Multimodal Treatment of Nasopharyngeal Carcinoma in Children, Adolescents and Young Adults-Extended Follow-Up of the NPC-2003-GPOH Study Cohort and Patients of the Interim Cohort
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Diagnosis, Staging, and Response Assessment
2.3. Treatment
2.4. Late Effects
2.5. Statistics
3. Results
3.1. Patient Characteristics
3.2. Treatment and Outcome
3.2.1. NPC-2003 Study Cohort
3.2.2. Interim Cohort
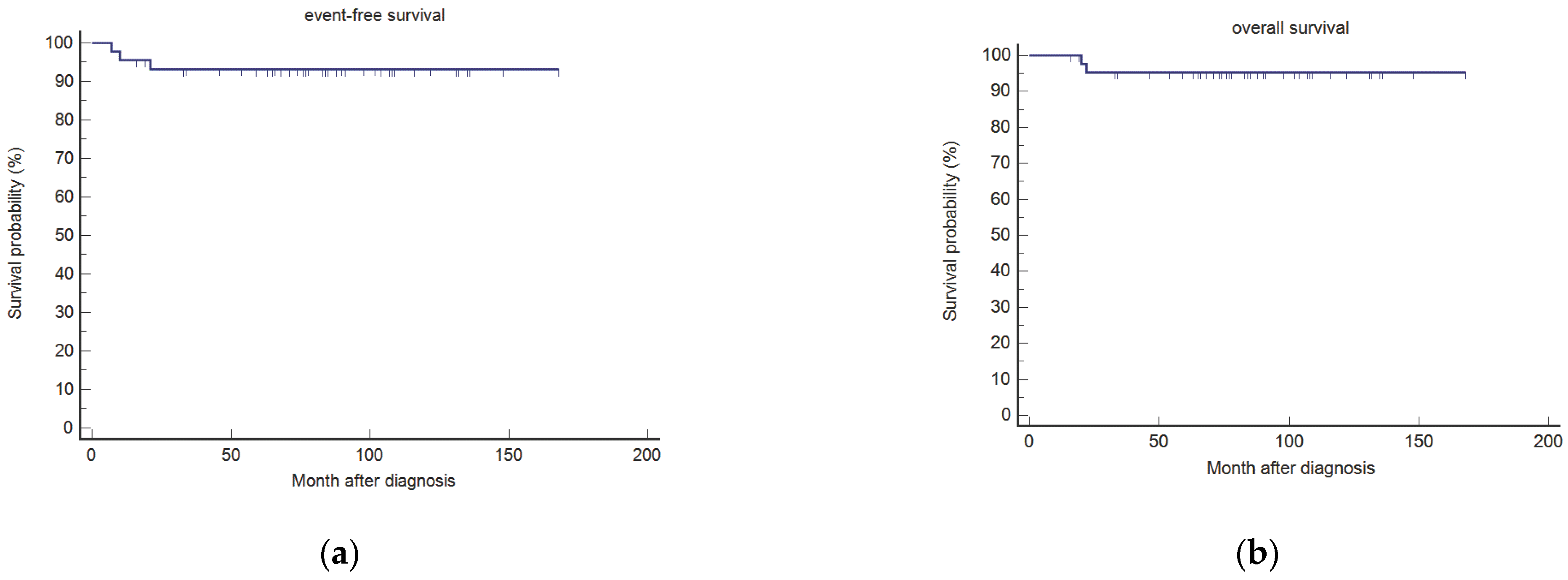
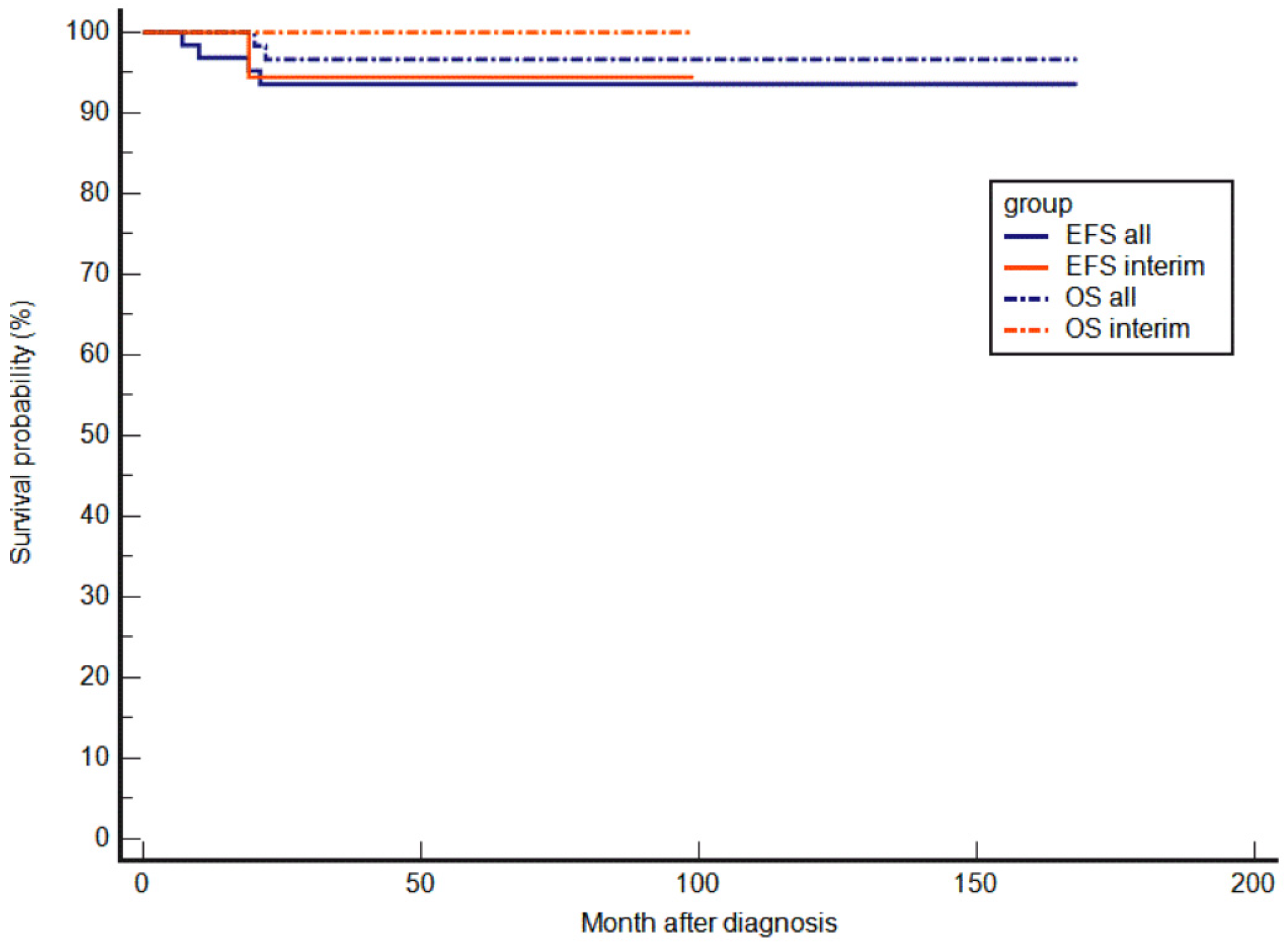
3.2.3. EFS and OS
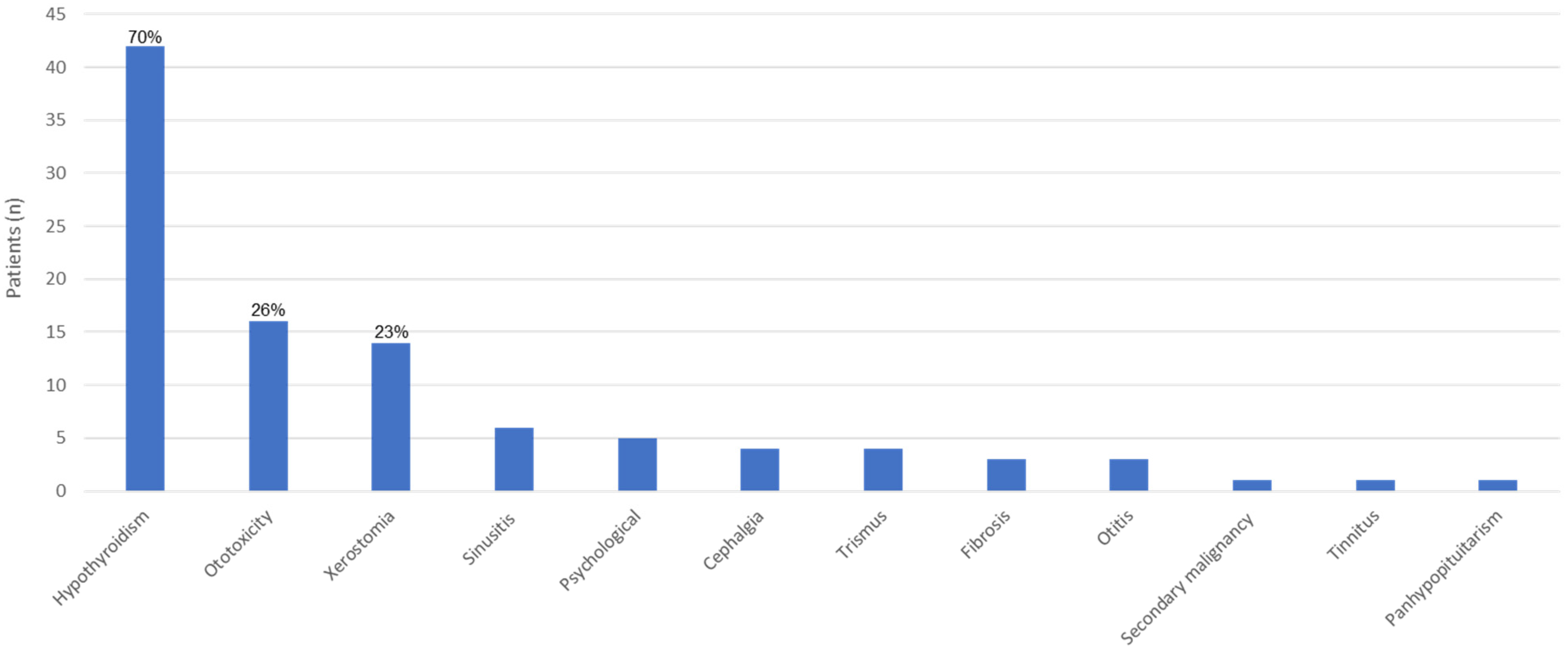
3.3. Late Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erdmann, F.; Kaatsch, P.; Grabow, D.; Spix, C. German Childhood Cancer Registry—Annual Report 2019 (1980–2018); Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI) at the University Medical Center of the Johannes Gutenberg University: Mainz, Germany, 2020. [Google Scholar]
- Tsao, S.W.; Tsang, C.M.; To, K.F.; Lo, K.W. The role of Epstein-Barr virus in epithelial malignancies. J. Pathol. 2015, 235, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Mertens, R.; Granzen, B.; Lassay, L.; Bucsky, P.; Hundgen, M.; Stetter, G.; Heimann, G.; Weiss, C.; Hess, C.F.; Gademann, G. Treatment of nasopharyngeal carcinoma in children and adolescents: Definitive results of a multicenter study. Cancer 2005, 104, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Buehrlen, M.; Zwaan, C.M.; Granzen, B.; Lassay, L.; Deutz, P.; Vorwerk, P.; Staatz, G.; Gadmann, G.; Christiansen, P.; Oldenburger, F.; et al. Multimodal treatment, including interferon beta, of nasopharyngeal carcinoma in children and young adults: Preliminary results from the prospective, multicenter study NPC-2003-GPOH/DCOG. Cancer 2012, 118, 4892–4900. [Google Scholar] [CrossRef] [PubMed]
- Kontny, H.U.; Franzen, S.; Behrends, U.; Bührlen, M.; Christiansen, H.; Delecluse, H.-J.; Eble, M.J.; Feuchtinger, T.; Gademann, G.; Granzen, B.; et al. Diagnosis and treatment of nasopharyngeal carcinoma in children and adolescents-recommendations of the GPOH-NPC study group. Klin. Padiatr. 2016, 228, 105–112. [Google Scholar] [CrossRef]
- Ben-Ami, T.; Kontny, U.; Surun, A.; Brecht, I.B.; López Almaraz, R.; Dragomir, M.; Pourtsidis, A.; Casanova, M.; Fresneau, B.; Bisogno, G.; et al. Nasopharyngeal carcinoma in children and adolescents: The EXPeRT/PARTNER diagnostic and therapeutic recommendations. Pediatr. Blood Cancer 2021, 68, e29018. [Google Scholar] [CrossRef]
- Krueger, G.R.F.; Wustrow, J. Current classification of nasopharyngeal carcinoma at Cologne University. In Nasopharyngeal Carcinoma; Grundmann, E., Krueger, G.R.F., Ablashi, D.V., Eds.; Gustav Fischer Verlag: Stuttgart, Germany; New York, NY, USA, 1981; Volume 5, pp. 11–15. [Google Scholar]
- American Joint Committee for Cancer Staging and End Results Reporting. Manual for Staging of Cancer, 4th ed.; American Joint Committee: Chicago, IL, USA, 1987; Volume 111, pp. 30–44. [Google Scholar]
- Edge, S.B.; Byrd, D.R.; Compton, C.C. AJCC Cancer Staging Manual, 7th ed.; Lippincott-Raven: Philadelphia, PA, USA, 2009. [Google Scholar]
- Chua, D.T.; Sham, J.S.; Kwong, D.L.; Au, G.K. Treatment outcome after radiotherapy alone for patients with Stage I-II nasopharyngeal carcinoma. Cancer 2003, 98, 74–80. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Kalbfleisch, J.D.; Prentice, R.L. The Statistical Analysis of Failure Time Data; John Wiley & Sons, Inc.: New York, NY, USA, 1980. [Google Scholar]
- Treuner, J.; Niethammer, D.; Dannecker, G.; Hagmann, R.; Neef, V.; Hofschneider, P.H. Successful treatment of nasopharyngeal carcinoma with interferon. Lancet 1980, 1, 817–818. [Google Scholar] [CrossRef]
- Mertens, R.; Lassay, L.; Heimann, G. Combined treatment of nasopharyngeal cancer in children and adolescents—Concept of a study. Klin. Padiatr. 1993, 205, 241–248. [Google Scholar] [CrossRef]
- Makowska, A.; Wahab, L.; Braunschweig, T.; Kapetanakis, N.-I.; Vokuhl, C.; Denecke, B.; Shen, L.; Busson, P.; Kontny, U. Interferon beta induces apoptosis in nasopharyngeal carcinoma cells via the TRAIL-signaling pathway. Oncotarget 2018, 9, 14228–14250. [Google Scholar] [CrossRef]
- Makowska, A.; Franzen, S.; Braunschweig, T.; Denecke, B.; Shen, L.; Baloche, V.; Busson, P.; Kontny, U. Interferon-beta increases NK cell cytotoxicity against tumor cells in patients with nasopharyngeal carcinoma via tumor necrosis factor apoptosis-inducing ligand. Cancer Immunol. Immunother. 2019, 68, 1317–1329. [Google Scholar] [CrossRef]
- Casanova, M.; Bisogno, G.; Gandola, L.; Cecchetto, G.; Di Cataldo, A.; Basso, E.; Indolfi, P.; D’Angelo, P.; Favini, F.; Collini, P.; et al. A prospective protocol for nasopharyngeal carcinoma in children and adolescents: The Italian Rare Tumors in Pediatric Age (TREP) project. Cancer 2012, 118, 2718–2725. [Google Scholar] [CrossRef]
- Rodriguez-Galindo, C.; Krailo, M.D.; Krasin, M.J.; Huang, L.; McCarville, M.B.; Hicks, J.; Panshankar, F.; Pappo, A.S. Treatment of Childhood Nasopharyngeal Carcinoma with Induction Chemotherapy and Concurrent Chemoradiotherapy: Results of the Children’s Oncology Group ARAR0331 Study. J. Clin. Oncol. 2019, 37, 3369–3376. [Google Scholar] [CrossRef] [PubMed]
- PLoSker, G.L. Interferon-β-1b: A review of its use in multiple sclerosis. CNS Drugs 2011, 25, 67–88. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, Y.D.; Comini-Frota, E.; Lopes, J.S.; Noal, J.S.; Giacomo, M.C.; Gomes, S.; Gonçalves, M.V.M.; da Gama, P.D.; Finkelsztejn, A. Severe depression, suicide attempts, and ideation during the use of interferon beta by patients with multiple sclerosis. Clin. Neuropharmacol. 2010, 33, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Lana-Peixoto, M.A.; Teixeira, A.L., Jr.; Haase, V.G. Interferon beta-1a-induced depression and suicidal ideation in multiple sclerosis. Arq. Neuropsiquiatr. 2002, 60, 721–724. [Google Scholar] [CrossRef][Green Version]
- Nikfar, S.; Rahimi, R.; Abdollahi, M. A meta-analysis of the efficacy and tolerability of interferon-β in multiple sclerosis, overall and by drug and disease type. Clin. Ther. 2010, 32, 1871–1888. [Google Scholar] [CrossRef]
- Goeb, J.L.; Even, C.; Nicolas, G.; Gohier, B.; Dubas, F.; Garré, J.B. Psychiatric side effects of interferon-beta in multiple sclerosis. Eur. Psychiatry 2006, 21, 186–193. [Google Scholar] [CrossRef]
- Gold, R.; Rieckmann, P.; Chang, P.; Abdalla, J.; PRISMS Study Group. The long-term safety and tolerability of high-dose interferon beta-1a in relapsing-remitting multiple sclerosis: 4-year data from the PRISMS study. Eur. J. Neurol. 2005, 12, 649–656. [Google Scholar] [CrossRef]
- Siegert, R.J.; Abernethy, D.A. Depression in multiple sclerosis: A review. J. Neurol. Neurosurg. Psychiatry 2005, 76, 469–475. [Google Scholar] [CrossRef]
- Ghim, T.T.; Briones, M.; Mason, P.; Crocker, I.; Davis, P.; Bell, B.; Vega, R.; Corden, B.; Meacham, L.; Alvarado, C.S. Effective adjuvant chemotherapy for advanced nasopharyngeal carcinoma in children: A final update of a long-term prospective study in a single institution. Pediatr. Hematol. Oncol. 1998, 20, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Galindo, C.; Wofford, M.; Castleberry, R.P.; Swanson, G.P.; London, W.B.; Fontanesi, J.; Pappo, A.S.; Douglass, E.C. Preradiation chemotherapy with methotrexate, cisplatin, 5-fluorouracil, and leucovorin for pediatric nasopharyngeal carcinoma. Cancer 2005, 103, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Orbach, D.; Brisse, H.; Helfre, S.; Klijanienko, J.; Bours, D.; Mosseri, V.; Rodriguez, J. Radiation and chemotherapy combination for nasopharyngeal carcinoma in children: Radiotherapy dose adaptation after chemotherapy response to minimize late effects. Pediatr. Blood Cancer 2008, 50, 849–853. [Google Scholar] [CrossRef]
- Lee, A.W.M.; Tung, S.Y.; Chua, D.T.T.; Ngan, R.K.; Chappell, R.; Tung, R.; Siu, L.; Ng, W.T.; Sze, W.K.; Au, G.K.H.; et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J. Natl. Cancer Inst. 2010, 102, 1188–1198. [Google Scholar] [CrossRef]
- Pow, E.H.; Kwong, D.L.; McMillan, A.S.; Wong, M.C.; Sham, J.S.; Leung, L.H.; Leung, K.W. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: Initial report on a randomized controlled clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Kam, M.K.; Leung, S.F.; Zee, B.; Chau, R.M.; Suen, J.J.; Mo, F.; Lai, M.; Ho, R.; Cheung, K.Y.; Yu, B.K.; et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J. Clin. Oncol. 2007, 25, 4873–4879. [Google Scholar] [CrossRef]
- Peng, G.; Wang, T.; Yang, K.Y.; Zhang, S.; Zhang, T.; Li, Q.; Han, J.; Wu, G. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother. Oncol. 2012, 104, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.; Özyar, E.; Patte, C.; Orbach, D.; Ferrari, A.; Veyrat-Follet, C.; Errihani, H.; Pan, J.; Zhang, L.; Shen, L.; et al. International randomized phase 2 study on the addition of docetaxel to the combination of cisplatin and 5-fluorouracil in the induction treatment for nasopharyngeal carcinoma in children and adolescents. Cancer Chemother. Pharmacol. 2016, 77, 289–298. [Google Scholar] [CrossRef]
- Zaghloul, M.S.; Eldebawy, E.; Ahmed, S.; Ammar, H.; Khalil, E.; Abdelrahman, H.; Zekri, W.; Elzomor, H.; Taha, H.; Elnashar, A. Does primary tumor volume predict the outcome of pediatric nasopharyngeal carcinoma?: A prospective single-arm study using neoadjuvant chemotherapy and concomitant chemotherapy with intensity modulated radiotherapy. Asia Pac. J. Clin. Oncol. 2016, 12, 143–150. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Hong, S.; Yang, Y.; Yu, G.; Jia, J.; Peng, P.; Wu, X.; Lin, Q.; Xi, X.; et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: A multicentre, randomised, open-label, phase 3 trial. Lancet 2016, 388, 1883–1892, Erratum in Lancet 2016, 388, 1882. [Google Scholar] [CrossRef]
- Hong, S.; Zhang, Y.; Yu, G.; Peng, P.; Peng, J.; Jia, J.; Wu, X.; Huang, Y.; Yang, Y.; Lin, Q.; et al. Gemcitabine Plus Cisplatin Versus Fluorouracil Plus Cisplatin as First-Line Therapy for Recurrent or Metastatic Nasopharyngeal Carcinoma: Final Overall Survival Analysis of GEM20110714 Phase III Study. J. Clin. Oncol. 2021, 39, 3273–3282. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.-Y.; Lu, J.-X.; Yu, X.-W.; Zhang, J.; Xu, Q.-L.; Zhang, R.-J.; Mi, J.-L.; Liao, S.-F.; Fan, J.-F.; Qin, X.-L.; et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin as a first-line concurrent chemotherapy regimen in nasopharyngeal carcinoma: A prospective, multi-institution, randomized controlled phase II study. Cancer Chemother. Pharmacol. 2019, 84, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chuner, J.; Lei, W.; Fengqin, Y.; Zhimin, Y.; Quanquan, S.; Tongxin, L.; Zhenfu, F.; Yangming, J. Optimal induction chemotherapeutic regimen followed by concurrent chemotherapy plus intensity-modulated radiotherapy as first-line therapy for locoregionally advanced nasopharyngeal carcinoma. Medicine 2020, 99, e22283. [Google Scholar] [CrossRef] [PubMed]
- Colevas, A.D.; Yom, S.S.; Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Brizel, D.M.; Burtness, B.; Busse, P.M.; Caudell, J.L.; et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 1.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Ismaila, N.; Chua, M.L.K.; Colevas, A.D.; Haddad, R.; Huang, S.H.; Wee, J.T.S.; Whitley, A.C.; Yi, J.-L.; Yom, S.S.; et al. Chemotherapy in Combination with Radiotherapy for Definitive-Intent Treatment of Stage II-IVA Nasopharyngeal Carcinoma: CSCO and ASCO Guideline. J. Clin. Oncol. 2021, 39, 840–859. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.J.; Chapuy, B.; Ouyang, J.; Sun, H.H.; Roemer, M.G.; Xu, M.L.; Yu, H.; Fletcher, C.D.M.; Freeman, G.J.; Shipp, M.A.; et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin. Cancer. Res. 2013, 19, 3462–3473. [Google Scholar] [CrossRef]
- Hsu, C.; Lee, S.H.; Ejadi, S.; Even, C.; Cohen, R.B.; Le Tourneau, C.; Mehnert, J.M.; Algazi, A.; van Brummelen, E.M.J.; Saraf, S.; et al. Safety and Antitumor Activity of Pembrolizumab in Patients with Programmed Death-Ligand 1-Positive Nasopharyngeal Carcinoma: Results of the KEYNOTE-028 Study. J. Clin. Oncol. 2017, 35, 4050–4056. [Google Scholar] [CrossRef]
- Ma, B.B.Y.; Lim, W.-T.; Goh, B.-C.; Hui, E.P.; Lo, K.-W.; Pettinger, A.; Foster, N.R.; Riess, J.W.; Agulnik, M.; Chang, A.Y.C.; et al. Antitumor Activity of Nivolumab in Recurrent and Metastatic Nasopharyngeal Carcinoma: An International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742). J. Clin. Oncol. 2018, 36, 1412–1418. [Google Scholar] [CrossRef]
- Langer, C.J.; Gadgeel, S.M.; Borghaei, H.; A Papadimitrakopoulou, V.; Patnaik, A.; Powell, S.F.; Gentzler, R.; Martins, R.G.; Stevenson, J.P.; I Jalal, S.; et al. KEYNOTE-021 investigators. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: A randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016, 17, 1497–1508. [Google Scholar] [CrossRef]
- Rischin, D.; Harrington, K.; Greil, R.; Soulieres, D.; Tahara, M.; De Castro, G.; Psyrri, A.; Baste, N.; Neupane, P.C.; Bratland, A.; et al. Protocol-specified final analysis of the phase 3 KEYNOTE-048 trial of pembrolizumab (pembro) as first-line therapy for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J. Clin. Oncol. 2019, 37, 6000. [Google Scholar] [CrossRef]
- Cheuk, D.; Billups, C.A.; Martin, M.G.; Roland, C.R.; Ribeiro, R.C.; Krasin, M.J.; Rodriguez-Galindo, C. Prognostic Factors and Long-Term Outcomes of Childhood Nasopharyngeal Carcinoma. Cancer 2011, 117, 197–206. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All Patients (n = 66) | NPC-2003 Study Patients (n = 45) | Interim Patients (n = 21) | p Value f |
|---|---|---|---|---|
| Gender–no. (%) | 0.4112 | |||
| Female | 23 (34.8) | 14 (31.1) | 9 (42.9) | |
| Male | 43 (65.2) | 31 (68.9) | 12 (57.1) | |
| Age at diagnosis–year | 0.3119 | |||
| Median | 15 | 15 | 15 | |
| Range | 8–24 | 8–20 | 11–24 | |
| UICC stage a–no. (%) | <0.001 | |||
| I | 1 (1.5) | 1 (2.2) | 0 | |
| II | 1 (1.5) | 0 | 1 (4.8) | |
| III | 14 (21.2) | 4 (8.9) | 10 (47.6) | |
| IV | 50 (75.8) | 40 (88.9) | 10 (47.6) | |
| Histological type b–no. (%) | 0.2556 | |||
| I (squamous cell) | 0 | 0 | 0 | |
| II (non-keratinizing) | 6 (9.5) | 3 (6.7) | 3 (16.7) | |
| III (undifferentiated) | 57 (90.5) | 42 (93.3) | 15 (83.3) | |
| EBV detection in tumor tissue (antigen or DNA) c–no. (%) | 0.152 | |||
| Positive | 45 (90) | 28 (84.8) | 17 (100) | |
| Negative | 5 (10) | 5 (15.2) | 0 | |
| Response to induction chemotherapy–no. (%) d,e | <0.05 | |||
| CR | 12 (20) | 5 (11.4) | 7 (41.2) | |
| VGPR | 14 (23.3) | 12 (27.3) | 2 (11.8) | |
| PR | 33 (55) | 26 (59) | 8 (47) | |
| SD | 1 (1.7) | 1 (2.3) | 0 | |
| PD | 0 | 0 | 0 | |
| Response after radiochemotherapy–no. (%) d | 0.051 | |||
| CR | 25 (51) | 17 (44.7) | 8 (72.7) | |
| VGPR | 13 (26.5) | 13 (34.2) | 1 (9.1) | |
| PR | 11 (22.5) | 8 (21.1) | 2 (18.2) | |
| SD | 0 | 0 | 0 | |
| PD | 0 | 0 | 0 | |
| Response after end of interferon treatment–no. (%) d | 0.8992 | |||
| CR | 32 (69.6) | 25 (67.6) | 7 (77.8) | |
| VGPR | 12 (26.1) | 10 (27) | 2 (22.2) | |
| PR | 0 | 0 | 0 | |
| SD | 0 | 0 | 0 | |
| PD | 2 (4.3) | 2 (5.4) | 0 | |
| Follow up–mo | <0.05 | |||
| Median | 73 | 85 | 40 | |
| Range | 3–168 | 16–168 | 3–99 | |
| Relapse–no. (%) | 4 (6.1) | 3 (6.7) | 1 (4.8) | 0.1573 |
| No. | Cohort | Sex | Age at Initial Diagnosis–Year | UICC Stage | Histol-ogical Type | Total Radiation Dose to Primary Tumor and Locoregional Lymph Nodes–Gy | Response at End of Primary Treatment | Time of Relapse (After Initial Diagnosis)–Month | Relapse Site | Treatment and Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NPC-2003 | m | 11 | IV | III | 59.4 | PR | 6 (after RT, before IFN treatment) | Distant: bone | Multiple bone metastases; salvage chemotherapy, RT; died of PD 15 months after relapse diagnosis |
| 2 | NPC-2003 | m | 15 | IV | III | 59.4 | PR | 10 (4 months after start of IFN treatment) | Distant: bone, pleura | Salvage chemotherapy, RT; died of PD 10 months after relapse diagnosis |
| 3 | NPC-2003 | m | 19 | IV | III | 59.4 | CR | 21 (9 months after end of IFN treatment) | Distant: bone | Second CR after surgery, salvage chemotherapy and RT; alive in CR at follow-up 72 months |
| 4 | Interim | m | 17 | IV | III | 69.8 | PR | 17 (4 months after end of IFN treatment) | Distant: bone | Multiple relapses, extensive surgery, RT, repeated treatment with pembrolizumab, remission achieved after salvage chemotherapy with gemcitabine and docetaxel; alive in remission at follow-up 75 months |
| No. | Cohort | Sex | Age at Initial Diagnosis–Year | UICC Stage | Histological Type | Documented Radiation Dose–Gy | Follow-Up–Month | Status at Last Follow-Up |
|---|---|---|---|---|---|---|---|---|
| 1 | NPC-2003 | m | 12 | IV | n.s. | 54.0 | 131 | Alive in CR; learning disability, obesitas, hypothyroidism |
| 2 | NPC-2003 | m | 12 | IV | III | 54.0 | 122 | Alive in CR; chronic dental/parodontal disease |
| 3 | NPC-2003 | m | 12 | III | III | 54.0 | 108 | Alive in CR; no further information |
| 4 | NPC-2003 | m | 16 | IV | III | 54.0 | 76 | Alive in CR; hypothyroidism, chronic tinnitus |
| 5 | NPC-2003 | m | 13 | IV | III | 54.0 | 34 | Alive in CR; hypothyroidism, high-frequency hearing deficit |
| 6 | Interim | f | 19 | III | II | 54.0 | 72 | Alive in CR; restriction of mouth opening |
| 7 | Interim | m | 14 | III | II | 54.0 | 45 | Alive in CR; chronic sinusitis |
| 8 a | Interim | m | 11 | III | III | 43.2 | 17 | Alive in CR; hearing deficit, xerostomia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Römer, T.; Franzen, S.; Kravets, H.; Farrag, A.; Makowska, A.; Christiansen, H.; Eble, M.J.; Timmermann, B.; Staatz, G.; Mottaghy, F.M.; et al. Multimodal Treatment of Nasopharyngeal Carcinoma in Children, Adolescents and Young Adults-Extended Follow-Up of the NPC-2003-GPOH Study Cohort and Patients of the Interim Cohort. Cancers 2022, 14, 1261. https://doi.org/10.3390/cancers14051261
Römer T, Franzen S, Kravets H, Farrag A, Makowska A, Christiansen H, Eble MJ, Timmermann B, Staatz G, Mottaghy FM, et al. Multimodal Treatment of Nasopharyngeal Carcinoma in Children, Adolescents and Young Adults-Extended Follow-Up of the NPC-2003-GPOH Study Cohort and Patients of the Interim Cohort. Cancers. 2022; 14(5):1261. https://doi.org/10.3390/cancers14051261
Chicago/Turabian StyleRömer, Tristan, Sabrina Franzen, Hanna Kravets, Ahmed Farrag, Anna Makowska, Hans Christiansen, Michael J. Eble, Beate Timmermann, Gundula Staatz, Felix M. Mottaghy, and et al. 2022. "Multimodal Treatment of Nasopharyngeal Carcinoma in Children, Adolescents and Young Adults-Extended Follow-Up of the NPC-2003-GPOH Study Cohort and Patients of the Interim Cohort" Cancers 14, no. 5: 1261. https://doi.org/10.3390/cancers14051261
APA StyleRömer, T., Franzen, S., Kravets, H., Farrag, A., Makowska, A., Christiansen, H., Eble, M. J., Timmermann, B., Staatz, G., Mottaghy, F. M., Bührlen, M., Hagenah, U., Puzik, A., Driever, P. H., Greiner, J., Jorch, N., Tippelt, S., Schneider, D. T., Kropshofer, G., ... Kontny, U. (2022). Multimodal Treatment of Nasopharyngeal Carcinoma in Children, Adolescents and Young Adults-Extended Follow-Up of the NPC-2003-GPOH Study Cohort and Patients of the Interim Cohort. Cancers, 14(5), 1261. https://doi.org/10.3390/cancers14051261







