Development of Low-Grade Serous Ovarian Carcinoma from Benign Ovarian Serous Cystadenoma Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
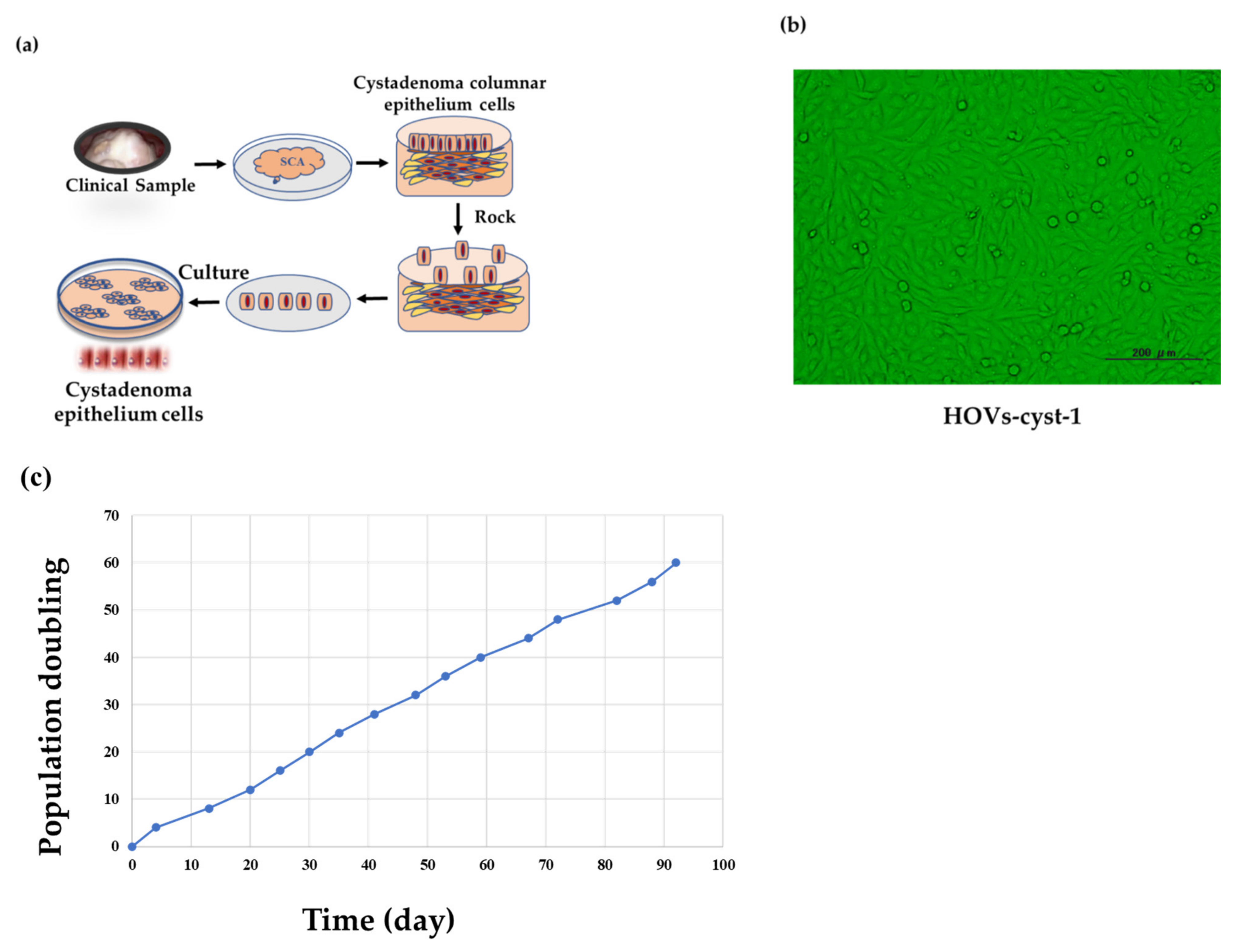
2.1. Purification and Isolation of Ovarian Serous Cystadenoma Epithelial Cells
2.2. Viral Vector Construction and Cell Transfection
2.3. Cell Culture and Cystadenoma Cell Lines
2.4. Population Doubling Assay
2.5. Whole-Exome Profiling
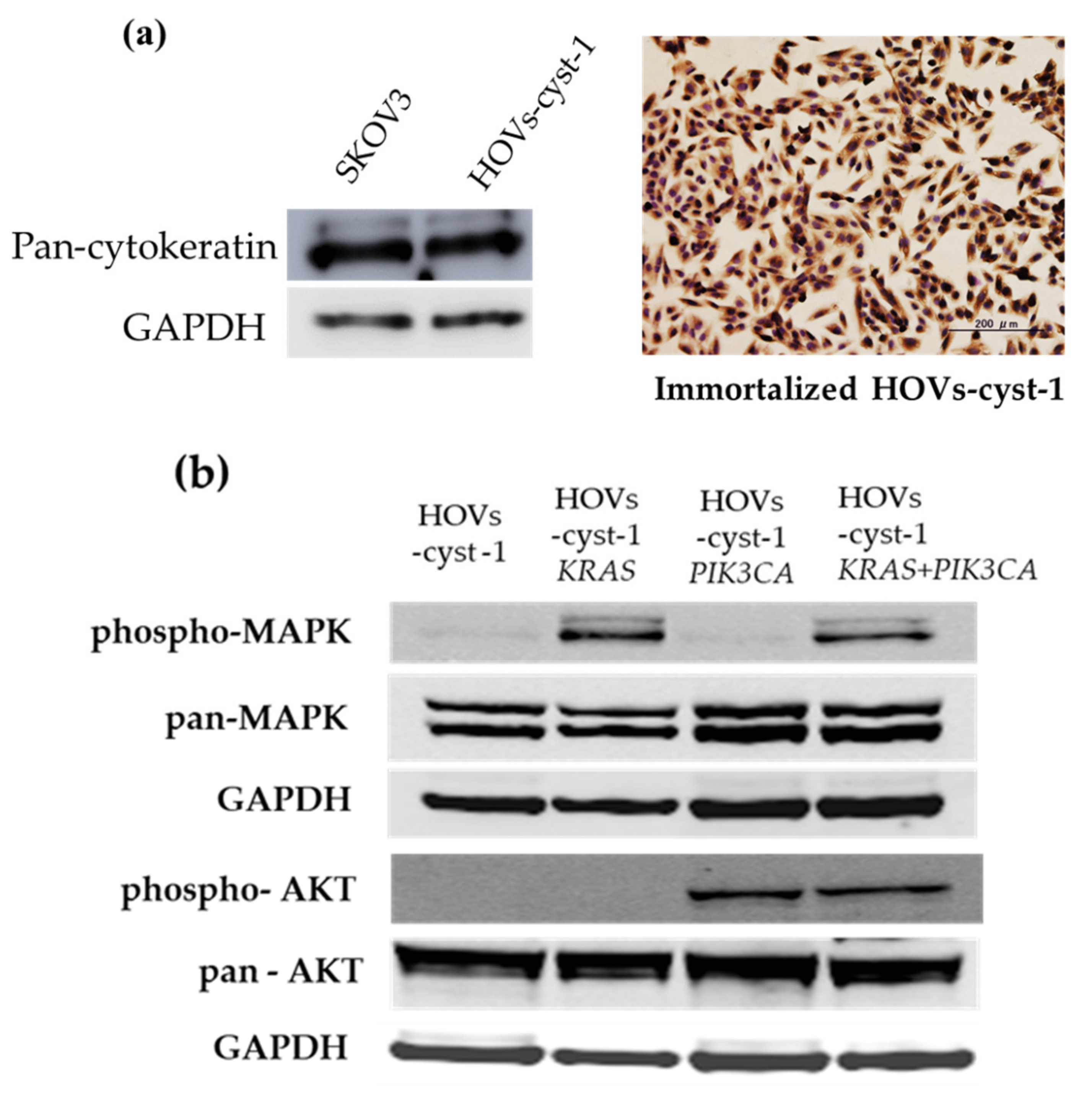
2.6. Immunocytochemistry (ICC)
2.7. Western Blot Analysis
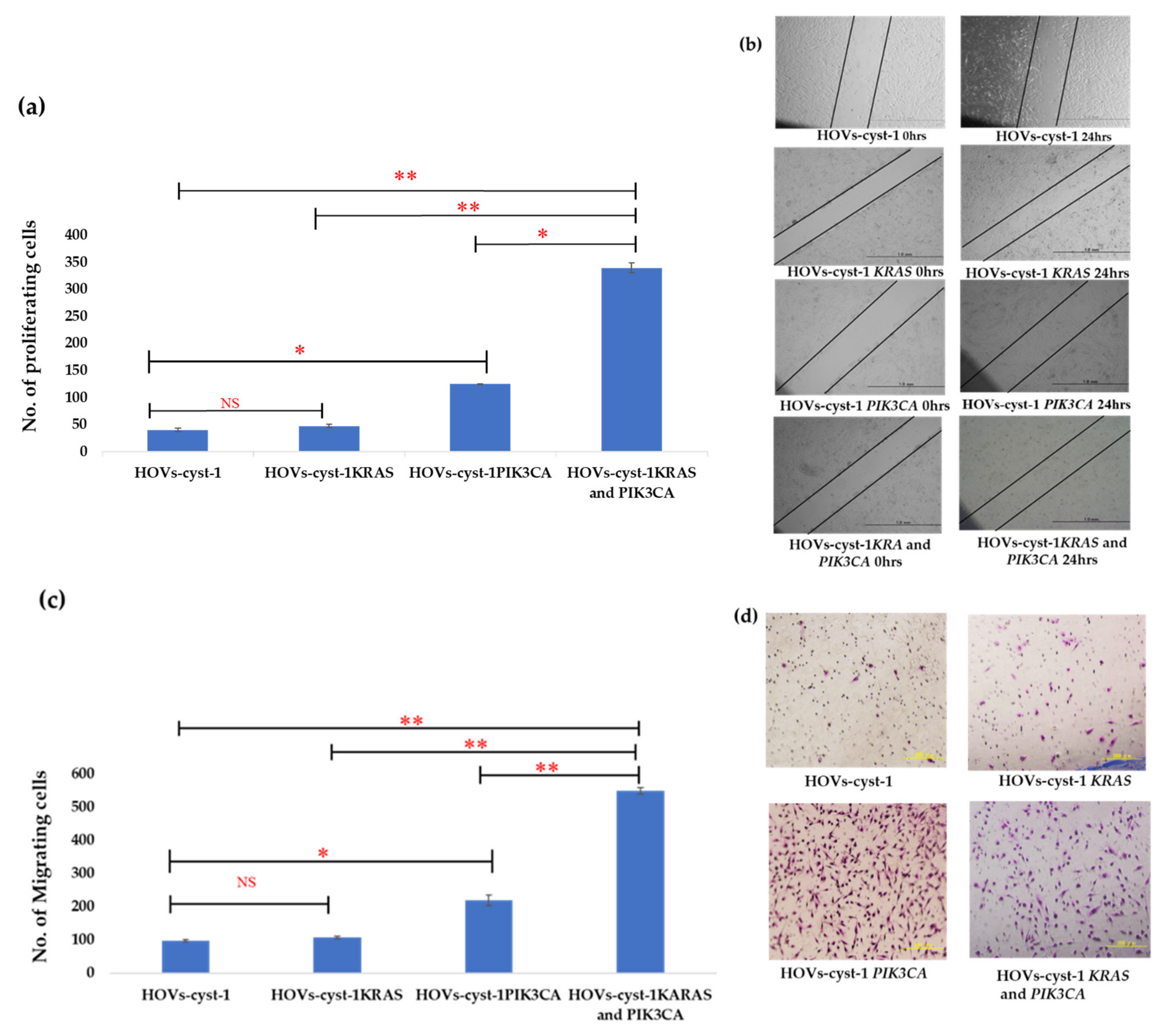
2.8. Cell Proliferation Assay
2.9. Wound Healing Assays
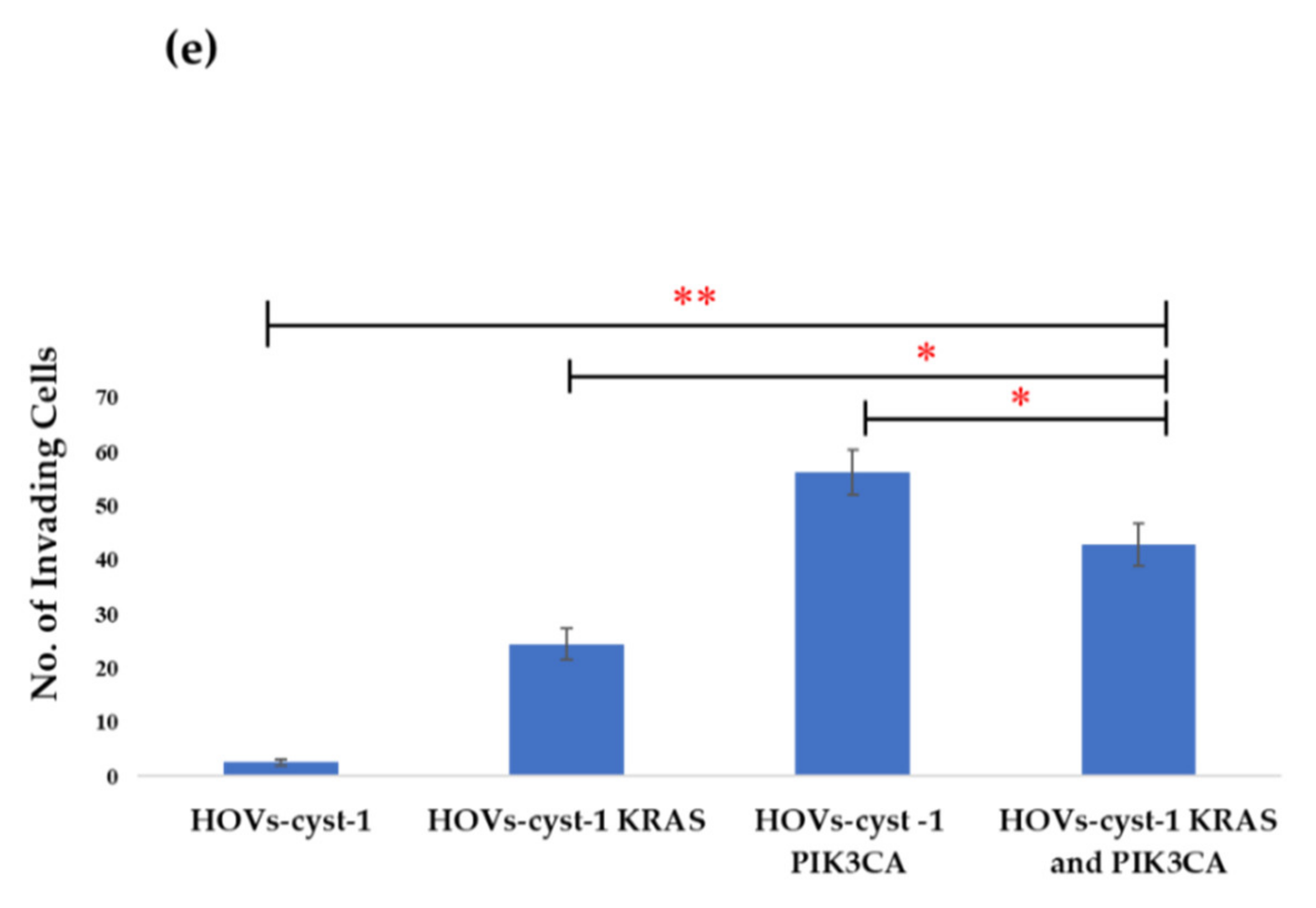
2.10. Matrigel Invasion Assay
2.11. Anchorage-Independent Assay
2.12. Nude Mouse Xenograft Experiments
2.13. Immunohistochemistry (IHC)
2.14. Statistical Analysis
3. Results
3.1. Development of Immortalized Serous Cystadenoma Epithelial Cell Lines
3.2. Immunocytochemical (ICC) and Western Blot Expression Pattern in Immortalized Serous Cystadenoma Epithelial Cell Lines
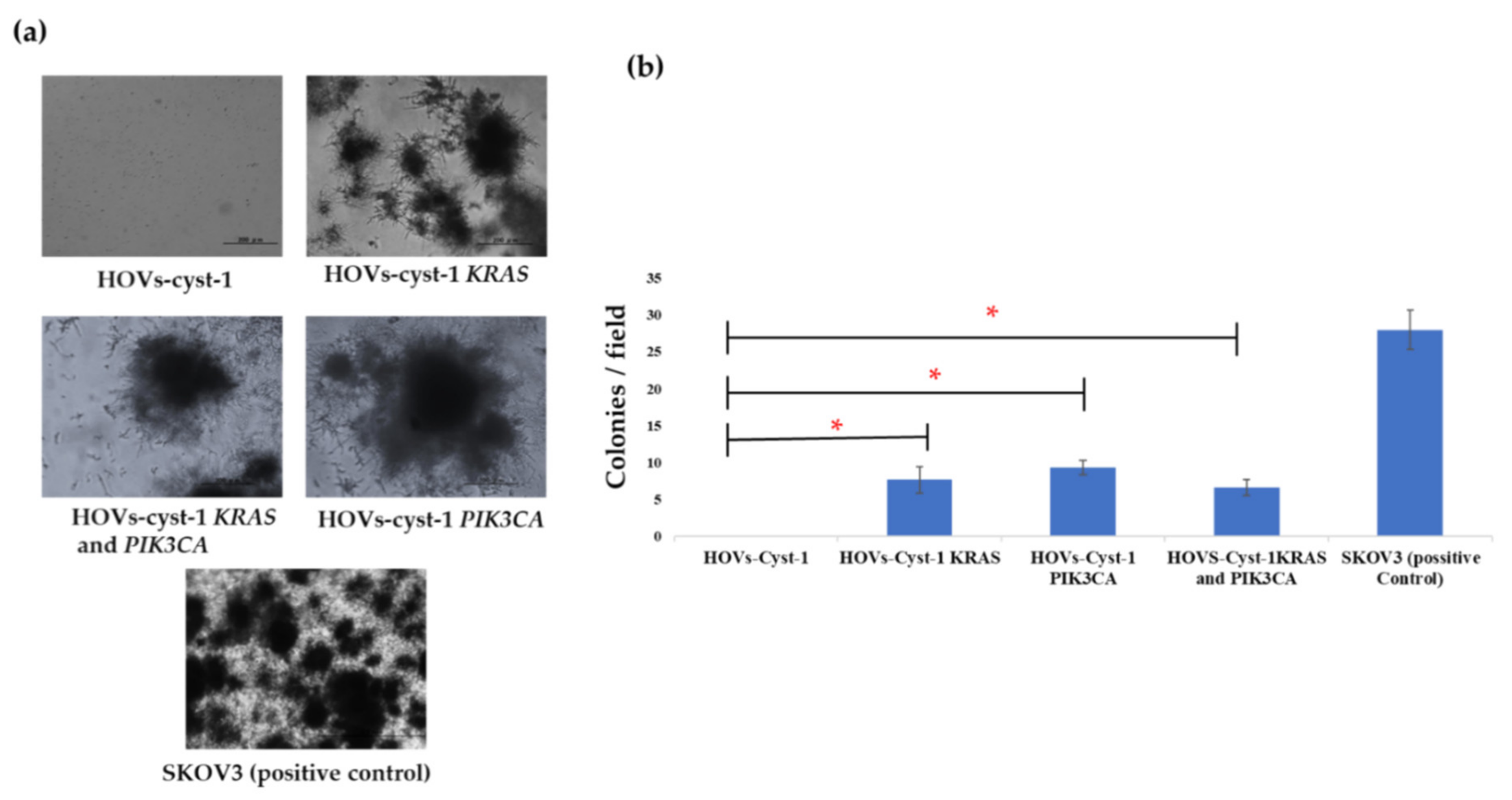
3.3. Proliferation, Migration, Invasion, and Anchorage-Independent Assays of a Series of HOVs-cyst-1 Mutant Cells
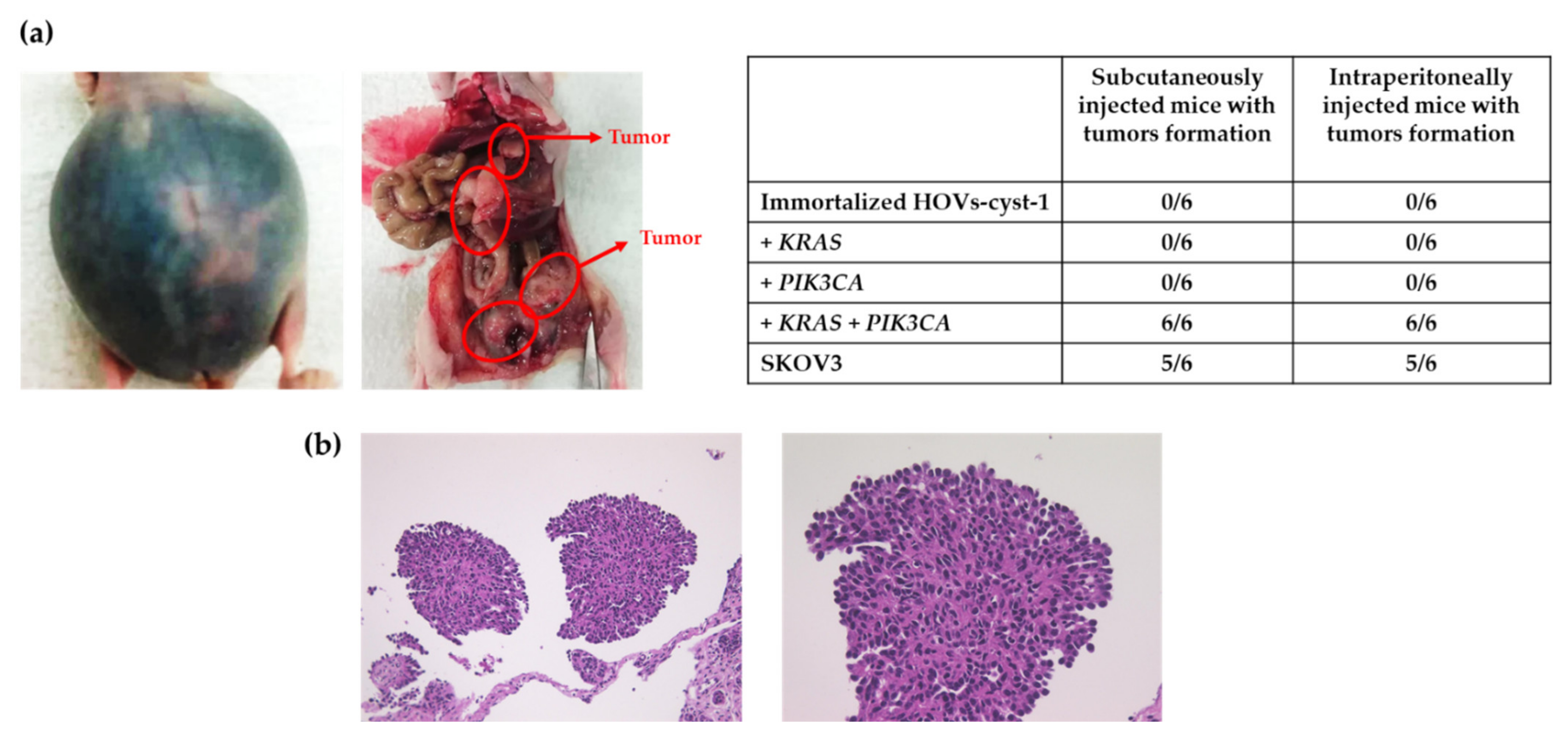
3.4. Tumorigenic Effect in HOVs-cyst-1 Both KRAS and PIK3CA Mutant Cells
3.5. Evaluation of Other Genetic Mutations in Immortalized HOVs-cyst-1 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banks, E.; Beral, V.; Reeves, G. The Epidemiology of Epithelial Ovarian Cancer: A Review. Int. J. Gynecol. Cancer 1997, 7, 425–438. [Google Scholar] [CrossRef]
- Parkin, D.M.; Muir, C.S.; Whelan, S.F. Cancer Incidence in Five Continents; International Arctic Research Center: Lyon, France, 1992. [Google Scholar]
- Shih, I.M.; Kurman, R.J. Ovarian Tumorigenesis—A Proposed Model Based on Morphological and Molecular Genetic Analysis. Am. J. Pathol. 2004, 164, 1511–1518. [Google Scholar] [CrossRef]
- Malpica, A.; Deavers, M.T.; Lu, K.; Bodurka, D.C.; Atkinson, E.N.; Gershenson, D.M.; Silva, E.G. Grading Ovarian Serous Carcinoma Using a Two-Tier System. Am. J. Surg. Pathol. 2004, 28, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Integrated Genomic Analyses of Ovarian Carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Chen, M.; Jin, Y.; Bi, Y.; Yin, J.; Wang, Y.; Pan, L. A Survival Analysis Comparing Women with Ovarian Low-Grade Serous Carcinoma to Those with High-Grade Histology. Onco. Targets Ther. 2014, 7, 1891–1899. [Google Scholar] [CrossRef]
- Wong, K.K.; Tsang, Y.T.; Deavers, M.T.; Mok, S.C.; Zu, Z.; Sun, C.; Malpica, A.; Wolf, J.K.; Lu, K.H.; Gershenson, D.M. BRAF Mutation Is Rare in Advanced-Stage Low-Grade Ovarian Serous Carcinomas. Am. J. Pathol. 2010, 177, 1611–1617. [Google Scholar] [CrossRef]
- Jones, S.; Wang, T.L.; Kurman, R.J.; Nakayama, K.; Velculescu, V.E.; Vogelstein, B.; Kinzler, K.W.; Papadopoulos, N.; Shih, I.M. Low-Grade Serous Carcinomas of the Ovary Contain Very Few Point Mutations. J. Pathol. 2012, 226, 413–420. [Google Scholar] [CrossRef]
- Singer, G.; Kurman, R.J.; Chang, H.W.; Cho, S.K.; Shih, I.M. Diverse Tumorigenic Pathways in Ovarian Serous Carcinoma. Am. J. Pathol. 2002, 160, 1223–1228. [Google Scholar] [CrossRef]
- Nakayama, K.; Nakayama, N.; Kurman, R.J.; Cope, L.; Pohl, G.; Samuels, Y.; Velculescu, V.E.; Wang, T.L.; Shih, I.M. Sequence Mutations and Amplification of PIK3CA and AKT2 Genes in Purified Ovarian Serous Neoplasms. Cancer Biol. Ther. 2006, 5, 779–785. [Google Scholar] [CrossRef]
- Nakamura, K.; Nakayama, K.; Ishibashi, T.; Ishikawa, N.; Ishikawa, M.; Katagiri, H.; Minamoto, T.; Sato, E.; Sanuki, K.; Yamashita, H.; et al. KRAS/BRAF Analysis in Ovarian Low-Grade Serous Carcinoma Having Synchronous All Pathological Precursor Regions. Int. J. Mol. Sci. 2016, 17, 625. [Google Scholar] [CrossRef]
- Ishibashi, T.; Nakayama, K.; Razia, S.; Ishikawa, M.; Nakamura, K.; Yamashita, H.; Dey, P.; Iida, K.; Kurioka, H.; Nakayama, S.; et al. High Frequency of PIK3CA Mutations in Low-Grade Serous Ovarian Carcinomas of Japanese Patients. Diagnostics 2019, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Bono, Y.; Kyo, S.; Takakura, M.; Maida, Y.; Mizumoto, Y.; Nakamura, M.; Nomura, K.; Kiyono, T.; Inoue, M. Creation of Immortalized Epithelial Cells From Ovarian Endometrioma. Br. J. Cancer. 2012, 106, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, T.; Hauer, M.; Schneider, J.; Serrano, M.; Wölfel, C.; Klehmann-Hieb, E.; De Plaen, E.; Hankeln, T.; Büschenfelde, K.H.; Beach, D. A p16INK4a-Insensitive CDK4 Mutant Targeted by Cytolytic T Lymphocytes in a Human Melanoma. Science 1995, 269, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, K.; Kiyono, T.; Okamura, K.; Uezumi, M.; Goto, Y.; Yasumoto, S.; Shimizu, S.; Hashimoto, N. CDK4 and Cyclin D1 Allow Human Myogenic Cells to Recapture Growth Property Without Compromising Differentiation Potential. Gene Ther. 2011, 18, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Blömer, U.; Takahashi, M.; Gage, F.H.; Verma, I.M. Development of a Self-Inactivating Lentivirus Vector. J. Virol. 1998, 72, 8150–8157. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.Y.; Fukuda, T.; Yang, L.; Zaha, H.; Akanuma, H.; Zeng, Q.; Yoshinaga, J.; Sone, H. Effects of Bisphenol A Exposure on the Proliferation and Senescence of Normal Human Mammary Epithelial Cells. Cancer Biol. Ther. 2012, 13, 296–306. [Google Scholar] [CrossRef]
- Nakamura, K.; Aimono, E.; Tanishima, S.; Imai, M.; Nagatsuma, A.K.; Hayashi, H.; Yoshimura, Y.; Nakayama, K.; Kyo, S.; Nishihara, H. Intratumoral Genomic Heterogeneity May Hinder Precision Medicine Strategies in Patients with Serous Ovarian Carcinoma. Diagnostics 2020, 10, 200. [Google Scholar] [CrossRef]
- Nakayama, K.; Miyazaki, K.; Kanzaki, A.; Fukumoto, M.; Takebayashi, Y. Expression and Cisplatin Sensitivity of Copper-Transporting P-Type Adenosine Triphosphates (ATP7B) in Human Solid Carcinoma Cell Lines. Oncol. Rep. 2001, 8, 1285–1287. [Google Scholar] [CrossRef]
- Grisham, R.N.; Iyer, G.; Garg, K.; Delair, D.; Hyman, D.M.; Zhou, Q.; Iasonos, A.; Berger, M.F.; Dao, F.; Spriggs, D.R.; et al. BRAF Mutation Is Associated with Early Stage Disease and Improved Outcome in Patients with Low-Grade Serous Ovarian Cancer. Cancer 2013, 119, 548–554. [Google Scholar] [CrossRef]
- Hunter, S.M.; Anglesio, M.S.; Ryland, G.L.; Sharma, R.; Chiew, Y.E.; Rowley, S.M.; Doyle, M.A.; Li, J.; Gilks, C.B.; Moss, P.; et al. Molecular Profiling of Low Grade Serous Ovarian Tumours Identies Novel Candidate Driver genes. Oncotarget 2015, 6, 37663–37677. [Google Scholar] [CrossRef]
- Etemadmoghadam, D.; Azar, W.J.; Lei, Y.; Moujaber, T.; Garsed, D.W.; Kennedy, C.J.; Fereday, S.; Mitchell, C.; Chiew, Y.E.; Hendley, J.; et al. EIF1AX and NRAS Mutations Co-Occur and Cooperate in Low-Grade Serous Ovarian Carcinomas. Cancer Res. 2017, 77, 4268–4278. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Narisawa-Saito, M.; Yugawa, T.; Fujita, M.; Tashiro, H.; Katabuchi, H. Kiyono, T. Oncogenic Transformation of Human Ovarian Surface Epithelial Cells with Defined Cellular Oncogenes. Carcinogenesis 2009, 30, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nakayama, K.; Ishikawa, N.; Ishikawa, M.; Sultana, R.; Kiyono, T.; Kyo, S. Reconstitution of High-Grade Serous Ovarian Carcinoma From Primary Fallopian Tube Secretory Epithelial Cells. Oncotarget 2017, 9, 12609–12619. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, M.; Ma, H.; Pan, Y.; Xiao, W.; Li, J.; Yu, J.; He, J. PAX2 and PAX8 Reliably Distinguishes Ovarian Serous Tumors from Mucinous Tumors. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 280–287. [Google Scholar] [CrossRef]
- Papadatos-Pastos, R.; Rabbie, P.; Ross, P.E.; Bonnot, G.; Passot, R.A.S. The Role of the PI3K Pathway in Colorectal Cancer. Crit. Rev. Oncol. Hematol. 2015, 94, 18–30. [Google Scholar] [CrossRef]
- Wang, G.M.; Wong, H.Y.; Konishi, H.; Blair, B.G.; Abukhdeir, A.M.; Gustin, J.P.; Rosen, D.M.; Denmeade, S.R.; Rasheed, Z.; Matsui, W.; et al. Single Copies of Mutant KRAS and Mutant PIK3CA Cooperate in Immortalized Human Epithelial Cells to Induce Tumor Formation. Cancer Res. 2013, 73, 3248–3261. [Google Scholar] [CrossRef]
- Hossain, M.M.; Nakayama, K.; Shanta, K.; Razia, S.; Ishikawa, M.; Ishibashi, T.; Yamashita, H.; Sato, S.; Iida, K.; Kanno, K.; et al. Establishment of a Novel In Vitro Model of Endometriosis with Oncogenic KRAS and PIK3CA Mutations for Understanding the Underlying Biology and Molecular Pathogenesis. Cancers 2021, 13, 3174. [Google Scholar] [CrossRef]
- Laury, R.A.; Hornick, L.J.; Perets, R.; Krane, F.J.; Corson, J.; Drapkin, R.; Hirsch, S.M. PAX8 reliably distinguishes ovarian serous tumors from malignant mesothelioma. Am. J. Surg. Pathol. 2010, 34, 627–635. [Google Scholar] [CrossRef]
- Tomasetti, C.; Marchionni, L.; Nowak, M.A.; Parmigiani, G.; Vogelstein, B. Only Three Driver Gene Mutations Are Required for the Development of Lung and Colorectal Cancers. Proc. Natl Acad. Sci. USA 2015, 112, 118–123. [Google Scholar] [CrossRef]
- Vogelstein, B.; Kinzler, K.W. The Path to Cancer –Three Strikes and You’re Out. N. Engl. J. Med. 2015, 373, 1895–1898. [Google Scholar] [CrossRef]
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in Cancer. Carcinogenesis 2010, 31, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.J.; Kurman, R.J.; Wang, M.; Oldt, R.; Wang, B.G.; Berman, D.M.; Shih, I.M. Molecular Genetic Analysis of Ovarian Serous cystadenomas. Lab. Investig. 2004, 84, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, K.D.; Marjoram, P.; Woo, Y.J.; Tavaré, S.; Shibata, D. Inferring Clonal Expansion and Cancer Stem Cell Dynamics from DNA Methylation Patterns in Colorectal Cancers. Proc. Natl. Acad. Sci. USA 2009, 106, 4828–4833. [Google Scholar] [CrossRef] [PubMed]
- Meir, Z.; Mukamel, Z.; Chomsky, E.; Lifshitz, A.; Tanay, A. Single-Cell Analysis of Clonal Maintenance of Transcriptional and Epigenetic States in Cancer Cells. Nat. Genet. 2020, 52, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Santillan, A.; Kim, Y.W.; Zahurak, M.L.; Gardner, G.J.; Giuntoli, R.L., 2nd; Shih, I.M.; Bristow, R.E. Differences of Chemoresistance Assay Between Invasive Micropapillary/low-grade Serous Ovarian Carcinoma and High-grade Serous Ovarian Carcinoma. Int. J. Gynecol. Cancer 2007, 17, 601–616. [Google Scholar] [CrossRef]
- Tang, M.; O’Connell, R.L.; Amant, F.; Beale, P.; McNally, O.; Sjoquist, K.M.; Grant, P.; Davis, A.; Sykes, P.; Mileshkin, L.; et al. PARAGON: A Phase II study of Anastrozole in Patients with Estrogen Receptor-positive Recurrent/metastatic Low-grade Ovarian Cancers and Serous Borderline Ovarian Tumors. Gynecol. Oncol. 2019, 154, 531–538. [Google Scholar] [CrossRef]
- Escobar, J.; Klimowicz, A.C.; Dean, M.; Chu, P.; Nation, J.G.; Nelson, G.S.; Ghatage, P.; Kalloger, S.E.; Köbel, M. Quantification of ER/PR Expression in Ovarian Low-grade Serous Carcinoma. Gynecol. Oncol. 2013, 128, 371–376. [Google Scholar] [CrossRef]
- Gershenson, D.M.; Sun, C.C.; Iyer, R.B.; Malpica, A.L.; Kavanagh, J.J.; Bodurka, D.C.; Schmeler, K.; Deavers, M. Hormonal Therapy for Recurrent Low-grade serous Carcinoma of the Ovary or Peritoneum. Gynecol. Oncol. 2012, 125, 661–666. [Google Scholar] [CrossRef]
- Fader, A.N.; Bergstrom, J.; Jernigan, A.; Tanner, E.J., 3rd; Roche, K.L.; Stone, R.L.; Levinson, K.L.; Ricci, S.; Wethingon, S.; Wang, T.L.; et al. Primary Cytoreductive Surgery and Adjuvant Hormonal Monotherapy in Women with Advanced Low-grade Serous Ovarian Carcinoma: Reducing Overtreatment Without Compromising Survival? Gynecol. Oncol. 2017, 147, 85–91. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, P.; Nakayama, K.; Razia, S.; Ishikawa, M.; Ishibashi, T.; Yamashita, H.; Kanno, K.; Sato, S.; Kiyono, T.; Kyo, S. Development of Low-Grade Serous Ovarian Carcinoma from Benign Ovarian Serous Cystadenoma Cells. Cancers 2022, 14, 1506. https://doi.org/10.3390/cancers14061506
Dey P, Nakayama K, Razia S, Ishikawa M, Ishibashi T, Yamashita H, Kanno K, Sato S, Kiyono T, Kyo S. Development of Low-Grade Serous Ovarian Carcinoma from Benign Ovarian Serous Cystadenoma Cells. Cancers. 2022; 14(6):1506. https://doi.org/10.3390/cancers14061506
Chicago/Turabian StyleDey, Puja, Kentaro Nakayama, Sultana Razia, Masako Ishikawa, Tomoka Ishibashi, Hitomi Yamashita, Kosuke Kanno, Seiya Sato, Tohru Kiyono, and Satoru Kyo. 2022. "Development of Low-Grade Serous Ovarian Carcinoma from Benign Ovarian Serous Cystadenoma Cells" Cancers 14, no. 6: 1506. https://doi.org/10.3390/cancers14061506
APA StyleDey, P., Nakayama, K., Razia, S., Ishikawa, M., Ishibashi, T., Yamashita, H., Kanno, K., Sato, S., Kiyono, T., & Kyo, S. (2022). Development of Low-Grade Serous Ovarian Carcinoma from Benign Ovarian Serous Cystadenoma Cells. Cancers, 14(6), 1506. https://doi.org/10.3390/cancers14061506





