The Role of Ferric Nitrilotriacetate in Renal Carcinogenesis and Cell Death: From Animal Models to Clinical Implications
Abstract
:Simple Summary
Abstract
1. Introduction
2. Iron-Induced Renal Carcinogenesis
2.1. Ferric Nitrilotriacetate (Fe-NTA) Induces Renal Oxidative Injury and Eventually Leads to Renal Cell Carcinoma (RCC)
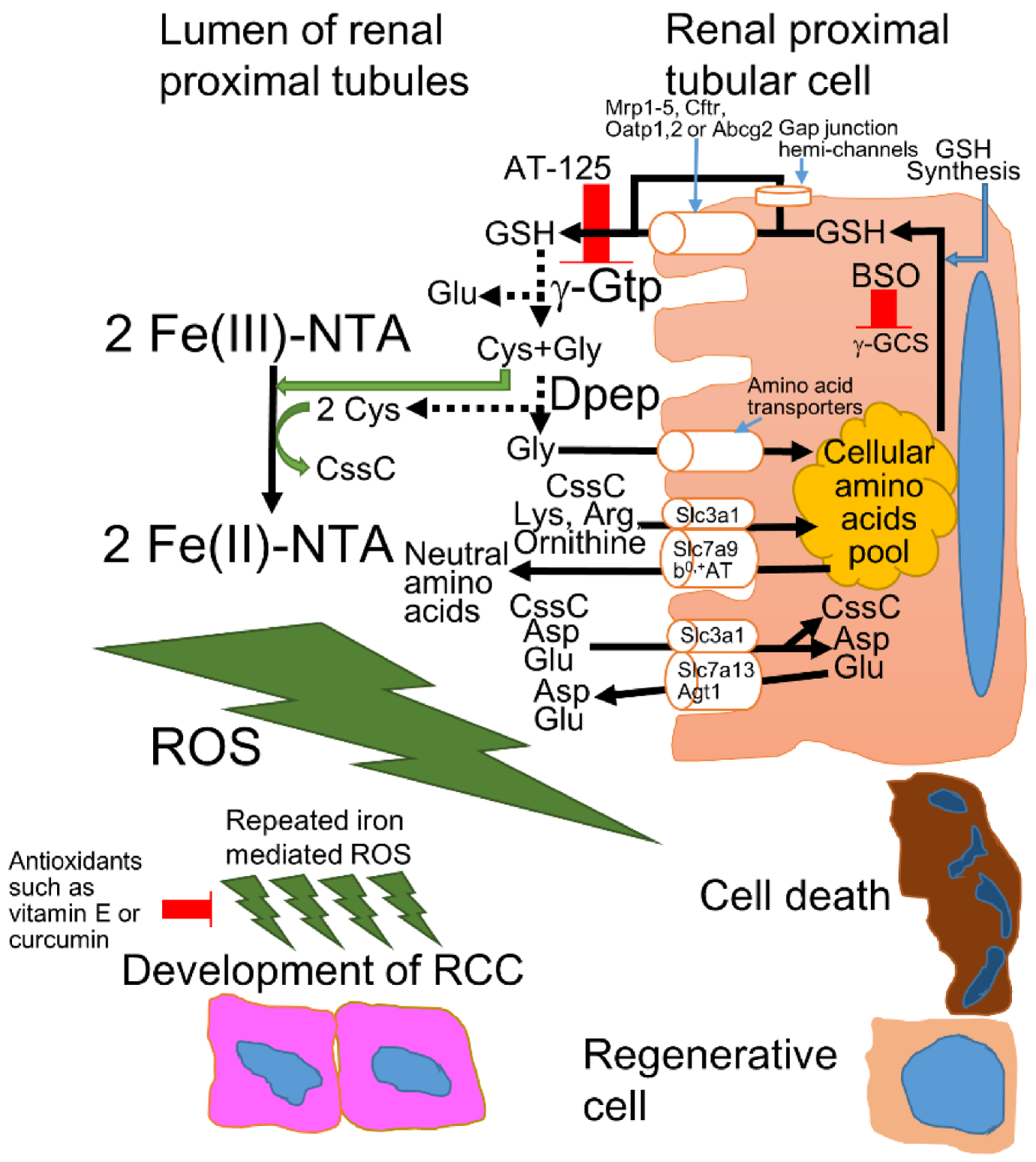
2.2. Mechanisms of Fe-NTA-Induced Renal Oxidative Injury
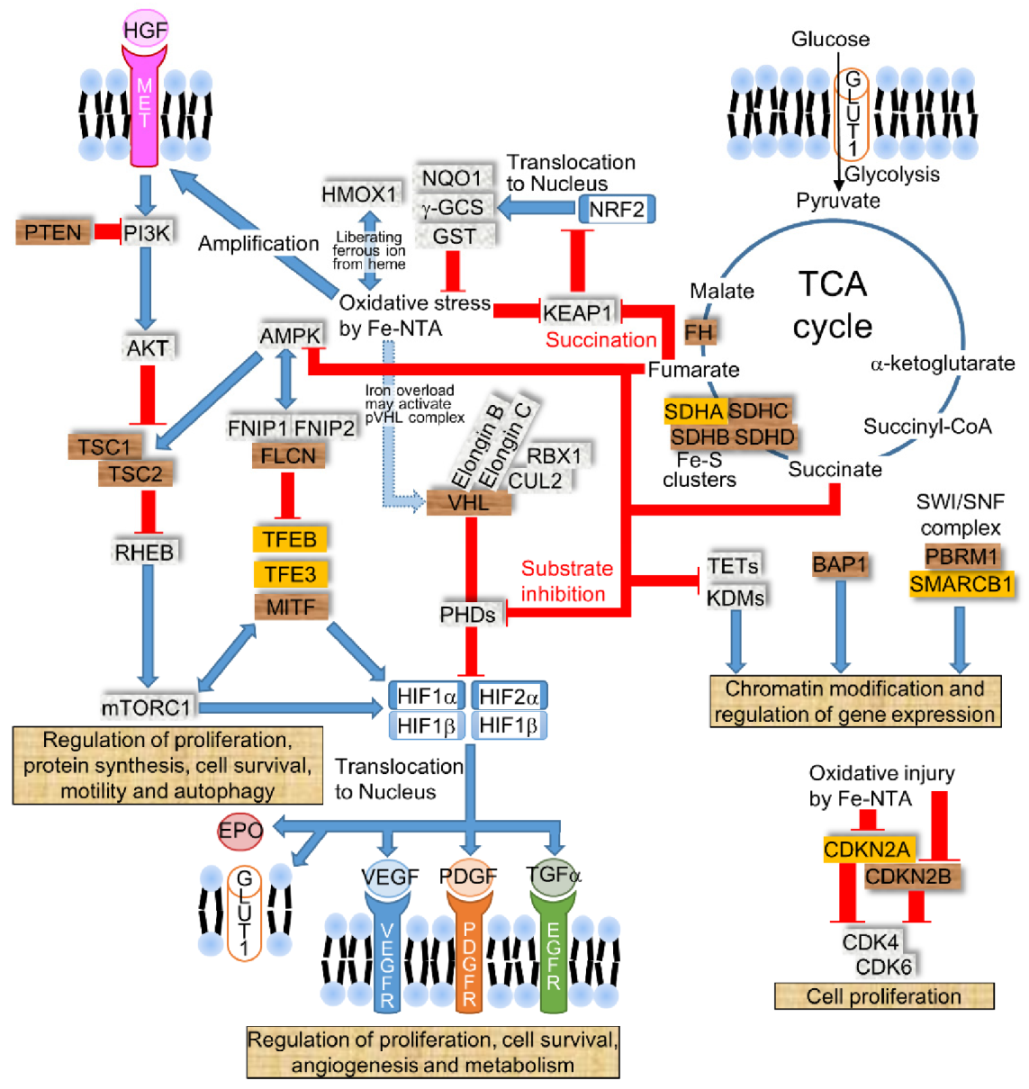
2.3. Molecular Mechanisms of Fe-NTA-Induced Carcinogenesis in Rodents Compared to Human RCC
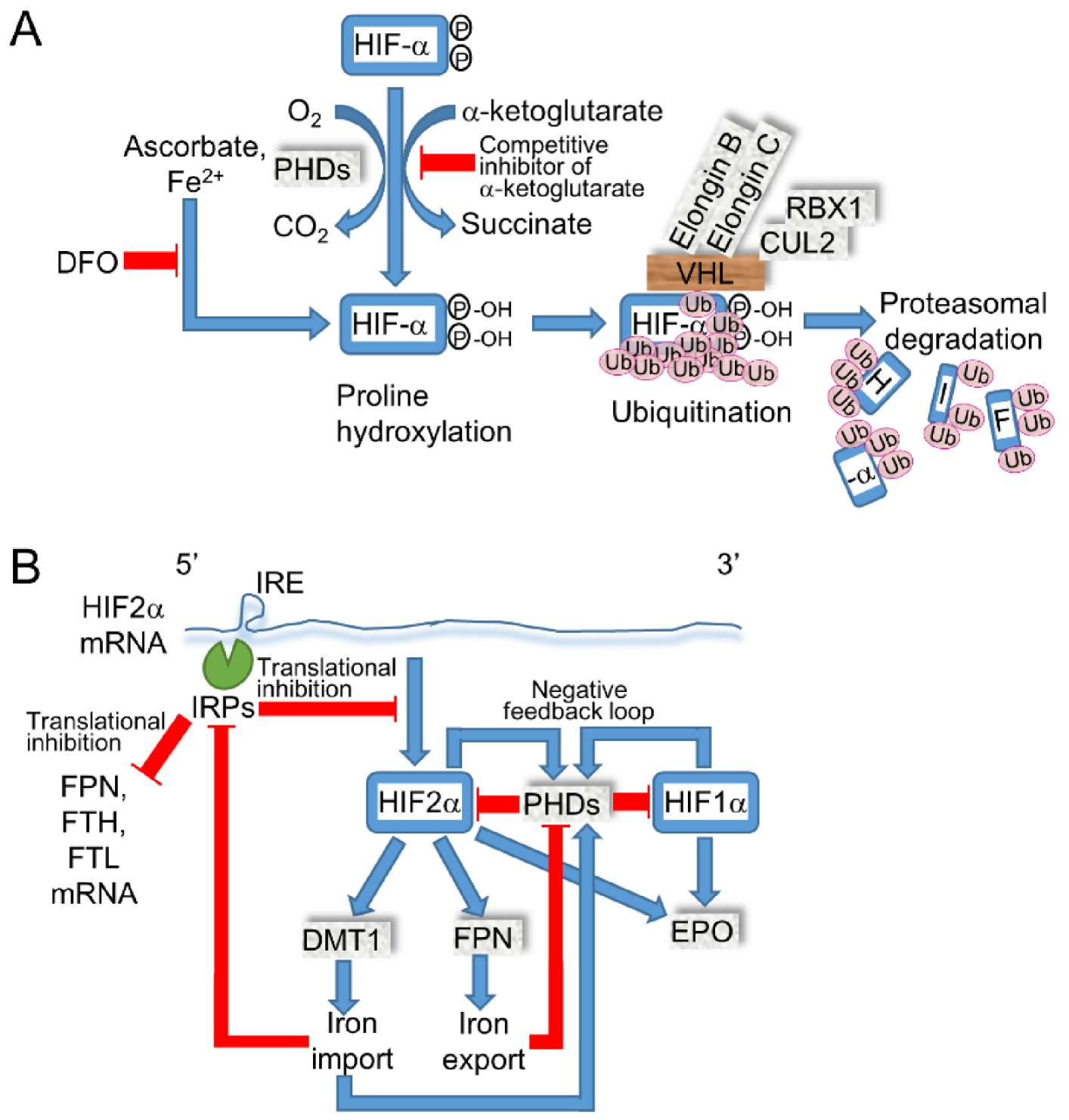
2.4. Iron Modulates the Expressions of HIF-α by PHDs and HIF2α by IRE/Iron Regulatory Protein (IRP)
2.5. Disruption in Succinate Dehydrogenase (SDH) or Fumarate Hydratase (FH) Causes RCC by Inhibiting PHDs
2.6. Ischemia Reperfusion and Iron-Mediated ROS Induces Renal Medullary Carcinoma
2.7. Histopathology of RCC
2.8. Iron-Induced Peritoneal Malignant Mesothelioma (MM) in Rats
3. Ferroptosis
3.1. Concept of Ferroptosis
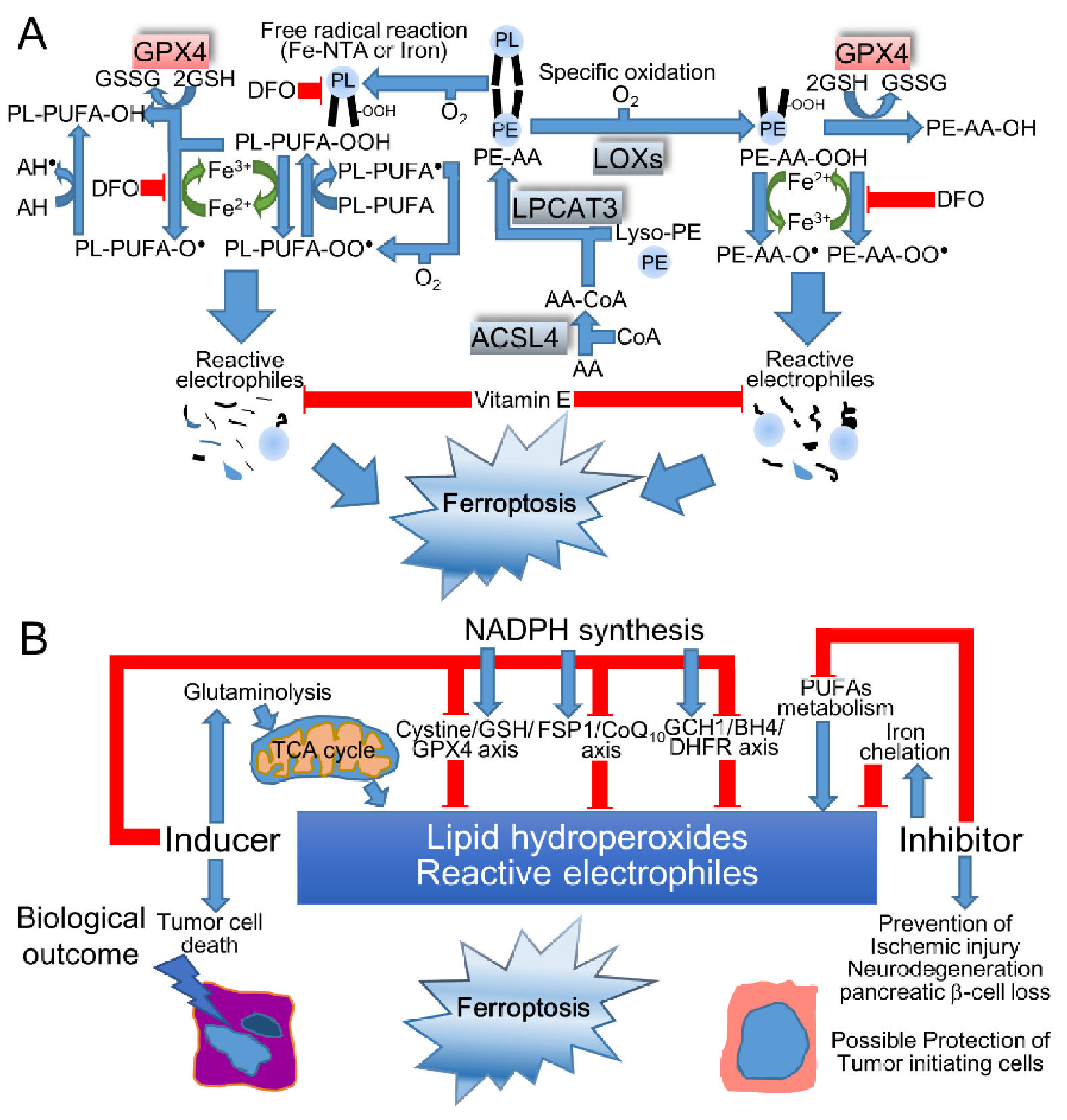
3.2. The Mechanisms of Ferroptosis
3.3. Ferroptosis in the Kidney and RCC
3.4. Induction of Oxidized Lipids and RCC by Fe-NTA
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AKI | acute kidney injury |
| BN/F344 | Brown Norway/Fischer344 |
| DFO | deferoxamine |
| DMT1 | divalent metal transporter 1 |
| Fe-NTA | ferric nitrilotriacetate |
| GSH | glutathione |
| GPX4 | glutathione peroxidase 4 |
| HCC | hepatocellular carcinoma |
| HD | homozygous deletion |
| IRE | iron-responsive element |
| IRI | ischemia-reperfusion injury |
| IRP | iron-regulatory protein |
| LOH | loss of heterozygosity |
| MM | malignant mesothelioma |
| RCC | renal cell carcinoma |
| ROS | reactive oxygen species |
| Wt | wild-type |
References
- Torti, S.V.; Manz, D.H.; Paul, B.T.; Blanchette-Farra, N.; Torti, F.M. Iron and Cancer. Annu. Rev. Nutr. 2018, 38, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Gunshin, H.; Fujiwara, Y.; Custodio, A.O.; Direnzo, C.; Robine, S.; Andrews, N.C. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J. Clin. Investig. 2005, 115, 1258–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badu-Boateng, C.; Naftalin, R.J. Ascorbate and ferritin interactions: Consequences for iron release in vitro and in vivo and implications for inflammation. Free Radic. Biol. Med. 2019, 133, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S.; Kong, Y.; Cheng, Z.; Sato, K.; Hayashi, S.; Ito, F.; Jiang, L.; Yanatori, I.; Okazaki, Y.; Akatsuka, S. Carcinogenesis as Side Effects of Iron and Oxygen Utilization: From the Unveiled Truth toward Ultimate Bioengineering. Cancers 2020, 12, 3320. [Google Scholar] [CrossRef]
- Awai, M.; Narasaki, M.; Yamanoi, Y.; Seno, S. Induction of diabetes in animals by parenteral administration of ferric nitrilotriacetate. A model of experimental hemochromatosis. Am. J. Pathol. 1979, 95, 663–673. [Google Scholar]
- Awai, M. Experimental iron overload using ferric nitrilotriacetate (Fe3+-NTA): A proposal model of the diseases in man caused by iron overload. Nihon Ketsueki Gakkai Zasshi 1986, 49, 1650–1659. [Google Scholar]
- Bates, G.W.; Wernicke, J. The kinetics and mechanism of iron(3) exchange between chelates and transferrin. IV. The reaction of transferrin with iron(3) nitrilotriacetate. J. Biol. Chem. 1971, 246, 3679–3685. [Google Scholar] [CrossRef]
- Okada, S. Iron-induced tissue damage and cancer: The role of reactive oxygen species-free radicals. Pathol. Int. 1996, 46, 311–332. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef]
- Uehara, T.; Pogribny, I.P.; Rusyn, I. The DEN and CCl4-Induced Mouse Model of Fibrosis and Inflammation-Associated Hepatocellular Carcinoma. Curr. Protoc. Pharmacol. 2014, 66, 14–30. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.; Shah, M.D.; Vun-Sang, S.; Okazaki, Y.; Okada, S. The therapeutic potential of curcumin in alleviating N-diethylnitrosamine and iron nitrilotriacetate induced renal cell tumours in mice via inhibition of oxidative stress: Implications for cancer chemoprevention. Biomed. Pharmacother. 2021, 139, 111636. [Google Scholar] [CrossRef]
- Athar, M.; Iqbal, M. Ferric nitrilotriacetate promotes N-diethylnitrosamine-induced renal tumorigenesis in the rat: Implications for the involvement of oxidative stress. Carcinogenesis 1998, 19, 1133–1139. [Google Scholar] [CrossRef] [Green Version]
- Toyokuni, S.; Okada, S.; Hamazaki, S.; Fujioka, M.; Li, J.L.; Midorikawa, O. Cirrhosis of the liver induced by cupric nitrilotriacetate in Wistar rats. An experimental model of copper toxicosis. Am. J. Pathol. 1989, 134, 1263–1274. [Google Scholar]
- Toyokuni, S.; Tanaka, T.; Nishiyama, Y.; Okamoto, K.; Nakashima, Y.; Hamazaki, S.; Okada, S.; Hiai, H. Induction of renal cell carcinoma in male Wistar rats treated with cupric nitrilotriacetate. Lab. Investig. 1996, 75, 239–248. [Google Scholar]
- Kitaura, K.; Chone, Y.; Satake, N.; Akagi, A.; Ohnishi, T.; Suzuki, Y.; Izumi, K. Role of copper accumulation in spontaneous renal carcinogenesis in Long-Evans Cinnamon rats. Jpn. J. Cancer Res. 1999, 90, 385–392. [Google Scholar] [CrossRef]
- Shribman, S.; Poujois, A.; Bandmann, O.; Czlonkowska, A.; Warner, T.T. Wilson’s disease: Update on pathogenesis, biomarkers and treatments. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1053–1061. [Google Scholar] [CrossRef]
- Ebina, Y.; Okada, S.; Hamazaki, S.; Ogino, F.; Li, J.L.; Midorikawa, O. Nephrotoxicity and renal cell carcinoma after use of iron- and aluminum-nitrilotriacetate complexes in rats. J. Natl. Cancer Inst. 1986, 76, 107–113. [Google Scholar]
- Hamazaki, S.; Okada, S.; Li, J.L.; Toyokuni, S.; Midorikawa, O. Oxygen reduction and lipid peroxidation by iron chelates with special reference to ferric nitrilotriacetate. Arch. Biochem. Biophys. 1989, 272, 10–17. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.; Wang, X.; Marshall, M.J.; Zachara, J.M.; Rosso, K.M.; Dupuis, M.; Fredrickson, J.K.; Heald, S.; Shi, L. Kinetics of reduction of Fe(III) complexes by outer membrane cytochromes MtrC and OmcA of Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 2008, 74, 6746–6755. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Croydon, UK, 2015. [Google Scholar]
- Liu, M.; Okada, S.; Kawabata, T. Radical-promoting “free” iron level in the serum of rats treated with ferric nitrilotriacetate: Comparison with other iron chelate complexes. Acta Med. Okayama 1991, 45, 401–408. [Google Scholar] [CrossRef]
- Engelmann, M.D.; Bobier, R.T.; Hiatt, T.; Cheng, I.F. Variability of the Fenton reaction characteristics of the EDTA, DTPA, and citrate complexes of iron. Biometals 2003, 16, 519–527. [Google Scholar] [CrossRef]
- Liu, M.; Okada, S. Induction of free radicals and tumors in the kidneys of Wistar rats by ferric ethylenediamine-N,N′-diacetate. Carcinogenesis 1994, 15, 2817–2821. [Google Scholar] [CrossRef]
- Mizuno, R.; Kawabata, T.; Sutoh, Y.; Nishida, Y.; Okada, S. Oxidative renal tubular injuries induced by aminocarboxylate-type iron (III) coordination compounds as candidate renal carcinogens. Biometals 2006, 19, 675–683. [Google Scholar] [CrossRef]
- Nishida, Y.; Yoshizawa, K.; Akamatsu, T. Preparation of Iron(III) Complex with Nitrilotriacetic Acid and Origin of Its Unique Reactivity. Chem. Lett. 1991, 20, 1521–1524. [Google Scholar] [CrossRef]
- Spear, N.; Aust, S.D. Thiol-mediated NTA-Fe(III) reduction and lipid peroxidation. Arch. Biochem. Biophys. 1994, 312, 198–202. [Google Scholar] [CrossRef]
- Bachhawat, A.K.; Thakur, A.; Kaur, J.; Zulkifli, M. Glutathione transporters. Biochim. Biophys. Acta 2013, 1830, 3154–3164. [Google Scholar] [CrossRef]
- Okada, S.; Minamiyama, Y.; Hamazaki, S.; Toyokuni, S.; Sotomatsu, A. Glutathione cycle dependency of ferric nitrilotriacetate-induced lipid peroxidation in mouse proximal renal tubules. Arch. Biochem. Biophys. 1993, 301, 138–142. [Google Scholar] [CrossRef]
- Nishida, Y.; Ito, Y.; Satoh, T. Origin of renal proximal tubular injuries by Fe(III)-nta chelate. Z. Nat. C J. Biosci. 2007, 62, 608–612. [Google Scholar] [CrossRef] [Green Version]
- Ekanayake, D.M.; Pham, D.; Probst, A.L.; Miller, J.R.; Popescu, C.V.; Fiedler, A.T. Electronic structures and spectroscopic signatures of diiron intermediates generated by O2 activation of nonheme iron(II)-thiolate complexes. Dalton Trans. 2021, 50, 14432–14443. [Google Scholar] [CrossRef]
- Toyokuni, S.; Masumizu, T.; Ozeki, M.; Kondo, S.; Hiroyasu, M.; Kohno, M.; Hiai, H. An electron spin resonance study on alkylperoxyl radical in thin-sliced renal tissues from ferric nitrilotriacetate-treated rats: The effect of alpha-tocopherol feeding. Free Radic. Res. 2001, 35, 245–255. [Google Scholar] [CrossRef]
- Akatsuka, S.; Yamashita, Y.; Ohara, H.; Liu, Y.T.; Izumiya, M.; Abe, K.; Ochiai, M.; Jiang, L.; Nagai, H.; Okazaki, Y.; et al. Fenton reaction induced cancer in wild type rats recapitulates genomic alterations observed in human cancer. PLoS ONE 2012, 7, e43403. [Google Scholar] [CrossRef] [PubMed]
- Corti, A.; Belcastro, E.; Dominici, S.; Maellaro, E.; Pompella, A. The dark side of gamma-glutamyltransferase (GGT): Pathogenic effects of an ‘antioxidant’ enzyme. Free Radic. Biol. Med. 2020, 160, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Broer, S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, Y.D.; Ganapathy, V. Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim. Biophys. Acta 2016, 1863, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Nagamori, S.; Wiriyasermkul, P.; Guarch, M.E.; Okuyama, H.; Nakagomi, S.; Tadagaki, K.; Nishinaka, Y.; Bodoy, S.; Takafuji, K.; Okuda, S.; et al. Novel cystine transporter in renal proximal tubule identified as a missing partner of cystinuria-related plasma membrane protein rBAT/SLC3A1. Proc. Natl. Acad. Sci. USA 2016, 113, 775–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.L.; Okada, S.; Hamazaki, S.; Deng, I.L.; Midorikawa, O. Sex differences in ferric nitrilotriacetate-induced lipid peroxidation and nephrotoxicity in mice. Biochim. Biophys. Acta 1988, 963, 82–87. [Google Scholar] [CrossRef]
- Toyokuni, S.; Okada, S.; Hamazaki, S.; Minamiyama, Y.; Yamada, Y.; Liang, P.; Fukunaga, Y.; Midorikawa, O. Combined histochemical and biochemical analysis of sex hormone dependence of ferric nitrilotriacetate-induced renal lipid peroxidation in ddY mice. Cancer Res. 1990, 50, 5574–5580. [Google Scholar]
- Deguchi, J.; Miyamoto, M.; Okada, S. Sex hormone-dependent renal cell carcinogenesis induced by ferric nitrilotriacetate in Wistar rats. Jpn. J. Cancer Res. 1995, 86, 1068–1071. [Google Scholar] [CrossRef]
- Hirayama, K.; Yasutake, A.; Inoue, M. Effect of sex hormones on the fate of methylmercury and on glutathione metabolism in mice. Biochem. Pharmacol. 1987, 36, 1919–1924. [Google Scholar] [CrossRef]
- Ma, Y.; Kawabata, T.; Hamazaki, S.; Ogino, T.; Okada, S. Sex differences in oxidative damage in ddY mouse kidney treated with a renal carcinogen, iron nitrilotriacetate. Carcinogenesis 1998, 19, 1983–1988. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.C.; Reisman, S.A.; Klaassen, C.D. Tissue distribution, hormonal regulation, ontogeny, diurnal expression, and induction of mouse cystine transporters Slc3a1 and Slc7a9. Free Radic. Res. 2020, 54, 525–534. [Google Scholar] [CrossRef]
- Xu, F.; Guan, Y.; Xue, L.; Zhang, P.; Li, M.; Gao, M.; Chong, T. The roles of ferroptosis regulatory gene SLC7A11 in renal cell carcinoma: A multi-omics study. Cancer Med. 2021, 10, 9078–9096. [Google Scholar] [CrossRef]
- Sato, H.; Shiiya, A.; Kimata, M.; Maebara, K.; Tamba, M.; Sakakura, Y.; Makino, N.; Sugiyama, F.; Yagami, K.; Moriguchi, T.; et al. Redox imbalance in cystine/glutamate transporter-deficient mice. J. Biol. Chem. 2005, 280, 37423–37429. [Google Scholar] [CrossRef] [Green Version]
- Harris, A.N.; Castro, R.A.; Lee, H.W.; Verlander, J.W.; Weiner, I.D. Role of the renal androgen receptor in sex differences in ammonia metabolism. Am. J. Physiol. Renal Physiol. 2021, 321, F629–F644. [Google Scholar] [CrossRef]
- Tarade, D.; Ohh, M. The HIF and other quandaries in VHL disease. Oncogene 2018, 37, 139–147. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Kobayashi, E.; Nishizawa, M.; Hamazaki, S.; Okada, S.; Hino, O. Cloning of the rat homologue of the von Hippel-Lindau tumor suppressor gene and its non-somatic mutation in rat renal cell carcinomas. Jpn. J. Cancer Res 1995, 86, 905–909. [Google Scholar] [CrossRef]
- Toyokuni, S.; Okada, K.; Kondo, S.; Nishioka, H.; Tanaka, T.; Nishiyama, Y.; Hino, O.; Hiai, H. Development of high-grade renal cell carcinomas in rats independently of somatic mutations in the Tsc2 and VHL tumor suppressor genes. Jpn. J. Cancer Res. 1998, 89, 814–820. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Suwa, H.; Okamoto, K.; Fukumoto, M.; Hiai, H.; Toyokuni, S. Low incidence of point mutations in H-, K- and N-ras oncogenes and p53 tumor suppressor gene in renal cell carcinoma and peritoneal mesothelioma of Wistar rats induced by ferric nitrilotriacetate. Jpn. J. Cancer Res. 1995, 86, 1150–1158. [Google Scholar] [CrossRef]
- Akiyama, T.; Hamazaki, S.; Okada, S. Absence of ras mutations and low incidence of p53 mutations in renal cell carcinomas induced by ferric nitrilotriacetate. Jpn. J. Cancer Res. 1995, 86, 1143–1149. [Google Scholar] [CrossRef]
- Harlander, S.; Schonenberger, D.; Toussaint, N.C.; Prummer, M.; Catalano, A.; Brandt, L.; Moch, H.; Wild, P.J.; Frew, I.J. Combined mutation in Vhl, Trp53 and Rb1 causes clear cell renal cell carcinoma in mice. Nat. Med. 2017, 23, 869–877. [Google Scholar] [CrossRef] [Green Version]
- Albers, J.; Rajski, M.; Schonenberger, D.; Harlander, S.; Schraml, P.; von Teichman, A.; Georgiev, S.; Wild, P.J.; Moch, H.; Krek, W.; et al. Combined mutation of Vhl and Trp53 causes renal cysts and tumours in mice. EMBO Mol. Med. 2013, 5, 949–964. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.F.; Cohn, S.; Christie, A.; McKenzie, T.; Wolff, N.; Do, Q.N.; Madhuranthakam, A.J.; Pedrosa, I.; Wang, T.; Dey, A.; et al. Modeling Renal Cell Carcinoma in Mice: Bap1 and Pbrm1 Inactivation Drive Tumor Grade. Cancer Discov. 2017, 7, 900–917. [Google Scholar] [CrossRef] [Green Version]
- Choueiri, T.K.; Bauer, T.M.; Papadopoulos, K.P.; Plimack, E.R.; Merchan, J.R.; McDermott, D.F.; Michaelson, M.D.; Appleman, L.J.; Thamake, S.; Perini, R.F.; et al. Inhibition of hypoxia-inducible factor-2alpha in renal cell carcinoma with belzutifan: A phase 1 trial and biomarker analysis. Nat. Med. 2021, 27, 802–805. [Google Scholar] [CrossRef]
- Henske, E.P.; Cornejo, K.M.; Wu, C.L. Renal Cell Carcinoma in Tuberous Sclerosis Complex. Genes 2021, 12, 1585. [Google Scholar] [CrossRef]
- Satake, N.; Urakami, S.; Hirayama, Y.; Izumi, K.; Hino, O. Biallelic mutations of the Tsc2 gene in chemically induced rat renal cell carcinoma. Int. J. Cancer. 1998, 77, 895–900. [Google Scholar] [CrossRef]
- Hino, O.; Kobayashi, E.; Hirayama, Y.; Kobayashi, T.; Kubo, Y.; Tsuchiya, H.; Kikuchi, Y.; Mitani, H. Molecular genetic basis of renal carcinogenesis in the Eker rat model of tuberous sclerosis (Tsc2). Mol. Carcinog. 1995, 14, 23–27. [Google Scholar] [CrossRef]
- Knapek, D.F.; Serrano, M.; Beach, D.; Trono, D.; Walker, C.L. Association of rat p15INK4B/p16INK4 deletions with monosomy 5 in kidney epithelial cell lines but not primary renal tumors. Cancer Res. 1995, 55, 1607–1612. [Google Scholar]
- Tanaka, T.; Iwasa, Y.; Kondo, S.; Hiai, H.; Toyokuni, S. High incidence of allelic loss on chromosome 5 and inactivation of p15INK4B and p16INK4A tumor suppressor genes in oxystress-induced renal cell carcinoma of rats. Oncogene 1999, 18, 3793–3797. [Google Scholar] [CrossRef] [Green Version]
- Hiroyasu, M.; Ozeki, M.; Kohda, H.; Echizenya, M.; Tanaka, T.; Hiai, H.; Toyokuni, S. Specific allelic loss of p16 (INK4A) tumor suppressor gene after weeks of iron-mediated oxidative damage during rat renal carcinogenesis. Am. J. Pathol. 2002, 160, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Jafri, M.; Wake, N.C.; Ascher, D.B.; Pires, D.E.; Gentle, D.; Morris, M.R.; Rattenberry, E.; Simpson, M.A.; Trembath, R.C.; Weber, A.; et al. Germline Mutations in the CDKN2B Tumor Suppressor Gene Predispose to Renal Cell Carcinoma. Cancer Discov. 2015, 5, 723–729. [Google Scholar] [CrossRef] [Green Version]
- Deguchi, J.; Kawabata, T.; Kondo, A.; Okada, S. Transforming growth factor-alpha expression of renal proximal tubules in Wistar rats treated with ferric and aluminum nitrilotriacetate. Jpn. J. Cancer Res. 1993, 84, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Akatsuka, S.; Li, G.H.; Mori, K.; Takahashi, T.; Toyokuni, S. Ferroptosis resistance determines high susceptibility of murine A/J strain to iron-induced renal carcinogenesis. Cancer Sci. 2021, 113, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Akatsuka, S.; Li, G.H.; Toyokuni, S. Superiority of rat over murine model for studies on the evolution of cancer genome. Free Radic. Res. 2018, 52, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Kanki, K.; Umemura, T.; Kitamura, Y.; Ishii, Y.; Kuroiwa, Y.; Kodama, Y.; Itoh, K.; Yamamoto, M.; Nishikawa, A.; Hirose, M. A possible role of nrf2 in prevention of renal oxidative damage by ferric nitrilotriacetate. Toxicol. Pathol. 2008, 36, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Aleksunes, L.M.; Goedken, M.J.; Chen, C.; Reisman, S.A.; Manautou, J.E.; Klaassen, C.D. Coordinated induction of Nrf2 target genes protects against iron nitrilotriacetate (FeNTA)-induced nephrotoxicity. Toxicol. Appl. Pharmacol. 2008, 231, 364–373. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.; Sharma, S.D.; Okazaki, Y.; Fujisawa, M.; Okada, S. Dietary supplementation of curcumin enhances antioxidant and phase II metabolizing enzymes in ddY male mice: Possible role in protection against chemical carcinogenesis and toxicity. Pharmacol. Toxicol. 2003, 92, 33–38. [Google Scholar] [CrossRef]
- Okazaki, Y.; Iqbal, M.; Okada, S. Suppressive effects of dietary curcumin on the increased activity of renal ornithine decarboxylase in mice treated with a renal carcinogen, ferric nitrilotriacetate. Biochim. Biophys. Acta 2005, 1740, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Okada, S.; Hamazaki, S.; Ebina, Y.; Li, J.L.; Midorikawa, O. Nephrotoxicity and its prevention by vitamin E in ferric nitrilotriacetate-promoted lipid peroxidation. Biochim. Biophys. Acta 1987, 922, 28–33. [Google Scholar] [CrossRef]
- Zhang, D.; Okada, S.; Yu, Y.; Zheng, P.; Yamaguchi, R.; Kasai, H. Vitamin E inhibits apoptosis, DNA modification, and cancer incidence induced by iron-mediated peroxidation in Wistar rat kidney. Cancer Res. 1997, 57, 2410–2414. [Google Scholar]
- Mizote, A.; Hida, A.I.; Hosako, M.; Fujisawa, M.; Kamekawa, M.; Okada, S. Effects of phlebotomy on the growth of ferric nitrilotriacetate-induced renal cell carcinoma. Acta Med. Okayama 2002, 56, 199–204. [Google Scholar] [CrossRef]
- Shirase, T.; Mori, K.; Okazaki, Y.; Itoh, K.; Yamamoto, M.; Tabuchi, M.; Kishi, F.; Jiang, L.; Akatsuka, S.; Nakao, K.; et al. Suppression of SLC11A2 expression is essential to maintain duodenal integrity during dietary iron overload. Am. J. Pathol. 2010, 177, 677–685. [Google Scholar] [CrossRef]
- Tong, W.H.; Sourbier, C.; Kovtunovych, G.; Jeong, S.Y.; Vira, M.; Ghosh, M.; Romero, V.V.; Sougrat, R.; Vaulont, S.; Viollet, B.; et al. The glycolytic shift in fumarate-hydratase-deficient kidney cancer lowers AMPK levels, increases anabolic propensities and lowers cellular iron levels. Cancer Cell 2011, 20, 315–327. [Google Scholar] [CrossRef] [Green Version]
- Mastrogiannaki, M.; Matak, P.; Keith, B.; Simon, M.C.; Vaulont, S.; Peyssonnaux, C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J. Clin. Investig. 2009, 119, 1159–1166. [Google Scholar] [CrossRef]
- Hirota, K. HIF-alpha Prolyl Hydroxylase Inhibitors and Their Implications for Biomedicine: A Comprehensive Review. Biomedicines 2021, 9, 468. [Google Scholar] [CrossRef]
- Shawki, A.; Anthony, S.R.; Nose, Y.; Engevik, M.A.; Niespodzany, E.J.; Barrientos, T.; Ohrvik, H.; Worrell, R.T.; Thiele, D.J.; Mackenzie, B. Intestinal DMT1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G635–G647. [Google Scholar] [CrossRef] [Green Version]
- Pacak, K.; Jochmanova, I.; Prodanov, T.; Yang, C.; Merino, M.J.; Fojo, T.; Prchal, J.T.; Tischler, A.S.; Lechan, R.M.; Zhuang, Z. New syndrome of paraganglioma and somatostatinoma associated with polycythemia. J. Clin. Oncol. 2013, 31, 1690–1698. [Google Scholar] [CrossRef] [Green Version]
- Rosenblum, J.S.; Wang, H.; Dmitriev, P.M.; Cappadona, A.J.; Mastorakos, P.; Xu, C.; Jha, A.; Edwards, N.; Donahue, D.R.; Munasinghe, J.; et al. Developmental vascular malformations in EPAS1 gain-of-function syndrome. JCI Insight 2021, 6, e144368. [Google Scholar] [CrossRef]
- Saxena, N.; Maio, N.; Crooks, D.R.; Ricketts, C.J.; Yang, Y.; Wei, M.H.; Fan, T.W.; Lane, A.N.; Sourbier, C.; Singh, A.; et al. SDHB-Deficient Cancers: The Role of Mutations That Impair Iron Sulfur Cluster Delivery. J. Natl. Cancer Inst. 2016, 108, djv287. [Google Scholar] [CrossRef] [Green Version]
- Yakirevich, E.; Ali, S.M.; Mega, A.; McMahon, C.; Brodsky, A.S.; Ross, J.S.; Allen, J.; Elvin, J.A.; Safran, H.; Resnick, M.B. A Novel SDHA-deficient Renal Cell Carcinoma Revealed by Comprehensive Genomic Profiling. Am. J. Surg. Pathol. 2015, 39, 858–863. [Google Scholar] [CrossRef]
- Kamai, T.; Higashi, S.; Murakami, S.; Arai, K.; Namatame, T.; Kijima, T.; Abe, H.; Jamiyan, T.; Ishida, K.; Shirataki, H.; et al. Single nucleotide variants of succinate dehydrogenase A gene in renal cell carcinoma. Cancer Sci. 2021, 112, 3375–3387. [Google Scholar] [CrossRef]
- Linehan, W.M.; Schmidt, L.S.; Crooks, D.R.; Wei, D.; Srinivasan, R.; Lang, M.; Ricketts, C.J. The Metabolic Basis of Kidney Cancer. Cancer Discov. 2019, 9, 1006–1021. [Google Scholar] [CrossRef] [Green Version]
- Nath, K.A.; Hebbel, R.P. Sickle cell disease: Renal manifestations and mechanisms. Nat. Rev. Nephrol. 2015, 11, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Msaouel, P.; Tannir, N.M.; Walker, C.L. A Model Linking Sickle Cell Hemoglobinopathies and SMARCB1 Loss in Renal Medullary Carcinoma. Clin. Cancer Res. 2018, 24, 2044–2049. [Google Scholar] [CrossRef] [Green Version]
- Msaouel, P.; Malouf, G.G.; Su, X.; Yao, H.; Tripathi, D.N.; Soeung, M.; Gao, J.; Rao, P.; Coarfa, C.; Creighton, C.J.; et al. Comprehensive Molecular Characterization Identifies Distinct Genomic and Immune Hallmarks of Renal Medullary Carcinoma. Cancer Cell 2020, 37, 720–734. [Google Scholar] [CrossRef]
- Schmidt, L.S.; Linehan, W.M. Genetic predisposition to kidney cancer. Semin. Oncol. 2016, 43, 566–574. [Google Scholar] [CrossRef] [Green Version]
- Alaghehbandan, R.; Trpkov, K.; Tretiakova, M.; Luis, A.S.; Rogala, J.D.; Hes, O. Comprehensive Review of Numerical Chromosomal Aberrations in Chromophobe Renal Cell Carcinoma Including Its Variant Morphologies. Adv. Anat. Pathol. 2021, 28, 8–20. [Google Scholar] [CrossRef]
- Lang, M.; Vocke, C.D.; Ricketts, C.J.; Metwalli, A.R.; Ball, M.W.; Schmidt, L.S.; Linehan, W.M. Clinical and Molecular Characterization of Microphthalmia-associated Transcription Factor (MITF)-related Renal Cell Carcinoma. Urology 2021, 149, 89–97. [Google Scholar] [CrossRef]
- Gallan, A.J.; Parilla, M.; Segal, J.; Ritterhouse, L.; Antic, T. BAP1-Mutated Clear Cell Renal Cell Carcinoma. Am. J. Clin. Pathol. 2021, 155, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Hamazaki, S.; Toyokuni, S.; Midorikawa, O. Induction of mesothelioma by intraperitoneal injections of ferric saccharate in male Wistar rats. Br. J. Cancer 1989, 60, 708–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Q.; Akatsuka, S.; Yamashita, Y.; Ohara, H.; Nagai, H.; Okazaki, Y.; Takahashi, T.; Toyokuni, S. Homozygous deletion of CDKN2A/2B is a hallmark of iron-induced high-grade rat mesothelioma. Lab. Investig. 2010, 90, 360–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minami, D.; Takigawa, N.; Kato, Y.; Kudo, K.; Isozaki, H.; Hashida, S.; Harada, D.; Ochi, N.; Fujii, M.; Kubo, T.; et al. Downregulation of TBXAS1 in an iron-induced malignant mesothelioma model. Cancer Sci. 2015, 106, 1296–1302. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Conrad, M. The Metabolic Underpinnings of Ferroptosis. Cell Metab. 2020, 32, 920–937. [Google Scholar] [CrossRef]
- Nagai, H.; Okazaki, Y.; Chew, S.H.; Misawa, N.; Yasui, H.; Toyokuni, S. Deferasirox induces mesenchymal-epithelial transition in crocidolite-induced mesothelial carcinogenesis in rats. Cancer Prev. Res. 2013, 6, 1222–1230. [Google Scholar] [CrossRef] [Green Version]
- Ohara, Y.; Chew, S.H.; Shibata, T.; Okazaki, Y.; Yamashita, K.; Toyokuni, S. Phlebotomy as a preventive measure for crocidolite-induced mesothelioma in male rats. Cancer Sci. 2018, 109, 330–339. [Google Scholar] [CrossRef] [Green Version]
- Kato, J.; Miyanishi, K.; Kobune, M.; Nakamura, T.; Takada, K.; Takimoto, R.; Kawano, Y.; Takahashi, S.; Takahashi, M.; Sato, Y.; et al. Long-term phlebotomy with low-iron diet therapy lowers risk of development of hepatocellular carcinoma from chronic hepatitis C. J. Gastroenterol. 2007, 42, 830–836. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [Green Version]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef]
- Miyazawa, T. Lipid hydroperoxides in nutrition, health, and diseases. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2021, 97, 161–196. [Google Scholar] [CrossRef]
- Ratan, R.R. The Chemical Biology of Ferroptosis in the Central Nervous System. Cell Chem. Biol. 2020, 27, 479–498. [Google Scholar] [CrossRef]
- Ward, R.J.; Dexter, D.T.; Martin-Bastida, A.; Crichton, R.R. Is Chelation Therapy a Potential Treatment for Parkinson’s Disease? Int. J. Mol. Sci. 2021, 22, 3338. [Google Scholar] [CrossRef]
- Kagan, V.E.; Mao, G.; Qu, F.; Angeli, J.P.; Doll, S.; Croix, C.S.; Dar, H.H.; Liu, B.; Tyurin, V.A.; Ritov, V.B.; et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017, 13, 81–90. [Google Scholar] [CrossRef]
- Bayir, H.; Anthonymuthu, T.S.; Tyurina, Y.Y.; Patel, S.J.; Amoscato, A.A.; Lamade, A.M.; Yang, Q.; Vladimirov, G.K.; Philpott, C.C.; Kagan, V.E. Achieving Life through Death: Redox Biology of Lipid Peroxidation in Ferroptosis. Cell Chem. Biol. 2020, 27, 387–408. [Google Scholar] [CrossRef]
- Ran, Q.; Van Remmen, H.; Gu, M.; Qi, W.; Roberts, L.J., 2nd; Prolla, T.; Richardson, A. Embryonic fibroblasts from Gpx4+/− mice: A novel model for studying the role of membrane peroxidation in biological processes. Free Radic. Biol. Med. 2003, 35, 1101–1109. [Google Scholar] [CrossRef]
- Thomas, J.P.; Maiorino, M.; Ursini, F.; Girotti, A.W. Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damaging lipid peroxidation. In situ reduction of phospholipid and cholesterol hydroperoxides. J. Biol. Chem. 1990, 265, 454–461. [Google Scholar] [CrossRef]
- Gao, M.; Monian, P.; Quadri, N.; Ramasamy, R.; Jiang, X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell 2015, 59, 298–308. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of Mitochondria in Ferroptosis. Mol. Cell 2019, 73, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Shimada, K.; Hayano, M.; Pagano, N.C.; Stockwell, B.R. Cell-Line Selectivity Improves the Predictive Power of Pharmacogenomic Analyses and Helps Identify NADPH as Biomarker for Ferroptosis Sensitivity. Cell Chem. Biol. 2016, 23, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef] [Green Version]
- Belavgeni, A.; Meyer, C.; Stumpf, J.; Hugo, C.; Linkermann, A. Ferroptosis and Necroptosis in the Kidney. Cell Chem. Biol. 2020, 27, 448–462. [Google Scholar] [CrossRef]
- Linkermann, A.; Skouta, R.; Himmerkus, N.; Mulay, S.R.; Dewitz, C.; De Zen, F.; Prokai, A.; Zuchtriegel, G.; Krombach, F.; Welz, P.S.; et al. Synchronized renal tubular cell death involves ferroptosis. Proc. Natl. Acad. Sci. USA 2014, 111, 16836–16841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonnus, W.; Meyer, C.; Steinebach, C.; Belavgeni, A.; von Massenhausen, A.; Gonzalez, N.Z.; Maremonti, F.; Gembardt, F.; Himmerkus, N.; Latk, M.; et al. Dysfunction of the key ferroptosis-surveilling systems hypersensitizes mice to tubular necrosis during acute kidney injury. Nat. Commun. 2021, 12, 4402. [Google Scholar] [CrossRef] [PubMed]
- Miess, H.; Dankworth, B.; Gouw, A.M.; Rosenfeldt, M.; Schmitz, W.; Jiang, M.; Saunders, B.; Howell, M.; Downward, J.; Felsher, D.W.; et al. The glutathione redox system is essential to prevent ferroptosis caused by impaired lipid metabolism in clear cell renal cell carcinoma. Oncogene 2018, 37, 5435–5450. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Sato, M.; Mishima, E.; Sato, H.; Proneth, B.; Conrad, M. Sorafenib fails to trigger ferroptosis across a wide range of cancer cell lines. Cell Death Dis. 2021, 12, 698. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S.; Luo, X.P.; Tanaka, T.; Uchida, K.; Hiai, H.; Lehotay, D.C. Induction of a wide range of C(2–12) aldehydes and C(7–12) acyloins in the kidney of Wistar rats after treatment with a renal carcinogen, ferric nitrilotriacetate. Free Radic. Biol. Med. 1997, 22, 1019–1027. [Google Scholar] [CrossRef]
- Deiana, M.; Aruoma, O.I.; Rosa, A.; Crobu, V.; Casu, V.; Piga, R.; Dessi, M.A. The effect of ferric-nitrilotriacetic acid on the profile of polyunsaturated fatty acids in the kidney and liver of rats. Toxicol. Lett. 2001, 123, 125–133. [Google Scholar] [CrossRef]
- Yasuda, S.; Watanabe, S.; Kobayashi, T.; Hata, N.; Misawa, Y.; Utsumi, H.; Okuyama, H. Dietary docosahexaenoic acid enhances ferric nitrilotriacetate-induced oxidative damage in mice but not when additional alpha-tocopherol is supplemented. Free Radic. Res. 1999, 30, 199–205. [Google Scholar] [CrossRef]
- Okazaki, Y.; Iqbal, M.; Kawakami, N.; Yamamoto, Y.; Toyokuni, S.; Okada, S. A beverage containing fermented black soybean ameliorates ferric nitrilotriacetate-induced renal oxidative damage in rats. J. Clin. Biochem. Nutr. 2010, 47, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Ran, Q.; Liang, H.; Ikeno, Y.; Qi, W.; Prolla, T.A.; Roberts, L.J., 2nd; Wolf, N.; Van Remmen, H.; Richardson, A. Reduction in glutathione peroxidase 4 increases life span through increased sensitivity to apoptosis. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 932–942. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; La Vecchia, C.; Negri, E.; DesMeules, M.; Mery, L.; Canadian Cancer Registries Epidemiology Research, G. Dietary vitamin C, E, and carotenoid intake and risk of renal cell carcinoma. Cancer Causes Control 2009, 20, 1451–1458. [Google Scholar] [CrossRef]
- Liao, Z.; Fang, Z.; Gou, S.; Luo, Y.; Liu, Y.; He, Z.; Li, X.; Peng, Y.; Fu, Z.; Li, D.; et al. The role of diet in renal cell carcinoma incidence: An umbrella review of meta-analyses of observational studies. BMC Med. 2022, 20, 39. [Google Scholar] [CrossRef]
| Syndrome | Predisposing Gene | Chromosomal Location | Predominant Histopathology | Activated Signaling Pathway |
|---|---|---|---|---|
| von Hippel–Lindau (VHL) disease | VHL | 3p25 | Clear cell | HIF pathway |
| Hereditary papillary renal cell carcinoma | MET | 7q31 | Papillary (type 1) | MET kinase pathway |
| Birt–Hogg–Dubé syndrome | FLCN (Folliculin) | 17p11 | Chromophobe, hybrid oncocytic, and clear cell | mTOR pathway |
| Hereditary leiomyomatosis and renal cell carcinoma (HLRCC) | FH (Fumarate hydratase) | 1q43 | Papillary (type 2) | HIF pathway, NRF2 pathway, and TCA cycle pathway |
| Succinate dehydrogenase-deficient renal cell carcinoma (SDH-deficient RCC) | SDHB SDHC SDHD | 1p36 1q23 11q23 | Oncocytic is most common and clear cell is less frequent | HIF pathway and TCA cycle pathway |
| Tuberous sclerosis complex (TSC) | TSC1 TSC2 | 9q34 16p13 | Heterogeneous; chromophobe, oncocytic, and cystic | mTOR pathway |
| Cowden syndrome | PTEN | 10q23 | Chromophobe | PI3/AKT/mTOR pathway |
| Inherited RCC | CDKN2B | 9q21 | Clear cell | Cell cycle regulation |
| BAP1 tumor predisposition syndrome | BAP1 (BRCA1-associated Protein 1) | 3p21 | # Clear cell or eosinophilic, high-grade nuclei | Dysregulated BAP1 transcriptional activity, including metabolic reprogramming |
| MiTF-associated cancer syndrome | MiTF (Microphthalmia-associated transcription factor) | 3p14 | ## Papillary (type 1) and/or clear cell or demonstrate TFE3-positive Xp11 translocation RCC | Dysregulated MiTF transcriptional activity, including mTOR pathway |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okazaki, Y. The Role of Ferric Nitrilotriacetate in Renal Carcinogenesis and Cell Death: From Animal Models to Clinical Implications. Cancers 2022, 14, 1495. https://doi.org/10.3390/cancers14061495
Okazaki Y. The Role of Ferric Nitrilotriacetate in Renal Carcinogenesis and Cell Death: From Animal Models to Clinical Implications. Cancers. 2022; 14(6):1495. https://doi.org/10.3390/cancers14061495
Chicago/Turabian StyleOkazaki, Yasumasa. 2022. "The Role of Ferric Nitrilotriacetate in Renal Carcinogenesis and Cell Death: From Animal Models to Clinical Implications" Cancers 14, no. 6: 1495. https://doi.org/10.3390/cancers14061495
APA StyleOkazaki, Y. (2022). The Role of Ferric Nitrilotriacetate in Renal Carcinogenesis and Cell Death: From Animal Models to Clinical Implications. Cancers, 14(6), 1495. https://doi.org/10.3390/cancers14061495






