Impact of Histotripsy on Development of Intrahepatic Metastases in a Rodent Liver Tumor Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. HCC Cell Culture
2.3. Animals
2.4. Orthotopic Tumor Implantation
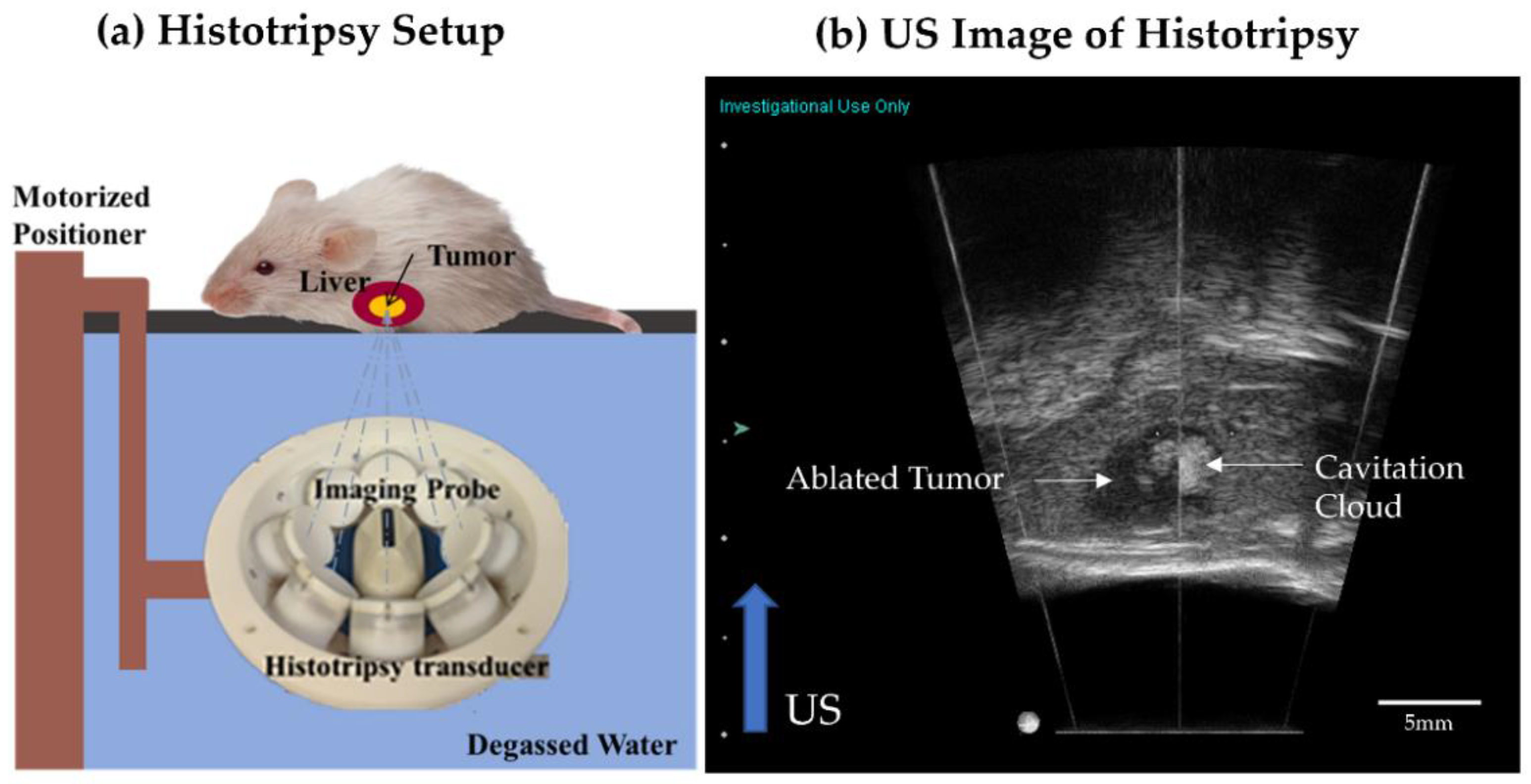
2.5. Histotripsy Ablation
2.6. MR Imaging
2.7. Histology
2.8. Immunofluorescence Staining
2.9. Statistical Analysis
3. Results
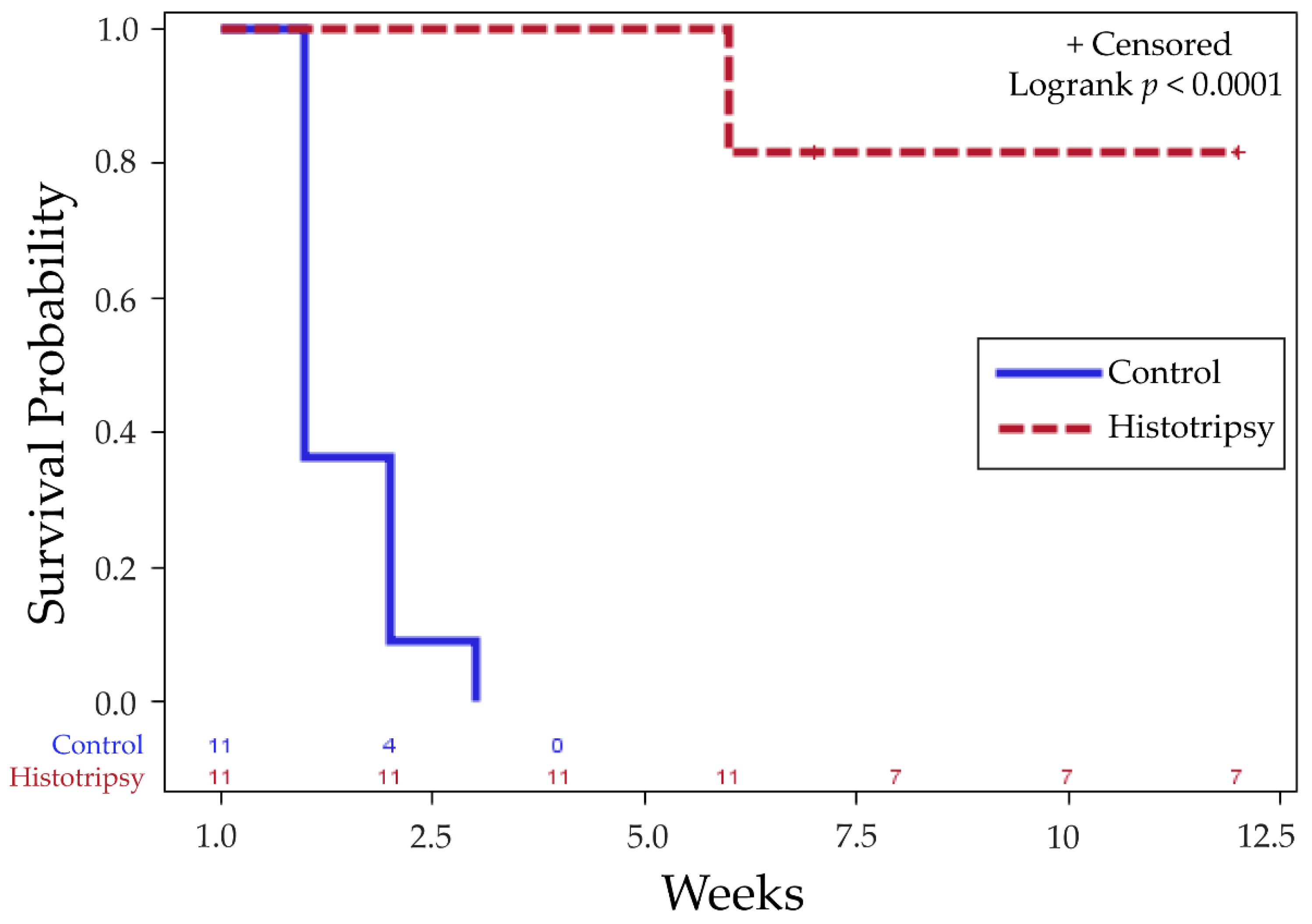
3.1. Tumor Response to Histotripsy and Survival Outcomes
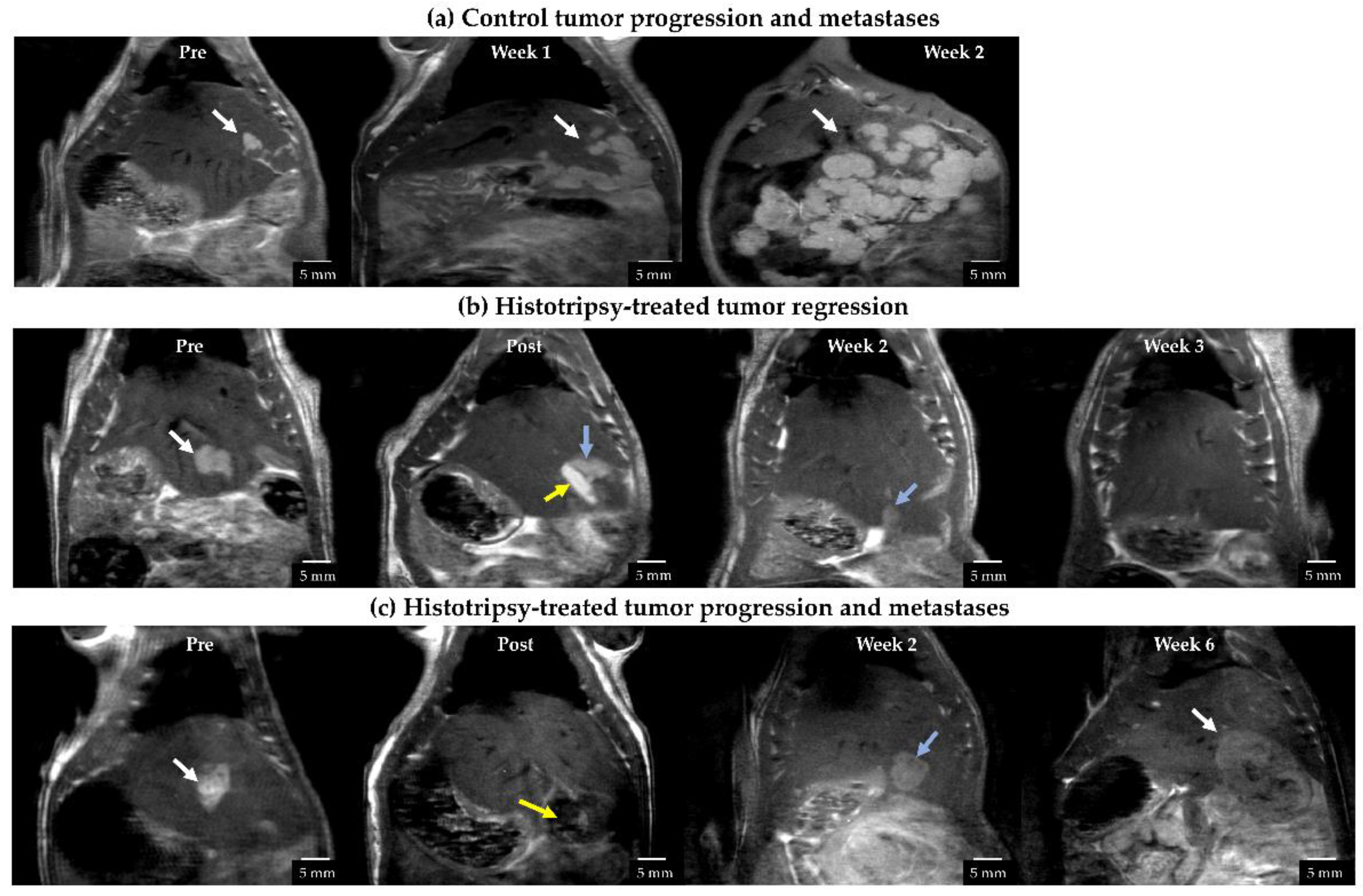
3.2. Radiology Observations
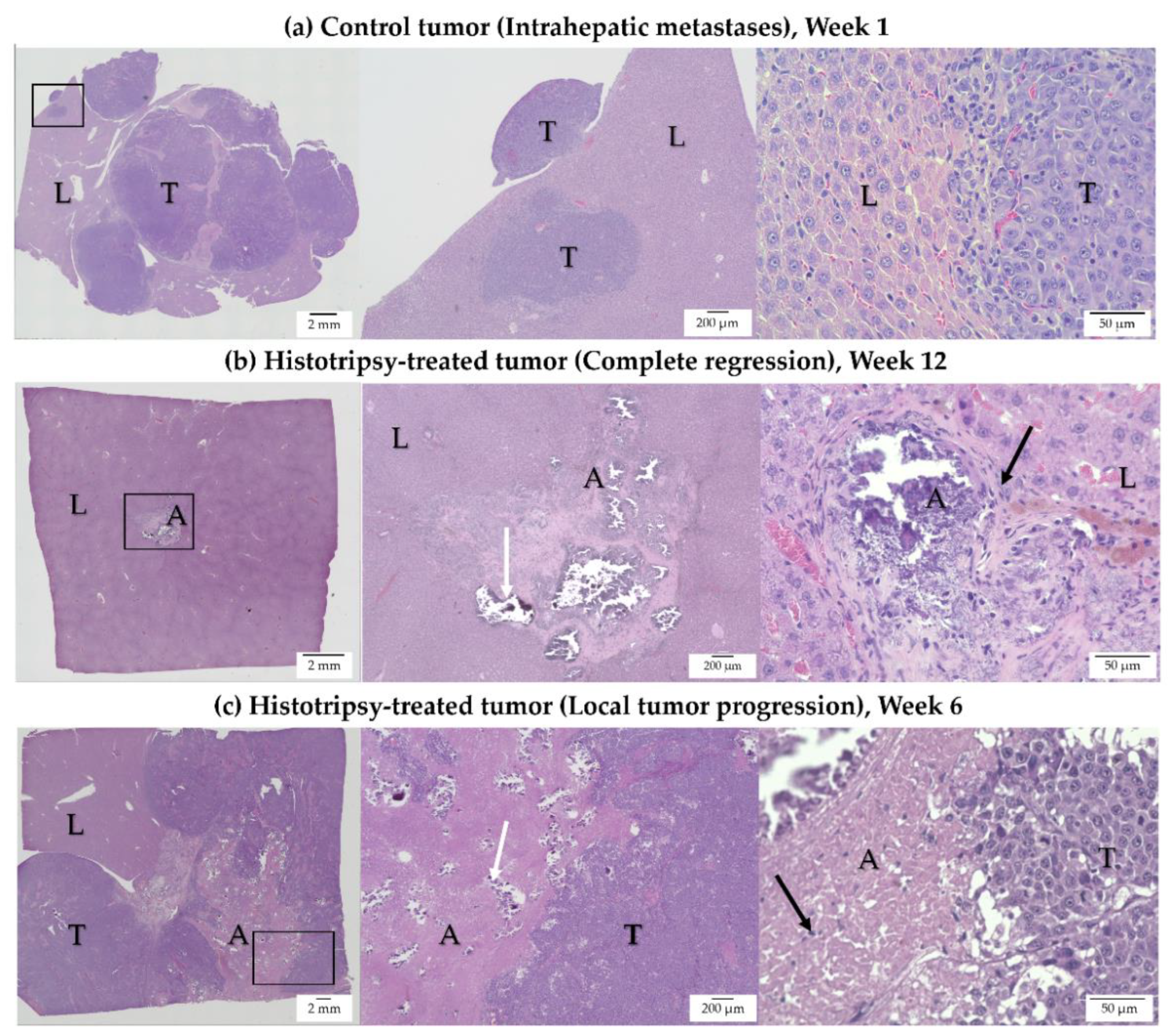
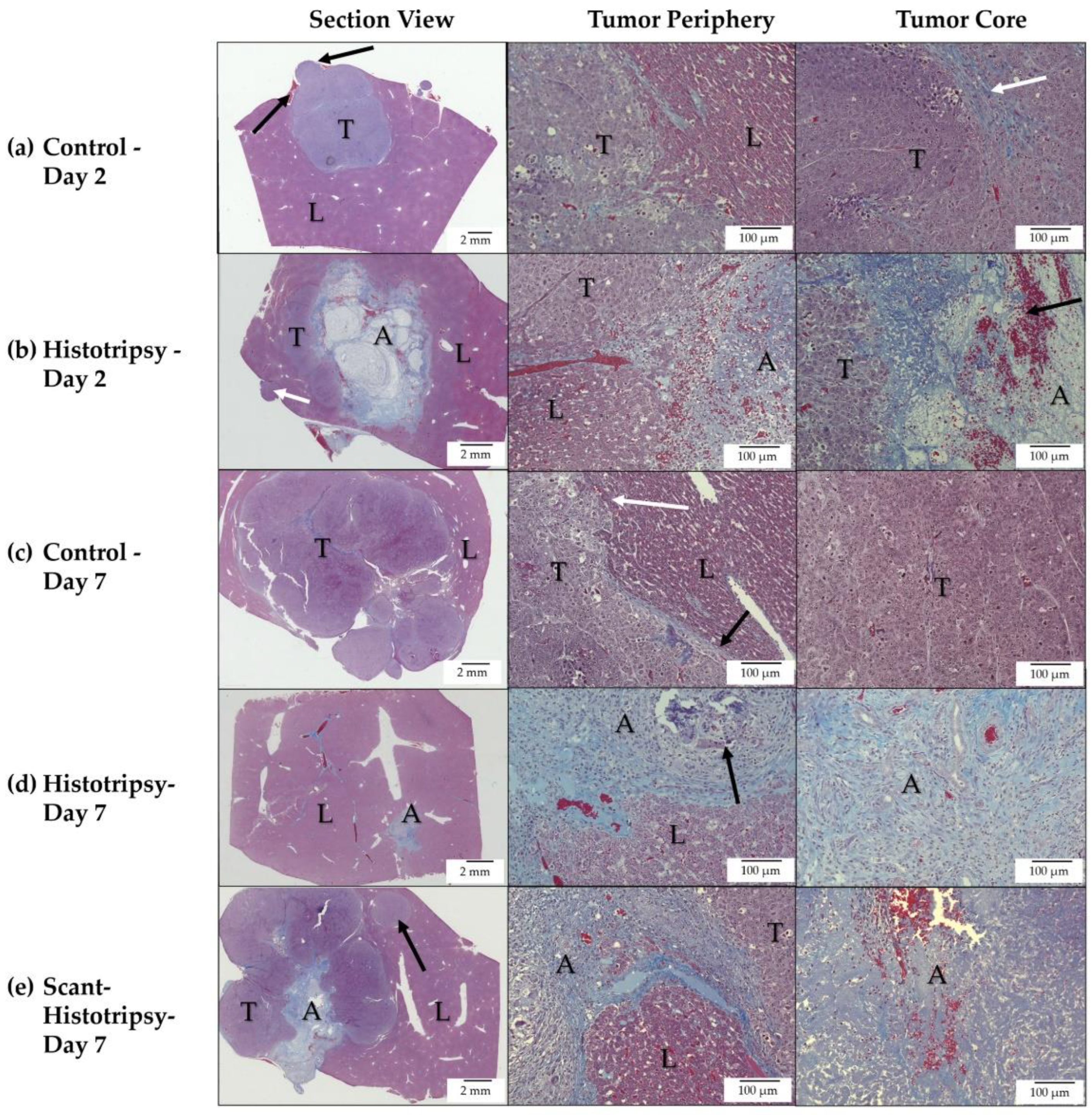
3.3. Histology Observations in Survival Groups
3.4. Histology Observations in Early Timepoint Groups
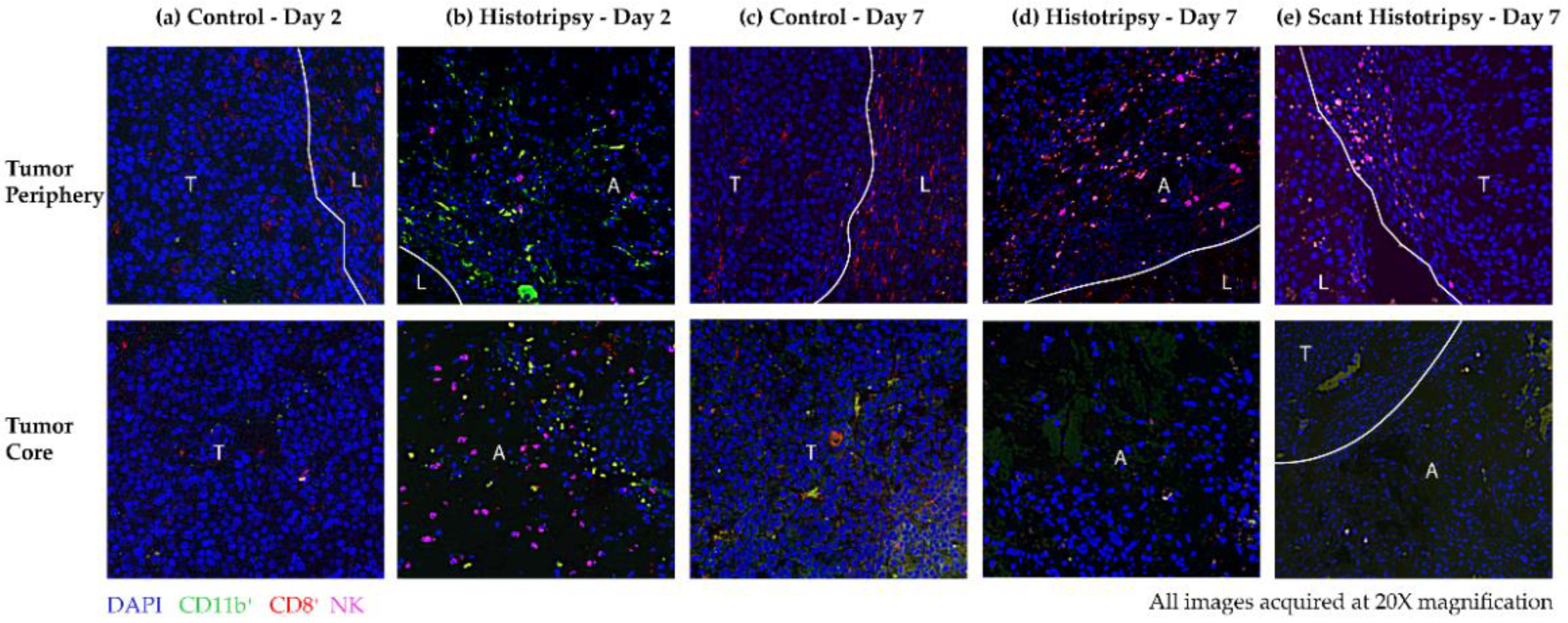
3.5. Observations from Immunoflourescence Staining of Immune Cells
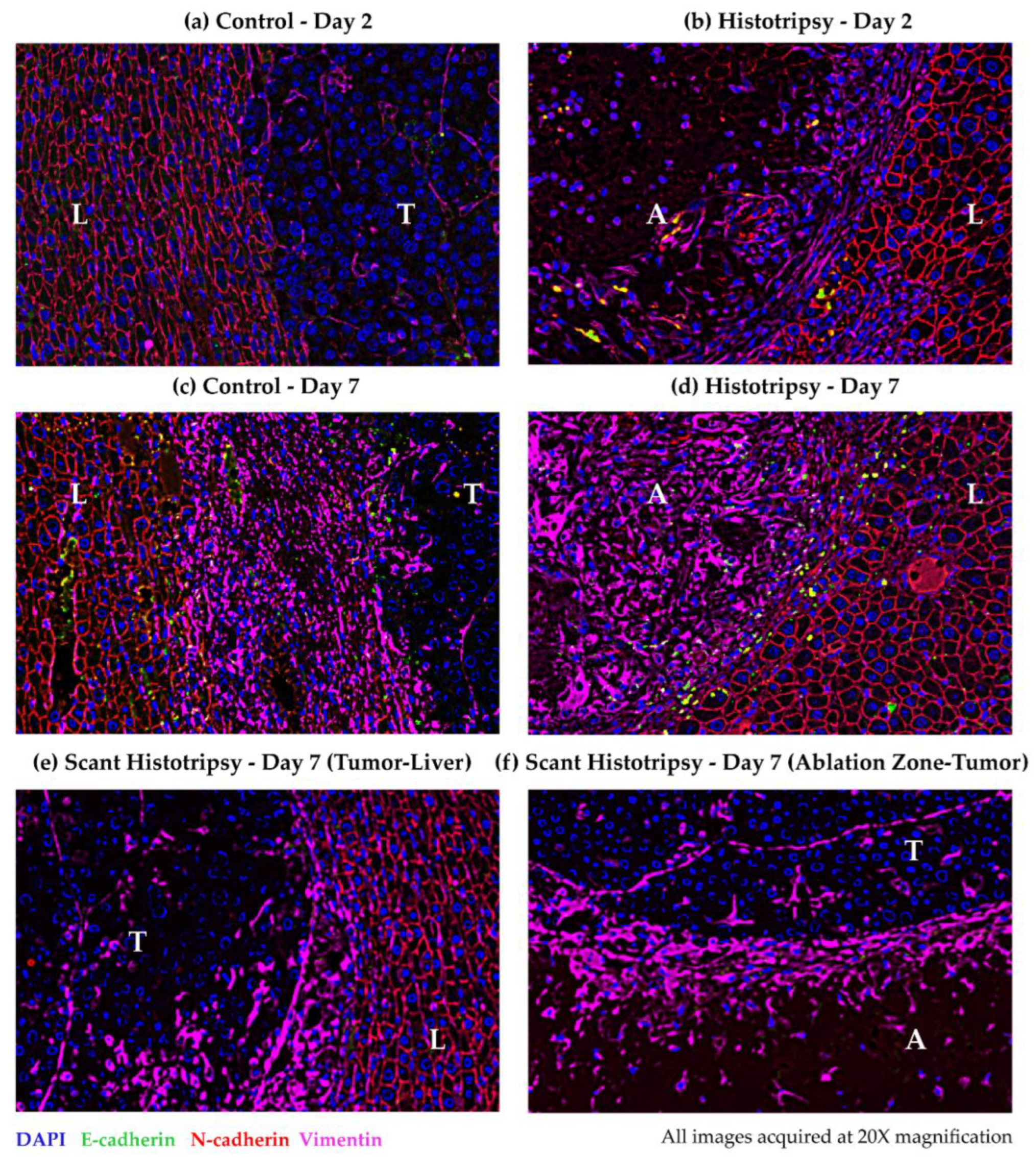
3.6. Observations for Immunoflourescence Staining for Epithelial and Mesenchymal Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef] [PubMed]
- de Ridder, J.; de Wilt, J.H.; Simmer, F.; Overbeek, L.; Lemmens, V.; Nagtegaal, I. Incidence and origin of histologically confirmed liver metastases: An explorative case-study of 23,154 patients. Oncotarget 2016, 7, 55368–55376. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e1. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures 2021; 2021; Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf (accessed on 10 October 2021).
- Portolani, N.; Coniglio, A.; Ghidoni, S.; Giovanelli, M.; Benetti, A.; Tiberio, G.A.; Giulini, S.M. Early and late recurrence after liver resection for hepatocellular carcinoma: Prognostic and therapeutic implications. Ann. Surg. 2006, 243, 229–235. [Google Scholar] [CrossRef]
- Leone, K.; Poggiana, C.; Zamarchi, R. The Interplay between Circulating Tumor Cells and the Immune System: From Immune Escape to Cancer Immunotherapy. Diagnostics 2018, 8, 59. [Google Scholar] [CrossRef]
- Steeg, P.S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006, 12, 895–904. [Google Scholar] [CrossRef]
- Xu, Z.; Ludomirsky, A.; Eun, L.Y.; Hall, T.L.; Tran, B.C.; Fowlkes, J.B.; Cain, C.A. Controlled ultrasound tissue erosion. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 726–736. [Google Scholar] [CrossRef]
- Parsons, J.E.; Cain, C.A.; Abrams, G.D.; Fowlkes, J.B. Pulsed cavitational ultrasound therapy for controlled tissue homogenization. Ultrasound Med. Biol. 2006, 32, 115–129. [Google Scholar] [CrossRef]
- Roberts, W.W.; Hall, T.L.; Ives, K.; Wolf, J.S.; Fowlkes, J.B., Jr.; Cain, C.A. Pulsed cavitational ultrasound: A noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J. Urol. 2006, 175, 734–738. [Google Scholar] [CrossRef]
- Wang, Y.N.; Khokhlova, T.; Bailey, M.; Hwang, J.H.; Khokhlova, V. Histological and biochemical analysis of mechanical and thermal bioeffects in boiling histotripsy lesions induced by high intensity focused ultrasound. Ultrasound Med. Biol. 2013, 39, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Winterroth, F.; Xu, Z.; Wang, T.Y.; Wilkinson, J.E.; Fowlkes, J.B.; Roberts, W.W.; Cain, C.A. Examining and analyzing subcellular morphology of renal tissue treated by histotripsy. Ultrasound Med. Biol. 2011, 37, 78–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hall, T.L.; Kieran, K.; Ives, K.; Fowlkes, J.B.; Cain, C.A.; Roberts, W.W. Histotripsy of rabbit renal tissue in vivo: Temporal histologic trends. J. Endourol. 2007, 21, 1159–1166. [Google Scholar] [CrossRef]
- Vlaisavljevich, E.; Kim, Y.; Allen, S.; Owens, G.; Pelletier, S.; Cain, C.; Ives, K.; Xu, Z. Image-guided non-invasive ultrasound liver ablation using histotripsy: Feasibility study in an in vivo porcine model. Ultrasound Med. Biol. 2013, 39, 1398–1409. [Google Scholar] [CrossRef]
- Smolock, A.R.; Cristescu, M.M.; Vlaisavljevich, E.; Gendron-Fitzpatrick, A.; Green, C.; Cannata, J.; Ziemlewicz, T.J.; Lee, F.T. Robotically Assisted Sonic Therapy as a Noninvasive Nonthermal Ablation Modality: Proof of Concept in a Porcine Liver Model. Radiology 2018, 287, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Knott, E.A.; Swietlik, J.F.; Longo, K.C.; Watson, R.F.; Green, C.M.; Abel, E.J.; Lubner, M.G.; Hinshaw, J.L.; Smolock, A.R.; Xu, Z.; et al. Robotically-Assisted Sonic Therapy for Renal Ablation in a Live Porcine Model: Initial Preclinical Results. J. Vasc. Interv. Radiol. 2019, 30, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Longo, K.C.; Knott, E.A.; Watson, R.F.; Swietlik, J.F.; Vlaisavljevich, E.; Smolock, A.R.; Xu, Z.; Cho, C.S.; Mao, L.; Lee, F.T.; et al. Robotically Assisted Sonic Therapy (RAST) for Noninvasive Hepatic Ablation in a Porcine Model: Mitigation of Body Wall Damage with a Modified Pulse Sequence. Cardiovasc. Intervent. Radiol. 2019, 42, 1016–1023. [Google Scholar] [CrossRef]
- Vlaisavljevich, E.; Owens, G.; Lundt, J.; Teofilovic, D.; Ives, K.; Duryea, A.; Bertolina, J.; Welling, T.H.; Xu, Z. Non-Invasive Liver Ablation Using Histotripsy: Preclinical Safety Study in an In Vivo Porcine Model. Ultrasound Med. Biol. 2017, 43, 1237–1251. [Google Scholar] [CrossRef]
- Qu, S.; Worlikar, T.; Felsted, A.E.; Ganguly, A.; Beems, M.V.; Hubbard, R.; Pepple, A.L.; Kevelin, A.A.; Garavaglia, H.; Dib, J.; et al. Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000200. [Google Scholar] [CrossRef]
- Worlikar, T.; Vlaisavljevich, E.; Gerhardson, T.; Greve, J.; Wan, S.; Kuruvilla, S.; Lundt, J.; Ives, K.; Hall, T.L.; Welling, T.H.; et al. Histotripsy for Non-Invasive Ablation of Hepatocellular Carcinoma (HCC) Tumor in a Subcutaneous Xenograft Murine Model. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2018, 2018, 6064–6067. [Google Scholar] [CrossRef]
- Worlikar, T.; Mendiratta-Lala, M.; Vlaisavljevich, E.; Hubbard, R.; Shi, J.; Hall, T.L.; Cho, C.S.; Lee, F.T.; Greve, J.M.; Xu, Z. Effects of Histotripsy on Local Tumor Progression in an in vivo Orthotopic Rodent Liver Tumor Model. BME Front. 2020, 2020, 9830304. [Google Scholar] [CrossRef] [PubMed]
- Styn, N.R.; Hall, T.L.; Fowlkes, J.B.; Cain, C.A.; Roberts, W.W. Histotripsy of renal implanted VX-2 tumor in a rabbit model: Investigation of metastases. Urology 2012, 80, 724–729. [Google Scholar] [CrossRef]
- Schade, G.R.; Keller, J.; Ives, K.; Cheng, X.; Rosol, T.J.; Keller, E.; Roberts, W.W. Histotripsy focal ablation of implanted prostate tumor in an ACE-1 canine cancer model. J. Urol. 2012, 188, 1957–1964. [Google Scholar] [CrossRef] [PubMed]
- Mancia, L.; Vlaisavljevich, E.; Xu, Z.; Johnsen, E. Predicting Tissue Susceptibility to Mechanical Cavitation Damage in Therapeutic Ultrasound. Ultrasound Med. Biol. 2017, 43, 1421–1440. [Google Scholar] [CrossRef]
- Vidal-Jove, J.; Serres-Creixams, X.; Ziemlewicz, T.J.; Cannata, J.M. Liver Histotripsy Mediated Abscopal Effect-Case Report. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 3001–3005. [Google Scholar] [CrossRef]
- Vlaisavljevich, E.; Greve, J.; Cheng, X.; Ives, K.; Shi, J.; Jin, L.; Arvidson, A.; Hall, T.L.; Welling, T.H.; Owens, G.; et al. Non-Invasive Ultrasound Liver Ablation Using Histotripsy: Chronic Study in an In Vivo Rodent Model. Ultrasound Med. Biol. 2016, 42, 1890–1902. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, A.D.; Cain, C.A.; Hall, T.L.; Fowlkes, J.B.; Xu, Z. Probability of cavitation for single ultrasound pulses applied to tissues and tissue-mimicking materials. Ultrasound Med. Biol. 2013, 39, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Vlaisavljevich, E.; Maxwell, A.; Warnez, M.; Johnsen, E.; Cain, C.; Xu, Z. Histotripsy-induced cavitation cloud initiation thresholds in tissues of different mechanical properties. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 341–352. [Google Scholar] [CrossRef]
- Zhang, W.; Qian, S.; Yang, G.; Zhu, L.; Zhou, B.; Qu, X.; Yan, Z.; Liu, R.; Wang, J. Establishment and characterization of McA-RH7777 cells using virus-mediated stable overexpression of enhanced green fluorescent protein. Exp. Ther. Med. 2018, 16, 3149–3154. [Google Scholar] [CrossRef]
- Wang, G.Z.; Fang, Z.T.; Zhang, W.; Qu, X.D.; Qian, S.; Liu, R.; Wang, J.H. Increased metastatic potential of residual carcinoma after transarterial embolization in rat with McA-RH7777 hepatoma. Oncol. Rep. 2014, 31, 95–102. [Google Scholar] [CrossRef][Green Version]
- van Zijl, F.; Krupitza, G.; Mikulits, W. Initial steps of metastasis: Cell invasion and endothelial transmigration. Mutat. Res. 2011, 728, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Paget, S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar] [CrossRef]
- van Zijl, F.; Zulehner, G.; Petz, M.; Schneller, D.; Kornauth, C.; Hau, M.; Machat, G.; Grubinger, M.; Huber, H.; Mikulits, W. Epithelial-mesenchymal transition in hepatocellular carcinoma. Future Oncol. 2009, 5, 1169–1179. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T.; Jung, A.; Reu, S.; Porzner, M.; Hlubek, F.; Kunz-Schughart, L.A.; Knuechel, R.; Kirchner, T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl. Acad. Sci. USA 2001, 98, 10356–10361. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 2021, 10, 1587. [Google Scholar] [CrossRef]
- Yu, K.; Li, Q.; Shi, G.; Li, N. Involvement of epithelial-mesenchymal transition in liver fibrosis. Saudi J. Gastroenterol. 2018, 24, 5–11. [Google Scholar] [CrossRef]
- Zeisberg, M.; Yang, C.; Martino, M.; Duncan, M.B.; Rieder, F.; Tanjore, H.; Kalluri, R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J. Biol. Chem. 2007, 282, 23337–23347. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Zhu, R.T.; Sun, Y.L. Epithelial-mesenchymal transition in liver fibrosis. Biomed. Rep. 2016, 4, 269–274. [Google Scholar] [CrossRef]
- Walker, J.L.; Bleaken, B.M.; Romisher, A.R.; Alnwibit, A.A.; Menko, A.S. In wound repair vimentin mediates the transition of mesenchymal leader cells to a myofibroblast phenotype. Mol. Biol. Cell 2018, 29, 1555–1570. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomiccanic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Vakkila, J.; Lotze, M.T. Inflammation and necrosis promote tumour growth. Nat. Rev. Immunol. 2004, 4, 641–648. [Google Scholar] [CrossRef]
- Smyth, M.J.; Dunn, G.P.; Schreiber, R.D. Cancer immunosurveillance and immunoediting: The roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv. Immunol. 2006, 90, 1–50. [Google Scholar] [CrossRef]
- Lin, W.W.; Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Investig. 2007, 117, 1175–1183. [Google Scholar] [CrossRef]
- Schmid, M.C.; Khan, S.Q.; Kaneda, M.M.; Pathria, P.; Shepard, R.; Louis, T.L.; Anand, S.; Woo, G.; Leem, C.; Faridi, M.H.; et al. Integrin CD11b activation drives anti-tumor innate immunity. Nat. Commun. 2018, 9, 5379. [Google Scholar] [CrossRef]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell. Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef]
- Wu, J.; Lanier, L.L. Natural killer cells and cancer. Adv. Cancer Res. 2003, 90, 127–156. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Fares, Y. Immune checkpoint inhibitors: Advances and impact in neuro-oncology. Surg. Neurol. Int. 2019, 10, 9. [Google Scholar] [CrossRef]
- Mlecnik, B.; Bindea, G.; Kirilovsky, A.; Angell, H.K.; Obenauf, A.C.; Tosolini, M.; Church, S.E.; Maby, P.; Vasaturo, A.; Angelova, M.; et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci. Transl. Med. 2016, 8, 327ra26. [Google Scholar] [CrossRef]
- Takaki, H.; Cornelis, F.; Kako, Y.; Kobayashi, K.; Kamikonya, N.; Yamakado, K. Thermal ablation and immunomodulation: From preclinical experiments to clinical trials. Diagn. Interv. Imaging 2017, 98, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Slovak, R.; Ludwig, J.M.; Gettinger, S.N.; Herbst, R.S.; Kim, H.S. Immuno-thermal ablations—Boosting the anticancer immune response. J. Immunother. Cancer 2017, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Shen, Y.; Xie, J.; Meng, Z. Immunomodulatory effects of ablation therapy on tumors: Potentials for combination with immunotherapy. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188385. [Google Scholar] [CrossRef]
- Haen, S.P.; Pereira, P.L.; Salih, H.R.; Rammensee, H.G.; Gouttefangeas, C. More than just tumor destruction: Immunomodulation by thermal ablation of cancer. Clin. Dev. Immunol. 2011, 2011, 160250. [Google Scholar] [CrossRef] [PubMed]
- Rozenblum, N.; Zeira, E.; Bulvik, B.; Gourevitch, S.; Yotvat, H.; Galun, E.; Goldberg, S.N. Radiofrequency Ablation: Inflammatory Changes in the Periablative Zone Can Induce Global Organ Effects, including Liver Regeneration. Radiology 2015, 276, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, J.; Ding, N.; Zhang, Y.; Zhu, Y.; Dong, S.; Wang, X.; Peng, C.; Zhou, C.; Zhou, L.; et al. Inflammation induced by incomplete radiofrequency ablation accelerates tumor progression and hinders PD-1 immunotherapy. Nat. Commun. 2019, 10, 5421. [Google Scholar] [CrossRef]
- Diehl, R.; Ferrara, F.; Müller, C.; Dreyer, A.Y.; McLeod, D.D.; Fricke, S.; Boltze, J. Immunosuppression for in vivo research: State-of-the-art protocols and experimental approaches. Cell Mol. Immunol. 2017, 14, 146–179. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Worlikar, T.; Zhang, M.; Ganguly, A.; Hall, T.L.; Shi, J.; Zhao, L.; Lee, F.T.; Mendiratta-Lala, M.; Cho, C.S.; Xu, Z. Impact of Histotripsy on Development of Intrahepatic Metastases in a Rodent Liver Tumor Model. Cancers 2022, 14, 1612. https://doi.org/10.3390/cancers14071612
Worlikar T, Zhang M, Ganguly A, Hall TL, Shi J, Zhao L, Lee FT, Mendiratta-Lala M, Cho CS, Xu Z. Impact of Histotripsy on Development of Intrahepatic Metastases in a Rodent Liver Tumor Model. Cancers. 2022; 14(7):1612. https://doi.org/10.3390/cancers14071612
Chicago/Turabian StyleWorlikar, Tejaswi, Man Zhang, Anutosh Ganguly, Timothy L. Hall, Jiaqi Shi, Lili Zhao, Fred T. Lee, Mishal Mendiratta-Lala, Clifford S. Cho, and Zhen Xu. 2022. "Impact of Histotripsy on Development of Intrahepatic Metastases in a Rodent Liver Tumor Model" Cancers 14, no. 7: 1612. https://doi.org/10.3390/cancers14071612
APA StyleWorlikar, T., Zhang, M., Ganguly, A., Hall, T. L., Shi, J., Zhao, L., Lee, F. T., Mendiratta-Lala, M., Cho, C. S., & Xu, Z. (2022). Impact of Histotripsy on Development of Intrahepatic Metastases in a Rodent Liver Tumor Model. Cancers, 14(7), 1612. https://doi.org/10.3390/cancers14071612






