Rintatolimod (Ampligen®) Enhances Numbers of Peripheral B Cells and Is Associated with Longer Survival in Patients with Locally Advanced and Metastasized Pancreatic Cancer Pre-Treated with FOLFIRINOX: A Single-Center Named Patient Program
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Definitions of Patient Subgroups According to Survival
2.3. Treatment Specifics
2.4. Endpoints of Study
2.5. Flow Cytometry Analysis
2.6. Statistical Analysis
3. Results
3.1. Patient Treatments
3.2. Rintatolimod Was Well-Tolerated
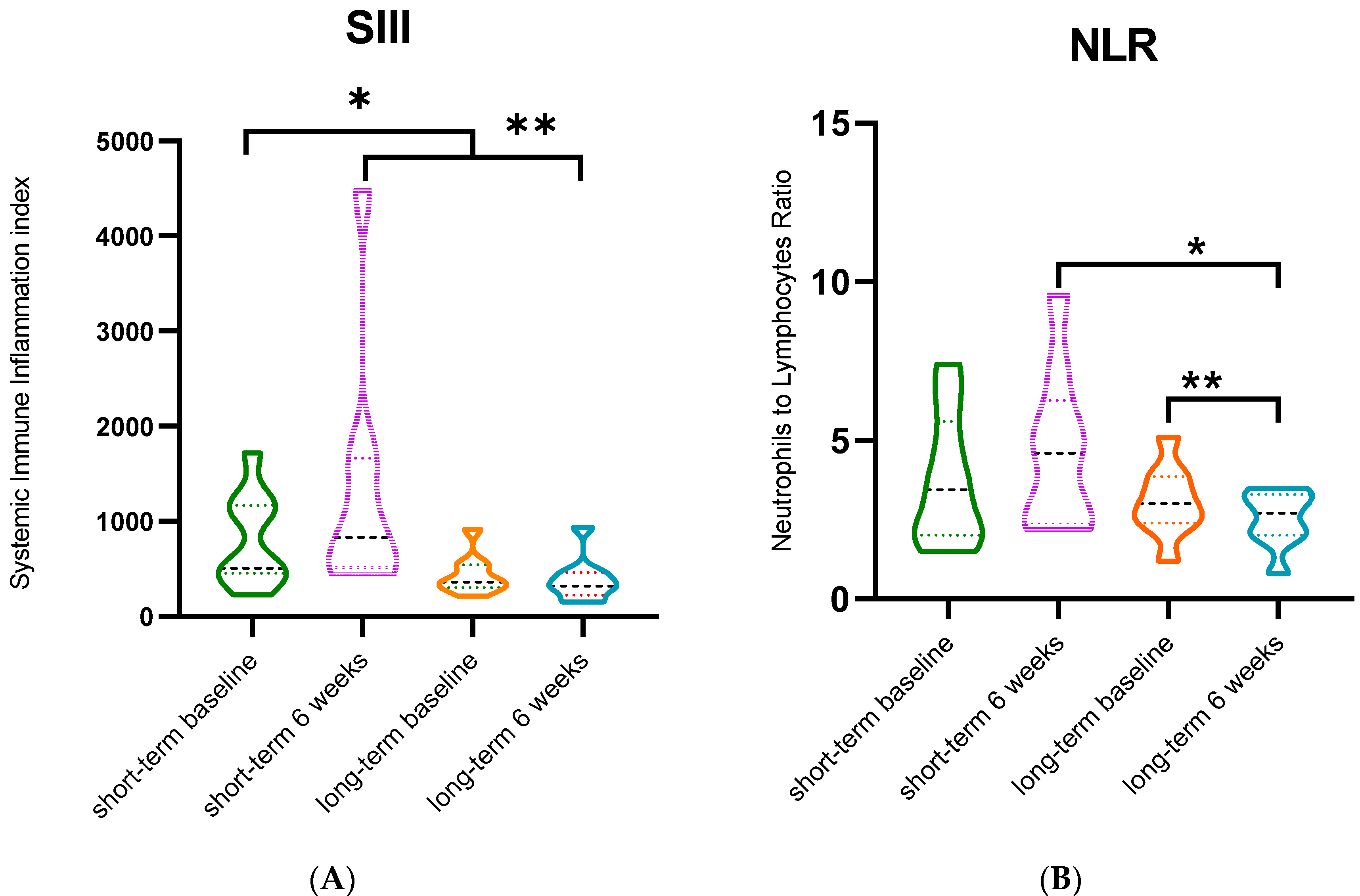
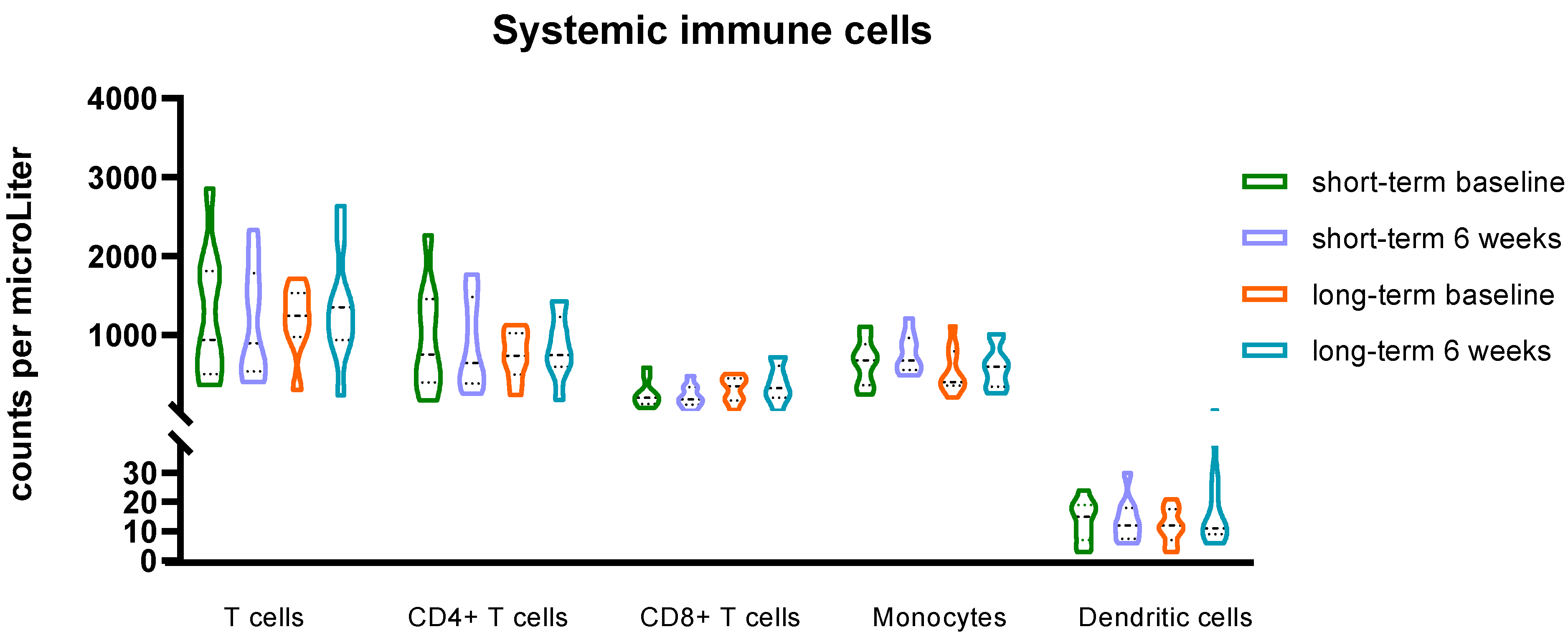
3.3. Rintatolimod Changes Indexes of Immune Effector Cells in the Circulation
3.4. Rintatolimod Enhances Numbers of B Cells in the Circulation
3.5. Rintatolimod Treatment Associate with Extended Progression-Free and Overall Survival
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suker, M.; Beumer, B.R.; Sadot, E.; Marthey, L.; Faris, J.E.; Mellon, E.A.; El-Rayes, B.F.; Wang-Gillam, A.; Lacy, J.; Hosein, P.J.; et al. FOLFIRINOX for locally advanced pancreatic cancer: A systematic review and patient-level meta-analysis. Lancet Oncol. 2016, 17, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Rombouts, S.J.; Walma, M.S.; Vogel, J.A.; van Rijssen, L.B.; Wilmink, J.W.; Mohammad, N.H.; van Santvoort, H.C.; Molenaar, I.Q.; Besselink, M.G. Systematic Review of Resection Rates and Clinical Outcomes After FOLFIRINOX-Based Treatment in Patients with Locally Advanced Pancreatic Cancer. Ann. Surg. Oncol. 2016, 23, 4352–4360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Zhou, F.; Hong, J.; Ng, D.M.; Yang, T.; Zhou, X.; Jin, J.; Zhou, F.; Chen, P.; Xu, Y. The role of FOLFIRINOX in metastatic pancreatic cancer: A meta-analysis. World J. Surg. Oncol. 2021, 19, 182. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [Green Version]
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The tumour microenvironment in pancreatic cancer—Clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef]
- Hosein, A.N.; Brekken, R.A.; Maitra, A. Pancreatic cancer stroma: An update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 487–505. [Google Scholar] [CrossRef]
- Huber, M.; Brehm, C.U.; Gress, T.M.; Buchholz, M.; Alashkar Alhamwe, B.; von Strandmann, E.P.; Slater, E.P.; Bartsch, J.W.; Bauer, C.; Lauth, M. The Immune Microenvironment in Pancreatic Cancer. Int. J. Mol. Sci. 2020, 21, 7307. [Google Scholar] [CrossRef]
- Steele, N.G.; Carpenter, E.S.; Kemp, S.B.; Sirihorachai, V.; The, S.; Delrosario, L.; Lazarus, J.; Amir, E.D.; Gunchick, V.; Espinoza, C.; et al. Multimodal Mapping of the Tumor and Peripheral Blood Immune Landscape in Human Pancreatic Cancer. Nat. Cancer 2020, 1, 1097–1112. [Google Scholar] [CrossRef]
- Leulier, F.; Lemaitre, B. Toll-like receptors--taking an evolutionary approach. Nat. Rev. Genet. 2008, 9, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Brennan, J.J.; Gilmore, T.D. Evolutionary Origins of Toll-like Receptor Signaling. Mol. Biol. Evol. 2018, 35, 1576–1587. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, L.; Peng, J.; Li, Z.; Wong, F.S. The effect of innate immunity on autoimmune diabetes and the expression of Toll-like receptors on pancreatic islets. J. Immunol. 2004, 172, 3173–3180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, T.; Akira, S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicodemus, C.F.; Wang, L.; Lucas, J.; Varghese, B.; Berek, J.S. Toll-like receptor-3 as a target to enhance bioactivity of cancer immunotherapy. Am. J. Obstet. Gynecol. 2010, 202, 608.e1–608.e8. [Google Scholar] [CrossRef]
- Jasani, B.; Navabi, H.; Adams, M. Ampligen: A potential toll-like 3 receptor adjuvant for immunotherapy of cancer. Vaccine 2009, 27, 3401–3404. [Google Scholar] [CrossRef]
- Fujisawa, M.; Kanda, T.; Shibata, T.; Sasaki, R.; Masuzaki, R.; Matsumoto, N.; Nirei, K.; Imazu, H.; Kuroda, K.; Sugitani, M.; et al. Involvement of the Interferon Signaling Pathways in Pancreatic Cancer Cells. Anticancer Res. 2020, 40, 4445–4455. [Google Scholar] [CrossRef]
- Booy, S.; Hofland, L.; van Eijck, C. Potentials of interferon therapy in the treatment of pancreatic cancer. J. Interferon. Cytokine Res. 2015, 35, 327–339. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Xu, F. Anticancer function of polyinosinic-polycytidylic acid. Cancer Biol. Ther. 2010, 10, 1219–1223. [Google Scholar] [CrossRef] [Green Version]
- Salaun, B.; Lebecque, S.; Matikainen, S.; Rimoldi, D.; Romero, P. Toll-like receptor 3 expressed by melanoma cells as a target for therapy? Clin. Cancer Res. 2007, 13 Pt 1, 4565–4574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, F.; Pretto, S.; Tagliabue, E.; Balsari, A.; Sfondrini, L. Exploiting poly(I:C) to induce cancer cell apoptosis. Cancer Biol. Ther. 2017, 18, 747–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strayer, D.R.; Carter, W.; Strauss, K.I.; Brodsky, I.; Suhadolnik, R.; Ablashi, D.; Henry, B.; Mitchell, W.M.; Bastien, S.; Peterson, D. Long Term Improvements in Patients with Chronic Fatigue Syndrome Treated with Ampligen. J. Chronic Fatigue Syndr. 1995, 1, 35–53. [Google Scholar] [CrossRef]
- Strayer, D.R.; Carter, W.A.; Brodsky, I.; Cheney, P.; Peterson, D.; Salvato, P.; Thompson, C.; Loveless, M.; Shapiro, D.E.; Elsasser, W. A controlled clinical trial with a specifically configured RNA drug, poly(I).poly(C12U), in chronic fatigue syndrome. Clin. Infect. Dis. 1994, 18 (Suppl. S1), S88–S95. [Google Scholar] [CrossRef] [PubMed]
- Strayer, D.R.; Carter, W.A.; Stouch, B.C.; Stevens, S.R.; Bateman, L.; Cimoch, P.J.; Lapp, C.W.; Peterson, D.L. A double-blind, placebo-controlled, randomized, clinical trial of the TLR-3 agonist rintatolimod in severe cases of chronic fatigue syndrome. PLoS ONE 2012, 7, e31334. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, R.; Choudry, H.; Jones, H.; Girgis, M.; Gooding, W.; Kalinski, P.; Bartlett, D. Phase II Trial of Adjuvant Dendritic Cell Vaccine in Combination with Celecoxib, Interferon-α, and Rintatolimod in Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Metastases. Ann. Surg. Oncol. 2021, 28, 4637–4646. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.Gov. 15 Studies Found for: Rintatolimod | Cancer: U.S. National Library of Medicine 2022 [Updated 2022]. Available online: https://clinicaltrials.gov/ct2/results?cond=cancer&term=rintatolimod&cntry=&state=&city=&dist= (accessed on 12 January 2021).
- Aziz, M.H.; Sideras, K.; Aziz, N.A.; Mauff, K.; Haen, R.; Roos, D.; Saida, L.; Suker, M.; van der Harst, E.; Mieog, J.S.; et al. The Systemic-immune-inflammation Index Independently Predicts Survival and Recurrence in Resectable Pancreatic Cancer and its Prognostic Value Depends on Bilirubin Levels: A Retrospective Multicenter Cohort Study. Ann. Surg. 2019, 270, 139–146. [Google Scholar] [CrossRef]
- Allen, J.; Cernik, C.; Bajwa, S.; Al-Rajabi, R.; Saeed, A.; Baranda, J.; Williamson, S.; Sun, W.; Kasi, A. Association of Neutrophil, Platelet, and Lymphocyte Ratios with the Prognosis in Unresectable and Metastatic Pancreatic Cancer. J. Clin. Med. 2020, 9, 3283. [Google Scholar] [CrossRef]
- National Cancer Institute. Cancer Therapy Evaluation Program (CTEP): CTEP. Updated 2022. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50 (accessed on 12 January 2021).
- Kunert, A.; Basak, E.A.; Hurkmans, D.P.; Balcioglu, H.E.; Klaver, Y.; van Brakel, M.; Oostvogels, A.A.M.; Lamers, C.H.J.; Bins, S.; Koolen, S.L.W.; et al. CD45RA(+)CCR7(-) CD8 T cells lacking co-stimulatory receptors demonstrate enhanced frequency in peripheral blood of NSCLC patients responding to nivolumab. J. Immunother. Cancer 2019, 7, 149. [Google Scholar] [CrossRef] [Green Version]
- Tsou, P.; Katayama, H.; Ostrin, E.J.; Hanash, S.M. The Emerging Role of B Cells in Tumor Immunity. Cancer Res. 2016, 76, 5597–5601. [Google Scholar] [CrossRef] [Green Version]
- Hua, Z.; Hou, B. TLR signaling in B-cell development and activation. Cell. Mol. Immunol. 2013, 10, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Ligeiro, D.; Rao, M.; Maia, A.; Castillo, M.; Beltran, A.; Maeurer, M. B Cells in the Gastrointestinal Tumor Microenvironment with a Focus on Pancreatic Cancer: Opportunities for Precision Medicine? Adv. Exp. Med. Biol. 2020, 1273, 175–195. [Google Scholar] [PubMed]
- Burger, J.A.; Wiestner, A. Targeting B cell receptor signalling in cancer: Preclinical and clinical advances. Nat. Rev. Cancer 2018, 18, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Castino, G.F.; Cortese, N.; Capretti, G.; Serio, S.; Di Caro, G.; Mineri, R.; Magrini, E.; Grizzi, F.; Cappello, P.; Novelli, F.; et al. Spatial distribution of B cells predicts prognosis in human pancreatic adenocarcinoma. Oncoimmunology 2016, 5, e1085147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunner, M.; Maier, K.; Rümmele, P.; Jacobsen, A.; Merkel, S.; Benard, A.; Krautz, C.; Kersting, S.; Grützmann, R.; Weber, G.F. Upregulation of CD20 Positive B-Cells and B-Cell Aggregates in the Tumor Infiltration Zone is Associated with Better Survival of Patients with Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2020, 21, 1779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navabi, H.; Jasani, B.; Reece, A.; Clayton, A.; Tabi, Z.; Donninger, C.; Mason, M.; Adams, M. A clinical grade poly I:C-analogue (Ampligen) promotes optimal DC maturation and Th1-type T cell responses of healthy donors and cancer patients in vitro. Vaccine 2009, 27, 107–115. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yanagimoto, H.; Satoi, S.; Toyokawa, H.; Yamao, J.; Kim, S.; Terakawa, N.; Takahashi, K.; Kwon, A.H. Circulating myeloid dendritic cells as prognostic factors in patients with pancreatic cancer who have undergone surgical resection. J. Surg. Res. 2012, 173, 299–308. [Google Scholar] [CrossRef]
- Shojaei, H.; Oberg, H.H.; Juricke, M.; Marischen, L.; Kunz, M.; Mundhenke, C.; Gieseler, F.; Kabelitz, D.; Wesch, D. Toll-like receptors 3 and 7 agonists enhance tumor cell lysis by human gammadelta T cells. Cancer Res. 2009, 69, 8710–8717. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.; Foster, R.E.; Horgan, K.; Mounsey, K.; Nixon, H.; Smalle, N.; Hughes, T.A.; Carter, C.R. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Res. 2016, 18, 10. [Google Scholar] [CrossRef] [Green Version]
- Mozaffari, F.; Lindemalm, C.; Choudhury, A.; Granstam-Björneklett, H.; Lekander, M.; Nilsson, B.; Ojutkangas, M.L.; Osterborg, A.; Bergkvist, L.; Mellstedt, H. Systemic immune effects of adjuvant chemotherapy with 5-fluorouracil, epirubicin and cyclophosphamide and/or radiotherapy in breast cancer: A longitudinal study. Cancer Immunol. Immunother. 2009, 58, 111–120. [Google Scholar] [CrossRef]




| Cell Type | Marker | |
|---|---|---|
| Granulocytes | Eosinophils | CD15+, CD16- |
| Mature neutrophils | CD15high, CD16high | |
| Immature neutrophils | CD15+, CD16+ | |
| Monocytes | Monocytes Dendritic cells | CD14+, CD16- CD14-, CD16-, CD11c+ |
| Lymphocytes | B cells | CD3-, CD19+ |
| NK cells | CD3-, CD56+, CD16+/- | |
| T cells | CD3+ | |
| T helper cells | CD3+, CD4+ | |
| Killer T cells | CD3+, CD8+ |
| N = 27 Rintatolimod Group | N = 54 Matched Controls | N = 27 Subset Matched Controls | p-Value | |
|---|---|---|---|---|
| Age, median (range) | 63 (44–73) | 62 (44–78) | 65 (46–78) | 0.684 * [1] 0.563 * [2] |
| Male, n (%) Female, n (%) | 19 (70.4) 8 (29.6) | 38 (70.4) 16 (29.6) | 18 (66.7) 9 (33.3) | 1.000 ** [1] 0.770 ** [2] |
| Disease stage n (%) | ||||
| LAPC | 5 (18.5) | 10 (18.5) | 5 (18.5) | 1.000 ** [1] 0.634 ** [2] |
| Metastatic disease | 16 (59.3) | 32 (59.3) | 13 (48.2) | |
| Tumor recurrence after surgery | 6 (22.2) | 12 (22.2) | 9 (33.3) | |
| FOLFIRINOX cycles, median (range) | 8 (1–12) | 8 (1–12) | 8 (1–12) | 0.241 * [1] 0.727 * [2] |
| Time in months from last FOLFIRINOX dose to start Rintatolimod, median (range) | 3.9 (0.4–28) | NA | NA | 0.009 Ɨ [1] 0.478 Ɨ [2] |
| Progression-free interval from last FOLFIRINOX to progression, median months (range) | NA | 2.2 (0.0–20.8) | 3.4 (2.3–20.8) | |
| Total N = 27 | ||
|---|---|---|
| CTCAE* Grade 1–2 n(%) | CTCAE Grade 3–5 n(%) | |
| Musculoskeletal and connective tissue disorders | 9(33.3) | 0(0) |
| 8(29.6) | |
| 1(3.7) | |
| General disorders and administration site conditions | 22(81.5) | 0(0) |
| 8(29.6) | |
| 14(51.8) | |
| Vascular disorders | 2(7.4) | 0(0) |
| ||
| Gastrointestinal disorders | 3(11.1) | 0(0) |
| ||
| Nervous system disorders | 3(11.1) | 0(0) |
| ||
| Immune system disorders | 2(7.4) | 0(0) |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
el Haddaoui, H.; Brood, R.; Latifi, D.; Oostvogels, A.A.; Klaver, Y.; Moskie, M.; Mustafa, D.A.; Debets, R.; van Eijck, C.H.J. Rintatolimod (Ampligen®) Enhances Numbers of Peripheral B Cells and Is Associated with Longer Survival in Patients with Locally Advanced and Metastasized Pancreatic Cancer Pre-Treated with FOLFIRINOX: A Single-Center Named Patient Program. Cancers 2022, 14, 1377. https://doi.org/10.3390/cancers14061377
el Haddaoui H, Brood R, Latifi D, Oostvogels AA, Klaver Y, Moskie M, Mustafa DA, Debets R, van Eijck CHJ. Rintatolimod (Ampligen®) Enhances Numbers of Peripheral B Cells and Is Associated with Longer Survival in Patients with Locally Advanced and Metastasized Pancreatic Cancer Pre-Treated with FOLFIRINOX: A Single-Center Named Patient Program. Cancers. 2022; 14(6):1377. https://doi.org/10.3390/cancers14061377
Chicago/Turabian Styleel Haddaoui, Hassana, Rianne Brood, Diba Latifi, Astrid A. Oostvogels, Yarne Klaver, Miranda Moskie, Dana A. Mustafa, Reno Debets, and Casper H. J. van Eijck. 2022. "Rintatolimod (Ampligen®) Enhances Numbers of Peripheral B Cells and Is Associated with Longer Survival in Patients with Locally Advanced and Metastasized Pancreatic Cancer Pre-Treated with FOLFIRINOX: A Single-Center Named Patient Program" Cancers 14, no. 6: 1377. https://doi.org/10.3390/cancers14061377
APA Styleel Haddaoui, H., Brood, R., Latifi, D., Oostvogels, A. A., Klaver, Y., Moskie, M., Mustafa, D. A., Debets, R., & van Eijck, C. H. J. (2022). Rintatolimod (Ampligen®) Enhances Numbers of Peripheral B Cells and Is Associated with Longer Survival in Patients with Locally Advanced and Metastasized Pancreatic Cancer Pre-Treated with FOLFIRINOX: A Single-Center Named Patient Program. Cancers, 14(6), 1377. https://doi.org/10.3390/cancers14061377









