The Prognostic Value of miR-125b, miR-200c and miR-205 in Primary Cutaneous Malignant Melanoma Is Independent of BRAF Mutational Status
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Melanoma Tissues
2.2. RNA Extraction
2.3. RT-PCR and Sanger Sequencing
2.4. miRNA Quantification
2.5. Statistical Analysis
3. Results
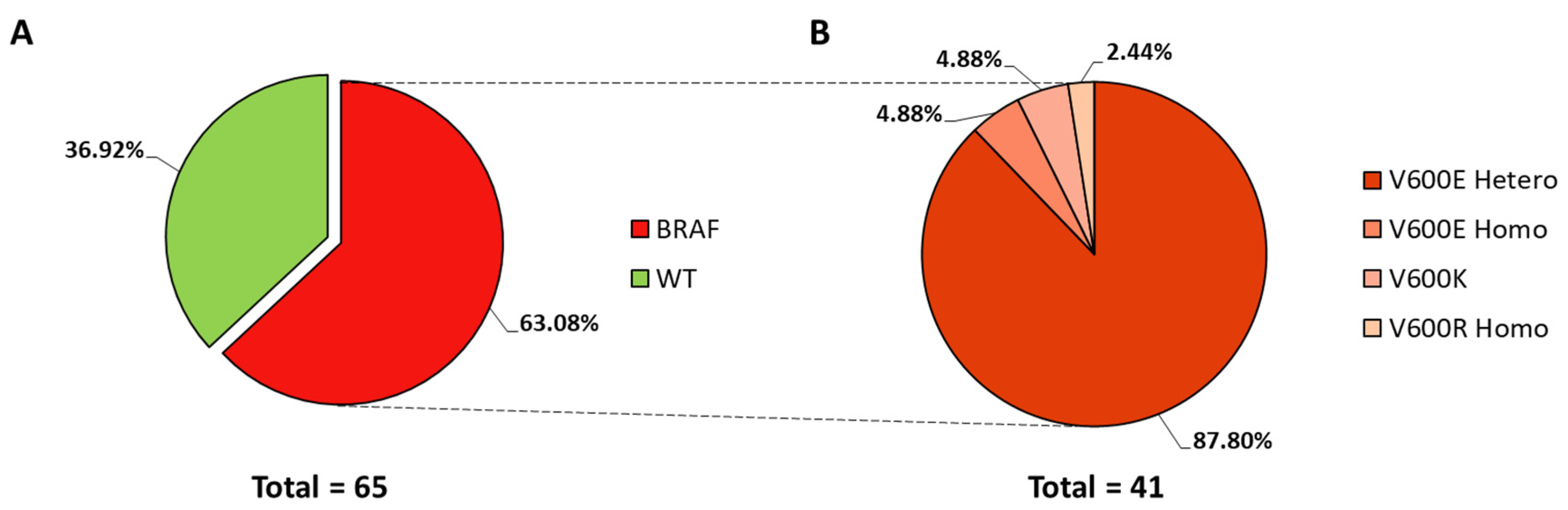
3.1. BRAF Mutational Status in Primary Melanomas
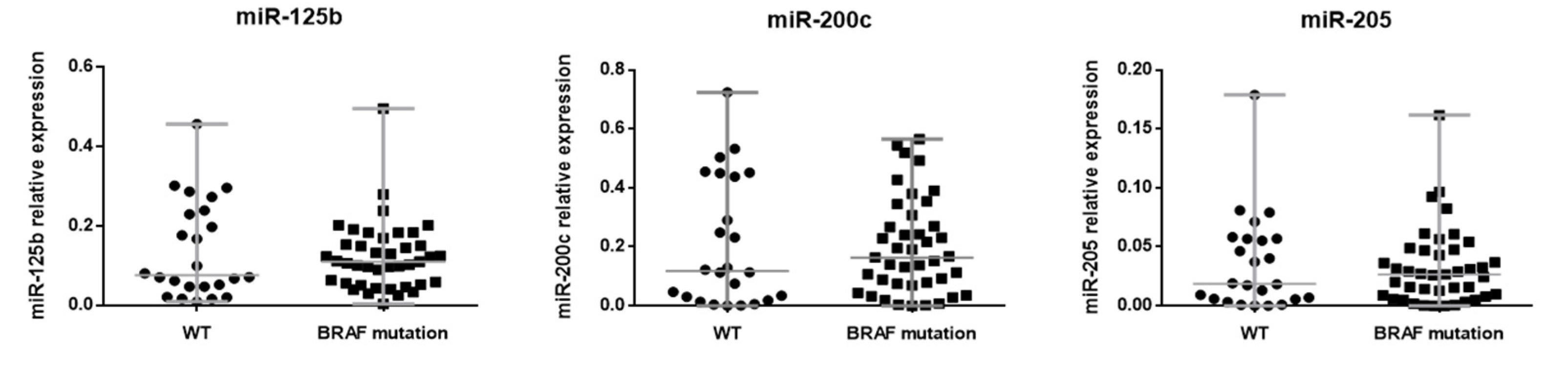
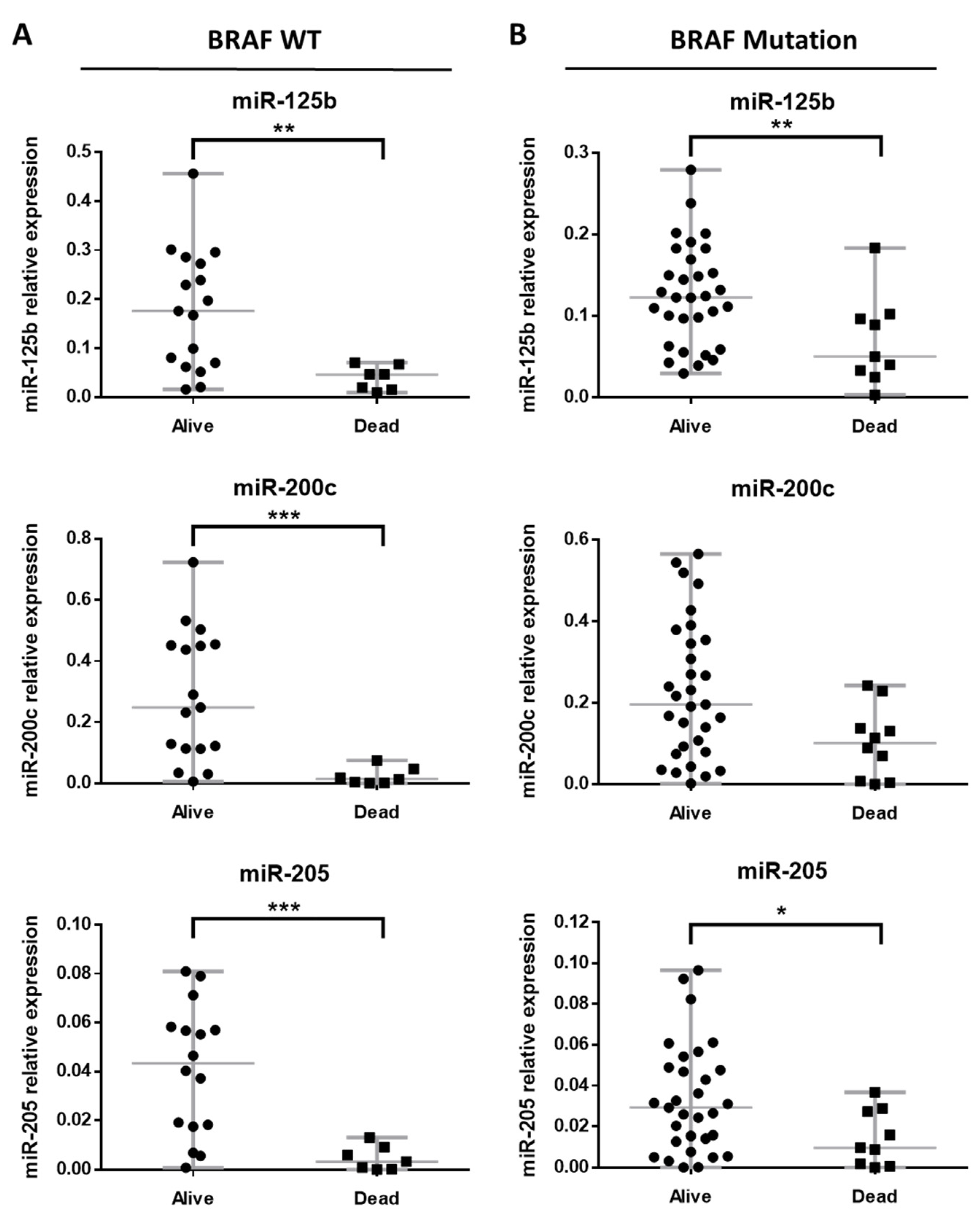
3.2. miRNA Expression Profile in BRAF Mutated and BRAF Wild-Type Primary Melanomas
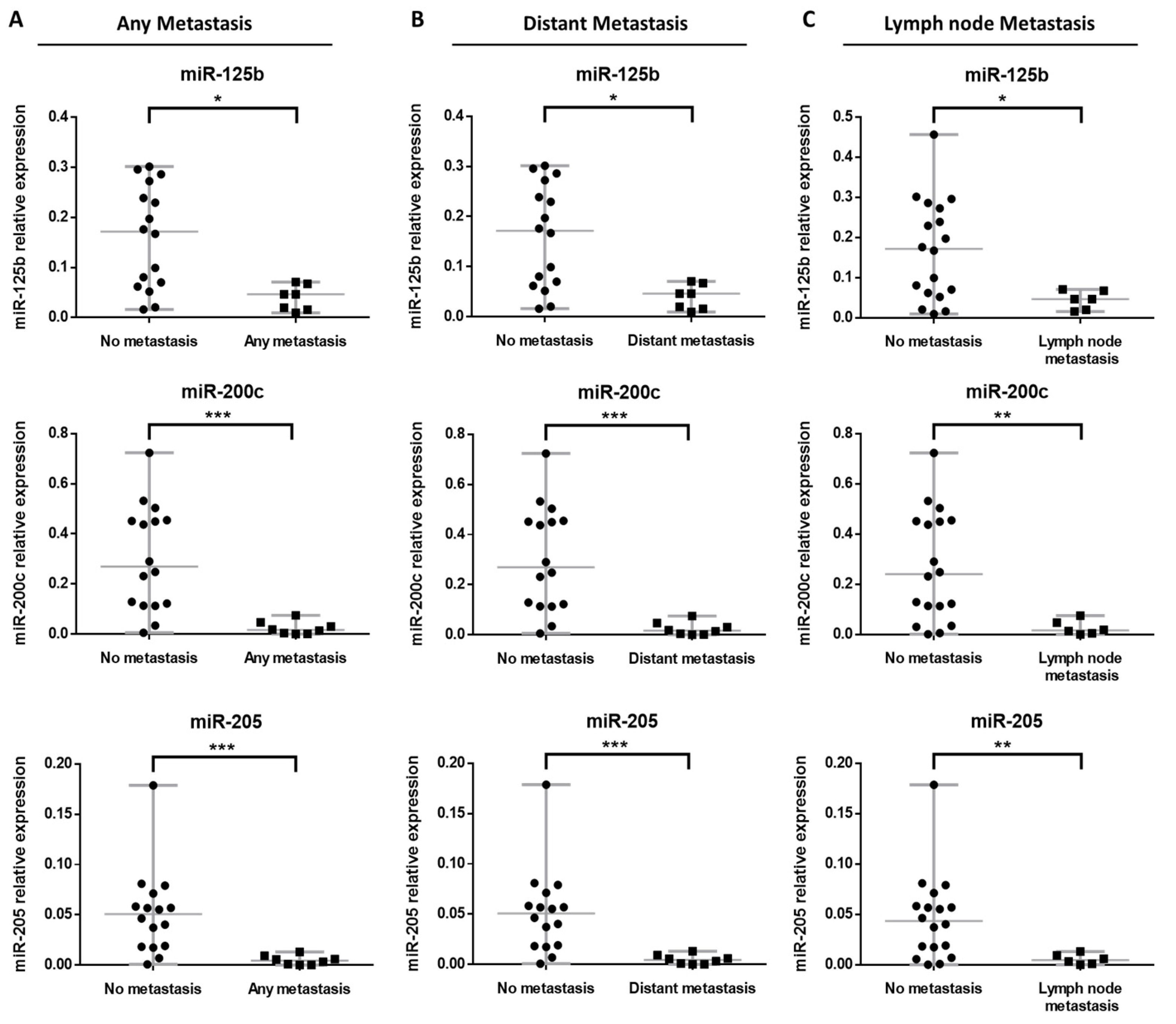
3.3. miRNA Expression Profile in BRAF Wild-Type Primary Metastatic Melanoma
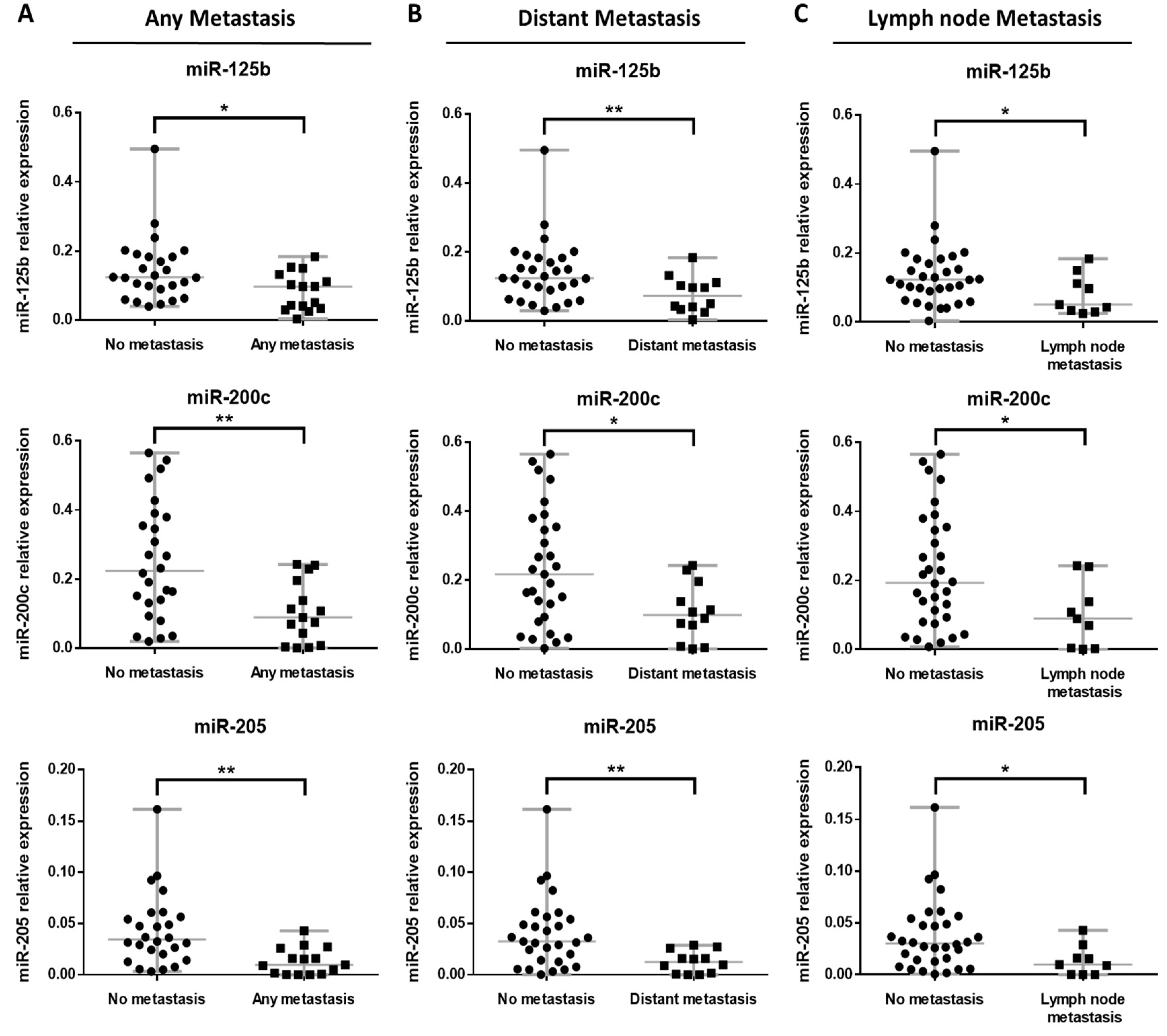
3.4. miRNA Expression Profile in BRAF Mutated Primary Metastatic Melanoma
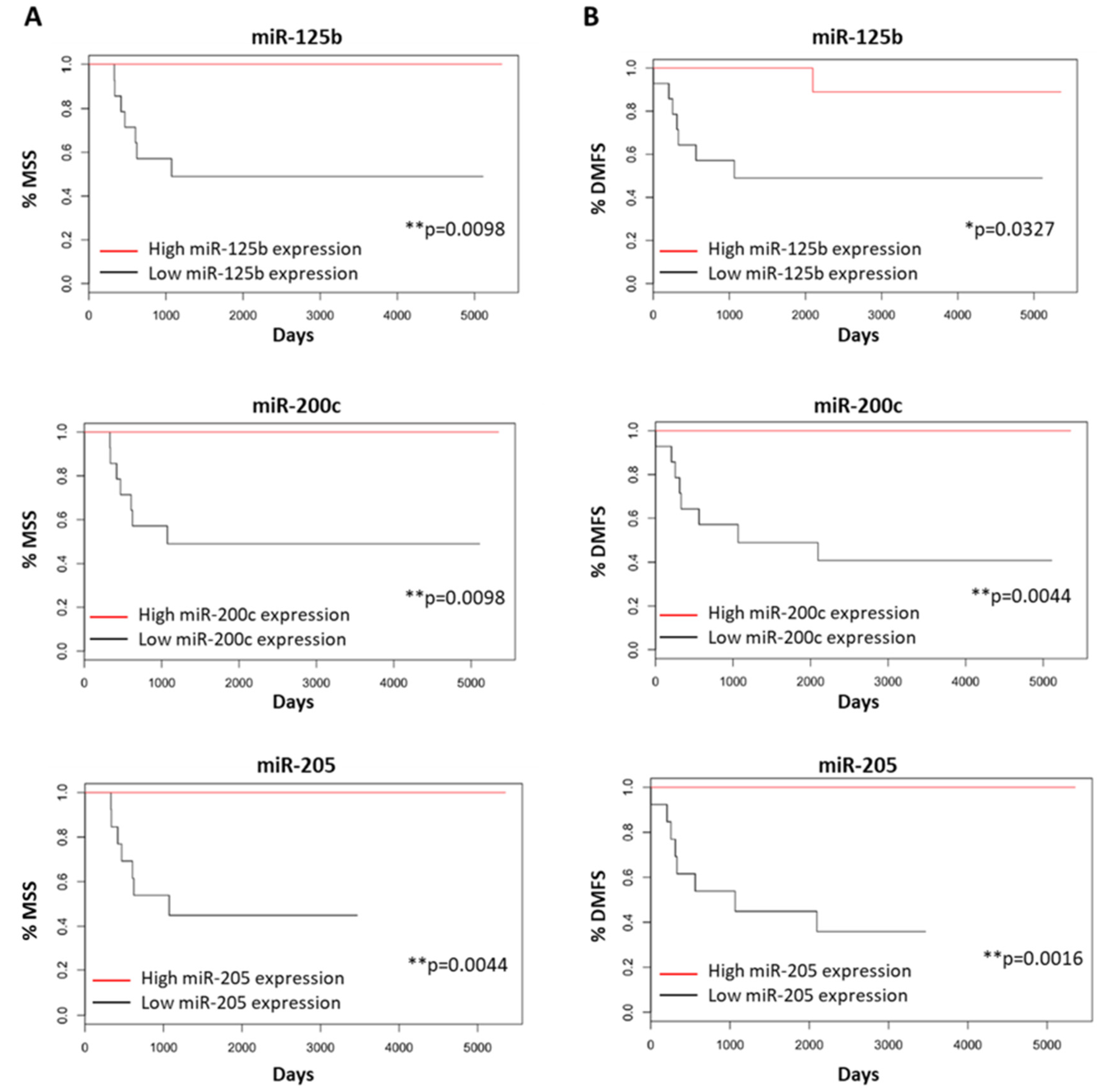
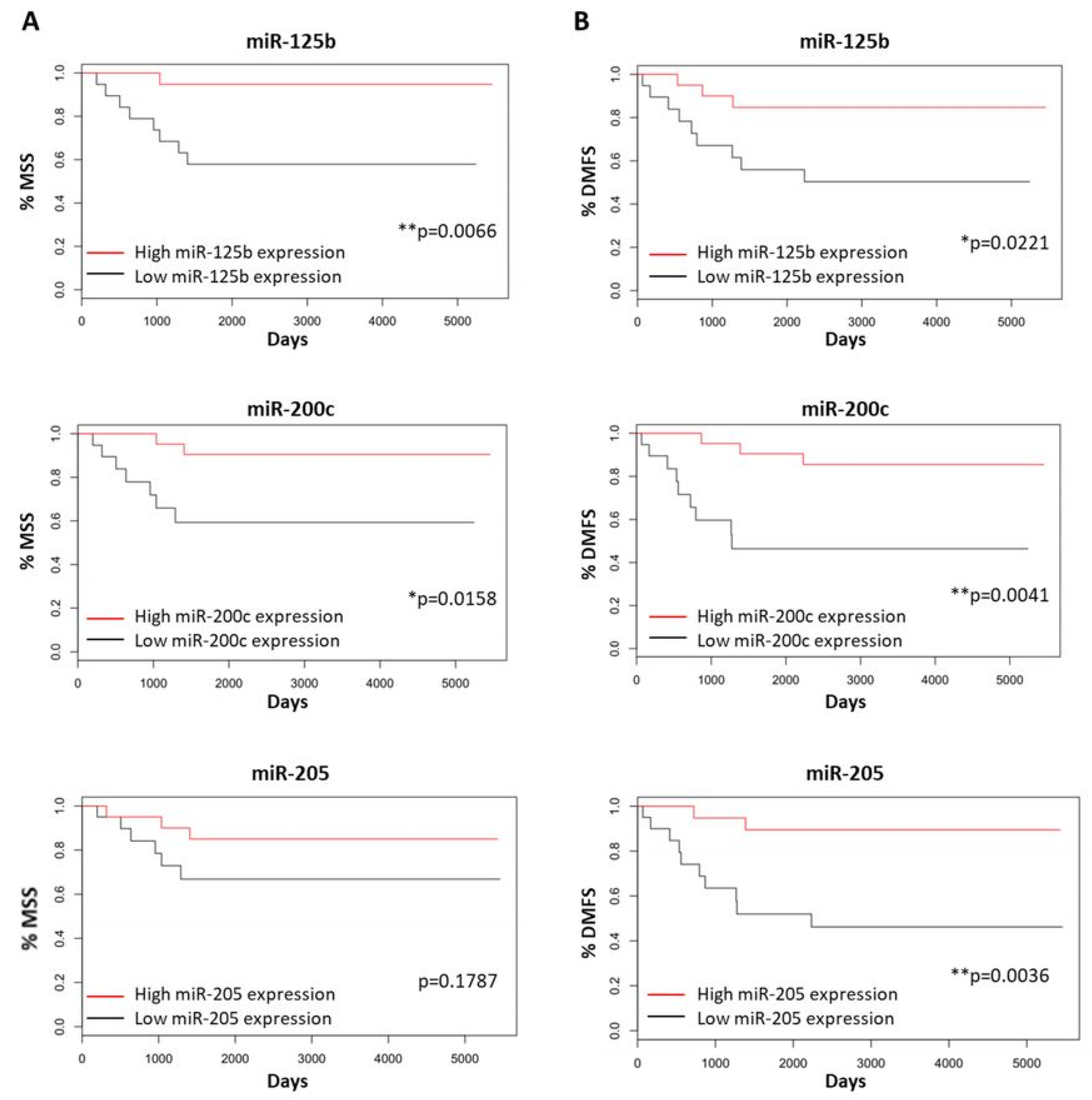
3.5. Melanoma-Specific and Distant Metastasis-Free Survival for Primary Melanomas
3.6. miRNAs as Potential Independent Biomarkers for Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dréno, B.; Fargnoli, M.C.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics—Update 2019. Eur. J. Cancer 2020, 126, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.; Garraway, L.A.; Fisher, D.E. Malignant melanoma: Genetics and therapeutics in the genomic era. Genes Dev. 2006, 20, 2149–2182. [Google Scholar] [CrossRef]
- Lovly, C.M.; Dahlman, K.B.; Fohn, L.E.; Su, Z.; Dias-Santagata, D.; Hicks, D.J.; Hucks, D.; Berry, E.; Terry, C.; Duke, M.; et al. Routine multiplex mutational profiling of melanomas enables enrollment in genotype-driven therapeutic trials. PLoS ONE 2012, 7, e35309. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.Y.; Miller, D.M.; Tsao, H. Somatic driver mutations in melanoma. Cancer 2017, 123, 2104–2117. [Google Scholar] [CrossRef]
- Davis, E.J.; Johnson, D.B.; Sosman, J.A.; Chandra, S. Melanoma: What do all the mutations mean? Cancer 2018, 124, 3490–3499. [Google Scholar] [CrossRef]
- Improta, G.; Pelosi, G.; Tamborini, E.; Donia, M.; Santinami, M.; de Braud, F.; Fraggetta, F. Biological insights into BRAFV600 mutations in melanoma patient: Not mere therapeutic targets. Oncoimmunology 2013, 2, e25594. [Google Scholar] [CrossRef][Green Version]
- Revythis, A.; Shah, S.; Kutka, M.; Moschetta, M.; Ozturk, M.A.; Pappas-Gogos, G.; Ioannidou, E.; Sheriff, M.; Rassy, E.; Boussios, S. Unraveling the wide spectrum of melanoma biomarkers. Diagnostics 2021, 11, 1341. [Google Scholar] [CrossRef]
- Mann, G.J.; Pupo, G.M.; Campain, A.E.; Carter, C.D.; Schramm, S.J.; Pianova, S.; Gerega, S.K.; De Silva, C.; Lai, K.; Wilmott, J.S.; et al. BRAF mutation, NRAS mutation, and the absence of an immune-related expressed gene profile predict poor outcome in patients with stage III melanoma. J. Investig. Dermatol. 2013, 133, 509–517. [Google Scholar] [CrossRef]
- Owsley, J.; Stein, M.K.; Porter, J.; In, G.K.; Salem, M.; O’Day, S.; Elliott, A.; Poorman, K.; Gibney, G.; VanderWalde, A. Prevalence of class I-III BRAF mutations among 114,662 cancer patients in a large genomic database. Exp. Biol. Med. 2021, 246, 31–39. [Google Scholar] [CrossRef]
- Louveau, B.; Jouenne, F.; Reger de Moura, C.; Sadoux, A.; Baroudjian, B.; Delyon, J.; Herms, F.; De Masson, A.; Da Meda, L.; Battistella, M.; et al. Baseline genomic features in BRAFV600-mutated metastatic melanoma patients treated with BRAF inhibitor + MEK inhibitor in routine care. Cancers 2019, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Leung, E.Y.; Baguley, B.C.; Finlay, G.J.; Askarian-Amiri, M.E. Epigenetic regulation in human melanoma: Past and future. Epigenetics 2015, 10, 103–121. [Google Scholar] [CrossRef]
- Mirzaei, H.; Gholamin, S.; Shahidsales, S.; Sahebkar, A.; Jaafari, M.R.; Mirzaei, H.R.; Hassanian, S.M.; Avan, A. MicroRNAs as potential diagnostic and prognostic biomarkers in melanoma. Eur. J. Cancer 2016, 53, 25–32. [Google Scholar] [CrossRef]
- Latchana, N.; Ganju, A.; Howard, J.H.; Carson, W.E. MicroRNA dysregulation in melanoma. Surg. Oncol. 2016, 25, 184–189. [Google Scholar] [CrossRef]
- Sánchez-Sendra, B.; Martinez-Ciarpaglini, C.; González-Muñoz, J.F.; Murgui, A.; Terrádez, L.; Monteagudo, C. Downregulation of intratumoral expression of miR-205, miR-200c and miR-125b in primary human cutaneous melanomas predicts shorter survival. Sci. Rep. 2018, 8, 17076. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Bennett, P.E.; Bemis, L.; Norris, D.A.; Shellman, Y.G. miR in melanoma development: miRNAs and acquired hallmarks of cancer in melanoma. Physiol. Genomics 2013, 45, 1049–1059. [Google Scholar] [CrossRef]
- Fattore, L.; Mancini, R.; Ascierto, P.A.; Ciliberto, G. The potential of BRAF-associated non-coding RNA as a therapeutic target in melanoma. Expert. Opin. Ther. Targets 2019, 23, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Couts, K.L.; Anderson, E.M.; Gross, M.M.; Sullivan, K.; Ahn, N.G. Oncogenic B-Raf signaling in melanoma cells controls a network of microRNAs with combinatorial functions. Oncogene 2013, 32, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Marranci, A.; D’Aurizio, R.; Vencken, S.; Mero, S.; Guzzolino, E.; Rizzo, M.; Pitto, L.; Pellegrini, M.; Chiorino, G.; Greene, C.M.; et al. Systematic evaluation of the microRNAome through miR-CATCHv2.0 identifies positive and negative regulators of BRAF-X1 mRNA. RNA Biol. 2019, 16, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Caramuta, S.; Egyházi, S.; Rodolfo, M.; Witten, D.; Hansson, J.; Larsson, C.; Lui, W.O. MicroRNA expression profiles associated with mutational status and survival in malignant melanoma. J. Investig. Dermatol. 2010, 130, 2062–2070. [Google Scholar] [CrossRef] [PubMed]
- Valentini, V.; Zelli, V.; Gaggiano, E.; Silvestri, V.; Rizzolo, P.; Bucalo, A.; Calvieri, S.; Grassi, S.; Frascione, P.; Donati, P.; et al. miRNAs as Potential Prognostic Biomarkers for Metastasis in Thin and Thick Primary Cutaneous Melanomas. Anticancer Res. 2019, 39, 4085–4093. [Google Scholar] [CrossRef]
- Pinto, R.; Strippoli, S.; De Summa, S.; Albano, A.; Azzariti, A.; Guida, G.; Popescu, O.; Lorusso, V.; Guida, M.; Tommasi, S. MicroRNA expression in BRAF-mutated and wild-type metastatic melanoma and its correlation with response duration to BRAF inhibitors. Expert. Opin. Ther. Targets 2015, 19, 1027–1035. [Google Scholar] [CrossRef]
- Zaman, A.; Wu, W.; Bivona, T.G. Targeting oncogenic BRAF: Past, present and future. Cancers 2019, 11, 1197. [Google Scholar] [CrossRef]
- Khaliq, M.; Fallahi-Sichani, M. Epigenetic mechanisms of escape from BRAF oncongene dependency. Cancers 2019, 11, 1480. [Google Scholar] [CrossRef]
- Fedorenko, I.V.; Gibney, G.T.; Sondak, V.K.; Smalley, K.S.M. Beyond BRAF: Where next for melanoma therapy? Br. J. Cancer 2015, 112, 217–226. [Google Scholar] [CrossRef]
- Tas, F.; Ertunk, K. BRAF V600E mutations as a prognostic factor in cutaneous melanoma patients. Dermatol. Ther. 2020, 33, e13270. [Google Scholar] [CrossRef] [PubMed]
- Heppt, M.V.; Siepmann, T.; Engel, J.; Schubert-Fritschle, G.; Eckel, R.; Mirlach, L.; Kirchner, T.; Jung, A.; Gesierich, A.; Ruzicka, T.; et al. Prognostic significance of BRAF and NRAS mutations in melanoma: A German study from routine care. BMC Cancer 2017, 17, 536. [Google Scholar] [CrossRef]
- Mar, V.J.; Liu, W.; Devitt, B.; Wong, S.Q.; Dobrovic, A.; McArthur, G.A.; Wolfe, R.; Kelly, J.W. The role of BRAF mutations in primary melanoma growth rate and survival. Br. J. Dermatol. 2015, 173, 76–82. [Google Scholar] [CrossRef]
- Akslen, L.A.; Angelini, S.; Straume, O.; Bachmann, I.M.; Molven, A.; Hemminki, K.; Kumar, R. BRAF and NRAS mutations are frequent in nodular melanoma but are not associated with tumor cell proliferation or patient survival. J. Investig. Dermatol. 2005, 125, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, M.; Fujimoto, A.; Morton, D.L.; Hoon, D.S. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin. Cancer Res. 2004, 10, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Thyagarajan, A.; Tsai, K.Y.; Sahu, R.P. MicroRNA heterogeneity in melanoma progression. Semin. Cancer Biol. 2019, 59, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Varrone, F.; Caputo, E. The miRNAs Role in Melanoma and in Its Resistance to Therapy. Int. J. Mol. Sci. 2020, 21, 878. [Google Scholar] [CrossRef]
- Wang, J.K.; Wang, Z.; Li, G. MicroRNA-125 in immunity and cancer. Cancer Lett. 2019, 454, 134–145. [Google Scholar] [CrossRef]
- Yan, J.; Jiang, Q.; Lu, H.; Na, S.; Long, S.; Xin, Y.; Zhang, C.; Zhang, J. Association between microRNA-125b expression in formalin-fixed paraffin-embedded tumor tissues and prognosis in patients with melanoma. Oncol. Lett. 2019, 18, 1856–1862. [Google Scholar] [CrossRef]
- Vergani, E.; Di Guardo, L.; Dugo, M.; Rigoletto, S.; Tragni, G.; Ruggeri, R.; Perrone, F.; Tamborini, E.; Gloghini, A.; Arienti, F.; et al. Overcoming melanoma resistance to vemurafenib by targeting CCL2-induced miR-34a, miR-100 and miR-125b. Oncotarget 2016, 7, 4428–4441. [Google Scholar] [CrossRef]
- Lorusso, C.; De Summa, S.; Pinto, R.; Danza, K.; Tommasi, S. miRNAs as Key Players in the Management of Cutaneous Melanoma. Cells 2020, 9, 415. [Google Scholar] [CrossRef] [PubMed]
- Wagenseller, A.G.; Shada, A.; D’Auria, K.M.; Murphy, C.; Sun, D.; Molhoek, K.R.; Papin, J.A.; Dutta, A.; Slingluff, C.L., Jr. MicroRNAs induced in melanoma treated with combination targeted therapy of Temsirolimus and Bevacizumab. J. Transl. Med. 2013, 18, 218. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.L.; Kaushik, S.; Valdes-Rodriguez, R.; Anvekar, R. MicroRNAs in cutaneous melanoma: Role as diagnostic and prognostic biomarkers. J. Cell Physiol. 2018, 233, 5133–5141. [Google Scholar] [CrossRef] [PubMed]
- Glud, M.; Rossing, M.; Hother, C.; Holst, L.; Hastrup, N.; Nielsen, F.C.; Gniadecki, R.; Drzewiecki, K.T. Downregulation of miR-125b in metastatic cutaneous malignant melanoma. Melanoma Res. 2010, 20, 479–484. [Google Scholar] [CrossRef]
- Xu, Y.; Brenn, T.; Brown, E.R.; Doherty, V.; Melton, D.W. Differential expression of microRNAs during melanoma progression: miR-200c, miR-205 and miR-211 are downregulated in melanoma and act as tumour suppressors. Br. J. Cancer 2012, 106, 553–561. [Google Scholar] [CrossRef]
| Primary Melanomas (Total Number: 65) | ||
|---|---|---|
| Variable | Number of Patients | (%) |
| Breslow thickness (mm) | ||
| ≤1 | 26 | 40.0 |
| 1.01–2.00 | 14 | 21.5 |
| 2.01–4.00 | 8 | 12.3 |
| >4 | 17 | 26.2 |
| Ulceration | ||
| Absent | 48 | 73.8 |
| Present | 17 | 26.2 |
| Mitosis/mm2 | ||
| 0 | 19 | 29.2 |
| ≥1 | 46 | 70.8 |
| Growth phase | ||
| Radial | 20 | 30.8 |
| Vertical | 45 | 69.2 |
| Location | ||
| Head and neck | 7 | 10.8 |
| Limbs | 23 | 35.4 |
| Trunk | 34 | 52.3 |
| Gender | ||
| Female | 42 | 64.6 |
| Male | 23 | 35.4 |
| Histological type * | ||
| ALM | 5 | 7.7 |
| LMM | 5 | 7.7 |
| NM | 9 | 13.8 |
| SSM | 46 | 70.8 |
| Age at diagnosis (years) | ||
| ≤65 | 34 | 52.3 |
| >65 | 31 | 47.7 |
| In-transit metastasis | ||
| Absent | 55 | 84.6 |
| Present | 10 | 15.4 |
| Lymph node metastasis | ||
| Absent | 50 | 76.9 |
| Present | 15 | 23.1 |
| Distant metastasis | ||
| Absent | 45 | 69.2 |
| Present | 20 | 30.8 |
| Any metastasis | ||
| Absent | 42 | 64.6 |
| Present | 23 | 35.4 |
| Melanoma-specific survival | ||
| Alive | 49 | 75.4 |
| Dead | 16 | 24.6 |
| Follow-up (months) | ||
| Mean, Range | 93 | (7–188) |
| Variables | Estimate | Std. Error | z Value | Pr (>|z|) |
|---|---|---|---|---|
| Distant Metastasis | ||||
| Intercept | −0.754 | 0.429 | −1.76 | 0.079 |
| BRAF mutational status | −0.195 | 0.547 | −0.36 | 0.721 |
| Exitus | ||||
| Intercept | −0.945 | 0.445 | −2.12 | 0.034 * |
| BRAF mutational status | −3.385 | 0.582 | −0.66 | 0.509 |
| miRNAs | HR (exp(b)) | 95% CI | p-Value |
|---|---|---|---|
| BRAF WT | |||
| Distant metastasis-free survival (DMFS) | |||
| miR-125b | 0.995 | 0.987–1.002 | 0.148 |
| miR-200c | 0.980 | 0.961–1 | 0.052 |
| miR-205 | 0.904 | 0.818–0.999 | 0.046 * |
| Melanoma-specific survival (MSS) | |||
| miR-125b | 0.982 | 0.963–1 | 0.055 |
| miR-200c | 0.981 | 0.961–1.002 | 0.070 |
| miR-205 | 0.909 | 0.823–1.005 | 0.063 |
| BRAF Mutated | |||
| Distant metastasis-free survival (DMFS) | |||
| miR-125b | 0.986 | 0.975–0.998 | 0.017 * |
| miR-200c | 0.994 | 0.988–0.999 | 0.016 * |
| miR-205 | 0.944 | 0.905–0.985 | 0.008 ** |
| Melanoma-specific survival (MSS) | |||
| miR-125b | 0.984 | 0.970–0.998 | 0.025 * |
| miR-200c | 0.995 | 0.989–1 | 0.066 |
| miR-205 | 0.957 | 0.916–0.999 | 0.047 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Sendra, B.; González-Muñoz, J.F.; Pérez-Debén, S.; Monteagudo, C. The Prognostic Value of miR-125b, miR-200c and miR-205 in Primary Cutaneous Malignant Melanoma Is Independent of BRAF Mutational Status. Cancers 2022, 14, 1532. https://doi.org/10.3390/cancers14061532
Sánchez-Sendra B, González-Muñoz JF, Pérez-Debén S, Monteagudo C. The Prognostic Value of miR-125b, miR-200c and miR-205 in Primary Cutaneous Malignant Melanoma Is Independent of BRAF Mutational Status. Cancers. 2022; 14(6):1532. https://doi.org/10.3390/cancers14061532
Chicago/Turabian StyleSánchez-Sendra, Beatriz, José F. González-Muñoz, Silvia Pérez-Debén, and Carlos Monteagudo. 2022. "The Prognostic Value of miR-125b, miR-200c and miR-205 in Primary Cutaneous Malignant Melanoma Is Independent of BRAF Mutational Status" Cancers 14, no. 6: 1532. https://doi.org/10.3390/cancers14061532
APA StyleSánchez-Sendra, B., González-Muñoz, J. F., Pérez-Debén, S., & Monteagudo, C. (2022). The Prognostic Value of miR-125b, miR-200c and miR-205 in Primary Cutaneous Malignant Melanoma Is Independent of BRAF Mutational Status. Cancers, 14(6), 1532. https://doi.org/10.3390/cancers14061532






