Different Nerve-Sparing Techniques during Radical Prostatectomy and Their Impact on Functional Outcomes
Abstract
Simple Summary
Abstract
1. Introduction
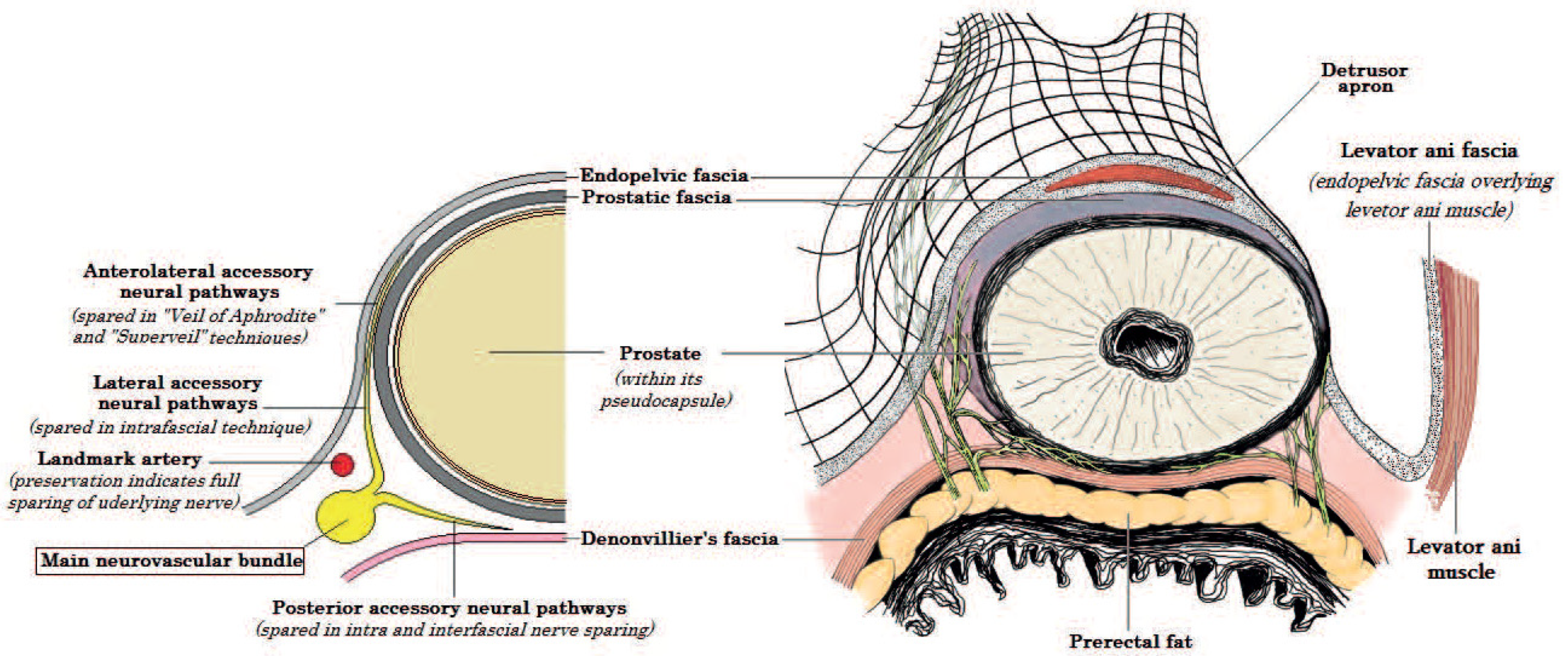
2. Anatomy of the NVBs
3. Basic Principles of NS Techniques
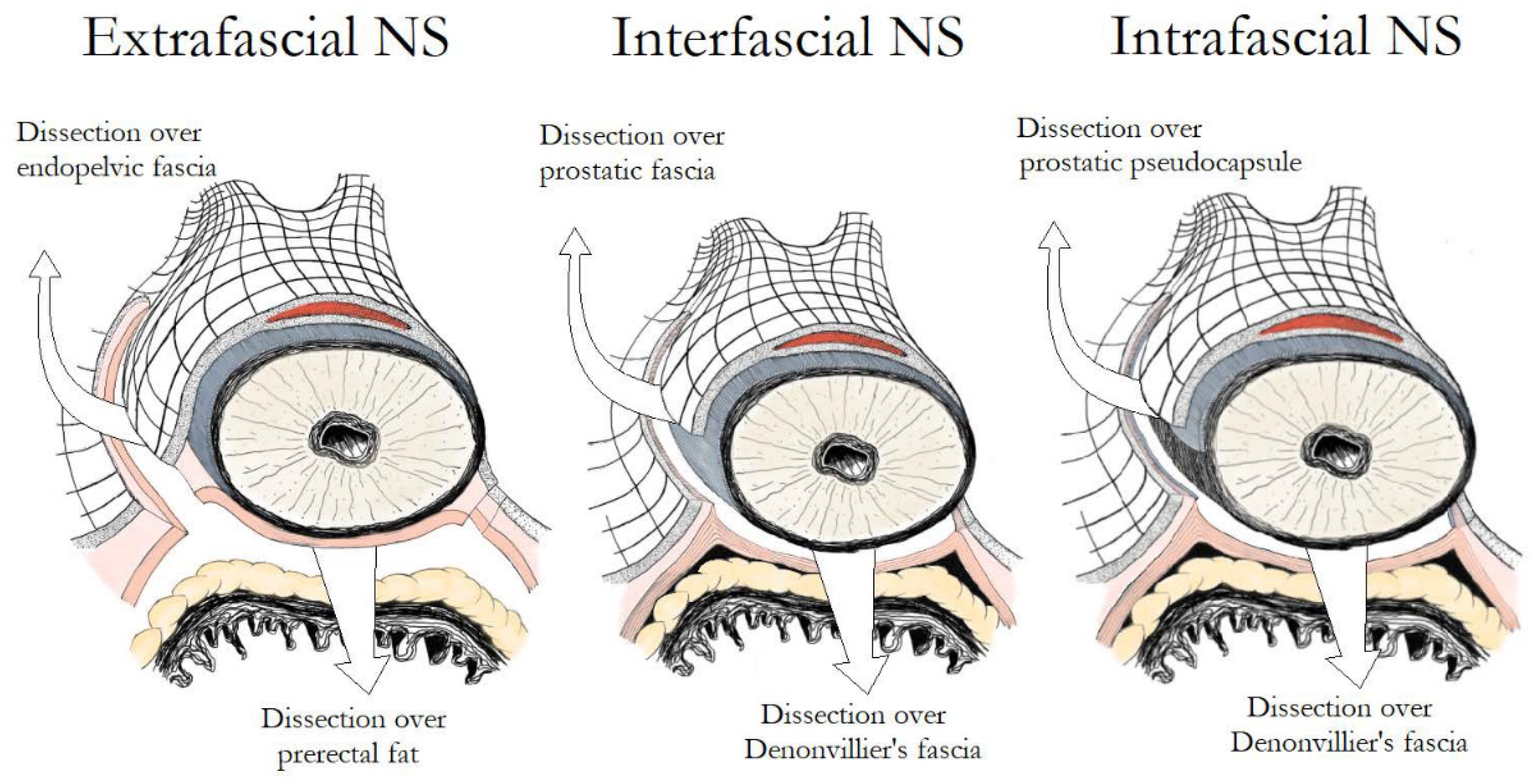
4. The Fascial Planes for NS
4.1. Intrafascial vs. Interfascial NS
4.2. Extrafascial vs. Non-Extrafascial (Intrafascial and Interfascial) NS
5. Different NS Grading Systems
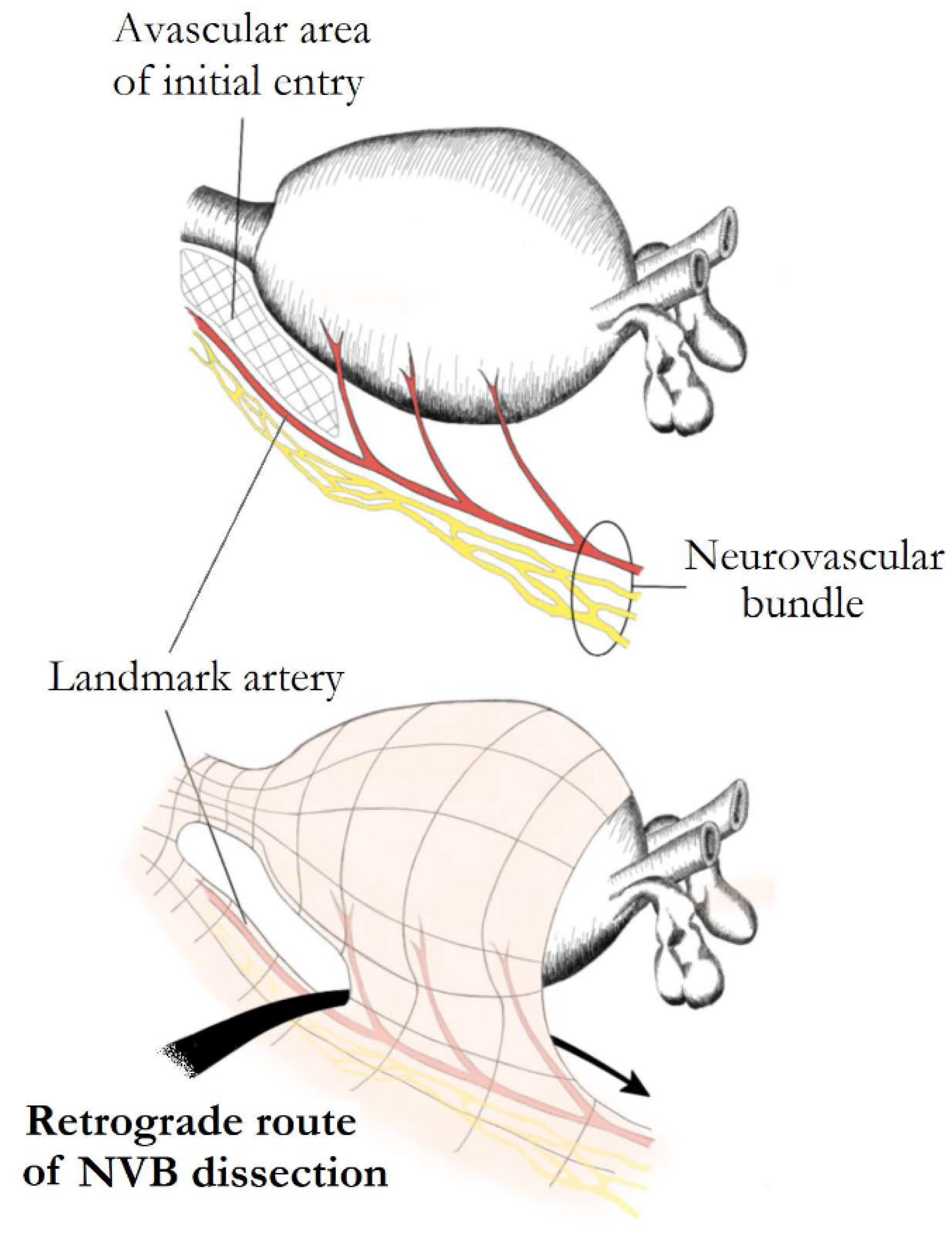
6. The Direction of NS
7. Unilateral versus Bilateral NS
8. The Functional Impact of Using Energy and Nerve Traction during NS RP
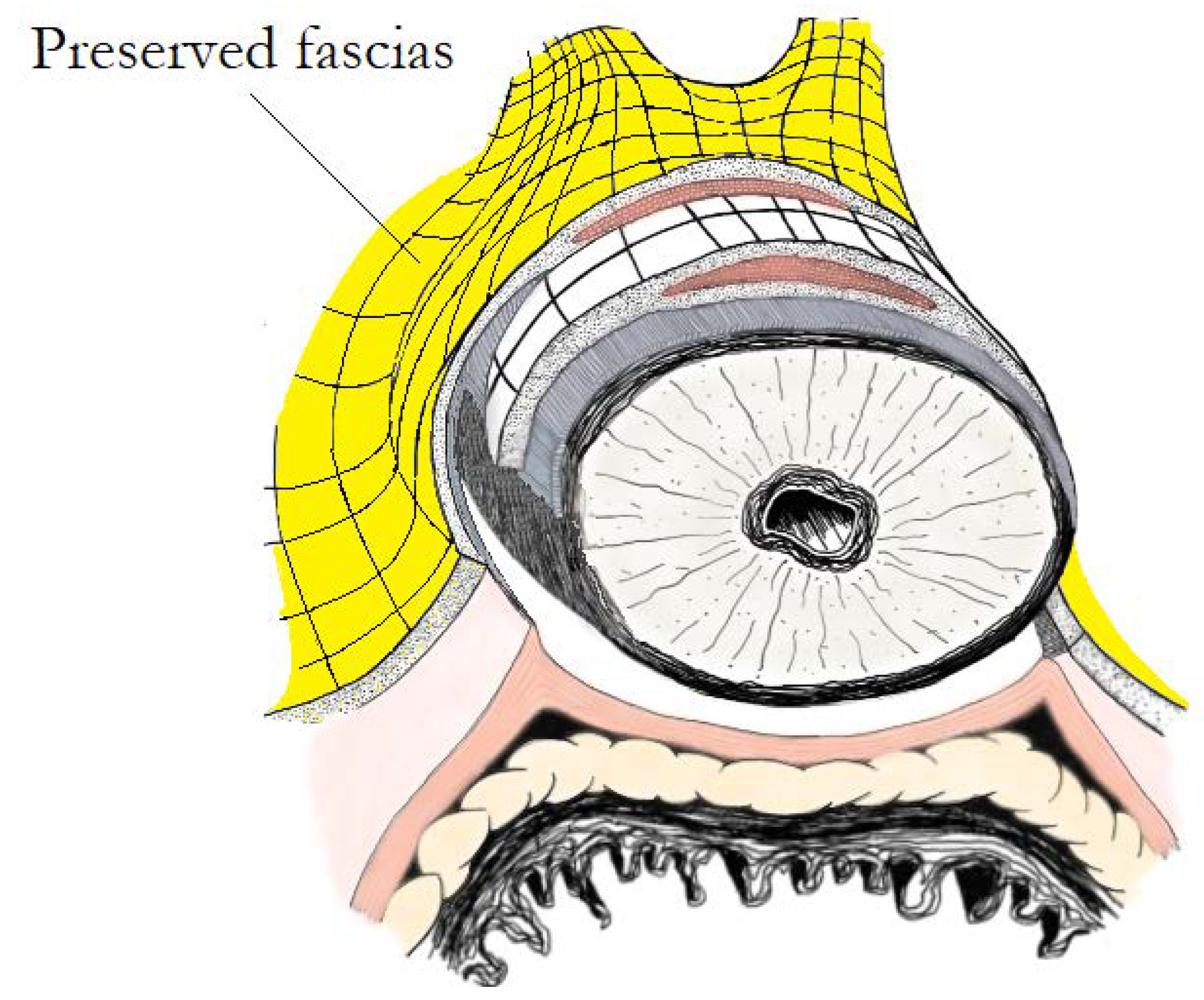
9. “Veil of Aphrodite” and “Super Veil” Technique
10. Hydrodissection of the NVBs
11. The Role of Lasers in NS
12. Retzius-Sparing RARP
13. Neurovascular Structure Adjacent Frozen Section Examination (NeuroSAFE) in NS
14. The Role of Near-Infrared Fluorescence (NIRF) and Indocyanine Green (ICG)
15. Bioengineering in NS
16. Devices for Identifying the Cavernous Nerves Intraoperatively
17. Discussion
18. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Eckhard, C. Untersuchungen über die Erection des beim Hunde. Anat. Physiol. 1863, 3, 123–166. [Google Scholar]
- Lepor, H.; Gregerman, M.; Crosby, R.; Mostofi, F.K.; Walsh, P.C. Precise localization of the autonomic nerves from the pelvic plexus to the corpora cavernosa: A detailed anatomical study of the adult male pelvis. J. Urol. 1985, 133, 207–212. [Google Scholar] [CrossRef]
- Walsh, P.C.; Donker, P.J. Impotence following radical prostatectomy: Insight into etiology and prevention. J. Urol. 1982, 128, 492–497. [Google Scholar] [CrossRef]
- Walsh, P.C.; Lepor, H.; Eggleston, J.C. Radical prostatectomy with preservation of sexual function: Anatomical and pathological considerations. Prostate 1983, 4, 473–485. [Google Scholar] [CrossRef]
- Stolzenburg, J.U.; Schwalenberg, T.; Horn, L.C.; Neuhaus, J.; Constantinides, C.; Liatsikos, E.N. Anatomical landmarks of radical prostatecomy. Eur. Urol. 2007, 51, 629–639. [Google Scholar] [CrossRef]
- Kumar, A.; Patel, V.R.; Panaiyadiyan, S.; Seetharam Bhat, K.R.; Moschovas, M.C.; Nayak, B. Nerve-sparing robot-assisted radical prostatectomy: Current perspectives. Asian J. Urol. 2021, 8, 2–13. [Google Scholar] [CrossRef]
- Costello, A.J.; Brooks, M.; Cole, O.J. Anatomical studies of the neurovascular bundle and cavernosal nerves. BJU Int. 2004, 94, 1071–1076. [Google Scholar] [CrossRef]
- Tewari, A.; Takenaka, A.; Mtui, E.; Horninger, W.; Peschel, R.; Bartsch, G.; Vaughan, E.D. The proximal neurovascular plate and the tri-zonal neural architecture around the prostate gland: Importance in the athermal robotic technique of nerve-sparing prostatectomy. BJU Int. 2006, 98, 314–323. [Google Scholar] [CrossRef]
- Savera, A.T.; Kaul, S.; Badani, K.; Stark, A.T.; Shah, N.L.; Menon, M. Robotic radical prostatectomy with the “Veil of Aphrodite” technique: Histologic evidence of enhanced nerve sparing. Eur. Urol. 2006, 49, 1065–1073. [Google Scholar] [CrossRef]
- Eichelberg, C.; Erbersdobler, A.; Michl, U.; Schlomm, T.; Salomon, G.; Graefen, M.; Huland, H. Nerve distribution along the prostatic capsule. Eur. Urol. 2007, 51, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.; Melamud, O.; Ahlering, T.E. Nerve-sparing techniques in open and laparoscopic prostatectomy. Expert Rev. Anticancer Ther. 2008, 8, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Coelho, R.F.; Rocco, B.; Palmer, K.J.; Orvieto, M.A.; Patel, V.R. Techniques of nerve-sparing and potency outcomes following robot-assisted laparoscopic prostatectomy. Int. Braz. J. Off. J. Braz. Soc. Urol. 2010, 36, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Stolzenburg, J.U.; Kallidonis, P.; Do, M.; Dietel, A.; Hafner, T.; Rabenalt, R.; Sakellaropoulos, G.; Ganzer, R.; Paasch, U.; Horn, L.C.; et al. A comparison of outcomes for interfascial and intrafascial nerve-sparing radical prostatectomy. Urology 2010, 76, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Khoder, W.Y.; Waidelich, R.; Buchner, A.; Becker, A.J.; Stief, C.G. Prospective comparison of one year follow-up outcomes for the open complete intrafascial retropubic versus interfascial nerve-sparing radical prostatectomy. SpringerPlus 2014, 3, 335. [Google Scholar] [CrossRef][Green Version]
- Potdevin, L.; Ercolani, M.; Jeong, J.; Kim, I.Y. Functional and oncologic outcomes comparing interfascial and intrafascial nerve sparing in robot-assisted laparoscopic radical prostatectomies. J. Endourol. 2009, 23, 1479–1484. [Google Scholar] [CrossRef]
- Curto, F.; Benijts, J.; Pansadoro, A.; Barmoshe, S.; Hoepffner, J.L.; Mugnier, C.; Piechaud, T.; Gaston, R. Nerve sparing laparoscopic radical prostatectomy: Our technique. Eur. Urol. 2006, 49, 344–352. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Guo, J.; Chen, H.; Weng, X.; Liu, X. Oncological safety of intrafascial nerve-sparing radical prostatectomy compared with conventional process: A pooled review and meta-regression analysis based on available studies. BMC Urol. 2019, 19, 41. [Google Scholar] [CrossRef]
- Weng, H.; Zeng, X.T.; Li, S.; Meng, X.Y.; Shi, M.J.; He, D.L.; Wang, X.H. Intrafascial versus interfascial nerve sparing in radical prostatectomy for localized prostate cancer: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 11454. [Google Scholar] [CrossRef]
- Shikanov, S.; Woo, J.; Al-Ahmadie, H.; Katz, M.H.; Zagaja, G.P.; Shalhav, A.L.; Zorn, K.C. Extrafascial versus interfascial nerve-sparing technique for robotic-assisted laparoscopic prostatectomy: Comparison of functional outcomes and positive surgical margins characteristics. Urology 2009, 74, 611–616. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhu, H.; Yu, H.; Kong, Q.; Fan, C.; Meng, L.; Liu, C.; Ding, X. Comparison of intrafascial and non-intrafascial radical prostatectomy for low risk localized prostate cancer. Sci. Rep. 2017, 7, 17604. [Google Scholar] [CrossRef] [PubMed]
- Montorsi, F.; Wilson, T.G.; Rosen, R.C.; Ahlering, T.E.; Artibani, W.; Carroll, P.R.; Costello, A.; Eastham, J.A.; Ficarra, V.; Guazzoni, G.; et al. Best practices in robot-assisted radical prostatectomy: Recommendations of the Pasadena Consensus Panel. Eur. Urol. 2012, 62, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Tewari, A.K.; Srivastava, A.; Huang, M.W.; Robinson, B.D.; Shevchuk, M.M.; Durand, M.; Sooriakumaran, P.; Grover, S.; Yadav, R.; Mishra, N.; et al. Anatomical grades of nerve sparing: A risk-stratified approach to neural-hammock sparing during robot-assisted radical prostatectomy (RARP). BJU Int. 2011, 108, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Tavukcu, H.H.; Aytac, O.; Atug, F. Nerve-sparing techniques and results in robot-assisted radical prostatectomy. Investig. Clin. Urol. 2016, 57, S172–S184. [Google Scholar] [CrossRef]
- Carini, M.; Masieri, L.; Minervini, A.; Lapini, A.; Serni, S. Oncological and functional results of antegrade radical retropubic prostatectomy for the treatment of clinically localised prostate cancer. Eur. Urol. 2008, 53, 554–561. [Google Scholar] [CrossRef]
- Carrerette, F.B.; Carvalho, E.; Machado, H.; Freire, F.C.; Damiao, R. Open anterograde anatomic radical retropubic prostatectomy technique: Description of the first fiftyfive procedures. Int. Braz. J. Off. J. Braz. Soc. Urol. 2019, 45, 1071–1072. [Google Scholar] [CrossRef]
- Rassweiler, J.; Wagner, A.A.; Moazin, M.; Gozen, A.S.; Teber, D.; Frede, T.; Su, L.M. Anatomic nerve-sparing laparoscopic radical prostatectomy: Comparison of retrograde and antegrade techniques. Urology 2006, 68, 587–591. [Google Scholar] [CrossRef]
- Ko, Y.H.; Coelho, R.F.; Sivaraman, A.; Schatloff, O.; Chauhan, S.; Abdul-Muhsin, H.M.; Carrion, R.J.; Palmer, K.J.; Cheon, J.; Patel, V.R. Retrograde versus antegrade nerve sparing during robot-assisted radical prostatectomy: Which is better for achieving early functional recovery? Eur. Urol. 2013, 63, 169–177. [Google Scholar] [CrossRef]
- Finley, D.S.; Rodriguez, E., Jr.; Skarecky, D.W.; Ahlering, T.E. Quantitative and qualitative analysis of the recovery of potency after radical prostatectomy: Effect of unilateral vs bilateral nerve sparing. BJU Int. 2009, 104, 1484–1489. [Google Scholar] [CrossRef]
- Nilsson, A.E.; Carlsson, S.; Jonsson, N.M.; Onelov, E.; Steineck, G.; Wiklund, N.P. Erectile function after robotic nerve sparing and semi-sparing of the neurovascular bundles. J. Robot. Surg. 2007, 1, 191–195. [Google Scholar] [CrossRef]
- Greco, F.; Hoda, M.R.; Wagner, S.; Reichelt, O.; Inferrera, A.; Magno, C.; Fornara, P. Bilateral vs unilateral laparoscopic intrafascial nerve-sparing radical prostatectomy: Evaluation of surgical and functional outcomes in 457 patients. BJU Int. 2011, 108, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Avulova, S.; Zhao, Z.; Lee, D.; Huang, L.C.; Koyama, T.; Hoffman, K.E.; Conwill, R.M.; Wu, X.C.; Chen, V.; Cooperberg, M.R.; et al. The Effect of Nerve Sparing Status on Sexual and Urinary Function: 3-Year Results from the CEASAR Study. J. Urol. 2018, 199, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Alsaid, B.; Karam, I.; Bessede, T.; Abdlsamad, I.; Uhl, J.F.; Delmas, V.; Benoit, G.; Droupy, S. Tridimensional computer-assisted anatomic dissection of posterolateral prostatic neurovascular bundles. Eur. Urol. 2010, 58, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Reeves, F.; Battye, S.; Borin, J.F.; Corcoran, N.M.; Costello, A.J. High-resolution Map of Somatic Periprostatic Nerves. Urology 2016, 97, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Jeon, S.H.; Park, Y.H.; Jang, W.H.; Lee, J.Y.; Kim, K.H. Visualization of prostatic nerves by polarization-sensitive optical coherence tomography. Biomed. Opt. Express 2016, 7, 3170–3183. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Y.; Sarikcioglu, L. Sir Herbert Seddon (1903–1977) and his classification scheme for peripheral nerve injury. Child’s Nerv. Syst. 2015, 31, 177–180. [Google Scholar] [CrossRef]
- Seddon, H.J. Three types of nerve injury. Brain 1943, 66, 237–288. [Google Scholar] [CrossRef]
- Chhabra, A.; Ahlawat, S.; Belzberg, A.; Andreseik, G. Peripheral nerve injury grading simplified on MR neurography: As referenced to Seddon and Sunderland classifications. Indian J. Radiol. Imaging 2014, 24, 217–224. [Google Scholar] [CrossRef]
- Ahlering, T.E.; Eichel, L.; Chou, D.; Skarecky, D.W. Feasibility study for robotic radical prostatectomy cautery-free neurovascular bundle preservation. Urology 2005, 65, 994–997. [Google Scholar] [CrossRef]
- Gill, I.S.; Ukimura, O.; Rubinstein, M.; Finelli, A.; Moinzadeh, A.; Singh, D.; Kaouk, J.; Miki, T.; Desai, M. Lateral pedicle control during laparoscopic radical prostatectomy: Refined technique. Urology 2005, 65, 23–27. [Google Scholar] [CrossRef]
- Ong, A.M.; Su, L.M.; Varkarakis, I.; Inagaki, T.; Link, R.E.; Bhayani, S.B.; Patriciu, A.; Crain, B.; Walsh, P.C. Nerve sparing radical prostatectomy: Effects of hemostatic energy sources on the recovery of cavernous nerve function in a canine model. J. Urol. 2004, 172, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Rodriguez, E.; Finley, D.S.; Skarecky, D.W.; Ahlering, T.E. Spread of thermal energy and heat sinks: Implications for nerve-sparing robotic prostatectomy. J. Endourol. 2007, 21, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Huang, E.; Huynh, L.M.; Kaler, K.; Vernez, S.; Gordon, A.; Morales, B.; Skarecky, D.; Ahlering, T.E. Retrospective Concomitant Nonrandomized Comparison of “Touch” Cautery versus Athermal Dissection of the Prostatic Vascular Pedicles and Neurovascular Bundles During Robot-assisted Radical Prostatectomy. Eur. Urol. 2022, 81, 104–109. [Google Scholar] [CrossRef]
- Ahlering, T.E.; Rodriguez, E.; Skarecky, D.W. Overcoming obstacles: Nerve-sparing issues in radical prostatectomy. J. Endourol. 2008, 22, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Ahlering, T.E.; Skarecky, D.; Borin, J. Impact of cautery versus cautery-free preservation of neurovascular bundles on early return of potency. J. Endourol. 2006, 20, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Chien, G.W.; Mikhail, A.A.; Orvieto, M.A.; Zagaja, G.P.; Sokoloff, M.H.; Brendler, C.B.; Shalhav, A.L. Modified clipless antegrade nerve preservation in robotic-assisted laparoscopic radical prostatectomy with validated sexual function evaluation. Urology 2005, 66, 419–423. [Google Scholar] [CrossRef]
- Fagin, R. Da Vinci prostatectomy: Athermal nerve sparing and effect of the technique on erectile recovery and negative margins. J. Robot. Surg. 2007, 1, 139–143. [Google Scholar] [CrossRef][Green Version]
- Pagliarulo, V.; Alba, S.; Gallone, M.F.; Zingarelli, M.; Lorusso, A.; Minafra, P.; Ludovico, G.M.; Di Stasi, S.; Ditonno, P. Athermal versus ultrasonic nerve-sparing laparoscopic radical prostatectomy: A comparison of functional and oncological outcomes. World J. Urol. 2021, 39, 1453–1462. [Google Scholar] [CrossRef]
- Pastore, A.L.; Palleschi, G.; Silvestri, L.; Leto, A.; Sacchi, K.; Pacini, L.; Petrozza, V.; Carbone, A. Prospective randomized study of radiofrequency versus ultrasound scalpels on functional outcomes of laparoscopic radical prostatectomy. J. Endourol. 2013, 27, 989–993. [Google Scholar] [CrossRef]
- Kowalczyk, K.J.; Huang, A.C.; Hevelone, N.D.; Lipsitz, S.R.; Yu, H.Y.; Ulmer, W.D.; Kaplan, J.R.; Patel, S.; Nguyen, P.L.; Hu, J.C. Stepwise approach for nerve sparing without countertraction during robot-assisted radical prostatectomy: Technique and outcomes. Eur. Urol. 2011, 60, 536–547. [Google Scholar] [CrossRef]
- Masterson, T.A.; Serio, A.M.; Mulhall, J.P.; Vickers, A.J.; Eastham, J.A. Modified technique for neurovascular bundle preservation during radical prostatectomy: Association between technique and recovery of erectile function. BJU Int. 2008, 101, 1217–1222. [Google Scholar] [CrossRef]
- Mattei, A.; Naspro, R.; Annino, F.; Burke, D.; Guida, R., Jr.; Gaston, R. Tension and energy-free robotic-assisted laparoscopic radical prostatectomy with interfascial dissection of the neurovascular bundles. Eur. Urol. 2007, 52, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Savera, A.; Badani, K.; Fumo, M.; Bhandari, A.; Menon, M. Functional outcomes and oncological efficacy of Vattikuti Institute prostatectomy with Veil of Aphrodite nerve-sparing: An analysis of 154 consecutive patients. BJU Int. 2006, 97, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Badani, K.K. High prostatic fascia release or standard nerve sparing? A viewpoint from Columbia University Medical Center. J. Robot. Surg. 2008, 2, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.; Shrivastava, A.; Kaul, S.; Badani, K.K.; Fumo, M.; Bhandari, M.; Peabody, J.O. Vattikuti Institute prostatectomy: Contemporary technique and analysis of results. Eur. Urol. 2007, 51, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.; Shrivastava, A.; Bhandari, M.; Satyanarayana, R.; Siva, S.; Agarwal, P.K. Vattikuti Institute prostatectomy: Technical modifications in 2009. Eur. Urol. 2009, 56, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Guru, K.A.; Perlmutter, A.E.; Butt, Z.M.; Peabody, J.O. Hydrodissection for preservation of neurovascular bundle during robot-assisted radical prostatectomy. Can. J. Urol. 2008, 15, 4000–4003. [Google Scholar]
- Cheetham, P.J.; Truesdale, M.D.; Lee, D.J.; Landman, J.M.; Badani, K.K. Use of a flexible carbon dioxide laser fiber for precise dissection of the neurovascular bundle during robot-assisted laparoscopic prostatectomy. J. Endourol. 2010, 24, 1091–1096. [Google Scholar] [CrossRef]
- Gianduzzo, T.R.; Colombo, J.R., Jr.; Haber, G.P.; Magi-Galluzzi, C.; Dall’Oglio, M.F.; Ulchaker, J.; Gill, I.S. KTP laser nerve sparing radical prostatectomy: Comparison of ultrasonic and cold scissor dissection on cavernous nerve function. J. Urol. 2009, 181, 2760–2766. [Google Scholar] [CrossRef]
- Galfano, A.; Ascione, A.; Grimaldi, S.; Petralia, G.; Strada, E.; Bocciardi, A.M. A new anatomic approach for robot-assisted laparoscopic prostatectomy: A feasibility study for completely intrafascial surgery. Eur. Urol. 2010, 58, 457–461. [Google Scholar] [CrossRef]
- Checcucci, E.; Veccia, A.; Fiori, C.; Amparore, D.; Manfredi, M.; Di Dio, M.; Morra, I.; Galfano, A.; Autorino, R.; Bocciardi, A.M.; et al. Retzius-sparing robot-assisted radical prostatectomy vs the standard approach: A systematic review and analysis of comparative outcomes. BJU Int. 2020, 125, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Galfano, A.; Di Trapani, D.; Sozzi, F.; Strada, E.; Petralia, G.; Bramerio, M.; Ascione, A.; Gambacorta, M.; Bocciardi, A.M. Beyond the learning curve of the Retzius-sparing approach for robot-assisted laparoscopic radical prostatectomy: Oncologic and functional results of the first 200 patients with ≥1 year of follow-up. Eur. Urol. 2013, 64, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, H.Y.; Goh, H.J.; Heo, J.E.; Almujalhem, A.; Alqahtani, A.A.; Chung, D.Y.; Chang, K.; Choi, Y.D.; Rha, K.H. Retzius Sparing Robot-Assisted Radical Prostatectomy Conveys Early Regain of Continence over Conventional Robot-Assisted Radical Prostatectomy: A Propensity Score Matched Analysis of 1,863 Patients. J. Urol. 2020, 203, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Egan, J.; Marhamati, S.; Carvalho, F.L.F.; Davis, M.; O’Neill, J.; Lee, H.; Lynch, J.H.; Hankins, R.A.; Hu, J.C.; Kowalczyk, K.J. Retzius-sparing Robot-assisted Radical Prostatectomy Leads to Durable Improvement in Urinary Function and Quality of Life versus Standard Robot-assisted Radical Prostatectomy without Compromise on Oncologic Efficacy: Single-surgeon Series and Step-by-step Guide. Eur. Urol. 2021, 79, 839–857. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.; Dalela, D.; Jamil, M.; Diaz, M.; Tallman, C.; Abdollah, F.; Sood, A.; Lehtola, L.; Miller, D.; Jeong, W. Functional Recovery, Oncologic Outcomes and Postoperative Complications after Robot-Assisted Radical Prostatectomy: An Evidence-Based Analysis Comparing the Retzius Sparing and Standard Approaches. J. Urol. 2018, 199, 1210–1217. [Google Scholar] [CrossRef]
- Nyarangi-Dix, J.N.; Gortz, M.; Gradinarov, G.; Hofer, L.; Schutz, V.; Gasch, C.; Radtke, J.P.; Hohenfellner, M. Retzius-sparing robot-assisted laparoscopic radical prostatectomy: Functional and early oncologic results in aggressive and locally advanced prostate cancer. BMC Urol. 2019, 19, 113. [Google Scholar] [CrossRef]
- Schlomm, T.; Tennstedt, P.; Huxhold, C.; Steuber, T.; Salomon, G.; Michl, U.; Heinzer, H.; Hansen, J.; Budaus, L.; Steurer, S.; et al. Neurovascular structure-adjacent frozen-section examination (NeuroSAFE) increases nerve-sparing frequency and reduces positive surgical margins in open and robot-assisted laparoscopic radical prostatectomy: Experience after 11,069 consecutive patients. Eur. Urol. 2012, 62, 333–340. [Google Scholar] [CrossRef]
- Dinneen, E.; Haider, A.; Allen, C.; Freeman, A.; Briggs, T.; Nathan, S.; Brew-Graves, C.; Grierson, J.; Williams, N.R.; Persad, R.; et al. NeuroSAFE robot-assisted laparoscopic prostatectomy versus standard robot-assisted laparoscopic prostatectomy for men with localised prostate cancer (NeuroSAFE PROOF): Protocol for a randomised controlled feasibility study. BMJ Open 2019, 9, e028132. [Google Scholar] [CrossRef]
- Kumar, A.; Tandon, S.; Samavedi, S.; Mouraviev, V.; Bates, A.S.; Patel, V.R. Current status of various neurovascular bundle-sparing techniques in robot-assisted radical prostatectomy. J. Robot. Surg. 2016, 10, 187–200. [Google Scholar] [CrossRef]
- Kumar, A.; Samavedi, S.; Bates, A.; Coelho, R.; Rocco, B.; Marquinez, J.; Palmer, K.J.; Patel, V. Using indocyanine green and near-infrared fluores-cence technology to identify the “landmark artery” during robot-assisted radical prostatectomy. Videourology 2015. [Google Scholar] [CrossRef]
- Patel, V.R.; Samavedi, S.; Bates, A.S.; Kumar, A.; Coelho, R.; Rocco, B.; Palmer, K. Dehydrated Human Amnion/Chorion Membrane Allograft Nerve Wrap Around the Prostatic Neurovascular Bundle Accelerates Early Return to Continence and Potency Following Robot-assisted Radical Prostatectomy: Propensity Score-matched Analysis. Eur. Urol. 2015, 67, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Ogaya-Pinies, G.; Palayapalam-Ganapathi, H.; Rogers, T.; Hernandez-Cardona, E.; Rocco, B.; Coelho, R.F.; Jenson, C.; Patel, V.R. Can dehydrated human amnion/chorion membrane accelerate the return to potency after a nerve-sparing robotic-assisted radical prostatectomy? Propensity score-matched analysis. J. Robot. Surg. 2018, 12, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Porpiglia, F.; Manfredi, M.; Checcucci, E.; Garrou, D.; De Cillis, S.; Amparore, D.; De Luca, S.; Fregnan, F.; Stura, I.; Migliaretti, G.; et al. Use of chitosan membranes after nerve-sparing radical prostatectomy improves early recovery of sexual potency: Results of a comparative study. BJU Int. 2019, 123, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Hinata, N.; Bando, Y.; Chiba, K.; Furukawa, J.; Harada, K.; Ishimura, T.; Nakano, Y.; Fujisawa, M. Application of hyaluronic acid/carboxymethyl cellulose membrane for early continence after nerve-sparing robot-assisted radical prostatectomy. BMC Urol. 2019, 19, 25. [Google Scholar] [CrossRef]
- Haga, N.; Miyazaki, T.; Tsubouchi, K.; Okabe, Y.; Shibayama, K.; Emoto, D.; Matsuoka, W.; Maruta, H.; Aoyagi, C.; Matsuzaki, H.; et al. Comprehensive approach for preserving cavernous nerves and erectile function after radical prostatectomy in the era of robotic surgery. Int. J. Urol. 2021, 28, 360–368. [Google Scholar] [CrossRef]
- Patel, V.R.; Sivaraman, A.; Coelho, R.F.; Chauhan, S.; Palmer, K.J.; Orvieto, M.A.; Camacho, I.; Coughlin, G.; Rocco, B. Pentafecta: A new concept for reporting outcomes of robot-assisted laparoscopic radical prostatectomy. Eur. Urol. 2011, 59, 702–707. [Google Scholar] [CrossRef]
- Arroyo, C.; Martini, A.; Wang, J.; Tewari, A.K. Anatomical, surgical and technical factors influencing continence after radical prostatectomy. Ther. Adv. Urol. 2019, 11, 1756287218813787. [Google Scholar] [CrossRef]

| Nerve-Sparing Grading System | References | Anatomical Landmark | Number of Grades | Description of Different Grades |
|---|---|---|---|---|
| Fascial planes | Stolzenburg et al. [14] | Periprostatic fasciae | 3 |
|
| Extent of nerve-sparing | Montorsi et al. [22] | Neurovascular bundles | 3 |
|
| 4-degree approach for preservation of the neurovascular bundles | Tewari et al. [23] | The veins which are situated on the lateral aspect of the prostate | 4 |
|
| 5-degree approach for the definition of the dissection planes | Schatloff et al. [24] | The “landmark artery” (LA), which runs on the lateral aspect of the prostate | 5 |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyriazis, I.; Spinos, T.; Tsaturyan, A.; Kallidonis, P.; Stolzenburg, J.U.; Liatsikos, E. Different Nerve-Sparing Techniques during Radical Prostatectomy and Their Impact on Functional Outcomes. Cancers 2022, 14, 1601. https://doi.org/10.3390/cancers14071601
Kyriazis I, Spinos T, Tsaturyan A, Kallidonis P, Stolzenburg JU, Liatsikos E. Different Nerve-Sparing Techniques during Radical Prostatectomy and Their Impact on Functional Outcomes. Cancers. 2022; 14(7):1601. https://doi.org/10.3390/cancers14071601
Chicago/Turabian StyleKyriazis, Iason, Theodoros Spinos, Arman Tsaturyan, Panagiotis Kallidonis, Jens Uwe Stolzenburg, and Evangelos Liatsikos. 2022. "Different Nerve-Sparing Techniques during Radical Prostatectomy and Their Impact on Functional Outcomes" Cancers 14, no. 7: 1601. https://doi.org/10.3390/cancers14071601
APA StyleKyriazis, I., Spinos, T., Tsaturyan, A., Kallidonis, P., Stolzenburg, J. U., & Liatsikos, E. (2022). Different Nerve-Sparing Techniques during Radical Prostatectomy and Their Impact on Functional Outcomes. Cancers, 14(7), 1601. https://doi.org/10.3390/cancers14071601







