Role of Radiosurgery and Stereotactic Ablative Radiotherapy for Oligometastatic Non-Oncogene Addicted NSCLC
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Patient Population
2.2. Outcomes/Endpoints
2.3. Treatment Details
2.4. Response Assessment and Follow-Up
2.5. Statistical Analyses
3. Results
3.1. Population Characteristics
3.2. Treatment Characteristics
3.3. Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic Non-Small Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. JCO 1995, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Lievens, Y.; Guckenberger, M.; Gomez, D.; Hoyer, M.; Iyengar, P.; Kindts, I.; Méndez Romero, A.; Nevens, D.; Palma, D.; Park, C.; et al. Defining Oligometastatic Disease from a Radiation Oncology Perspective: An ESTRO-ASTRO Consensus Document. Radiother. Oncol. 2020, 148, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; deSouza, N.M.; Dingemans, A.-M.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and Classification of Oligometastatic Disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer Consensus Recommendation. Lancet Oncol. 2020, 21, e18–e28. [Google Scholar] [CrossRef] [Green Version]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol. Lab. Med. 2018, 142, 321–346. [Google Scholar] [CrossRef] [Green Version]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or Chemotherapy for Non-Small-Cell Lung Cancer with Mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrelli, F.; Ghidini, A.; Cabiddu, M.; Tomasello, G.; De Stefani, A.; Bruschieri, L.; Vitali, E.; Ghilardi, M.; Borgonovo, K.; Barni, S.; et al. Addition of Radiotherapy to the Primary Tumour in Oligometastatic NSCLC: A Systematic Review and Meta-Analysis. Lung Cancer 2018, 126, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gomez, D.; Iyengar, P. Local Ablative Therapy in Oligometastatic NSCLC. Semin Radiat. Oncol. 2021, 31, 235–241. [Google Scholar] [CrossRef]
- Román-Jobacho, A.; Hernández-Miguel, M.; García-Anaya, M.J.; Gómez-Millán, J.; Medina-Carmona, J.A.; Otero-Romero, A. Oligometastatic Non-Small Cell Lung Cancer: Current Management. J. Clin. Transl. Res. 2021, 7, 311–319. [Google Scholar] [PubMed]
- Mentink, J.F.; Paats, M.S.; Dumoulin, D.W.; Cornelissen, R.; Elbers, J.B.W.; Maat, A.P.W.M.; von der Thüsen, J.H.; Dingemans, A.-M.C. Defining Oligometastatic Non-Small Cell Lung Cancer: Concept versus Biology, a Literature Review. Transl. Lung Cancer Res. 2021, 10, 3329–3338. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, P.; Wardak, Z.; Gerber, D.E.; Tumati, V.; Ahn, C.; Hughes, R.S.; Dowell, J.E.; Cheedella, N.; Nedzi, L.; Westover, K.D.; et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018, 4, e173501. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.R.; Tang, C.; Zhang, J.; Blumenschein, G.R.; Hernandez, M.; Lee, J.J.; Ye, R.; Palma, D.A.; Louie, A.V.; Camidge, D.R.; et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J. Clin. Oncol. 2019, 37, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.C.; Raben, D.; Formenti, S.C. The Integration of Radiotherapy with Immunotherapy for the Treatment of Non-Small Cell Lung Cancer. Clin. Cancer Res. 2018, 24, 5792–5806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theelen, W.S.M.E.; Chen, D.; Verma, V.; Hobbs, B.P.; Peulen, H.M.U.; Aerts, J.G.J.V.; Bahce, I.; Niemeijer, A.L.N.; Chang, J.Y.; de Groot, P.M.; et al. Pembrolizumab with or without Radiotherapy for Metastatic Non-Small-Cell Lung Cancer: A Pooled Analysis of Two Randomised Trials. Lancet Respir. Med. 2021, 9, 467–475. [Google Scholar] [CrossRef]
- Ricardi, U.; Frezza, G.; Filippi, A.R.; Badellino, S.; Levis, M.; Navarria, P.; Salvi, F.; Marcenaro, M.; Trovò, M.; Guarneri, A.; et al. Stereotactic Ablative Radiotherapy for Stage I Histologically Proven Non-Small Cell Lung Cancer: An Italian Multicenter Observational Study. Lung Cancer 2014, 84, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Badellino, S.; Muzio, J.D.; Schivazappa, G.; Guarneri, A.; Ragona, R.; Bartoncini, S.; Trino, E.; Filippi, A.R.; Fonio, P.; Ricardi, U. No Differences in Radiological Changes after 3D Conformal vs VMAT-Based Stereotactic Radiotherapy for Early Stage Non-Small Cell Lung Cancer. Br. J. Radiol. 2017, 90, 20170143. [Google Scholar] [CrossRef] [PubMed]
- Filippi, A.R.; Badellino, S.; Guarneri, A.; Levis, M.; Botticella, A.; Mantovani, C.; Ragona, R.; Racca, P.; Buffoni, L.; Novello, S.; et al. Outcomes of Single Fraction Stereotactic Ablative Radiotherapy for Lung Metastases. Technol. Cancer Res. Treat. 2014, 13, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.; Scott, C.; Souhami, L.; Dinapoli, R.; Kline, R.; Loeffler, J.; Farnan, N. Single Dose Radiosurgical Treatment of Recurrent Previously Irradiated Primary Brain Tumors and Brain Metastases: Final Report of RTOG Protocol 90-05. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 291–298. [Google Scholar] [CrossRef]
- Mantovani, C.; Gastino, A.; Cerrato, M.; Badellino, S.; Ricardi, U.; Levis, M. Modern Radiation Therapy for the Management of Brain Metastases From Non-Small Cell Lung Cancer: Current Approaches and Future Directions. Front Oncol. 2021, 11, 772789. [Google Scholar] [CrossRef] [PubMed]
- Benedict, S.H.; Yenice, K.M.; Followill, D.; Galvin, J.M.; Hinson, W.; Kavanagh, B.; Keall, P.; Lovelock, M.; Meeks, S.; Papiez, L.; et al. Stereotactic Body Radiation Therapy: The Report of AAPM Task Group 101. Med. Phys. 2010, 37, 4078–4101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, G.G.; Murray, L.; Patel, R.; Jain, S.; Aitken, K.L.; Franks, K.N.; van As, N.; Tree, A.; Hatfield, P.; Harrow, S.; et al. UK Consensus on Normal Tissue Dose Constraints for Stereotactic Radiotherapy. Clin. Oncol. (R. Coll. Radiol.) 2018, 30, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aggarwal, C.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J. Natl. Compr. Canc. Netw. 2019, 17, 1464–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroeze, S.G.C.; Schaule, J.; Fritz, C.; Kaul, D.; Blanck, O.; Kahl, K.H.; Roeder, F.; Siva, S.; Verhoeff, J.J.C.; Adebahr, S.; et al. Metastasis Directed Stereotactic Radiotherapy in NSCLC Patients Progressing under Targeted- or Immunotherapy: Efficacy and Safety Reporting from the “TOaSTT” Database. Radiat. Oncol. 2021, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, A.B.; Senan, S.; Palma, D.A.; Riquet, M.; Ahn, Y.C.; Ricardi, U.; Congedo, M.T.; Gomez, D.R.; Wright, G.M.; Melloni, G.; et al. An Individual Patient Data Metaanalysis of Outcomes and Prognostic Factors after Treatment of Oligometastatic Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2014, 15, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Kissel, M.; Martel-Lafay, I.; Lequesne, J.; Faivre, J.-C.; Le Péchoux, C.; Stefan, D.; Barraux, V.; Loiseau, C.; Grellard, J.-M.; Danhier, S.; et al. Stereotactic Ablative Radiotherapy and Systemic Treatments for Extracerebral Oligometastases, Oligorecurrence, Oligopersistence and Oligoprogression from Lung Cancer. BMC Cancer 2019, 19, 1237. [Google Scholar] [CrossRef] [PubMed]
- Poon, I.; Erler, D.; Dagan, R.; Redmond, K.J.; Foote, M.; Badellino, S.; Biswas, T.; Louie, A.V.; Lee, Y.; Atenafu, E.G.; et al. Evaluation of Definitive Stereotactic Body Radiotherapy and Outcomes in Adults With Extracranial Oligometastasis. JAMA Netw. Open 2020, 3, e2026312. [Google Scholar] [CrossRef] [PubMed]
- Buglione, M.; Jereczek-Fossa, B.A.; Bonù, M.L.; Franceschini, D.; Fodor, A.; Zanetti, I.B.; Gerardi, M.A.; Borghetti, P.; Tomasini, D.; Di Muzio, N.G.; et al. Radiosurgery and Fractionated Stereotactic Radiotherapy in Oligometastatic/Oligoprogressive Non-Small Cell Lung Cancer Patients: Results of a Multi-Institutional Series of 198 Patients Treated with “Curative” Intent. Lung Cancer 2020, 141, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Hoang, C.D.; Kesarwala, A.H.; Schrump, D.S.; Guha, U.; Rajan, A. Role of Local Ablative Therapy in Patients with Oligometastatic and Oligoprogressive Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 179–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parikh, R.B.; Cronin, A.M.; Kozono, D.E.; Oxnard, G.R.; Mak, R.H.; Jackman, D.M.; Lo, P.C.; Baldini, E.H.; Johnson, B.E.; Chen, A.B. Definitive Primary Therapy in Patients Presenting with Oligometastatic Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 880–887. [Google Scholar] [CrossRef] [PubMed]
- De Ruysscher, D.; Wanders, R.; van Baardwijk, A.; Dingemans, A.-M.C.; Reymen, B.; Houben, R.; Bootsma, G.; Pitz, C.; van Eijsden, L.; Geraedts, W.; et al. Radical Treatment of Non-Small-Cell Lung Cancer Patients with Synchronous Oligometastases: Long-Term Results of a Prospective Phase II Trial (Nct01282450). J. Thorac. Oncol. 2012, 7, 1547–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Zhu, R.; Li, D.; Li, N.; Zhu, X. Prognostic Factors of Oligometastatic Non-Small Cell Lung Cancer: A Meta-Analysis. J. Thorac. Dis. 2018, 10, 3701–3713. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Shirato, H.; Nagata, Y.; Hiraoka, M.; Fujino, M.; Gomi, K.; Niibe, Y.; Karasawa, K.; Hayakawa, K.; Takai, Y.; et al. Hypofractionated Stereotactic Radiotherapy (HypoFXSRT) for Stage I Non-Small Cell Lung Cancer: Updated Results of 257 Patients in a Japanese Multi-Institutional Study. J. Thorac. Oncol. 2007, 2, S94–S100. [Google Scholar] [CrossRef] [Green Version]
- Lehrer, E.J.; Peterson, J.L.; Zaorsky, N.G.; Brown, P.D.; Sahgal, A.; Chiang, V.L.; Chao, S.T.; Sheehan, J.P.; Trifiletti, D.M. Single versus Multifraction Stereotactic Radiosurgery for Large Brain Metastases: An International Meta-Analysis of 24 Trials. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 618–630. [Google Scholar] [CrossRef]
- Amini, A.; Verma, V.; Simone, C.B.; Chetty, I.J.; Chun, S.G.; Donington, J.; Edelman, M.J.; Higgins, K.A.; Kestin, L.L.; Movsas, B.; et al. American Radium Society Appropriate Use Criteria for Radiation Therapy in Oligometastatic or Oligoprogressive Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Basler, L.; Kroeze, S.G.C.; Guckenberger, M. SBRT for Oligoprogressive Oncogene Addicted NSCLC. Lung Cancer 2017, 106, 50–57. [Google Scholar] [CrossRef]
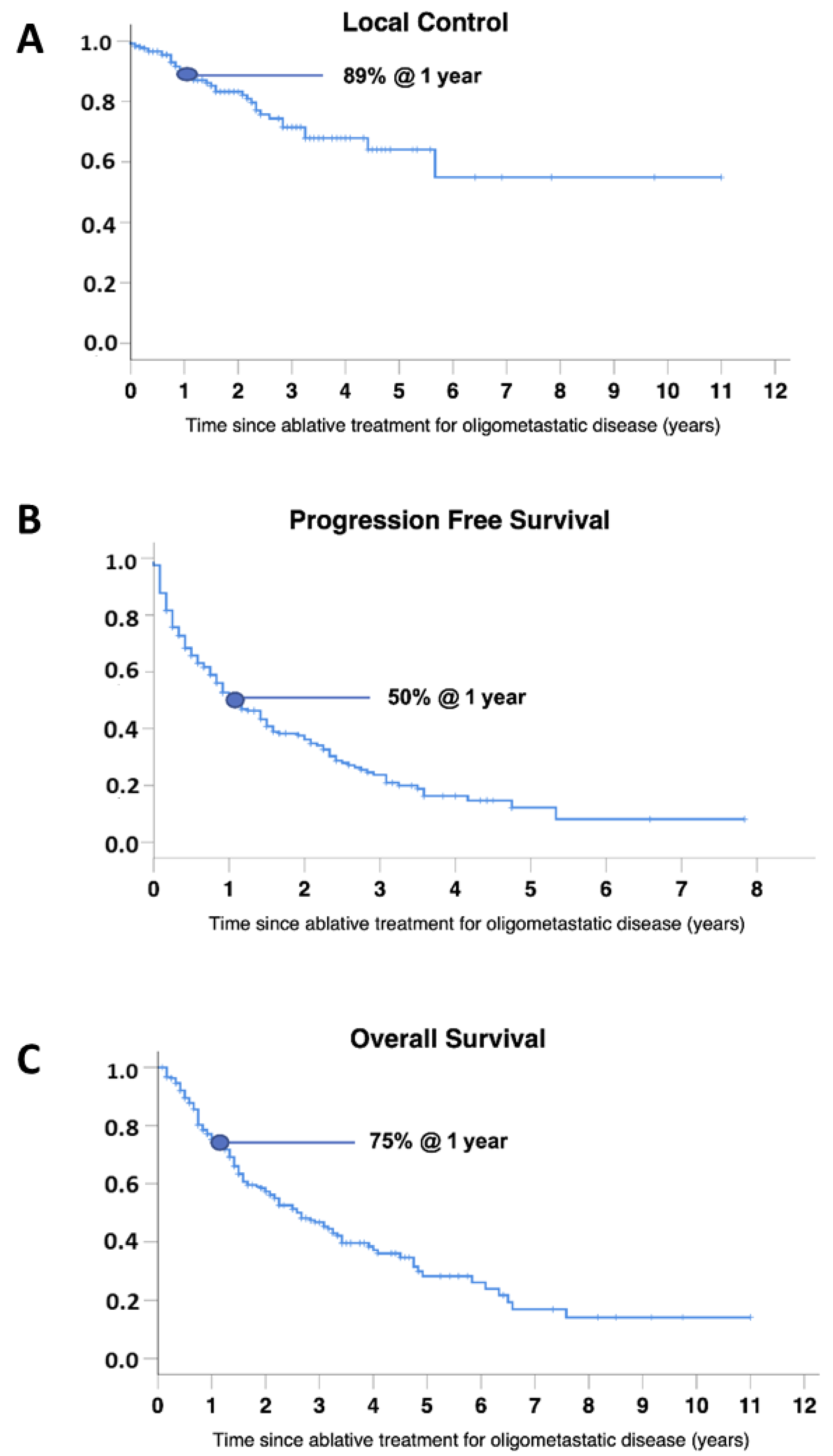
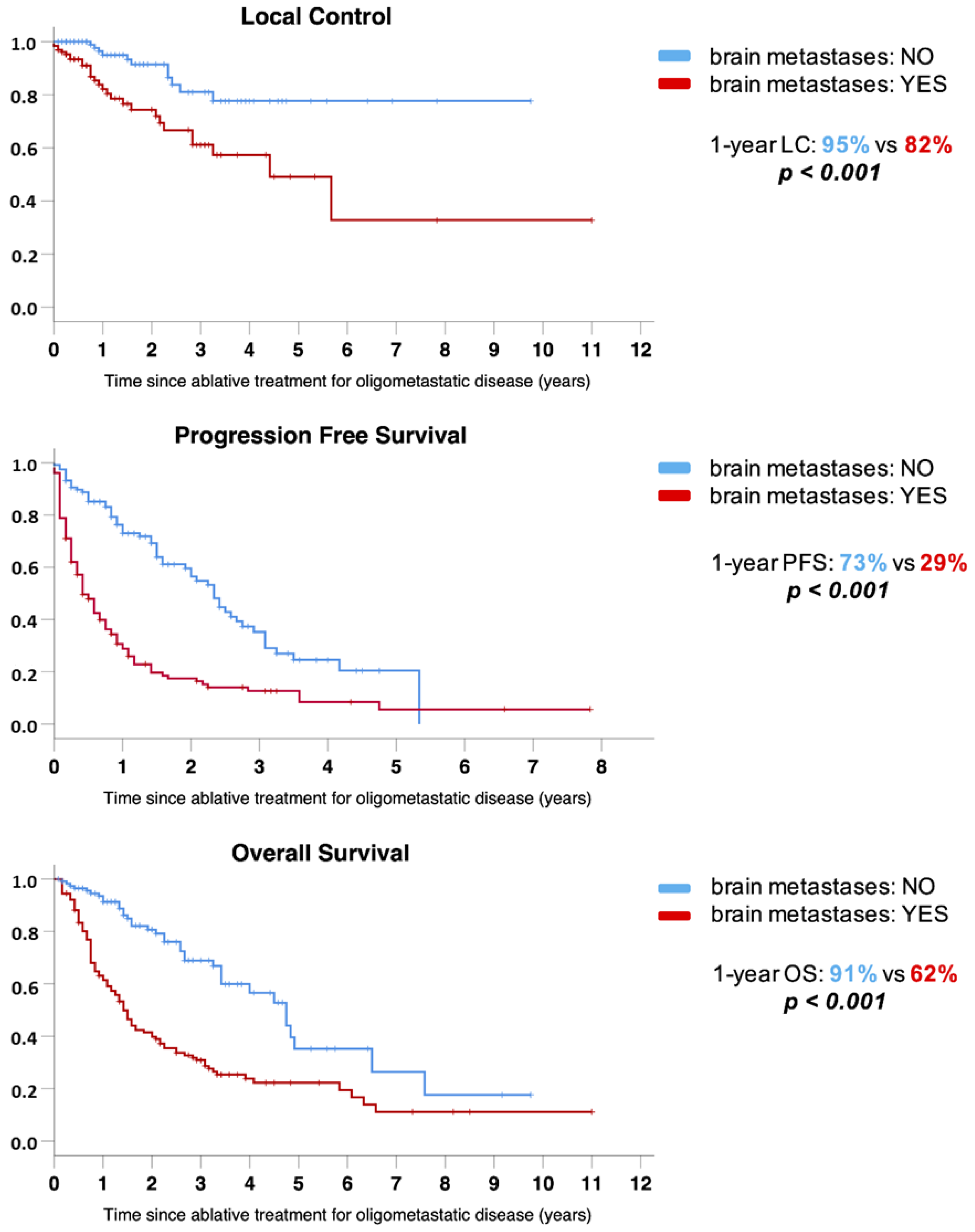
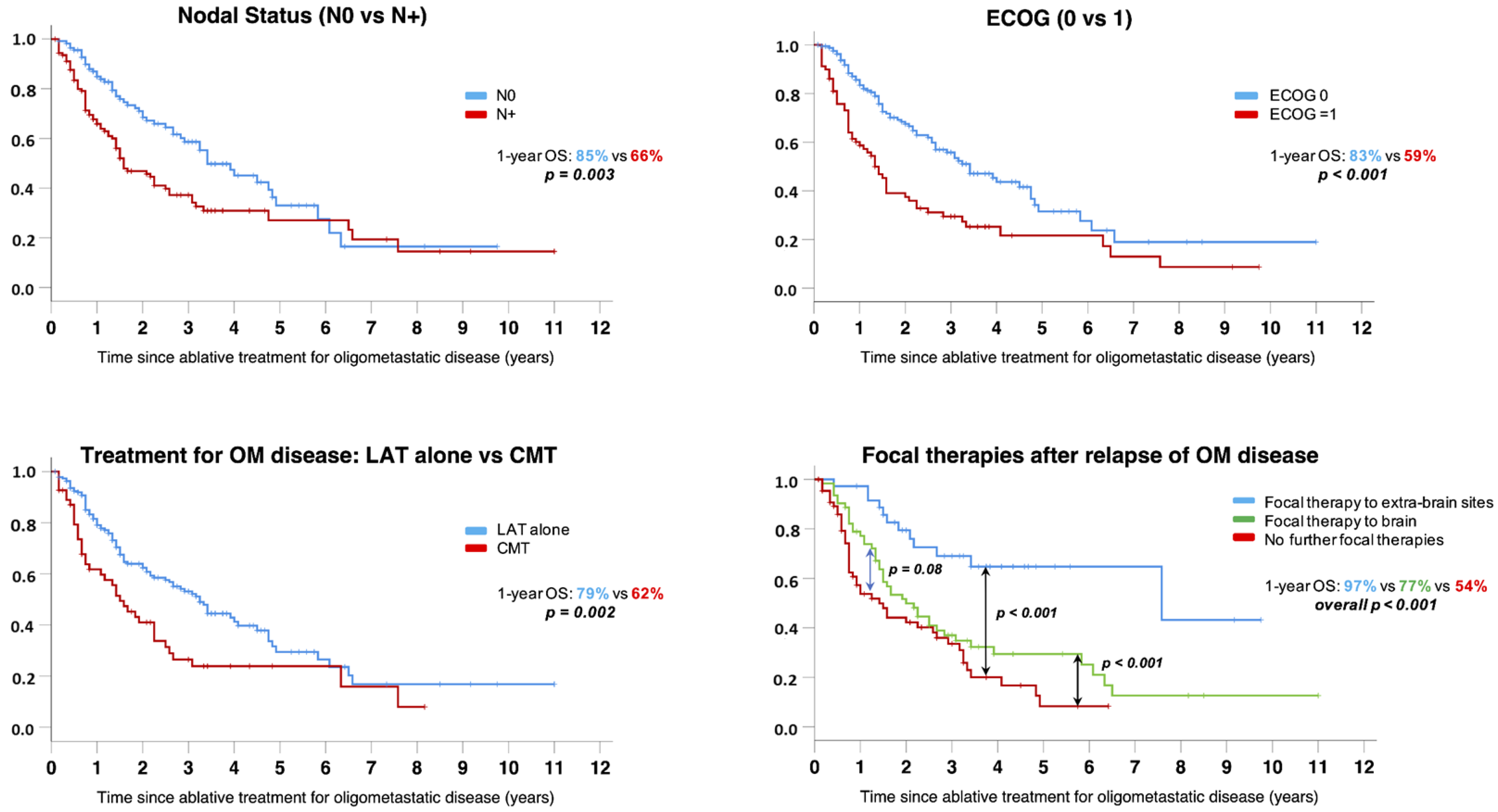
| All Patients, n = 245 No. (%) | |
|---|---|
| Gender | |
| Male | 174 (71) |
| Female | 71 (29) |
| Age (years) | |
| <70 | 125 (51) |
| ≥70 | 120 (49) |
| ECOG PS | |
| 0 | 166 (68) |
| 1 | 79 (32) |
| Histology | |
| Adenocarcinoma | 181 (74) |
| Squamous Cell | 34 (14) |
| Other | 16 (6) |
| NSCLC Unknown/Not specified | 14 (6) |
| PD-L1 | |
| 0% | 21 (8) |
| >1% | 15 (6) |
| >50% | 14 (6) |
| Unknown | 195 (80) |
| Stage at NSCLC diagnosis | |
| Early | 65 (27) |
| Locally Advanced | 106 (43) |
| Metastatic | 74 (30) |
| N stage at NSCLC diagnosis | |
| 0 | 114 (47) |
| 1 | 39 (16) |
| 2 | 66 (27) |
| 3 | 20 (8) |
| Unknown | 6 (2) |
| Type of OMD | |
| Synchronous | 53 (22) |
| Oligorecurrent | 165 (67) |
| Oligoprogressive | 27 (11) |
| Lesion(s) at OMD diagnosis | |
| 1 | 154 (63) |
| 2 | 63 (25) |
| 3 | 19 (8) |
| 4 | 4 (2) |
| 5 | 5 (2) |
| No. of involved organ(s) at OMD diagnosis | |
| 1 | 218 (89) |
| 2 | 25 (10) |
| 3–4 | 2 (1) |
| Type of involved organ(s) at OMD diagnosis | |
| Lung | 102 (41) |
| Brain | 108 (44) |
| Bone | 4 (2) |
| Adrenal | 5 (2) |
| Multiple organs with brain a | 21 (9) |
| Multiple organs extra brain b | 5 (2) |
| Treatment Characteristics | All Patients, n = 245 |
|---|---|
| No. (%) | |
| Treatment at NSCLC diagnosis | |
| Surgery alone | 71 (29) |
| SABR | 30 (12) |
| CT + RT | 24 (10) |
| Systemic therapy alone | 55 (22) |
| Surgery + adjuvant therapy | 60 (25) |
| Other treatment | 5 (2) |
| Systemic therapy at NSCLC diagnosis | |
| CT | 129 (53) |
| IT | 3 (1) |
| CT + IT | 2 (1) |
| None | 111 (45) |
| Response after NSCLC treatment | |
| CR | 97 (40) |
| PR | 91 (37) |
| SD | 36 (15) |
| PD | 21 (8) |
| Primary controlled at OMD diagnosis | |
| Yes | 195 (80) |
| No | 50 (20) |
| No. of treated lesion(s) with LAT | |
| 1 | 187 (77) |
| 2 | 48 (19) |
| 3 | 9 (3.5) |
| 4 | 1 (0.5) |
| No. of treated organ(s) with LAT at OMD diagnosis | |
| 1 | 243 (99) |
| 2 | 2 (1) |
| Cranial vs. Extracranial metastatic disease | |
| Cranial | 128 (53) |
| Extracranial | 117 (47) |
| Systemic treatment for metachronous OMD/OPD | |
| Yes | 18 (7) |
| No | 227 (93) |
| Additional non stereotactic local therapies | |
| Surgery | 10 (4) |
| RFA | 2 (1) |
| None | 233 (95) |
| Adjuvant SRS/SABR | |
| Yes | 8 (3) |
| No | 237 (97) |
| Variable | Progression-Free Survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age | 0.98 (0.96–0.99) | 0.009 | NS | - | NS | - | NS | - |
| Stage IV at diagnosis | 2.03 (1.47–2.81) | <0.001 | NS | - | 1.62 (1.14–2.30) | 0.007 | NS | - |
| Nodal involvement at baseline (N+) | 1.70 (1.24–2.33) | 0.001 | NS | - | 1.71 (1.20–2.45) | 0.003 | 1.6 (1.1–2.4) | 0.049 |
| No response after primary treatment | 2.04 (1.25–3.34) | 0.005 | NS | - | NS | - | NS | - |
| ECOG PS = 1 | 1.75 (1.27–2.41) | 0.001 | 1.9 (1.3–2.6) | <0.001 | 2.06 (1.46–2.92) | <0.001 | 1.7 (1.1–2.6) | 0.011 |
| Type of oligometastatic disease | 1.21 (1.07–1.37) | 0.002 | NS | - | NS | - | NS | - |
| No. of lesions at OMD diagnosis | 1.27 (1.09–1.48) | 0.003 | NS | - | 1.29 (1.07–1.55) | 0.007 | NS | - |
| Primary tumor uncontrolled | 1.78 (1.24–2.55) | 0.002 | NS | - | 1.65 (1.11–2.44) | 0.013 | NS | - |
| Intracranial metastatic disease | 2.78 (2.00–3.83) | <0.001 | 2.4 (1.7–3.4) | <0.001 | 2.79 (1.90–4.10) | <0.001 | 2.3 (1.4–3.9) | 0.001 |
| CMT for OMD | 2.5 (1.7–3.5) | <0.001 | 1.9 (1.3–2.6) | 0.002 | 1.77 (1.21–2.57) | 0.003 | 1.7 (1.1–2.6) | 0.020 |
| No response after LAT | 4.36 (2.27–8.38) | <0.001 | 4.4 (2.2–8.5) | <0.001 | NS | - | NS | - |
| New local treatment for PD | NS | - | NS | - | 2.01 (1.35–3.00) | 0.001 | NS | - |
| No local treatment for PD vs. Intracranial vs. Extracranial new site of local treatment | NA | - | NA | - | 1.85 (1.41–2.43) | <0.001 | 2.2 (1.6–3.1) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badellino, S.; Levis, M.; Cuffini, E.M.; Cerrato, M.; Orlandi, E.; Chiovatero, I.; Aprile, A.; Gastino, A.; Cavallin, C.; Iorio, G.C.; et al. Role of Radiosurgery and Stereotactic Ablative Radiotherapy for Oligometastatic Non-Oncogene Addicted NSCLC. Cancers 2022, 14, 1465. https://doi.org/10.3390/cancers14061465
Badellino S, Levis M, Cuffini EM, Cerrato M, Orlandi E, Chiovatero I, Aprile A, Gastino A, Cavallin C, Iorio GC, et al. Role of Radiosurgery and Stereotactic Ablative Radiotherapy for Oligometastatic Non-Oncogene Addicted NSCLC. Cancers. 2022; 14(6):1465. https://doi.org/10.3390/cancers14061465
Chicago/Turabian StyleBadellino, Serena, Mario Levis, Erica Maria Cuffini, Marzia Cerrato, Erika Orlandi, Ilaria Chiovatero, Arianna Aprile, Alessio Gastino, Chiara Cavallin, Giuseppe Carlo Iorio, and et al. 2022. "Role of Radiosurgery and Stereotactic Ablative Radiotherapy for Oligometastatic Non-Oncogene Addicted NSCLC" Cancers 14, no. 6: 1465. https://doi.org/10.3390/cancers14061465
APA StyleBadellino, S., Levis, M., Cuffini, E. M., Cerrato, M., Orlandi, E., Chiovatero, I., Aprile, A., Gastino, A., Cavallin, C., Iorio, G. C., Parise, R., Mantovani, C., & Ricardi, U. (2022). Role of Radiosurgery and Stereotactic Ablative Radiotherapy for Oligometastatic Non-Oncogene Addicted NSCLC. Cancers, 14(6), 1465. https://doi.org/10.3390/cancers14061465





