Multimodality Treatment including Surgery Related to the Type of N2 Involvement in Locally Advanced Non-Small Cell Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Stage IIIA-N2 Disease
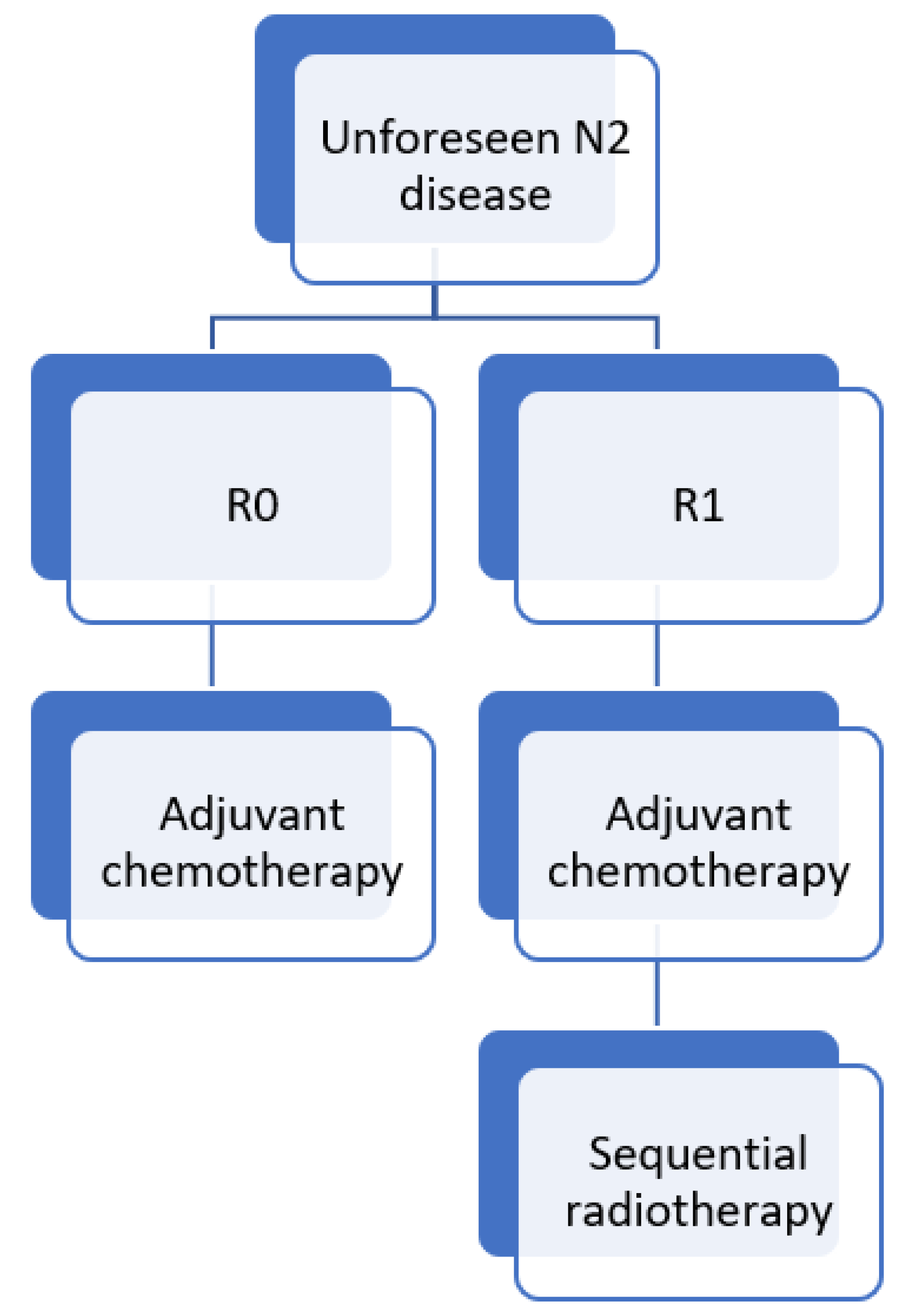
3.1. Unforeseen (Unexpected or Surprise) N2
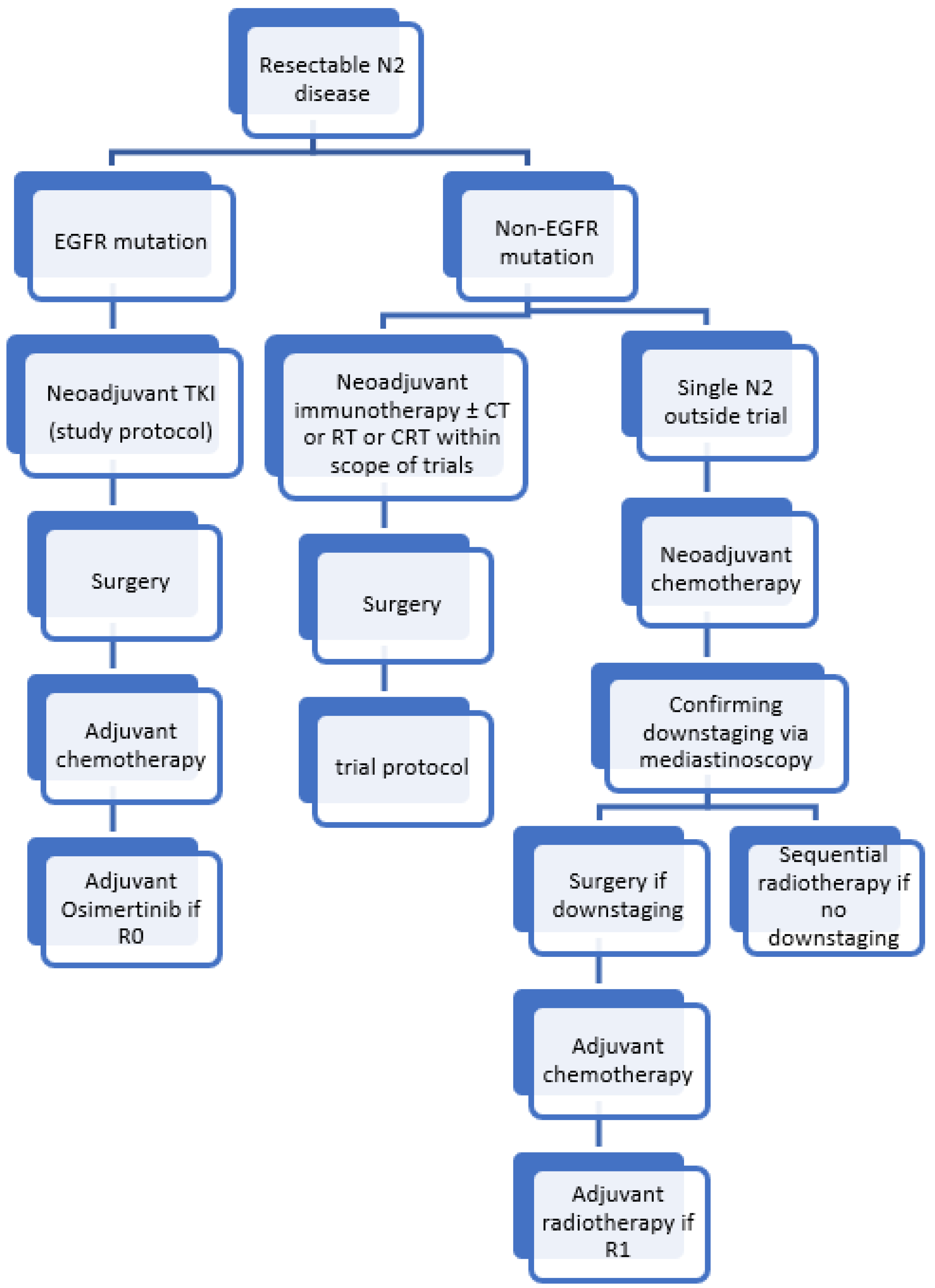
3.2. Resectable N2
3.2.1. Induction Chemo(radio)therapy
3.2.2. Adjuvant Radiotherapy
3.2.3. Induction Immunotherapy
3.2.4. Adjuvant Immunotherapy
3.2.5. Targeted Therapies
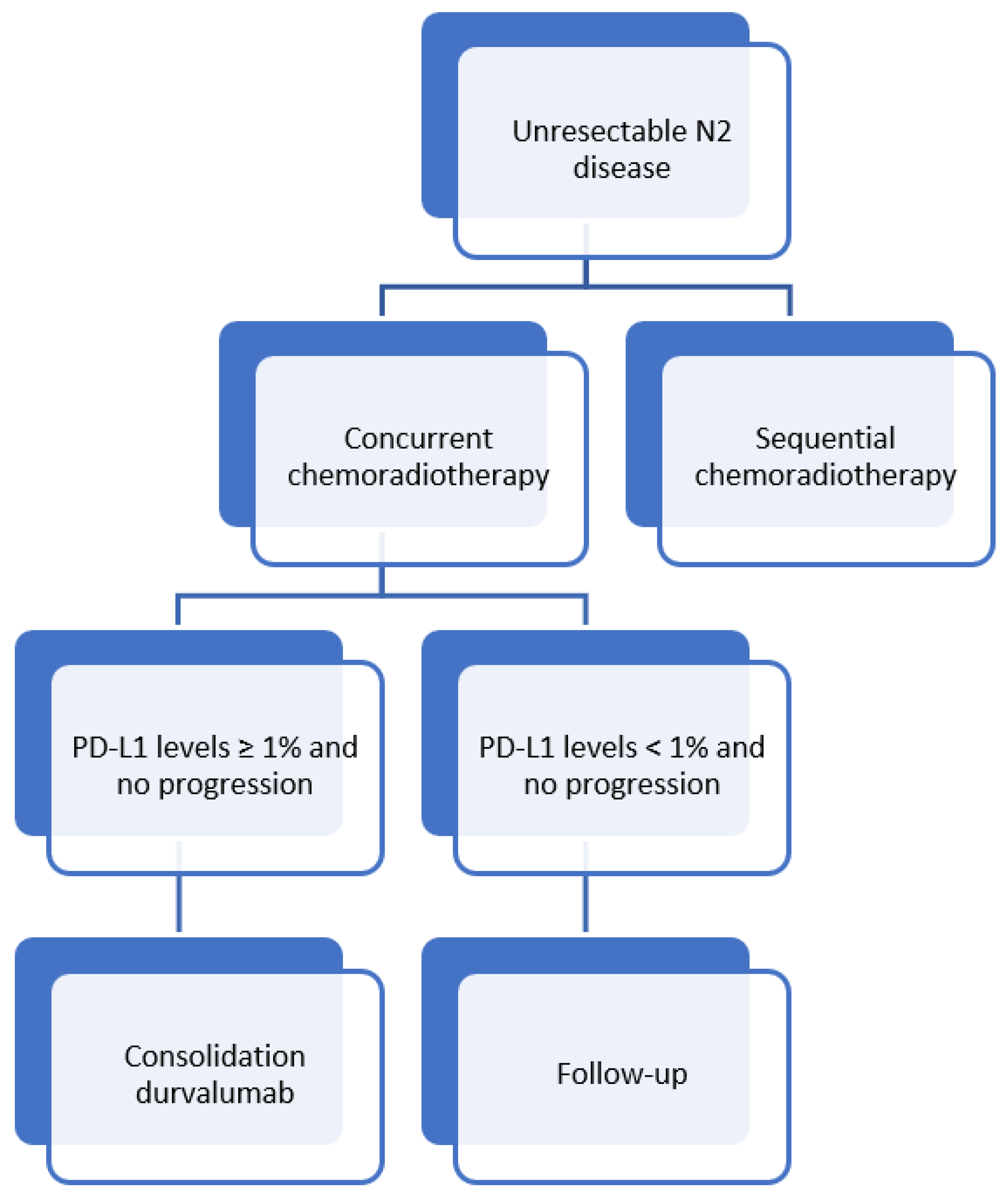
3.3. Unresectable N2
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5YS | 5-year survival |
| AJCC | American Joint Committee on Cancer |
| CRT | Chemoradiation therapy |
| EBUS | Endobronchial Ultrasound |
| DCR | Disease control rate |
| DFS | Disease-free survival |
| EFS | Event-free survival |
| EGFR | Epidermal growth factor receptor |
| ESMO | European Society for Medical Oncology |
| IASLC | International Association for the Study of Lung Cancer |
| ICI | Immune-checkpoint inhibitors |
| LNR | Lymph node ratio |
| MPR | Major pathological response |
| NCCN | National Comprehensive Cancer Network |
| NSCLC | Non-small cell lung cancer |
| ORR | Overall response rate |
| OS | Overall survival |
| pCR | Pathological complete response |
| PD-L1 | programmed death-ligand 1 |
| PFS | Progression-free survival |
| PORT | Postoperative radiotherapy |
| tAEs | Treatment-related adverse events |
| TKI | Tyrosine kinase inhibitor |
| TNM | Tumour, Node, and Metastasis |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lococo, F.; Sassorossi, C.; Mazzarella, C.; Vita, E.; Leoncini, F.; Martino, A.; Nachira, D.; Chiappetta, M.; Cesario, A.; Trisolini, R.; et al. Surgery after induction chemo or immunotherapy for locally advanced NSCLC. Curr. Chall. Thorac. Surg. 2020, 2, 1–6. [Google Scholar] [CrossRef]
- Eberhardt, W.E.; De Ruysscher, D.; Weder, W.; Le Péchoux, C.; De Leyn, P.; Hoffmann, H.; Westeel, V.; Stahel, R.; Felip, E.; Peters, S. 2nd ESMO Consensus Conference in Lung Cancer: Locally advanced stage III non-small-cell lung cancer. Ann. Oncol. 2015, 26, 1573–1588. [Google Scholar] [CrossRef]
- Remon, J.; Soria, J.C.; Peters, S. Early and locally advanced non-small-cell lung cancer: An update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann. Oncol. 2021, 32, 1637–1642. [Google Scholar] [CrossRef]
- Jeremić, B.; Casas, F.; Dubinsky, P.; Gomez-Caamano, A.; Čihorić, N.; Videtic, G.; Igrutinovic, I. Treatment-Related Predictive and Prognostic Factors in Trimodality Approach in Stage IIIA/N2 Non-Small Cell Lung Cancer. Front. Oncol. 2018, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN Guidelines Insights: Non–Small Cell Lung Cancer, Version 2.2021: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef]
- Allaeys, T.; Berzenji, L.; Van Schil, P.E. Surgery after Induction Targeted Therapy and Immunotherapy for Lung Cancer. Cancers 2021, 13, 2603. [Google Scholar] [CrossRef]
- Decaluwé, H.; De Leyn, P.; Vansteenkiste, J.; Dooms, C.; Van Raemdonck, D.; Nafteux, P.; Coosemans, W.; Lerut, T. Surgical multimodality treatment for baseline resectable stage IIIA-N2 non-small cell lung cancer. Degree of mediastinal lymph node involvement and impact on survival. Eur. J. Cardiothorac. Surg. 2009, 36, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Rusch, V.W.; Crowley, J.; Giroux, D.J.; Goldstraw, P.; Im, J.G.; Tsuboi, M.; Tsuchiya, R.; Vansteenkiste, J. The IASLC Lung Cancer Staging Project: Proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2007, 2, 603–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misthos, P.; Sepsas, E.; Kokotsakis, J.; Skottis, I.; Lioulias, A. The significance of one-station N2 disease in the prognosis of patients with nonsmall-cell lung cancer. Ann. Thorac. Surg. 2008, 86, 1626–1630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Hou, R.P.; Feng, W.; Fu, X.L. Lymph Node Parameters Predict Adjuvant Chemoradiotherapy Efficacy and Disease-Free Survival in Pathologic N2 Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 736892. [Google Scholar] [CrossRef] [PubMed]
- Asamura, H.; Chansky, K.; Crowley, J.; Goldstraw, P.; Rusch, V.W.; Vansteenkiste, J.F.; Watanabe, H.; Wu, Y.L.; Zielinski, M.; Ball, D.; et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2015, 10, 1675–1684. [Google Scholar] [CrossRef] [Green Version]
- Rami-Porta, R.; Wittekind, C.; Goldstraw, P. Complete resection in lung cancer surgery: Proposed definition. Lung Cancer 2005, 49, 25–33. [Google Scholar] [CrossRef]
- Bousema, J.E.; van Dorp, M.; Noyez, V.; Dijkgraaf, M.G.W.; Annema, J.T.; van den Broek, F.J.C. Unforeseen N2 Disease after Negative Endosonography Findings with or without Confirmatory Mediastinoscopy in Resectable Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. J. Thorac. Oncol. 2019, 14, 979–992. [Google Scholar] [CrossRef]
- Le Pechoux, C.; Pourel, N.; Barlesi, F.; Faivre-Finn, C.; Lerouge, D.; Zalcman, G.; Antoni, D.; Lamezec, B.; Nestle, U.; Boisselier, P.; et al. LBA3_PR An international randomized trial, comparing post-operative conformal radiotherapy (PORT) to no PORT, in patients with completely resected non-small cell lung cancer (NSCLC) and mediastinal N2 involvement: Primary end-point analysis of LungART (IFCT-0503, UK NCRI, SAKK) NCT00410683. Ann. Oncol. 2020, 31, S1178. [Google Scholar]
- Wang, E.H.; Corso, C.D.; Rutter, C.E.; Park, H.S.; Chen, A.B.; Kim, A.W.; Wilson, L.D.; Decker, R.H.; Yu, J.B. Postoperative Radiation Therapy Is Associated With Improved Overall Survival in Incompletely Resected Stage II and III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2015, 33, 2727–2734. [Google Scholar] [CrossRef]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. S4), iv1–iv21. [Google Scholar] [CrossRef]
- Albain, K.S.; Swann, R.S.; Rusch, V.W.; Turrisi, A.T., 3rd; Shepherd, F.A.; Smith, C.; Chen, Y.; Livingston, R.B.; Feins, R.H.; Gandara, D.R.; et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: A phase III randomised controlled trial. Lancet 2009, 374, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Van Meerbeeck, J.P.; Kramer, G.W.P.M.; Van Schil, P.E.Y.; Legrand, C.; Smit, E.F.; Schramel, F.; Tjan-Heijnen, V.C.; Biesma, B.; Debruyne, C.; van Zandwijk, N.; et al. Randomized Controlled Trial of Resection Versus Radiotherapy after Induction Chemotherapy in Stage IIIA-N2 Non–Small-Cell Lung Cancer. JNCI J. Natl. Cancer Inst. 2007, 99, 442–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eberhardt, W.E.E.; Pöttgen, C.; Gauler, T.C.; Friedel, G.; Veit, S.; Heinrich, V.; Welter, S.; Budach, W.; Spengler, W.; Kimmich, M.; et al. Phase III Study of Surgery Versus Definitive Concurrent Chemoradiotherapy Boost in Patients with Resectable Stage IIIA(N2) and Selected IIIB Non–Small-Cell Lung Cancer after Induction Chemotherapy and Concurrent Chemoradiotherapy (ESPATUE). J. Clin. Oncol. 2015, 33, 4194–4201. [Google Scholar] [CrossRef] [PubMed]
- Pless, M.; Stupp, R.; Ris, H.B.; Stahel, R.A.; Weder, W.; Thierstein, S.; Gerard, M.A.; Xyrafas, A.; Früh, M.; Cathomas, R.; et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: A phase 3 randomised trial. Lancet 2015, 386, 1049–1056. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; Liang, H.; Yang, C.J.; D’Amico, T.; Ng, C.S.H.; Liu, C.C.; Petersen, R.H.; Rocco, G.; Brunelli, A.; et al. The Optimal Treatment for Stage IIIA-N2 Non-Small Cell Lung Cancer: A Network Meta-Analysis. Ann. Thorac. Surg. 2019, 107, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- McElnay, P.J.; Choong, A.; Jordan, E.; Song, F.; Lim, E. Outcome of surgery versus radiotherapy after induction treatment in patients with N2 disease: Systematic review and meta-analysis of randomised trials. Thorax 2015, 70, 764–768. [Google Scholar] [CrossRef] [Green Version]
- Maconachie, R.; Mercer, T.; Navani, N.; McVeigh, G. Lung cancer: Diagnosis and management: Summary of updated NICE guidance. BMJ 2019, 364, l1049. [Google Scholar] [CrossRef]
- Gao, F.; Li, N.; Xu, Y.; Yang, G. Effects of Postoperative Radiotherapy on Survival of Patients With Stage IIIA Resected Non–Small Cell Lung Cancer: Analysis of the SEER Database. J. Natl. Compr. Cancer Netw. 2020, 18, 718–727. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Chaft, J.E.; William, W.N.; Rusch, V.; Pisters, K.M.W.; Kalhor, N.; Pataer, A.; Travis, W.D.; Swisher, S.G.; Kris, M.G. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: Proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014, 15, e42–e50. [Google Scholar] [CrossRef] [Green Version]
- Pataer, A.; Kalhor, N.; Correa, A.M.; Raso, M.G.; Erasmus, J.J.; Kim, E.S.; Behrens, C.; Lee, J.J.; Roth, J.A.; Stewart, D.J.; et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J. Thorac. Oncol. 2012, 7, 825–832. [Google Scholar] [CrossRef] [Green Version]
- Weissferdt, A.; Pataer, A.; Vaporciyan, A.A.; Correa, A.M.; Sepesi, B.; Moran, C.A.; Wistuba, I.I.; Roth, J.A.; Shewale, J.B.; Heymach, J.V.; et al. Agreement on Major Pathological Response in NSCLC Patients Receiving Neoadjuvant Chemotherapy. Clin. Lung Cancer 2020, 21, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Ma, K. Neoadjuvant Therapy in Lung Cancer: What Is Most Important: Objective Response Rate or Major Pathological Response? Curr. Oncol. 2021, 28, 4129–4138. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Xu, A.; Lin, Y.; Camidge, D.R.; Di Maio, M.; Califano, R.; Hida, T.; Rossi, A.; Guibert, N.; Zhu, C.; et al. A narrative review of primary research endpoints of neoadjuvant therapy for lung cancer: Past, present and future. Transl. Lung Cancer Res. 2021, 10, 3264–3275. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Cai, K.; Chen, C.; Chen, J.; Chen, K.-N.; Chen, Q.-X.; Cheng, C.; Dai, T.-Y.; Fan, J.; Fan, Z.; et al. Expert consensus on perioperative immunotherapy for local advanced non-small cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 3713–3736. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.; Lee, J.; Kris, M.; Wistuba, I.; Kwiatkowski, D.; Owen, D.; Bunn, P.; Johnson, B.; Oezkan, F.; Tang, Y.; et al. OA06.06 Clinical/Biomarker Data for Neoadjuvant Atezolizumab in Resectable Stage IB-IIIB NSCLC: Primary Analysis in the LCMC3 Study. J. Thorac. Oncol. 2021, 16 (Suppl. S3), S115–S116. [Google Scholar] [CrossRef]
- Rothschild, S.I.; Zippelius, A.; Eboulet, E.I.; Savic Prince, S.; Betticher, D.; Bettini, A.; Früh, M.; Joerger, M.; Lardinois, D.; Gelpke, H.; et al. SAKK 16/14: Durvalumab in Addition to Neoadjuvant Chemotherapy in Patients With Stage IIIA(N2) Non-Small-Cell Lung Cancer-A Multicenter Single-Arm Phase II Trial. J. Clin. Oncol 2021, 39, 2872–2880. [Google Scholar] [CrossRef]
- Provencio, M.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal-Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; De Castro Carpeño, J.; et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 1413–1422. [Google Scholar] [CrossRef]
- Spicer, J.; Wang, C.; Tanaka, F.; Saylors, G.B.; Chen, K.-N.; Liberman, M.; Vokes, E.E.; Girard, N.; Lu, S.; Provencio, M.; et al. Surgical outcomes from the phase 3 CheckMate 816 trial: Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs. chemo alone as neoadjuvant treatment for patients with resectable non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2021, 39 (Suppl. S15), 8503. [Google Scholar] [CrossRef]
- Shen, D.; Wang, J.; Wu, J.; Chen, S.; Li, J.; Liu, J.; Chen, Q.; Jiang, Y. Neoadjuvant pembrolizumab with chemotherapy for the treatment of stage IIB-IIIB resectable lung squamous cell carcinoma. J. Thorac. Dis. 2021, 13, 1760–1768. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Cai, L.; Chen, S.; Jiang, Y. The safety and efficacy of neoadjuvant programmed death 1 inhibitor therapy with surgical resection in stage IIIA non-small cell lung cancer. Ann. Transl. Med. 2021, 9, 486. [Google Scholar] [CrossRef]
- Shu, C.A.; Gainor, J.F.; Awad, M.M.; Chiuzan, C.; Grigg, C.M.; Pabani, A.; Garofano, R.F.; Stoopler, M.B.; Cheng, S.K.; White, A.; et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 786–795. [Google Scholar] [CrossRef]
- Zinner, R.; Axelrod, R.; Solomides, C.C.; Cowan, S.; Leiby, B.; Bhatia, A.K.; Sundermeyer, M.L.; Hooper, D.C.; Harshyne, L.; Lu-Yao, G.L.; et al. Neoadjuvant nivolumab (N) plus cisplatin (C)/pemetrexed (P) or cisplatin /gemcitabine (G) in resectable NSCLC. J. Clin. Oncol. 2020, 38, 9051. [Google Scholar] [CrossRef]
- Fernando, H.C.; Yang, J.; Ferraro, G.L.; Keller, S.M. Randomized, double-blind phase 3 study evaluating neoadjuvant platinum-based chemotherapy with perioperative pembrolizumab or placebo in resectable stage IIB or IIIA NSCLC: KEYNOTE-671. J. Clin. Oncol. 2018, 36, TPS8583. [Google Scholar] [CrossRef]
- Rizvi, N.; Gandara, D.; Solomon, B.; Kim, A.; Brunelli, A.; Sun, S.; Gitlitz, B.; Tajima, K.; Lin, W.; Sandler, A.; et al. P2.17-27 IMpower030: Phase III Study Evaluating Neoadjuvant Treatment of Resectable Stage II-IIIB NSCLC with Atezolizumab + Chemotherapy. J. Thorac. Oncol. 2018, 13, S863. [Google Scholar] [CrossRef] [Green Version]
- Cascone, T.; Provencio, M.; Sepesi, B.; Lu, S.; Aanur, N.; Li, S.; Spicer, J. Checkmate 77T: A phase III trial of neoadjuvant nivolumab (NIVO) plus chemotherapy (chemo) followed by adjuvant nivo in resectable early-stage NSCLC. J. Clin. Oncol. 2020, 38 (Suppl. S15), TPS9076. [Google Scholar] [CrossRef]
- Heymach, J.V.; Mitsudomi, T.; Harpole, D.; Aperghis, M.; Jones, S.; Mann, H.; Fouad, T.M.; Reck, M. Design and Rationale for a Phase III, Double-Blind, Placebo-Controlled Study of Neoadjuvant Durvalumab + Chemotherapy Followed by Adjuvant Durvalumab for the Treatment of Patients With Resectable Stages II and III non-small-cell Lung Cancer: The AEGEAN Trial. Clin. Lung Cancer 2021, S1525-7304(21)00265-5. [Google Scholar] [CrossRef]
- Cascone, T.; William, W.N.; Weissferdt, A.; Leung, C.H.; Lin, H.Y.; Pataer, A.; Godoy, M.C.B.; Carter, B.W.; Federico, L.; Reuben, A.; et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: The phase 2 randomized NEOSTAR trial. Nat. Med. 2021, 27, 504–514. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef]
- Taunk, N.K.; Rimner, A.; Culligan, M.; Friedberg, J.S.; Brahmer, J.; Chaft, J. Immunotherapy and radiation therapy for operable early stage and locally advanced non-small cell lung cancer. Transl. Lung Cancer Res. 2017, 6, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Von Reibnitz, D.; Wu, A.J.; Barker, C.A.; Panchoo, K.; Rimner, A. Safety of Combining Immune Checkpoint Inhibition and Thoracic Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96 (Suppl. S2), S156. [Google Scholar] [CrossRef]
- Altorki, N.K.; McGraw, T.E.; Borczuk, A.C.; Saxena, A.; Port, J.L.; Stiles, B.M.; Lee, B.E.; Sanfilippo, N.J.; Scheff, R.J.; Pua, B.B.; et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: A single-centre, randomised phase 2 trial. Lancet Oncol. 2021, 22, 824–835. [Google Scholar] [CrossRef]
- Bott, M.J.; Yang, S.C.; Park, B.J.; Adusumilli, P.S.; Rusch, V.W.; Isbell, J.M.; Downey, R.J.; Brahmer, J.R.; Battafarano, R.; Bush, E.; et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2019, 158, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Wislez, M.; Mazieres, J.; Lavole, A.; Zalcman, G.; Carre, O.; Egenod, T.; Caliandro, R.; Gervais, R.; Jeannin, G.; Molinier, O.; et al. 1214O Neoadjuvant durvalumab in resectable non-small cell lung cancer (NSCLC): Preliminary results from a multicenter study (IFCT-1601 IONESCO). Ann. Oncol. 2020, 31, S794. [Google Scholar] [CrossRef]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- Bai, R.; Li, L.; Chen, X.; Chen, N.; Song, W.; Cui, J. Neoadjuvant and Adjuvant Immunotherapy: Opening New Horizons for Patients With Early-Stage Non-small Cell Lung Cancer. Front. Oncol. 2020, 10, 575472. [Google Scholar] [CrossRef]
- Chaft, J.E.; Dahlberg, S.E.; Khullar, O.V.; Edelman, M.J.; Simone, C.B.; Heymach, J.; Rudin, C.M.; Ramalingam, S.S. EA5142 adjuvant nivolumab in resected lung cancers (ANVIL). J. Clin. Oncol. 2018, 36 (Suppl. S15), TPS8581. [Google Scholar] [CrossRef]
- O’Brien, M.E.R.; Hasan, B.; Dafni, U.; Menis, J.; Peters, S.; Waele, M.D.; Stahel, R.A.; Schil, P.V.; Coukos, G.; Lantuejoul, S.; et al. EORTC-ETOP randomized, phase 3 trial with anti-PD-1 monoclonal antibody pembrolizumab versus placebo for patients with early stage non-small cell lung cancer (NSCLC) after resection and standard adjuvant chemotherapy: PEARLS (NCT02504372). J. Clin. Oncol. 2016, 34 (Suppl. S15), TPS8571. [Google Scholar] [CrossRef]
- Ahn, M.-J.; Park, S.; Jung, H.A.; Cho, J.H.; Sun, J.-M.; Lee, S.-H.; Choi, Y.S.; Ahn, J.S.; Kim, J.; Park, K.; et al. Phase II, prospective single-arm study of adjuvant pembrolizumab in N2 positive non-small cell lung cancer (NSCLC) treated with neoadjuvant concurrent chemoradiotherapy followed by curative resection: Preliminary results. J. Clin. Oncol. 2019, 37 (Suppl. S15), 8520. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Zhong, W.; Chen, K.-N.; Chen, C.; Yang, F.; Yang, X.-N.; Gu, C.; Mao, W.; Wang, Q.; Qiao, G.-B.; et al. CTONG1103: Final overall survival analysis of the randomized phase 2 trial of erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of stage IIIA-N2 EGFR-mutant non–small cell lung cancer. J. Clin. Oncol. 2021, 39 (Suppl. S15), 8502. [Google Scholar] [CrossRef]
- Zhong, W.; Yang, X.; Yan, H.; Zhang, X.; Su, J.; Chen, Z.; Liao, R.; Nie, Q.; Dong, S.; Zhou, Q.; et al. Phase II study of biomarker-guided neoadjuvant treatment strategy for IIIA-N2 non-small cell lung cancer based on epidermal growth factor receptor mutation status. J. Hematol. Oncol. 2015, 8, 54. [Google Scholar] [CrossRef] [Green Version]
- Xiong, L.; Li, R.; Sun, J.; Lou, Y.; Zhang, W.; Bai, H.; Wang, H.; Shen, J.; Jing, B.; Shi, C.; et al. Erlotinib as Neoadjuvant Therapy in Stage IIIA (N2) EGFR Mutation-Positive Non-Small Cell Lung Cancer: A Prospective, Single-Arm, Phase II Study. Oncologist 2019, 24, e157–e164. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Guo, Y.-J.; Song, J.; Wang, Y.-R.; Zhang, S.-L.; Huang, L.-T.; Zhao, J.-Z.; Jing, W.; Han, C.-B.; Ma, J.-T. Neoadjuvant EGFR-TKI Therapy for EGFR-Mutant NSCLC: A Systematic Review and Pooled Analysis of Five Prospective Clinical Trials. Front. Oncol. 2021, 10, 3021. [Google Scholar] [CrossRef]
- Herbst, R.S.; Tsuboi, M.; John, T.; Grohé, C.; Majem, M.; Goldman, J.W.; Kim, S.-W.; Marmol, D.; Rukazenkov, Y.; Wu, Y.-L. Osimertinib as adjuvant therapy in patients (pts) with stage IB–IIIA EGFR mutation positive (EGFRm) NSCLC after complete tumor resection: ADAURA. J. Clin. Oncol. 2020, 38 (Suppl. S18), LBA5. [Google Scholar] [CrossRef]
- Tsuboi, M.; Weder, W.; Escriu, C.; Blakely, C.; He, J.; Dacic, S.; Yatabe, Y.; Zeng, L.; Walding, A.; Chaft, J.E. Neoadjuvant osimertinib with/without chemotherapy versus chemotherapy alone for EGFR-mutated resectable non-small-cell lung cancer: NeoADAURA. Future Oncol. 2021, 17, 4045–4055. [Google Scholar] [CrossRef]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.G.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-year survival outcomes with durvalumab after chemoradiotherapy in unresectable stage III NSCLC: An update from the PACIFIC trial. J. Clin. Oncol. 2021, 39 (Suppl. S15), 8511. [Google Scholar] [CrossRef]
- Durm, G.A.; Jabbour, S.K.; Althouse, S.K.; Liu, Z.; Sadiq, A.A.; Zon, R.T.; Jalal, S.I.; Kloecker, G.H.; Williamson, M.J.; Reckamp, K.L.; et al. A phase 2 trial of consolidation pembrolizumab following concurrent chemoradiation for patients with unresectable stage III non-small cell lung cancer: Hoosier Cancer Research Network LUN 14-179. Cancer 2020, 126, 4353–4361. [Google Scholar] [CrossRef]
- Jabbour, S.K.; Berman, A.T.; Decker, R.H.; Lin, Y.; Feigenberg, S.J.; Gettinger, S.N.; Aggarwal, C.; Langer, C.J.; Simone, C.B., 2nd; Bradley, J.D.; et al. Phase 1 Trial of Pembrolizumab Administered Concurrently With Chemoradiotherapy for Locally Advanced Non-Small Cell Lung Cancer: A Nonrandomized Controlled Trial. JAMA Oncol. 2020, 6, 848–855. [Google Scholar] [CrossRef]
- Peters, S.; Felip, E.; Dafni, U.; Tufman, A.; Guckenberger, M.; Álvarez, R.; Nadal, E.; Becker, A.; Vees, H.; Pless, M.; et al. Progression-Free and Overall Survival for Concurrent Nivolumab With Standard Concurrent Chemoradiotherapy in Locally Advanced Stage IIIA-B NSCLC: Results From the European Thoracic Oncology Platform NICOLAS Phase II Trial (European Thoracic Oncology Platform 6-14). J. Thorac. Oncol. 2021, 16, 278–288. [Google Scholar]
- Peters, S.; Felip, E.; Dafni, U.; Belka, C.; Guckenberger, M.; Irigoyen, A.; Nadal, E.; Becker, A.; Vees, H.; Pless, M.; et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer-The ETOP NICOLAS trial. Lung Cancer 2019, 133, 83–87. [Google Scholar] [CrossRef]
- Ross, H.J.; Kozono, D.E.; Urbanic, J.J.; Williams, T.M.; DuFrane, C.; Bara, I.; Schulze, K.; Brockman, J.M.; Wang, X.F.; Gao, J.; et al. AFT-16: Phase II trial of neoadjuvant and adjuvant atezolizumab and chemoradiation (CRT) for stage III non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2021, 39 (Suppl. S15), 8513. [Google Scholar] [CrossRef]
- Lin, S.H.; Lin, Y.; Yao, L.; Kalhor, N.; Carter, B.W.; Altan, M.; Blumenschein, G.; Byers, L.A.; Fossella, F.; Gibbons, D.L.; et al. Phase II Trial of Concurrent Atezolizumab With Chemoradiation for Unresectable NSCLC. J. Thorac. Oncol. 2020, 15, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Bradley, J.D.; Nishio, M.; Okamoto, I.; Newton, M.D.; Trani, L.; Shire, N.J.; Gu, Y.; Dennis, P.A.; Lee, K.H. PACIFIC-2: Phase 3 study of concurrent durvalumab and platinum-based chemoradiotherapy in patients with unresectable, stage III NSCLC. J. Clin. Oncol. 2019, 37 (Suppl. S15), TPS8573. [Google Scholar] [CrossRef]
- Varlotto, J.M.; Sun, Z.; Ramalingam, S.S.; Wakelee, H.A.; Lovly, C.M.; Oettel, K.R.; Masters, G.A.; Pennell, N. Randomized phase III Trial of MEDI4736 (durvalumab) as concurrent and consolidative therapy or consolidative therapy alone for unresectable stage 3 NSCLC: A trial of the ECOG-ACRIN Cancer Research Group (EA5181). J. Clin. Oncol. 2021, 39 (Suppl. S15), TPS8584. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Ramalingam, S.; Urbanic, J.; Gerber, D.E.; Tan, D.S.W.; Cai, J.; Li, A.; Peters, S. CheckMate 73L: A Phase 3 Study Comparing Nivolumab Plus Concurrent Chemoradiotherapy Followed by Nivolumab With or Without Ipilimumab Versus Concurrent Chemoradiotherapy Followed by Durvalumab for Previously Untreated, Locally Advanced Stage III Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2021, 75, P620. [Google Scholar]
- Käsmann, L.; Eze, C.; Taugner, J.; Roengvoraphoj, O.; Dantes, M.; Schmidt-Hegemann, N.-S.; Schiopu, S.; Belka, C.; Manapov, F. Chemoradioimmunotherapy of inoperable stage III non-small cell lung cancer: Immunological rationale and current clinical trials establishing a novel multimodal strategy. Radiat Oncol. 2020, 15, 167. [Google Scholar] [CrossRef]
- NCCN. Non-Small Cell Lung Cancer (Version 3, 2020). Available online: https://www2.tri-kobe.org/nccn/guideline/lung/english/non_small.pdf (accessed on 10 December 2021).
- Evison, M.; Clive, A.; Castle, L.; Powell, H.; Thomas, R.; Buttery, R.; Masani, V.; Harden, S.; West, D.; Woolhouse, I. Resectable Clinical N2 Non–Small Cell Lung Cancer; What Is the Optimal Treatment Strategy? An Update by the British Thoracic Society Lung Cancer Specialist Advisory Group. J. Thorac. Oncol. 2017, 12, 1434–1441. [Google Scholar] [CrossRef] [Green Version]
- Glatzer, M.; Leskow, P.; Caparrotti, F.; Elicin, O.; Furrer, M.; Gambazzi, F.; Dutly, A.; Gelpke, H.; Guckenberger, M.; Heuberger, J.; et al. Stage III N2 non-small cell lung cancer treatment: Decision-making among surgeons and radiation oncologists. Transl. Lung Cancer Res. 2021, 10, 1960–1968. [Google Scholar] [CrossRef]
- Reuss, J.E.; Anagnostou, V.; Cottrell, T.R.; Smith, K.N.; Verde, F.; Zahurak, M.; Lanis, M.; Murray, J.C.; Chan, H.Y.; McCarthy, C.; et al. Neoadjuvant nivolumab plus ipilimumab in resectable non-small cell lung cancer. J. Immunother. Cancer 2020, 8, e001282. [Google Scholar] [CrossRef]
| Clinical Trial | Phase | Stage | Intervention | (Neo)adjuvant | Estimated Enrollment | Primary Endpoint |
|---|---|---|---|---|---|---|
| Immunotherapy Monotherapy | ||||||
| NCT04560686 | II | I-IIIB | Neoadjuvant Bintrafusp alfa | Neoadjuvant | 23 | MPR |
| NCT03197467 | II | II-IIIA | Neoadjuvant pembrolizumab | Neoadjuvant | 30 | pCR, tAEs, radiological response |
| NCT02818920 | II | IB-IIIA | Neoadjuvant and adjuvant pembrolizumab | (neo)adjuvant | 35 | Surgical feasibility |
| NCT04062708 | II | IIIA-IIIB N2 | Neoadjuvant durvalumab + CT, adjuvant durvalumab | neoadjuvant | 55 | N2 nodal clearance |
| NCT04379739 | II | II-IIIA | Neoadjuvant camrelizumab | neoadjuvant | 82 | MPR |
| Combination immunotherapy | ||||||
| NCT03794544 | II | I-IIIA | Neoadjuvant durvalumab vs. durvalumab + oleclumab or monalizumab or danvatirsen | Neoadjuvant | 160 | MPR |
| Immunotherapy + CT | ||||||
| NCT04512430 | II | IIIA | Neoadjuvant atezolizumab + bevacizumab + CT, adjuvant atezolizumab | (neo)adjuvant | 26 | MPR |
| NCT04326153 | II | IIIA | Neoadjuvant and adjuvant sintilumab + CT | (neo)adjuvant | 40 | DFS |
| NCT04061590 | II | I-IIIA | Neoadjuvant pembrolizumab vs. pembrolizumab + CT | Neoadjuvant | 84 | Increase tumour-infiltrating cells |
| NCT03838159 | II | IIIA-IIIB | Neoadjuvant Nivolumab + CT vs. CT, adjuvant nivolumab | Neoadjuvant | 90 | pCR |
| NCT03800134 | III | II-III | Neoadjuvant Durvalumab + CT vs. CT, adjuvant durvalumab | (neo)adjuvant | 300 | MPR |
| NCT03456063 | III | II-IIIB | Neoadjuvant Atezolizumab + CT vs. placebo + CT | neoadjuvant | 374 | EFS |
| NCT04025879 | III | II-IIIB | Neoadjuvant Nivolumab + CT vs. placebo + CT, adjuvant nivolumab vs. placebo | (neo)adjuvant | 452 | EFS |
| NCT02998528 | III | IB-IIIA | Neoadjuvant CT + nivolumab vs. CT vs. nivolumab + ipilimumab | neoadjuvant | 350 | EFS |
| Immunotherapy + RT | ||||||
| NCT03237377 | II | IIIA | Neoadjuvant durvalumab + RT vs. durvalumab + tremelimumab + RT | Neoadjuvant | 32 | Toxicity and feasibility |
| NCT03217071 | II | I-IIIA | Neoadjuvant Pembrolizumab vs. pembrolizumab + RT | Neoadjuvant | 40 | Change in numbers of infiltrating CD3+ T-cells |
| NCT04245514 | II | III (N2) | Neoadjuvant durvalumab + RT + CT, adjuvant durvalumab + RT | (Neo)adjuvant | 90 | EFS |
| Immunotherapy + CRT | ||||||
| NCT03871153 | II | IIIA-N2 | Neoadjuvant durvalumab + CRT, adjuvant durvalumab | (neo)adjuvant | 25 | pCR |
| NCT02987998 | I | IIIA-N2 | Neoadjuvant pembrolizumab + CRT, adjuvant pembrolizumab | (neo)adjuvant | 9 | safety |
| NCT03694236 | II | III | Neoadjuvant durvalumab + CRT | Neoadjuvant | 39 | pCR |
| NCT04202809 | II | III | Neoadjuvant durvalumab + CRT vs. neoadjuvant CRT, adjuvant durvalumab | (neo)adjuvant | 90 | PFS |
| Targeted therapy | ||||||
| NCT03433469 | II | I-IIIA | Neoadjuvant osimertinib | Neoadjuvant | 27 | MPR |
| NCT04302025 | II | IIA-IIIB | Neoadjuvant and adjuvant alectinib or entrectinib or vemurafenib and cobimetinib or pralsetinib | (Neo)adjuvant | 60 | MPR |
| NCT04351555 | III | II-IIIB N2 | Neoadjuvant osimertinib vs. Osimertinib + CT vs. CT | Neoadjuvant | 328 | MPR |
| Clinical Trial | Phase | Stage | Intervention | Estimated Enrollment | Primary Endpoint |
|---|---|---|---|---|---|
| Immunotherapy + RT | |||||
| NCT04013542 | I | Unresectable II-III | nivolumab and ipilimumab + RT | 20 | Safety |
| NCT03523702 | II | Unresectable II-III | Pembrolizumab + RT (if PD-L1 > 50%) vs. CRT (if PD-L1 < 50%) | 63 | PFS |
| NCT03644823 | II | Unresectable III-IV | Atezolizumab + RT | 21 | safety |
| Immunotherapy + CRT | |||||
| NCT03631784 | II | Unresectable III | Pembrolizumab + CRT | 217 | Safety, ORR |
| NCT03102242 | II | Unresectable III | Atezolizumab + CRT | 64 | DCR |
| NCT04287894 | IB | Unresectable II or stage III | Durvalumab + tremelimumab + CRT | 34 | Safety |
| NCT03663166 | I/II | Unresectable III | Ipilimumab + CRT, consolidation nivolumab | 19 | Safety, PFS |
| NCT04026412 | III | Unresectable III | nivolumab + CRT, consolidation nivolumab and ipilimumab vs. nivolumab + CRT, consolidation nivolumab vs. CRT, consolidation durvalumab | 888 | PFS, OS |
| NCT03285321 | II | Unresectable III | CRT, consolidation nivolumab vs. nivolumab and ipilimumab | 108 | PFS |
| NCT03693300 | II | Unresectable III | CRT, consolidation durvalumab | 117 | Safety |
| NCT04380636 | III | Unresectable III | CRT + pembrolizumab vs. CRT + pembrolizumab + olaparib vs. CRT + durvalumab | 870 | PFS, OS |
| NCT03589547 | II | III | CRT, durvalumab, RT, durvalumab | 25 | Safety, PFS |
| NCT04085250 | II | Unresectable III | CT + nivolumab, CRT, consolidation nivolumab vs. observation | 264 | PFS |
| NCT04085250 | II | Unresectable III | CRT + atezolizumab vs. consolidation atezolizumab | 52 | PFS |
| NCT04092283 | III | Unresectable III | CRT + durvalumab vs. consolidation durvalumab | 660 | OS |
| NCT03519971 | III | Unresectable III | CRT + durvalumab vs. CRT + placebo | 328 | PFS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allaeys, T.; Berzenji, L.; Lauwers, P.; Yogeswaran, S.K.; Hendriks, J.M.H.; Billiet, C.; De Bondt, C.; Van Schil, P.E. Multimodality Treatment including Surgery Related to the Type of N2 Involvement in Locally Advanced Non-Small Cell Lung Cancer. Cancers 2022, 14, 1656. https://doi.org/10.3390/cancers14071656
Allaeys T, Berzenji L, Lauwers P, Yogeswaran SK, Hendriks JMH, Billiet C, De Bondt C, Van Schil PE. Multimodality Treatment including Surgery Related to the Type of N2 Involvement in Locally Advanced Non-Small Cell Lung Cancer. Cancers. 2022; 14(7):1656. https://doi.org/10.3390/cancers14071656
Chicago/Turabian StyleAllaeys, Toon, Lawek Berzenji, Patrick Lauwers, Suresh Krishan Yogeswaran, Jeroen M. H. Hendriks, Charlotte Billiet, Charlotte De Bondt, and Paul E. Van Schil. 2022. "Multimodality Treatment including Surgery Related to the Type of N2 Involvement in Locally Advanced Non-Small Cell Lung Cancer" Cancers 14, no. 7: 1656. https://doi.org/10.3390/cancers14071656
APA StyleAllaeys, T., Berzenji, L., Lauwers, P., Yogeswaran, S. K., Hendriks, J. M. H., Billiet, C., De Bondt, C., & Van Schil, P. E. (2022). Multimodality Treatment including Surgery Related to the Type of N2 Involvement in Locally Advanced Non-Small Cell Lung Cancer. Cancers, 14(7), 1656. https://doi.org/10.3390/cancers14071656







