HPV8 Reverses the Transcriptional Output in Lrig1 Positive Cells to Drive Skin Tumorigenesis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Global Gene Expression Profiling and Data Analysis of K14-HPV8-CER Murine Skin
2.2. UV Treatment of Murine Skin and RT-qPCR
2.3. IHC Staining
2.4. Dataset from Lrig1-EGFP-Ires-CreERT2 Mice
2.5. Data and Statistical Analysis
2.5.1. Differentially Expressed Genes
2.5.2. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analyses
2.5.3. Protein-Protein Interaction (PPI) Network Analysis
2.5.4. Transcriptional Factor Enrichment Analysis
3. Results
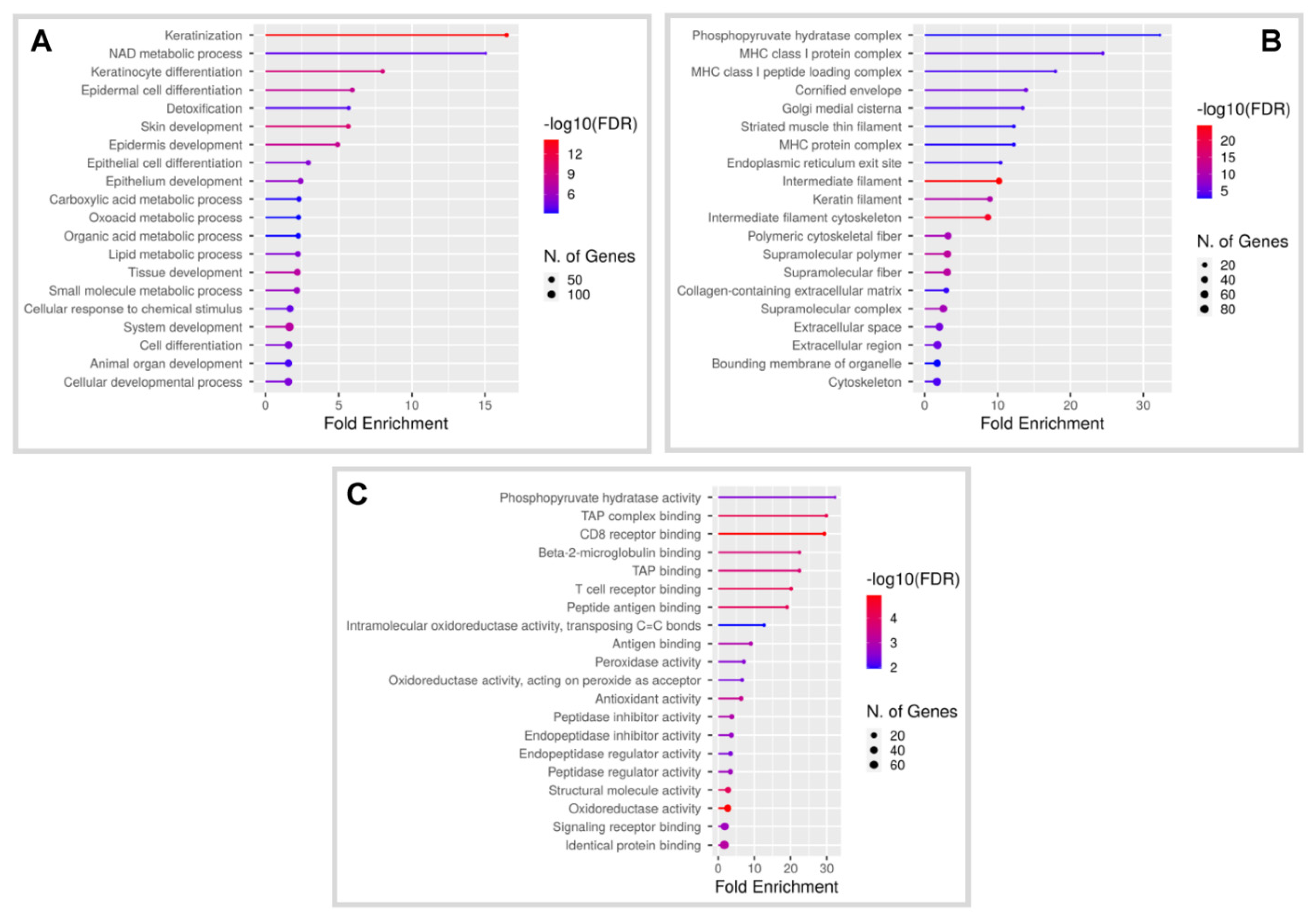
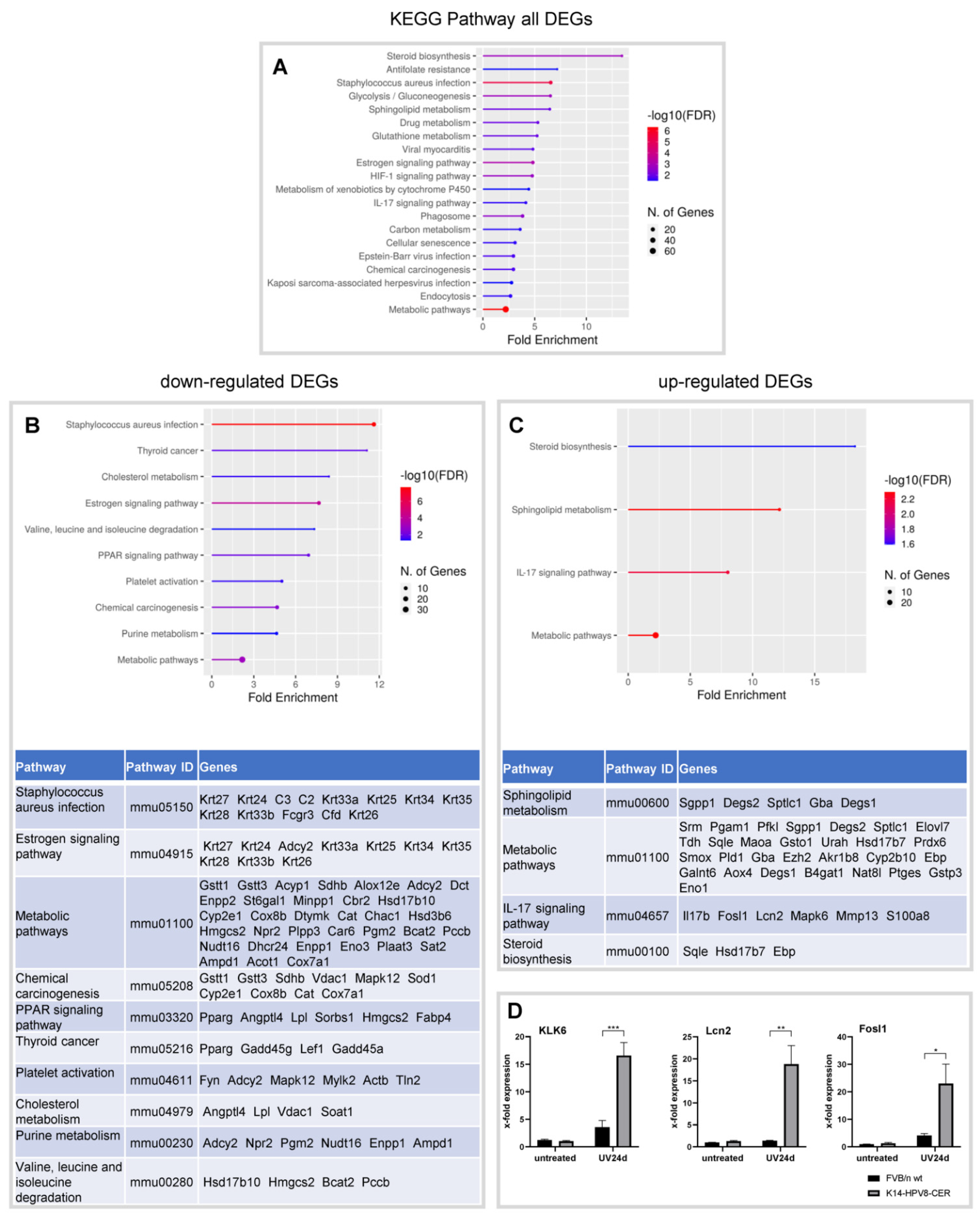
3.1. Characterization of Global Gene Expression in Skin Tumours of K14-HPV8-CER Mice
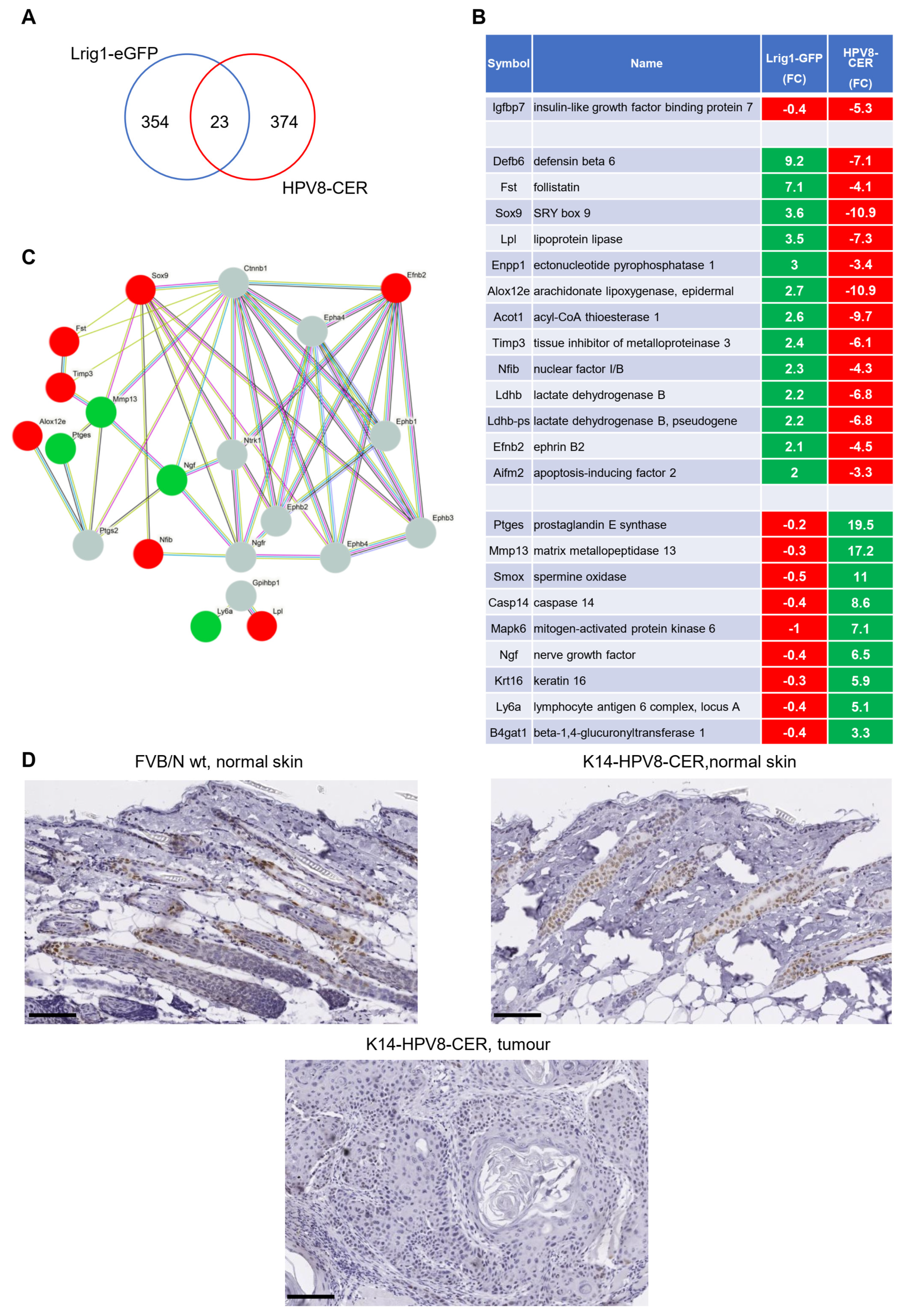
3.2. Identification of a Common Gene Set in Lrig1-EGFP-IRES-CreERT2 and K14-HPV8-CER Mice
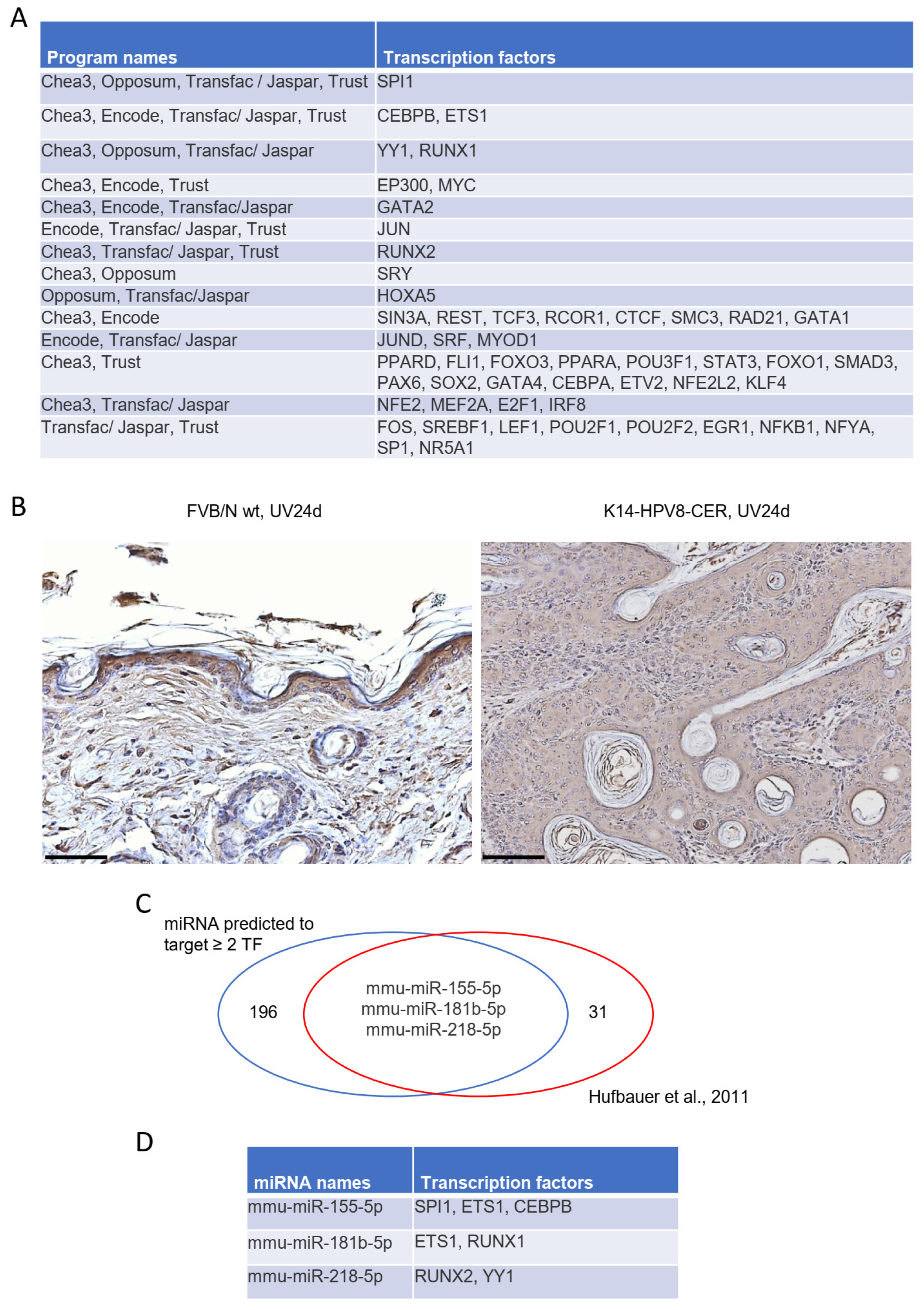
3.3. Identification of Potential Upstream Transcriptional Regulators
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hufbauer, M.; Akgül, B. Molecular Mechanisms of Human Papillomavirus Induced Skin Carcinogenesis. Viruses 2017, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Bandolin, L.; Borsetto, D.; Fussey, J.; Da Mosto, M.C.; Nicolai, P.; Menegaldo, A.; Calabrese, L.; Tommasino, M.; Boscolo-Rizzo, P. Beta human papillomaviruses infection and skin carcinogenesis. Rev. Med. Virol. 2020, 30, e2104. [Google Scholar] [CrossRef] [PubMed]
- Smola, S. Human Papillomaviruses and Skin Cancer. Adv. Exp. Med. Biol. 2020, 1268, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Dell'Oste, V.; Azzimonti, B.; De Andrea, M.; Mondini, M.; Zavattaro, E.; Leigheb, G.; Weissenborn, S.J.; Pfister, H.; Michael, K.M.; Waterboer, T.; et al. High beta-HPV DNA loads and strong seroreactivity are present in epidermodysplasia verruciformis. J. Investig Dermatol. 2009, 129, 1026–1034. [Google Scholar] [CrossRef]
- Bavinck, J.N.B.; Feltkamp, M.C.W.; Green, A.C.; Fiocco, M.; Euvrard, S.; Harwood, C.A.; Nasir, S.; Thomson, J.; Proby, C.M.; Naldi, L.; et al. Human papillomavirus and posttransplantation cutaneous squamous cell carcinoma: A multicenter, prospective cohort study. Am. J. Transplant. 2018, 18, 1220–1230. [Google Scholar] [CrossRef]
- Hasche, D.; Vinzon, S.E.; Rosl, F. Cutaneous Papillomaviruses and Non-melanoma Skin Cancer: Causal Agents or Innocent Bystanders? Front. Microbiol. 2018, 9, 874. [Google Scholar] [CrossRef]
- Howley, P.M.; Pfister, H.J. Beta genus papillomaviruses and skin cancer. Virology 2015, 479–480, 290–296. [Google Scholar] [CrossRef]
- Tommasino, M. The biology of beta human papillomaviruses. Virus Res. 2017, 231, 128–138. [Google Scholar] [CrossRef]
- Ramsay, H.M.; Fryer, A.A.; Hawley, C.M.; Smith, A.G.; Harden, P.N. Non-melanoma skin cancer risk in the Queensland renal transplant population. Br. J. Dermatol. 2002, 147, 950–956. [Google Scholar] [CrossRef]
- Olivero, C.; Lanfredini, S.; Borgogna, C.; Gariglio, M.; Patel, G.K. HPV-Induced Field Cancerisation: Transformation of Adult Tissue Stem Cell into Cancer Stem Cell. Front. Microbiol. 2018, 9, 546. [Google Scholar] [CrossRef]
- Schaper, I.D.; Marcuzzi, G.P.; Weissenborn, S.J.; Kasper, H.U.; Dries, V.; Smyth, N.; Fuchs, P.; Pfister, H. Development of skin tumors in mice transgenic for early genes of human papillomavirus type 8. Cancer Res. 2005, 65, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Akgül, B.; Pfefferle, R.; Marcuzzi, G.P.; Zigrino, P.; Krieg, T.; Pfister, H.; Mauch, C. Expression of matrix metalloproteinase (MMP)-2, MMP-9, MMP-13, and MT1-MMP in skin tumors of human papillomavirus type 8 transgenic mice. Exp. Dermatol. 2006, 15, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Hufbauer, M.; Lazic, D.; Akgül, B.; Brandsma, J.L.; Pfister, H.; Weissenborn, S.J. Enhanced human papillomavirus type 8 oncogene expression levels are crucial for skin tumorigenesis in transgenic mice. Virology 2010, 403, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Lazic, D.; Alborzi, F.; Marcuzzi, G.P.; Angel, P.; Hess, J.; Pfister, H.; Akgül, B. Enhanced StefinA and Sprr2 expression during papilloma formation in HPV8 transgenic mice. J. Dermatol. Sci. 2011, 62, 84–90. [Google Scholar] [CrossRef]
- Hufbauer, M.; Lazic, D.; Reinartz, M.; Akgül, B.; Pfister, H.; Weissenborn, S.J. Skin tumor formation in human papillomavirus 8 transgenic mice is associated with a deregulation of oncogenic miRNAs and their tumor suppressive targets. J. Dermatol. Sci. 2011, 64, 7–15. [Google Scholar] [CrossRef]
- Boxman, I.L.; Berkhout, R.J.; Mulder, L.H.; Wolkers, M.C.; Bavinck, J.N.B.; Vermeer, B.J.; Ter Schegget, J. Detection of human papillomavirus DNA in plucked hairs from renal transplant recipients and healthy volunteers. J. Investig. Dermatol. 1997, 108, 712–715. [Google Scholar] [CrossRef]
- Schmitt, A.; Rochat, A.; Zeltner, R.; Borenstein, L.; Barrandon, Y.; Wettstein, F.O.; Iftner, T. The primary target cells of the high-risk cottontail rabbit papillomavirus colocalize with hair follicle stem cells. J. Virol. 1996, 70, 1912–1922. [Google Scholar] [CrossRef]
- Nafz, J.; Kohler, A.; Ohnesorge, M.; Nindl, I.; Stockfleth, E.; Rosl, F. Persistence of Mastomys natalensis papillomavirus in multiple organs identifies novel targets for infection. J. Gen. Virol. 2007, 88, 2670–2678. [Google Scholar] [CrossRef]
- Egawa, N.; Egawa, K.; Griffin, H.; Doorbar, J. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses 2015, 7, 3863–3890. [Google Scholar] [CrossRef]
- Lanfredini, S.; Olivero, C.; Borgogna, C.; Calati, F.; Powell, K.; Davies, K.J.; De Andrea, M.; Harries, S.; Tang, H.K.C.; Pfister, H.; et al. HPV8 Field Cancerization in a Transgenic Mouse Model is due to Lrig1+ Keratinocyte Stem Cell Expansion. J. Investig. Dermatol. 2017, 137, 2208–2216. [Google Scholar] [CrossRef]
- Page, M.E.; Lombard, P.; Ng, F.; Gottgens, B.; Jensen, K.B. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell 2013, 13, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Dekoninck, S.; Rulands, S.; Lenglez, S.; Mascre, G.; Simons, B.D.; Blanpain, C. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat. Commun. 2017, 8, 14684. [Google Scholar] [CrossRef] [PubMed]
- Donati, G.; Rognoni, E.; Hiratsuka, T.; Liakath-Ali, K.; Hoste, E.; Kar, G.; Kayikci, M.; Russell, R.; Kretzschmar, K.; Mulder, K.W.; et al. Wounding induces dedifferentiation of epidermal Gata6+ cells and acquisition of stem cell properties. Nat. Cell Biol. 2017, 19, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Gur, G.; Rubin, C.; Katz, M.; Amit, I.; Citri, A.; Nilsson, J.; Amariglio, N.; Henriksson, R.; Rechavi, G.; Hedman, H.; et al. LRIG1 restricts growth factor signaling by enhancing receptor ubiquitylation and degradation. EMBO J. 2004, 23, 3270–3281. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Stange, D.E.; Page, M.E.; Buczacki, S.; Wabik, A.; Itami, S.; van de Wetering, M.; Poulsom, R.; Wright, N.A.; Trotter, M.W.; et al. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat. Cell Biol. 2012, 14, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Kumar, R.; Gokhale, A.; Chao, H.P.; Rycaj, K.; Chen, X.; Li, Q.; Tang, D.G. LRIG1, a regulator of stem cell quiescence and a pleiotropic feedback tumor suppressor. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar] [CrossRef]
- Hoesl, C.; Hundt, J.E.; Rose, C.; Wolf, R.; Schneider, M.R.; Dahlhoff, M. Epidermal overexpression of LRIG1 disturbs development and homeostasis in skin by disrupting the ERBB system. J. Dermatol. Sci. 2019, 96, 185–188. [Google Scholar] [CrossRef]
- Durchdewald, M.; Guinea-Viniegra, J.; Haag, D.; Riehl, A.; Lichter, P.; Hahn, M.; Wagner, E.F.; Angel, P.; Hess, J. Podoplanin is a novel fos target gene in skin carcinogenesis. Cancer Res. 2008, 68, 6877–6883. [Google Scholar] [CrossRef]
- Akgül, B.; Ghali, L.; Davies, D.; Pfister, H.; Leigh, I.M.; Storey, A. HPV8 early genes modulate differentiation and cell cycle of primary human adult keratinocytes. Exp. Dermatol. 2007, 16, 590–599. [Google Scholar] [CrossRef]
- Kazem, S.; van der Meijden, E.; Struijk, L.; de Gruijl, F.R.; Feltkamp, M.C. Human papillomavirus 8 E6 disrupts terminal skin differentiation and prevents pro-Caspase-14 cleavage. Virus Res. 2012, 163, 609–616. [Google Scholar] [CrossRef]
- Marthaler, A.M.; Podgorska, M.; Feld, P.; Fingerle, A.; Knerr-Rupp, K.; Grasser, F.; Smola, H.; Roemer, K.; Ebert, E.; Kim, Y.J.; et al. Identification of C/EBPalpha as a novel target of the HPV8 E6 protein regulating miR-203 in human keratinocytes. PLoS Pathog. 2017, 13, e1006406. [Google Scholar] [CrossRef]
- Meyers, J.M.; Uberoi, A.; Grace, M.; Lambert, P.F.; Munger, K. Cutaneous HPV8 and MmuPV1 E6 Proteins Target the NOTCH and TGF-beta Tumor Suppressors to Inhibit Differentiation and Sustain Keratinocyte Proliferation. PLoS Pathog. 2017, 13, e1006171. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Piao, Y.J.; Sohn, K.C.; Ha, J.M.; Im, M.; Seo, Y.J.; Whang, K.U.; Lee, J.H.; Lee, Y.; Kim, C.D. Sox9 is a beta-catenin-regulated transcription factor that enhances the colony-forming activity of squamous cell carcinoma cells. Mol. Med. Rep. 2016, 14, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Heuser, S.; Hufbauer, M.; Marx, B.; Tok, A.; Majewski, S.; Pfister, H.; Akgül, B. The levels of epithelial anchor proteins beta-catenin and zona occludens-1 are altered by E7 of human papillomaviruses 5 and 8. J. Gen. Virol. 2016, 97, 463–472. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Malanchi, I.; Peinado, H.; Kassen, D.; Hussenet, T.; Metzger, D.; Chambon, P.; Huber, M.; Hohl, D.; Cano, A.; Birchmeier, W.; et al. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature 2008, 452, 650–653. [Google Scholar] [CrossRef]
- Baritaki, S.; Sifakis, S.; Huerta-Yepez, S.; Neonakis, I.K.; Soufla, G.; Bonavida, B.; Spandidos, D.A. Overexpression of VEGF and TGF-beta1 mRNA in Pap smears correlates with progression of cervical intraepithelial neoplasia to cancer: Implication of YY1 in cervical tumorigenesis and HPV infection. Int. J. Oncol. 2007, 31, 69–79. [Google Scholar]
- Sperling, T.; Oldak, M.; Walch-Ruckheim, B.; Wickenhauser, C.; Doorbar, J.; Pfister, H.; Malejczyk, M.; Majewski, S.; Keates, A.C.; Smola, S. Human papillomavirus type 8 interferes with a novel C/EBPbeta-mediated mechanism of keratinocyte CCL20 chemokine expression and Langerhans cell migration. PLoS Pathog. 2012, 8, e1002833. [Google Scholar] [CrossRef]
- Podgorska, M.; Oldak, M.; Marthaler, A.; Fingerle, A.; Walch-Ruckheim, B.; Lohse, S.; Muller, C.S.L.; Vogt, T.; Ustav, M.; Wnorowski, A.; et al. Chronic Inflammatory Microenvironment in Epidermodysplasia Verruciformis Skin Lesions: Role of the Synergism Between HPV8 E2 and C/EBPbeta to Induce Pro-Inflammatory S100A8/A9 Proteins. Front. Microbiol 2018, 9, 392. [Google Scholar] [CrossRef]
- Müller, A.; Ritzkowsky, A.; Steger, G. Cooperative activation of human papillomavirus type 8 gene expression by the E2 protein and the cellular coactivator p300. J. Virol. 2002, 76, 11042–11053. [Google Scholar] [CrossRef]
- Hufbauer, M.; Cooke, J.; van der Horst, G.T.; Pfister, H.; Storey, A.; Akgül, B. Human papillomavirus mediated inhibition of DNA damage sensing and repair drives skin carcinogenesis. Mol. Cancer 2015, 14, 183. [Google Scholar] [CrossRef]
- Kastner, P.; Chan, S. PU.1: A crucial and versatile player in hematopoiesis and leukemia. Int. J. Biochem. Cell Biol. 2008, 40, 22–27. [Google Scholar] [CrossRef]
- Imperato, M.R.; Cauchy, P.; Obier, N.; Bonifer, C. The RUNX1-PU.1 axis in the control of hematopoiesis. Int. J. Hematol. 2015, 101, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Hoi, C.S.; Lee, S.E.; Lu, S.Y.; McDermitt, D.J.; Osorio, K.M.; Piskun, C.M.; Peters, R.M.; Paus, R.; Tumbar, T. Runx1 directly promotes proliferation of hair follicle stem cells and epithelial tumor formation in mouse skin. Mol. Cell. Biol. 2010, 30, 2518–2536. [Google Scholar] [CrossRef] [PubMed]
- De Andrea, M.; Ritta, M.; Landini, M.M.; Borgogna, C.; Mondini, M.; Kern, F.; Ehrenreiter, K.; Baccarini, M.; Marcuzzi, G.P.; Smola, S.; et al. Keratinocyte-specific stat3 heterozygosity impairs development of skin tumors in human papillomavirus 8 transgenic mice. Cancer Res. 2010, 70, 7938–7948. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.; Macias, E.; Carbajal, S.; Kiguchi, K.; DiGiovanni, J. Constitutive Stat3 activation alters behavior of hair follicle stem and progenitor cell populations. Mol. Carcinog. 2015, 54, 121–133. [Google Scholar] [CrossRef]
- Sano, S.; Itami, S.; Takeda, K.; Tarutani, M.; Yamaguchi, Y.; Miura, H.; Yoshikawa, K.; Akira, S.; Takeda, J. Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J. 1999, 18, 4657–4668. [Google Scholar] [CrossRef]
- Chan, K.S.; Sano, S.; Kiguchi, K.; Anders, J.; Komazawa, N.; Takeda, J.; DiGiovanni, J. Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. J. Clin. Investig. 2004, 114, 720–728. [Google Scholar] [CrossRef]
- Cao, Q.; Li, Y.Y.; He, W.F.; Zhang, Z.Z.; Zhou, Q.; Liu, X.; Shen, Y.; Huang, T.T. Interplay between microRNAs and the STAT3 signaling pathway in human cancers. Physiol. Genom. 2013, 45, 1206–1214. [Google Scholar] [CrossRef]
- Chen, M.; Li, M.; Zhang, N.; Sun, W.; Wang, H.; Wei, W. Mechanism of miR-218-5p in autophagy, apoptosis and oxidative stress in rheumatoid arthritis synovial fibroblasts is mediated by KLF9 and JAK/STAT3 pathways. J. Investig. Med. 2021, 69, 824–832. [Google Scholar] [CrossRef]
- Elton, T.S.; Selemon, H.; Elton, S.M.; Parinandi, N.L. Regulation of the MIR155 host gene in physiological and pathological processes. Gene 2013, 532, 1–12. [Google Scholar] [CrossRef]
- Wohlfahrt, T.; Rauber, S.; Uebe, S.; Luber, M.; Soare, A.; Ekici, A.; Weber, S.; Matei, A.E.; Chen, C.W.; Maier, C.; et al. PU.1 controls fibroblast polarization and tissue fibrosis. Nature 2019, 566, 344–349. [Google Scholar] [CrossRef]
- He, X.H.; Zhu, W.; Yuan, P.; Jiang, S.; Li, D.; Zhang, H.W.; Liu, M.F. miR-155 downregulates ErbB2 and suppresses ErbB2-induced malignant transformation of breast epithelial cells. Oncogene 2016, 35, 6015–6025. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cai, B.; Li, X.; Li, D.; Yin, G. MiR-125b-5p and miR-181b-5p inhibit keratinocyte proliferation in skin by targeting Akt3. Eur. J. Pharmacol. 2019, 862, 172659. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Chen, Y.; Yang, N.; Chen, Q.; Bao, Z.; Liu, M.; Hu, S.; Li, J.; Wu, X. miR-218-5p regulates skin and hair follicle development through Wnt/beta-catenin signaling pathway by targeting SFRP2. J. Cell. Physiol. 2019, 234, 20329–20341. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cai, G.; Liu, C.; Zhao, J.; Gu, C.; Wu, L.; Hamilton, T.A.; Zhang, C.J.; Ko, J.; Zhu, L.; et al. IL-17R-EGFR axis links wound healing to tumorigenesis in Lrig1(+) stem cells. J. Exp. Med. 2019, 216, 195–214. [Google Scholar] [CrossRef]
- Sahu, U.; Khare, P. Role of interleukin-17 in human papillomavirus infection and associated malignancies. Microb. Pathog. 2021, 161, 105294. [Google Scholar] [CrossRef]
- Wang, L.; Yi, T.; Zhang, W.; Pardoll, D.M.; Yu, H. IL-17 enhances tumor development in carcinogen-induced skin cancer. Cancer Res. 2010, 70, 10112–10120. [Google Scholar] [CrossRef]
- Nardinocchi, L.; Sonego, G.; Passarelli, F.; Avitabile, S.; Scarponi, C.; Failla, C.M.; Simoni, S.; Albanesi, C.; Cavani, A. Interleukin-17 and interleukin-22 promote tumor progression in human nonmelanoma skin cancer. Eur. J. Immunol. 2015, 45, 922–931. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syed, A.S.; Marcuzzi, G.P.; Miller-Lazic, D.; Hess, J.; Hufbauer, M.; Akgül, B. HPV8 Reverses the Transcriptional Output in Lrig1 Positive Cells to Drive Skin Tumorigenesis. Cancers 2022, 14, 1662. https://doi.org/10.3390/cancers14071662
Syed AS, Marcuzzi GP, Miller-Lazic D, Hess J, Hufbauer M, Akgül B. HPV8 Reverses the Transcriptional Output in Lrig1 Positive Cells to Drive Skin Tumorigenesis. Cancers. 2022; 14(7):1662. https://doi.org/10.3390/cancers14071662
Chicago/Turabian StyleSyed, Adnan Shahzad, Gian Paolo Marcuzzi, Daliborka Miller-Lazic, Jochen Hess, Martin Hufbauer, and Baki Akgül. 2022. "HPV8 Reverses the Transcriptional Output in Lrig1 Positive Cells to Drive Skin Tumorigenesis" Cancers 14, no. 7: 1662. https://doi.org/10.3390/cancers14071662
APA StyleSyed, A. S., Marcuzzi, G. P., Miller-Lazic, D., Hess, J., Hufbauer, M., & Akgül, B. (2022). HPV8 Reverses the Transcriptional Output in Lrig1 Positive Cells to Drive Skin Tumorigenesis. Cancers, 14(7), 1662. https://doi.org/10.3390/cancers14071662






