef]
- Whistance, R.N.; Conroy, T.; Chie, W.; Costantini, A.; Sezer, O.; Koller, M.; Johnson, C.D.; Pilkington, S.A.; Arraras, J.; Ben-Josef, E.; et al. Clinical and psychometric validation of the EORTC QLQ-CR29 questionnaire module to assess health-related quality of life in patients with colorectal cancer. Eur. J. Cancer 2009, 45, 3017–3026. [Google Scholar] [CrossRef] [PubMed]
- Hinz, A.; Mehnert, A.; Dégi, C.; Reissmann, D.R.; Schotte, D.; Schulte, T. The relationship between global and specific components of quality of life, assessed with the EORTC QLQ-C30 in a sample of 2019 cancer patients. Eur. J. Cancer Care 2017, 26, e12416. [Google Scholar] [CrossRef]
- Lindqvist Bagge, A.-S.; Carlander, A.; Fahlke, C.; Olofsson Bagge, R. Health-related quality of life (FACT-GP) in Sweden. Health Qual. Life Outcomes 2020, 18, 172. [Google Scholar] [CrossRef]
- Koskinen, S.; Lundqvist, A.; Ristiluoma, N. Health, Functional Capacity and Welfare in Finland in 2011; National Institute for Health and Welfare (THL): Helsinki, Finland, 2012. [Google Scholar]
- Aromaa, A.; Koskinen, S. Health and functional capacity in Finland. Baseline results of the Health 2000 Health Examination Survey. Publ. Natl. Public Health Inst. 2004, 2004, 171. [Google Scholar]
- Brooks, R. EuroQol: The current state of play. Health Policy 1996, 37, 53–72. [Google Scholar] [CrossRef]
- Alanne, S.; Roine, R.P.; Räsänen, P.; Vainiola, T.; Sintonen, H. Estimating the minimum important change in the 15D scores. Qual. Life Res. 2015, 24, 599–606. [Google Scholar] [CrossRef]
- EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Fayers, P.; Aaronson, N.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A. The EORTC QLQ-C30 Scoring Manual, 3rd ed.; European Organisation for Research and Treatment of Cancer: Brussels, Belgium, 2001. [Google Scholar]
- Cocks, K.; King, M.T.; Velikova, G.; Martyn St-James, M.; Fayers, P.M.; Brown, J.M. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J. Clin. Oncol. 2011, 29, 89–96. [Google Scholar] [CrossRef]
- Färkkilä, N.; Sintonen, H.; Saarto, T.; Järvinen, H.; Hänninen, J.; Taari, K.; Roine, R.P. Health-related quality of life in colorectal cancer. Color. Dis. 2013, 15, e215–e222. [Google Scholar] [CrossRef] [PubMed]
- Pate, A.; Lowery, J.; Kilbourn, K.; Blatchford, P.J.; McNulty, M.; Risendal, B. Quality of life and the negative impact of comorbidities in long-term colorectal cancer survivors: A population-based comparison. J. Cancer Surviv. 2020, 14, 653–659. [Google Scholar] [CrossRef]
- Husson, O.; de Rooij, B.H.; Kieffer, J.; Oerlemans, S.; Mols, F.; Aaronson, N.K.; van der Graaf, W.T.A.; van de Poll-Franse, L.V. The EORTC QLQ-C30 Summary Score as Prognostic Factor for Survival of Patients with Cancer in the “Real-World”: Results from the Population-Based PROFILES Registry. Oncologist 2020, 25, e722. [Google Scholar] [CrossRef] [Green Version]
- Ratjen, I.; Schafmayer, C.; Enderle, J.; di Giuseppe, R.; Waniek, S.; Koch, M.; Burmeister, G.; Nöthlings, U.; Hampe, J.; Schlesinger, S.; et al. Health-related quality of life in long-term survivors of colorectal cancer and its association with all-cause mortality: A German cohort study. BMC Cancer 2018, 18, 1156. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Berry, K.; Moinpour, C.; Giedzinska, A.; Andersen, M.R. Quality of life in long term survivors of colorectal cancer. Am. J. Gastroenterol. 2002, 97, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Hamidou, Z.; Chibaudel, B.; Hebbar, M.; Hug de Larauze, M.; André, T.; Louvet, C.; Brusquant, D.; Garcia-Larnicol, M.-L.; de Gramont, A.; Bonnetain, F. Time to Definitive Health-Related Quality of Life Score Deterioration in Patients with Resectable Metastatic Colorectal Cancer Treated with FOLFOX4 versus Sequential Dose-Dense FOLFOX7 followed by FOLFIRI: The MIROX Randomized Phase III Trial. PLoS ONE 2016, 11, e0157067. [Google Scholar] [CrossRef]
- Láng, I.; Köhne, C.-H.; Folprecht, G.; Rougier, P.; Curran, D.; Hitre, E.; Sartorius, U.; Griebsch, I.; Van Cutsem, E. Quality of life analysis in patients with KRAS wild-type metastatic colorectal cancer treated first-line with cetuximab plus irinotecan, fluorouracil and leucovorin. Eur. J. Cancer 2013, 49, 439–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, L.; Zhao, Z.; Barber, B.; Zhou, X.; Peeters, M.; Zhang, J.; Xu, F.; Wiezorek, J.; Douillard, J.-Y. Health-related quality of life in patients with metastatic colorectal cancer treated with panitumumab in first- or second-line treatment. Br. J. Cancer 2011, 105, 1495–1502. [Google Scholar] [CrossRef]
- Quidde, J.; Hegewisch-Becker, S.; Graeven, U.; Lerchenmüller, C.A.; Killing, B.; Depenbusch, R.; Steffens, C.-C.; Lange, T.; Dietrich, G.; Stoehlmacher, J.; et al. Quality of life assessment in patients with metastatic colorectal cancer receiving maintenance therapy after first-line induction treatment: A preplanned analysis of the phase III AIO KRK 0207 trial. Ann. Oncol. 2016, 27, 2203–2210. [Google Scholar] [CrossRef]
- Bertaut, A.; Touchefeu, Y.; Blanc, J.; Bouché, O.; François, E.; Conroy, T.; Artru, P.; Adenis, A.; Gobbo, J.; Borg, C.; et al. Health-Related Quality of Life Analysis in Metastatic Colorectal Cancer Patients Treated by Second-Line Chemotherapy, Associated With Either Cetuximab or Bevacizumab: The PRODIGE 18 Randomized Phase II Study. Clin. Colorectal Cancer, 2021; in press. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Komatsu, Y.; Yamada, Y.; Yamazaki, K.; Tsuji, A.; Ura, T.; Grothey, A.; Van Cutsem, E.; Wagner, A.; Cihon, F.; et al. Randomized phase III trial of regorafenib in metastatic colorectal cancer: Analysis of the CORRECT Japanese and non-Japanese subpopulations. Invest. New Drugs 2015, 33, 740–750. [Google Scholar] [CrossRef] [Green Version]
- Schuurhuizen, C.S.E.W.; Braamse, A.M.J.; Konings, I.R.H.M.; Sprangers, M.A.G.; Ket, J.C.F.; Dekker, J.; Verheul, H.M.W. Does severe toxicity affect global quality of life in patients with metastatic colorectal cancer during palliative systemic treatment? A systematic review. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 478–486. [Google Scholar] [CrossRef]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet. Oncol. 2013, 14, 1208–1215. [Google Scholar] [CrossRef]
- Meijerink, M.R.; Puijk, R.S.; van Tilborg, A.A.J.M.; Henningsen, K.H.; Fernandez, L.G.; Neyt, M.; Heymans, J.; Frankema, J.S.; de Jong, K.P.; Richel, D.J.; et al. Radiofrequency and Microwave Ablation Compared to Systemic Chemotherapy and to Partial Hepatectomy in the Treatment of Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cardiovasc. Intervent. Radiol. 2018, 41, 1189–1204. [Google Scholar] [CrossRef] [Green Version]
- Filippiadis, D.K.; Velonakis, G.; Kelekis, A.; Sofocleous, C.T. The Role of Percutaneous Ablation in the Management of Colorectal Cancer Liver Metastatic Disease. Diagnostics 2021, 11, 308. [Google Scholar] [CrossRef] [PubMed]
- Fonck, M.; Perez, J.-T.; Catena, V.; Becouarn, Y.; Cany, L.; Brudieux, E.; Vayre, L.; Texereau, P.; Le Brun-Ly, V.; Verger, V.; et al. Pulmonary Thermal Ablation Enables Long Chemotherapy-Free Survival in Metastatic Colorectal Cancer Patients. Cardiovasc. Intervent. Radiol. 2018, 41, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Kurilova, I.; Gonzalez-Aguirre, A.; Beets-Tan, R.G.; Erinjeri, J.; Petre, E.N.; Gonen, M.; Bains, M.; Kemeny, N.E.; Solomon, S.B.; Sofocleous, C.T. Microwave Ablation in the Management of Colorectal Cancer Pulmonary Metastases. Cardiovasc. Intervent. Radiol. 2018, 41, 1530–1544. [Google Scholar] [CrossRef] [PubMed]
- Kurilova, I.; Bendet, A.; Petre, E.; Boas, F.; Kaye, E.; Gonen, M.; Covey, A.; Brody, L.A.; Brown, K.T.; Kemeny, N.E.; et al. Factors Associated With Local Tumor Control and Complications After Thermal Ablation of Colorectal Cancer Liver Metastases: A 15-year Retrospective Cohort Study. Clin. Colorectal Cancer 2021, 20, e82–e95. [Google Scholar] [CrossRef]
- Osterlund, E.; Ristimäki, A.; Kytölä, S.; Kuopio, T.; Heervä, E.; Muhonen, T.; Halonen, P.; Kallio, R.; Soveri, L.-M.; Sundström, J.; et al. KRAS-G12C Mutation in One Real-Life and Three Population-Based Nordic Cohorts of Metastatic Colorectal Cancer. Front. Oncol. 2022, 12, 274. [Google Scholar] [CrossRef]
- Ruers, T.; Van Coevorden, F.; Punt, C.J.A.; Pierie, J.-P.E.N.; Borel-Rinkes, I.; Ledermann, J.A.; Poston, G.; Bechstein, W.; Lentz, M.-A.; Mauer, M.; et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J. Natl. Cancer Inst. 2017, 109, djx015. [Google Scholar] [CrossRef]
- Ruers, T.J.M.; Joosten, J.J.; Wiering, B.; Langenhoff, B.S.; Dekker, H.M.; Wobbes, T.; Oyen, W.J.G.; Krabbe, P.F.M.; Punt, C.J.A. Comparison between local ablative therapy and chemotherapy for non-resectable colorectal liver metastases: A prospective study. Ann. Surg. Oncol. 2007, 14, 1161–1169. [Google Scholar] [CrossRef]
- Färkkilä, N.; Torvinen, S.; Roine, R.P.; Sintonen, H.; Hänninen, J.; Taari, K.; Saarto, T. Health-related quality of life among breast, prostate, and colorectal cancer patients with end-stage disease. Qual. Life Res. 2014, 23, 1387–1394. [Google Scholar] [CrossRef]
- Flyum, I.R.; Mahic, S.; Grov, E.K.; Joranger, P. Health-related quality of life in patients with colorectal cancer in the palliative phase: A systematic review and meta-analysis. BMC Palliat. Care 2021, 20, 144. [Google Scholar] [CrossRef] [PubMed]
- Magaji, B.A.; Moy, F.M.; Law, C.W.; Sii, H.L.; Roslani, A.C. Pattern of Health-Related Quality of Life and its Association among Patients with Colorectal Cancer. Asian Pacific J. Cancer Care 2019, 4, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Schover, L.R.; van der Kaaij, M.; van Dorst, E.; Creutzberg, C.; Huyghe, E.; Kiserud, C.E. Sexual dysfunction and infertility as late effects of cancer treatment. EJC Suppl. 2014, 12, 41–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadovsky, R.; Basson, R.; Krychman, M.; Morales, A.M.; Schover, L.; Wang, R.; Incrocci, L. Cancer and Sexual Problems. J. Sex. Med. 2010, 7, 349–373. [Google Scholar] [CrossRef] [PubMed]
- Simard, S.; Thewes, B.; Humphris, G.; Dixon, M.; Hayden, C.; Mireskandari, S.; Ozakinci, G. Fear of cancer recurrence in adult cancer survivors: A systematic review of quantitative studies. J. Cancer Surviv. 2013, 7, 300–322. [Google Scholar] [CrossRef] [PubMed]
- Mullens, A.B.; McCaul, K.D.; Erickson, S.C.; Sandgren, A.K. Coping after cancer: Risk perceptions, worry, and health behaviors among colorectal cancer survivors. Psychooncology 2004, 13, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, J.; Chen, M.; Gong, J.; Xu, Y.; Li, Q. A literature review of post-treatment survivorship interventions for colorectal cancer survivors and/or their caregivers. Psychooncology 2021, 30, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Maisey, N.; Norman, A.; Watson, M.; Allen, M.; Hill, M.; Cunningham, D. Baseline quality of life predicts survival in patients with advanced colorectal cancer. Eur. J. Cancer 2002, 38, 1351–1357. [Google Scholar] [CrossRef]
- Quinten, C.; Martinelli, F.; Coens, C.; Sprangers, M.A.G.; Ringash, J.; Gotay, C.; Bjordal, K.; Greimel, E.; Reeve, B.B.; Maringwa, J.; et al. A global analysis of multitrial data investigating quality of life and symptoms as prognostic factors for survival in different tumor sites. Cancer 2014, 120, 302–311. [Google Scholar] [CrossRef]
- Park, S.; Eo, W.; Lee, S. The Relationship Between Health-Related Quality of Life and Survival in Metastatic Colorectal Cancer Patients Treated With Korean Medicine. Integr. Cancer Ther. 2018, 17, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osterlund, P.; Salminen, T.; Algars, A.; Soveri, L.-M.; Ristam€ Aki, R.; Kallio, R.S.; Lamminm€ Aki, A.; Halonen, P.M.; Poussa, T.; Lantto, E.; et al. Patient characteristics associated with poor performance status, ECOG 2-3, and effect on survival in 1086 Finnish metastatic colorectal cancers (mCRC) nationwide (prospective RAXO study). Ann. Oncol. 2019, 30, 245. [Google Scholar] [CrossRef]
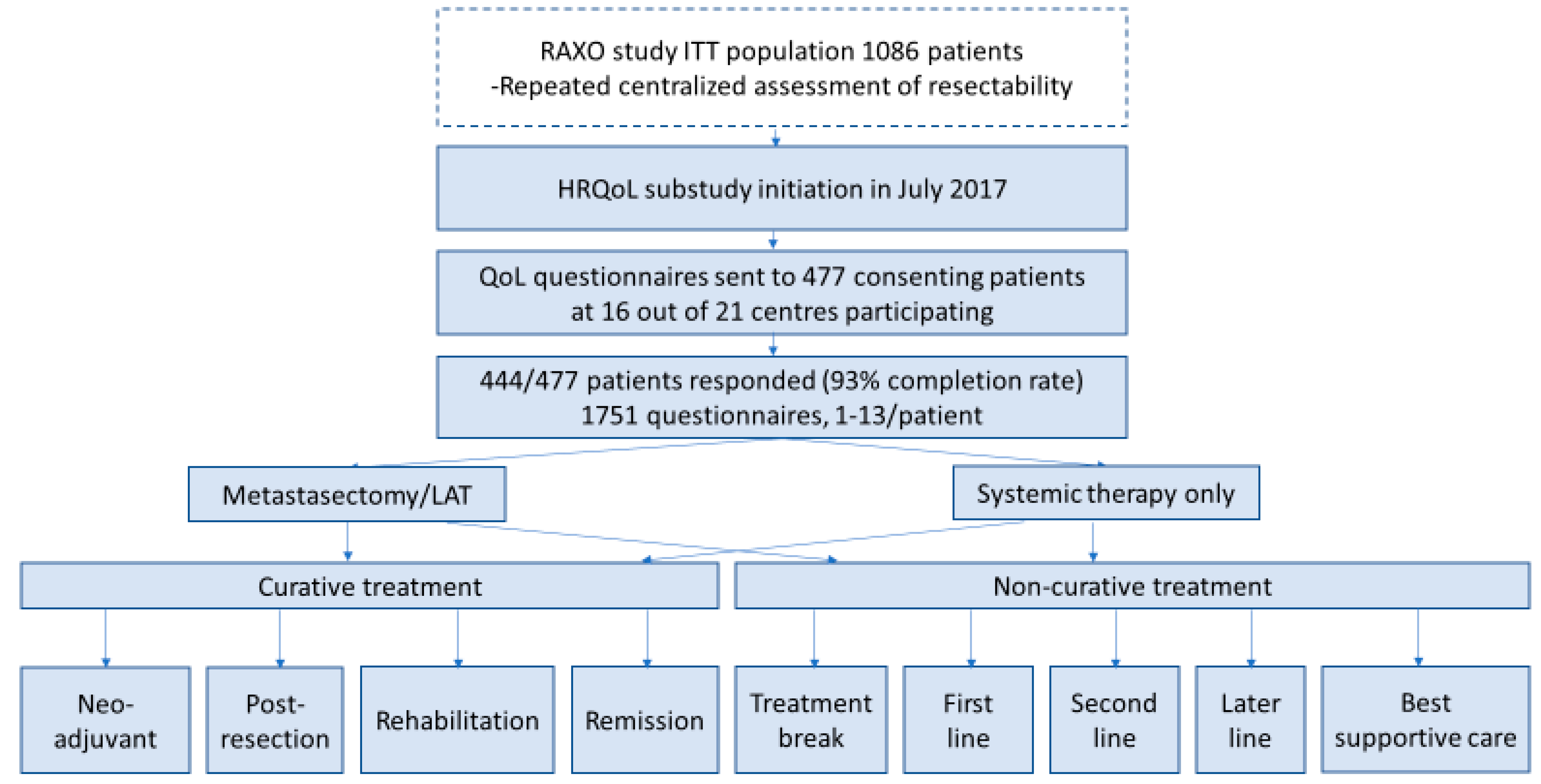

| All Patients | Curative Metastasectomy/LAT | Systemic Therapy Only | |||||
|---|---|---|---|---|---|---|---|
| n = 444 | n = 247 | n = 197 | |||||
| Age | Median (range) | 64.7 | (24.3–87.8) | 64.3 | (25.0–81.5) | 66.2 | (24.3–87.8) |
| ≤70 | 322 | 73% | 189 | 77% * | 133 | 68% | |
| >70 | 122 | 28% | 58 | 24% | 64 | 33% * | |
| Sex | Male | 258 | 58% | 142 | 58% | 116 | 59% |
| Female | 186 | 42% | 105 | 43% | 81 | 41% | |
| ECOG | PS 0 | 172 | 39% | 115 | 47% * | 57 | 29% |
| PS 1 | 235 | 53% | 120 | 49% | 115 | 58% * | |
| PS 2–3 | 37 | 8% | 12 | 5% | 25 | 13% * | |
| Charlson comorbidity index | 0 | 360 | 81% | 204 | 83% | 156 | 79% |
| 1 to 2 | 82 | 19% | 42 | 17% | 40 | 20% | |
| 3 to 5 | 2 | 1% | 1 | 0% | 1 | 1% | |
| Smoking | No | 284 | 86% | 159 | 87% | 125 | 83% |
| Yes | 48 | 15% | 23 | 13% | 25 | 17% | |
| BMI | ≥20 | 412 | 93% | 227 | 92% | 185 | 94% |
| <20 | 32 | 7% | 20 | 8% | 12 | 6% | |
| Presentation § | Synchronous | 298 | 67% | 155 | 63% | 143 | 73% * |
| Early metachronous | 38 | 9% | 21 | 9% | 17 | 9% | |
| Late metachronous | 108 | 24% | 71 | 29% * | 37 | 19% | |
| Primary tumour | Right colon | 109 | 25% | 50 | 20% | 59 | 30% * |
| Left colon | 181 | 41% | 116 | 47% * | 65 | 33% | |
| Rectum | 153 | 35% | 81 | 33% | 72 | 37% | |
| Multiple | 1 | 0% | 0 | 0% | 1 | 1% | |
| Surgery primary tumour | Surgery upfront | 314 | 71% | 196 | 79% * | 118 | 60% |
| No or later surgery | 130 | 29% | 51 | 21% | 79 | 40% * | |
| Prior adjuvant therapy | No adjuvant | 338 | 76% | 177 | 72% | 161 | 82% * |
| Fluoropyrimidine | 45 | 10% | 27 | 11% | 18 | 9% | |
| Oxaliplatin-containing | 61 | 14% | 43 | 17% * | 18 | 9% | |
| Radiotherapy for rectal cancer | No | 380 | 86% | 208 | 84% | 172 | 87% |
| Preop 5x5Gy | 27 | 6% | 22 | 9% * | 5 | 3% | |
| Chemoradiation | 30 | 7% | 13 | 5% | 17 | 9% | |
| Palliative | 7 | 2% | 4 | 2% | 3 | 2% | |
| Metastatic sites | 1 | 284 | 64% | 195 | 79% * | 89 | 45% |
| 2 | 113 | 26% | 43 | 17% | 70 | 36% * | |
| 3 to 6 | 47 | 11% | 9 | 4% | 38 | 19% * | |
| Liver | 326 | 73% | 201 | 81% * | 125 | 64% | |
| Lung | 118 | 27% | 46 | 19% | 72 | 37% * | |
| Peritoneal | 55 | 13% | 21 | 9% | 34 | 19% * | |
| Distant lymph nodes | 95 | 21% | 17 | 7% | 78 | 40% * | |
| Other | 53 | 12% | 25 | 10% | 28 | 14% | |
| Molecular status | RAS/BRAF wild-type | 153 | 38% | 94 | 43% * | 59 | 32% |
| KRAS/NRAS mutant | 222 | 55% | 120 | 54% | 102 | 56% | |
| BRAF mutant of tested | 29 | 10% | 7 | 4% | 22 | 15% * | |
| Treatment Phase | Patients | 15D | EQ-5D | VAS | GHS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | 95% CI | Mean | SD | 95% CI | Mean | SD | 95% CI | Mean | SD | 95% CI | |
| Curative | |||||||||||||
| Neoadjuvant | 56 | 0.889 | 0.096 | 0.864–0.915 | 0.834 | 0.204 | 0.776–0.893 | 67.8 | 16.2 | 63.1–72.5 | 68.3 | 20.2 | 62.5–74.1 |
| Post-resection | 58 | 0.876 | 0.090 | 0.853–0.900 | 0.848 | 0.148 | 0.808–0.888 | 75.0 | 14.5 | 71.0–78.9 | 75.6 | 17.9 | 70.8–80.5 |
| Rehabilitation | 61 | 0.909 | 0.069 | 0.891–0.927 | 0.872 | 0.125 | 0.839–0.905 | 80.0 | 12.1 | 76.8–83.3 | 78.8 | 15.1 | 74.8–82.8 |
| Remission | 132 | 0.895 | 0.092 | 0.879–0.911 | 0.874 | 0.139 | 0.849–0.898 | 77.9 | 15.0 | 75.2–80.5 | 75.5 | 18.8 | 72.2–78.8 |
| Non-curative | |||||||||||||
| Treatment break | 87 | 0.869 | 0.091 | 0.849–0.888 | 0.830 | 0.170 | 0.791–0.869 | 73.9 | 15.1 | 70.4–77.4 | 71.9 | 19.3 | 67.5–76.3 |
| First line | 169 | 0.860 | 0.090 | 0.850–0.880 | 0.810 | 0.160 | 0.780–0.840 | 69.2 | 16.0 | 66.4–72.0 | 66.8 | 17.3 | 63.8–69.9 |
| Second line | 115 | 0.857 | 0.088 | 0.841–0.874 | 0.812 | 0.168 | 0.779–0.845 | 71.1 | 15.6 | 68.0–74.2 | 67.6 | 18.1 | 64.1–71.2 |
| Later line | 106 | 0.853 | 0.095 | 0.834–0.871 | 0.790 | 0.159 | 0.759–0.820 | 68.4 | 16.5 | 65.2–71.6 | 67.1 | 17.8 | 63.6–70.5 |
| BSC | 35 | 0.763 | 0.123 | 0.720–0.805 | 0.653 | 0.279 | 0.555–0.750 | 56.8 | 19.2 | 49.9–63.8 | 54.2 | 20.7 | 47.0–61.4 |
| 15D | EQ-5D | VAS | GHS | Symptom Burden | Functioning Scale Sum | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ∆ | p Value | ∆ | p Value | ∆ | p Value | ∆ | p Value | ∆ | p Value | ∆ | p Value | |
| Curative vs. Curative | ||||||||||||
| Remission vs. Neoadjuvant | 0.005 | 0.683 | 0.039 | 0.537 | 10.07 | <0.001 | 7.14 | 0.019 | −86 | 0.002 | 23 | 0.071 |
| Remission vs. Post-resection | 0.018 | 0.115 | 0.025 | 0.263 | 2.9 | 0.106 | −0.16 | 0.693 | −92 | 0.052 | 16 | 0.331 |
| Remission vs. Rehabilitation | −0.014 | 0.666 | 0.002 | 0.771 | −2.17 | 0.670 | −3.3 | 0.550 | 29 | 0.649 | −5 | 0.486 |
| Rehabilitation vs. Post-resection | 0.033 | 0.063 | 0.024 | 0.367 | 5.07 | 0.06 | 3.15 | 0.373 | −121 | 0.029 | 21 | 0.177 |
| Curative vs. Non-curative | ||||||||||||
| Remission vs. Treatment break | 0.026 | 0.015 | 0.044 | 0.096 | 3.99 | 0.046 | 3.55 | 0.127 | −52 | 0.040 | 28 | 0.009 |
| Remission vs. First-line | 0.035 | 0.001 | 0.064 | 0.002 | 8.65 | <0.001 | 8.65 | <0.001 | −102 | <0.001 | 38 | <0.001 |
| Remission vs. Second-line | 0.038 | <0.001 | 0.061 | 0.006 | 6.76 | <0.001 | 7.85 | <0.001 | −108 | <0.001 | 40 | <0.001 |
| Remission vs. Later-line | 0.042 | <0.001 | 0.084 | <0.001 | 9.46 | <0.001 | 8.43 | <0.001 | −101 | <0.001 | 35 | <0.001 |
| Remission vs. BSC | 0.132 | <0.001 | 0.221 | <0.001 | 21.02 | <0.001 | 21.27 | <0.001 | −232 | <0.001 | 134 | <0.001 |
| Neoadjuvant vs. First-line | 0.029 | 0.019 | 0.024 | 0.125 | −1.42 | 0.576 | 1.51 | 0.291 | −16 | 0.531 | 15 | 0.079 |
| Non-curative vs. Non-curative | ||||||||||||
| Treatment break vs. First-line | 0.009 | 0.576 | 0.02 | 0.309 | 4.66 | 0.030 | 5.09 | 0.010 | −51 | 0.096 | 10 | 0.139 |
| First line vs. Second-line | 0.003 | 0.420 | −0.002 | 0.959 | −1.89 | 0.339 | −0.79 | 0.824 | −5 | 0.975 | 1 | 0.628 |
| First line vs. Later-line | 0.007 | 0.378 | 0.02 | 0.165 | 11.56 | 0.743 | −0.22 | 0.914 | 1 | 0.718 | −4 | 0.638 |
| Later line vs. BSC | 0.090 | <0.001 | 0.137 | 0.009 | 11.56 | 0.003 | 12.84 | 0.001 | −131 | 0.004 | 99 | <0.001 |
| Neoadjuvant | Post-Resection | Rehabilitation | Remission | Treatment Break | First-Line | Second-Line | Later-Line | BSC | |
|---|---|---|---|---|---|---|---|---|---|
| n = 56 | n = 58 | n = 60 | n = 126 | n = 87 | n = 169 | n = 114 | n = 105 | n = 35 | |
| EQ-5D score | 0.030 | 0.040 | 0.075 * | 0.070 * | 0.034 | 0.018 | 0.010 | −0.013 | −0.107 * |
| 15D score | −0.023 | −0.035 * | 0.002 | −0.014 | −0.039 * | −0.044 * | −0.052 * | −0.056 * | −0.123 * |
| Mobility | 0.034 * | −0.011 | 0.016 | 0.006 | −0.058 * | −0.017 | −0.028 * | −0.031 * | −0.166 * |
| Vision | −0.010 | −0.045 * | −0.012 | −0.001 | −0.008 | −0.019 * | −0.005 | −0.024 | −0.065 |
| Hearing | 0.021 | −0.005 | 0.002 | 0.010 | 0.010 | 0.015 | 0.016 | 0.008 | −0.023 |
| Breathing | −0.018 | −0.035 | −0.032 | −0.022 | −0.071 * | −0.053 * | −0.084 * | −0.094 * | −0.235 * |
| Sleeping | −0.022 | −0.055 | −0.001 | −0.036 * | −0.013 | −0.036 * | −0.033 * | −0.047 * | −0.047 |
| Eating | −0.015 | −0.009 | 0.004 * | −0.001 | −0.006 | −0.017 * | 0.000 | −0.015 * | −0.031 |
| Speech | 0.004 | −0.018 | 0.003 | 0.000 | −0.011 | −0.003 | −0.017 | −0.009 | −0.040 |
| Excretion | −0.045 | −0.065 * | −0.008 | −0.050 * | −0.047 * | −0.045 * | −0.078 * | −0.074 * | −0.133 * |
| Usual activities | −0.075 | −0.093 * | −0.004 | −0.021 | −0.100 * | −0.120 * | −0.130 * | −0.119 * | −0.251 * |
| Mental function | 0.013 | 0.010 | 0.054 * | 0.027 * | 0.020 | 0.021 | 0.009 | 0.036 * | 0.026 |
| Discomf. and sympt. | 0.014 | 0.004 | 0.059 * | 0.045 * | −0.007 | −0.019 | −0.025 | −0.043 * | −0.118 * |
| Depression | −0.054 * | −0.029 | −0.023 | −0.041 * | −0.043 * | −0.079 * | −0.082 * | −0.069 * | −0.105 * |
| Distress | −0.064 * | −0.049 * | −0.036 * | −0.053 * | −0.052 * | −0.076 * | −0.085 * | −0.079 * | −0.115 * |
| Vitality | −0.080 * | −0.061 * | −0.013 | −0.026 * | −0.078 * | −0.106 * | −0.112 * | −0.129 * | −0.219 * |
| Sexual activity | −0.163 * | −0.146 * | −0.098 * | −0.148 * | −0.179 * | −0.205 * | −0.204 * | −0.240 * | −0.371 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehtomäki, K.; Stedt, H.P.; Osterlund, E.; Muhonen, T.; Soveri, L.-M.; Halonen, P.; Salminen, T.K.; Kononen, J.; Kallio, R.; Ålgars, A.; et al. Health-Related Quality of Life in Metastatic Colorectal Cancer Patients Treated with Curative Resection and/or Local Ablative Therapy or Systemic Therapy in the Finnish RAXO-Study. Cancers 2022, 14, 1713. https://doi.org/10.3390/cancers14071713
Lehtomäki K, Stedt HP, Osterlund E, Muhonen T, Soveri L-M, Halonen P, Salminen TK, Kononen J, Kallio R, Ålgars A, et al. Health-Related Quality of Life in Metastatic Colorectal Cancer Patients Treated with Curative Resection and/or Local Ablative Therapy or Systemic Therapy in the Finnish RAXO-Study. Cancers. 2022; 14(7):1713. https://doi.org/10.3390/cancers14071713
Chicago/Turabian StyleLehtomäki, Kaisa, Hanna P. Stedt, Emerik Osterlund, Timo Muhonen, Leena-Maija Soveri, Päivi Halonen, Tapio K. Salminen, Juha Kononen, Raija Kallio, Annika Ålgars, and et al. 2022. "Health-Related Quality of Life in Metastatic Colorectal Cancer Patients Treated with Curative Resection and/or Local Ablative Therapy or Systemic Therapy in the Finnish RAXO-Study" Cancers 14, no. 7: 1713. https://doi.org/10.3390/cancers14071713
APA StyleLehtomäki, K., Stedt, H. P., Osterlund, E., Muhonen, T., Soveri, L.-M., Halonen, P., Salminen, T. K., Kononen, J., Kallio, R., Ålgars, A., Heervä, E., Lamminmäki, A., Uutela, A., Nordin, A., Lehto, J., Saarto, T., Sintonen, H., Kellokumpu-Lehtinen, P.-L., Ristamäki, R., ... Osterlund, P. (2022). Health-Related Quality of Life in Metastatic Colorectal Cancer Patients Treated with Curative Resection and/or Local Ablative Therapy or Systemic Therapy in the Finnish RAXO-Study. Cancers, 14(7), 1713. https://doi.org/10.3390/cancers14071713