Impact of Delaying the Addition of Anti-EGFR in First Line of RAS Wild-Type Metastatic Colorectal Cancer: A Propensity-Weighted Pooled Data Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
3.1. Patients
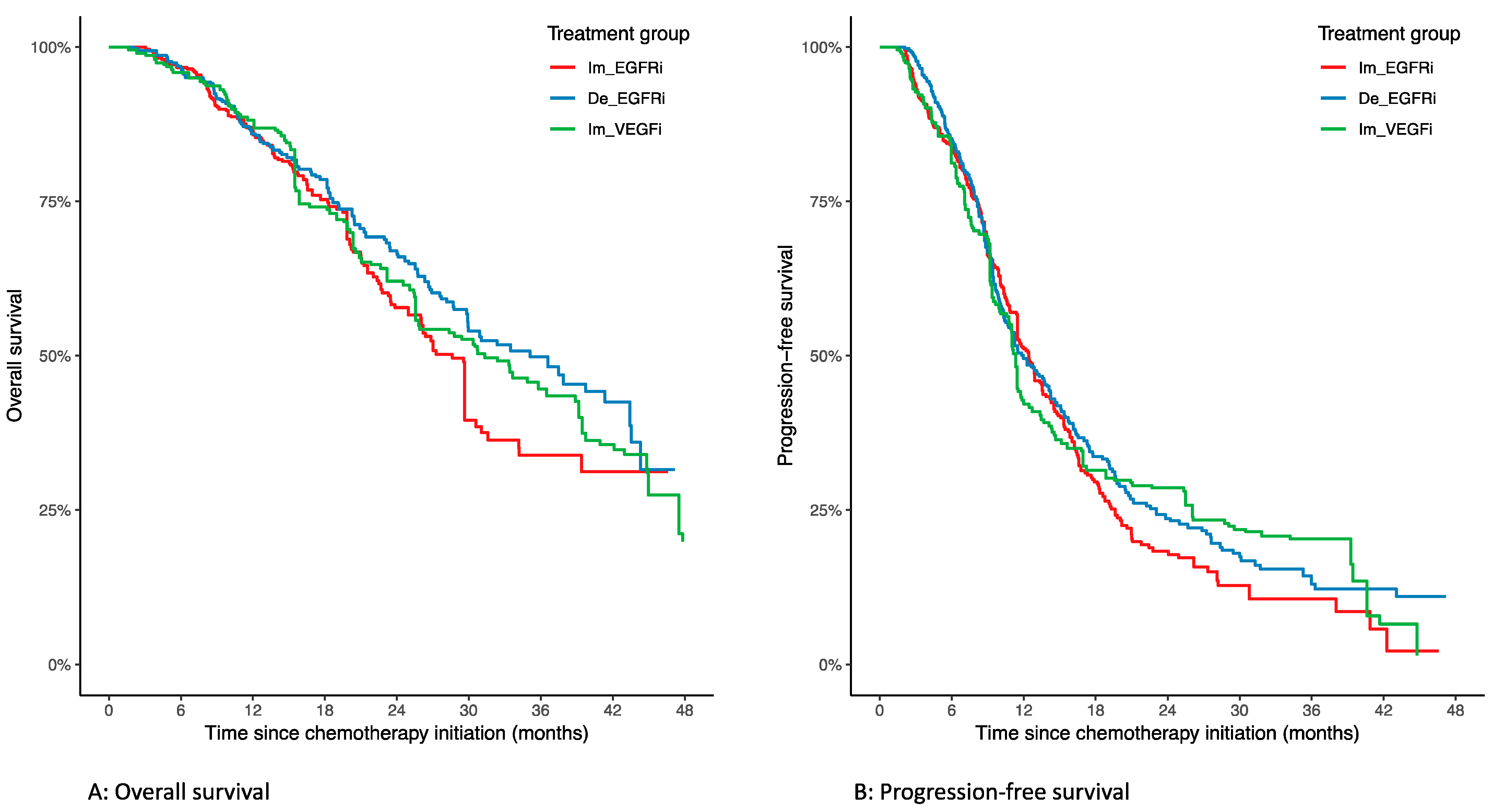
3.2. Overall Survival
3.3. Progression-Free Survival
3.4. Objective Response Rate
3.5. Tumor Localization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Survival According to Treatment Groups in the Unweighted Study Population

References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.-J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O’Neil, B.H.; Atkins, J.N.; et al. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2017, 317, 2392–2401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.-E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- Cercek, A.; Braghiroli, M.I.; Chou, J.F.; Hechtman, J.F.; Kemeny, N.; Saltz, L.; Capanu, M.; Yaeger, R. Clinical Features and Outcomes of Patients with Colorectal Cancers Harboring NRAS Mutations. Clin. Cancer Res. 2017, 23, 4753–4760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lièvre, A.; Bachet, J.-B.; Boige, V.; Cayre, A.; Le Corre, D.; Buc, E.; Ychou, M.; Bouché, O.; Landi, B.; Louvet, C.; et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J. Clin. Oncol. 2008, 26, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Lièvre, A.; Bachet, J.-B.; Le Corre, D.; Boige, V.; Landi, B.; Emile, J.-F.; Côté, J.-F.; Tomasic, G.; Penna, C.; Ducreux, M.; et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006, 66, 3992–3995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douillard, J.-Y.; Oliner, K.S.; Siena, S.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Panitumumab–FOLFOX4 Treatment and RAS Mutations in Colorectal Cancer. N. Engl. J. Med. 2013, 369, 1023–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allegra, C.J.; Rumble, R.B.; Hamilton, S.R.; Mangu, P.B.; Roach, N.; Hantel, A.; Schilsky, R.L. Extended RAS Gene Mutation Testing in Metastatic Colorectal Carcinoma to Predict Response to Anti–Epidermal Growth Factor Receptor Monoclonal Antibody Therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update 2015. JCO 2016, 34, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Lièvre, A.; Merlin, J.-L.; Sabourin, J.-C.; Artru, P.; Tong, S.; Libert, L.; Audhuy, F.; Gicquel, C.; Moureau-Zabotto, L.; Ossendza, R.-A.; et al. RAS mutation testing in patients with metastatic colorectal cancer in French clinical practice: A status report in 2014. Dig. Liver Dis. 2018, 50, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Fiala, O.; Veskrnova, V.; Chloupkova, R.; Poprach, A.; Kiss, I.; Kopeckova, K.; Dusek, L.; Slavicek, L.; Kohoutek, M.; Finek, J.; et al. Impact of Delayed Addition of Anti-EGFR Monoclonal Antibodies on the Outcome of First-Line Therapy in Metastatic Colorectal Cancer Patients: A Retrospective Registry-Based Analysis. Target Oncol. 2018, 13, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, L.-J.; Mineur, L.; Tougeron, D.; Rousseau, B.; Granger, V.; Gornet, J.-M.; Smith, D.; Lievre, A.; Galais, M.-P.; Doat, S.; et al. Withholding the Introduction of Anti-Epidermal Growth Factor Receptor: Impact on Outcomes in RAS Wild-Type Metastatic Colorectal Tumors: A Multicenter AGEO Study (the WAIT or ACT Study). Oncologist 2020, 25, e266–e275. [Google Scholar] [CrossRef] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009, 338, b2393. [Google Scholar] [CrossRef] [PubMed]
- Leyrat, C.; Seaman, S.R.; White, I.R.; Douglas, I.; Smeeth, L.; Kim, J.; Resche-Rigon, M.; Carpenter, J.R.; Williamson, E.J. Propensity score analysis with partially observed covariates: How should multiple imputation be used? Stat. Methods Med. Res. 2019, 28, 3–19. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, D.F.; Griffin, B.A.; Almirall, D.; Slaughter, M.E.; Ramchand, R.; Burgette, L.F. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Statist. Med. 2013, 32, 3388–3414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Statist. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 21 January 2021).
- Stintzing, S.; Modest, D.P.; Rossius, L.; Lerch, M.M.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.-E.; Heintges, T.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): A post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016, 17, 1426–1434. [Google Scholar] [CrossRef]
- Schwartzberg, L.S.; Rivera, F.; Karthaus, M.; Fasola, G.; Canon, J.-L.; Hecht, J.R.; Yu, H.; Oliner, K.S.; Go, W.Y. PEAK: A randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J. Clin. Oncol. 2014, 32, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, V.; Stintzing, S.; Modest, D.P.; Giessen-Jung, C.; Michl, M.; Mansmann, U.R. Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur. J. Cancer 2015, 51, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Holch, J.W.; Ricard, I.; Stintzing, S.; Modest, D.P.; Heinemann, V. The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta-analysis of first-line clinical trials. Eur. J. Cancer 2017, 70, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.; Muinelo, L.; Dalmases, A.; Jones, F.; Edelstein, D.; Iglesias, M.; Orrillo, M.; Abalo, A.; Rodríguez, C.; Brozos, E.; et al. Plasma ctDNA RAS mutation analysis for the diagnosis and treatment monitoring of metastatic colorectal cancer patients. Ann. Oncol. 2017, 28, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Bachet, J.B.; Bouché, O.; Taieb, J.; Dubreuil, O.; Garcia, M.L.; Meurisse, A.; Normand, C.; Gornet, J.M.; Artru, P.; Louafi, S.; et al. RAS mutation analysis in circulating tumor DNA from patients with metastatic colorectal cancer: The AGEO RASANC prospective multicenter study. Ann. Oncol. 2018, 29, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Normanno, N.; Esposito Abate, R.; Lambiase, M.; Forgione, L.; Cardone, C.; Iannaccone, A.; Sacco, A.; Rachiglio, A.M.; Martinelli, E.; Rizzi, D.; et al. RAS testing of liquid biopsy correlates with the outcome of metastatic colorectal cancer patients treated with first-line FOLFIRI plus cetuximab in the CAPRI-GOIM trial. Ann. Oncol. 2018, 29, 112–118. [Google Scholar] [CrossRef] [PubMed]
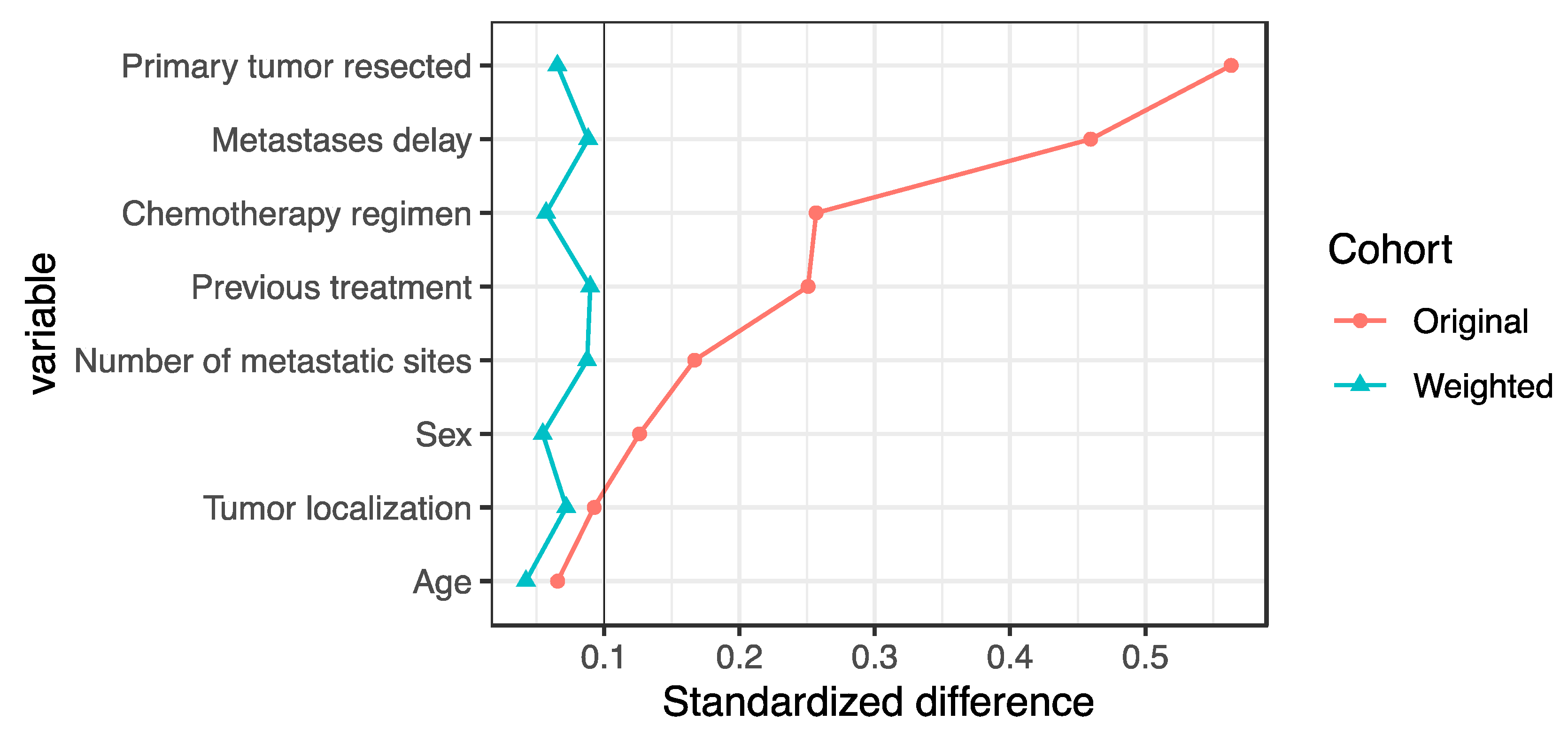
| Item | Overall | Immediate Anti-EGFR | Delayed Anti-EGFR | Immediate Anti-VEGF | Standardized Difference * | Missing (%) |
|---|---|---|---|---|---|---|
| Number of patients | 835 | 401 | 305 | 129 | ||
| Age, median (range), years | 64.0 [56.6, 69.7] | 64.0 [56.6, 68.9] | 63.9 [56.5, 70.1] | 64.0 [57.3, 73.8] | 0.145 | 0.0 |
| Sex | 0.126 | 0.0 | ||||
| Men | 550 (65.9) | 278 (69.3) | 184 (60.3) | 88 (68.2) | ||
| Women | 285 (34.1) | 123 (30.7) | 121 (39.7) | 41 (31.8) | ||
| Tumor localization | 0.093 | 3.4 | ||||
| Right/transverse | 150 (18.6) | 66 (17.1) | 64 (21.5) | 20 (16.1) | ||
| Left/Rectum | 657 (81.4) | 320 (82.9) | 233 (78.5) | 104 (83.9) | ||
| Primary tumor resected | 0.563 | 0.0 | ||||
| No | 249 (29.8) | 66 (16.5) | 113 (37.0) | 70 (54.3) | ||
| Yes | 586 (70.2) | 335 (83.5) | 192 (63.0) | 59 (45.7) | ||
| Previous treatment | 0.251 | 0.0 | ||||
| No | 557 (66.7) | 235 (58.6) | 224 (73.4) | 98 (76.0) | ||
| Yes | 278 (33.3) | 166 (41.4) | 81 (26.6) | 31 (24.0) | ||
| Metastases delay | 0.460 | 26.0 | ||||
| Synchronous | 496 (80.3) | 222 (91.0) | 192 (78.4) | 82 (63.6) | ||
| Metachronous | 122 (19.7) | 22 (9.0) | 53 (21.6) | 47 (36.4) | ||
| Metastatic sites | 0.167 | 30.8 | ||||
| 1 | 280 (48.4) | 100 (45.0) | 125 (55.1) | 55 (42.6) | ||
| >=2 | 298 (51.6) | 122 (55.0) | 102 (44.9) | 74 (57.4) | ||
| Chemotherapy regimen | 0.257 | 0.1 | ||||
| Oxaliplatin-based | 578 (69.3) | 263 (65.6) | 237 (78.0) | 78 (60.5) | ||
| Irinotecan-based | 256 (30.7) | 138 (34.4) | 67 (22.0) | 51 (39.5) | ||
| Country | NA | 0.0 | ||||
| Czech Republic | 573 (68.6) | 401 (100.0) | 172 (56.4) | 0 (0.0) | ||
| France | 262 (31.4) | 0 (0.0) | 133 (43.6) | 129 (100.0) |
| Item | Overall | Immediate Anti-EGFR | Delayed Anti-EGFR | Immediate Anti-VEGF | Standardized Difference * | Missing (%) |
|---|---|---|---|---|---|---|
| Number of patients | 833 | 409 | 310 | 114 | ||
| Age, years | 0.04 | 0.0 | ||||
| <60 years | 445 (53.5) | 214 (52.3) | 168 (54.3) | 63 (55.4) | ||
| ≥60years | 388 (46.6) | 195 (47.7) | 142 (45.7) | 51 (44.6) | ||
| Sex | 0.05 | 0.0 | ||||
| Men | 542 (65.1) | 267 (65.3) | 205 (66.0) | 71 (62.1) | ||
| Women | 291 (34.9) | 142 (34.7) | 105 (34.0) | 43 (38.0) | ||
| Tumor localization | 0.07 | 0.0 | ||||
| Right/transverse | 154 (18.5) | 72 (17.7) | 57 (18.2) | 25 (22.1) | ||
| Left/Rectum | 679 (81.5) | 336 (82.3) | 253 (81.8) | 89 (78.0) | ||
| Primary tumor resected | 0.07 | 0.0 | ||||
| No | 259 (31.1) | 129 (31.5) | 91 (29.4) | 39 (34.0) | ||
| Yes | 574 (68.9) | 280 (68.5) | 219 (70.6) | 75 (66.0) | ||
| Previous treatment | 0.09 | 0.0 | ||||
| No | 556 (66.8) | 271 (66.3) | 203 (65.5) | 82 (71.8) | ||
| Yes | 277 (33.2) | 138 (33.7) | 107 (34.5) | 32 (28.2) | ||
| Metastases delay | 0.09 | 0.0 | ||||
| Synchronous | 617 (74.1) | 302 (73.9) | 235 (75.9) | 80 (70.0) | ||
| Metachronous | 216 (25.9) | 107 (26.1) | 75 (24.1) | 34 (30.0) | ||
| Metastatic sites | 0.09 | 0.0 | ||||
| 1 | 405 (48.6) | 201 (49.2) | 154 (49.7) | 49 (43.2) | ||
| ≥2 | 428 (51.4) | 208 (50.8) | 156 (50.3) | 65 (56.8) | ||
| Chemotherapy regimen | 0.06 | 0.0 | ||||
| Oxaliplatin-based | 594 (71.3) | 292 (71.3) | 218 (70.3) | 85 (74.1) | ||
| Irinotecan-based | 239 (28.7) | 117 (28.7) | 92 (29.7) | 30 (25.9) |
| Cox Models | ||||||
| Delayed Versus Immediate Anti-EGFR | Immediate Anti-VEGF Versus Immediate Anti-EGFR | Immediate Anti-VEGF Versus Delayed Anti-EGFR | ||||
| aHR (95%CI) | p | aHR (95%CI) | p | aHR (95%CI) | p | |
| Overall survival | 0.78 [0.60–1.01] | 0.06 | 0.92 [0.69–1.24] | 0.61 | 1.19 [0.88–1.60] | 0.26 |
| Right/transverse tumor | 1.03 [0.59–1.79] | 0.93 | 0.86 [0.45–1.64] | 0.64 | 0.83 [0.43–1.60] | 0.58 |
| Left/Rectum tumor | 0.71 [0.53–0.97] | 0.03 | 0.89 [0.63–1.25] | 0.49 | 1.24 [0.87–1.75] | 0.23 |
| Progression-free survival | 0.91 [0.75–1.10] | 0.32 | 0.93 [0.73–1.18] | 0.55 | 1.02 [0.80–1.31] | 0.83 |
| Right/transverse tumor | 0.89 [0.57–1.40] | 0.62 | 0.84 [0.48–1.46] | 0.53 | 0.94 [0.53–1.64] | 0.82 |
| Left/Rectum tumor | 0.91 [0.74–1.13] | 0.40 | 0.92 [0.70–1.21] | 0.57 | 1.01 [0.77–1.33] | 0.92 |
| Logistic Regression Models | ||||||
| Delayed Versus Immediate Anti-EGFR | Immediate Anti-Vegf Versus Immediate Anti-EGFR | Immediate Anti-VEGF Versus Delayed Anti-EGFR | ||||
| aOR (95%CI) | p | aOR (95%CI) | p | aOR (95%CI) | p | |
| Objective response rate | 1.75 [1.20–2.54] | 0.004 | 1.24 [0.71–2.19] | 0.45 | 0.71 [0.41–1.25] | 0.23 |
| Right/transverse tumor | 1.69 [0.67–4.28] | 0.26 | 0.92 [0.21–3.98] | 0.91 | 0.54 [0.12–2.42] | 0.42 |
| Left/Rectum tumor | 1.78 [1.17–2.68] | 0.007 | 1.38 [0.77–2.46] | 0.28 | 0.78 [0.43–1.40] | 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmieri, L.-J.; Buchler, T.; Meyer, A.; Veskrnova, V.; Fiala, O.; Brabec, P.; Baranova, J.; Coriat, R. Impact of Delaying the Addition of Anti-EGFR in First Line of RAS Wild-Type Metastatic Colorectal Cancer: A Propensity-Weighted Pooled Data Analysis. Cancers 2022, 14, 1410. https://doi.org/10.3390/cancers14061410
Palmieri L-J, Buchler T, Meyer A, Veskrnova V, Fiala O, Brabec P, Baranova J, Coriat R. Impact of Delaying the Addition of Anti-EGFR in First Line of RAS Wild-Type Metastatic Colorectal Cancer: A Propensity-Weighted Pooled Data Analysis. Cancers. 2022; 14(6):1410. https://doi.org/10.3390/cancers14061410
Chicago/Turabian StylePalmieri, Lola-Jade, Tomas Buchler, Antoine Meyer, Veronika Veskrnova, Ondrej Fiala, Petr Brabec, Jana Baranova, and Romain Coriat. 2022. "Impact of Delaying the Addition of Anti-EGFR in First Line of RAS Wild-Type Metastatic Colorectal Cancer: A Propensity-Weighted Pooled Data Analysis" Cancers 14, no. 6: 1410. https://doi.org/10.3390/cancers14061410
APA StylePalmieri, L.-J., Buchler, T., Meyer, A., Veskrnova, V., Fiala, O., Brabec, P., Baranova, J., & Coriat, R. (2022). Impact of Delaying the Addition of Anti-EGFR in First Line of RAS Wild-Type Metastatic Colorectal Cancer: A Propensity-Weighted Pooled Data Analysis. Cancers, 14(6), 1410. https://doi.org/10.3390/cancers14061410






