Line-Field Confocal Optical Coherence Tomography: A New Tool for the Differentiation between Nevi and Melanomas?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. LC-OCT
2.2. RCM and OCT
2.3. Patients
2.4. Measurements
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. LC-OCT and RCM Image Quality and Confidence Level
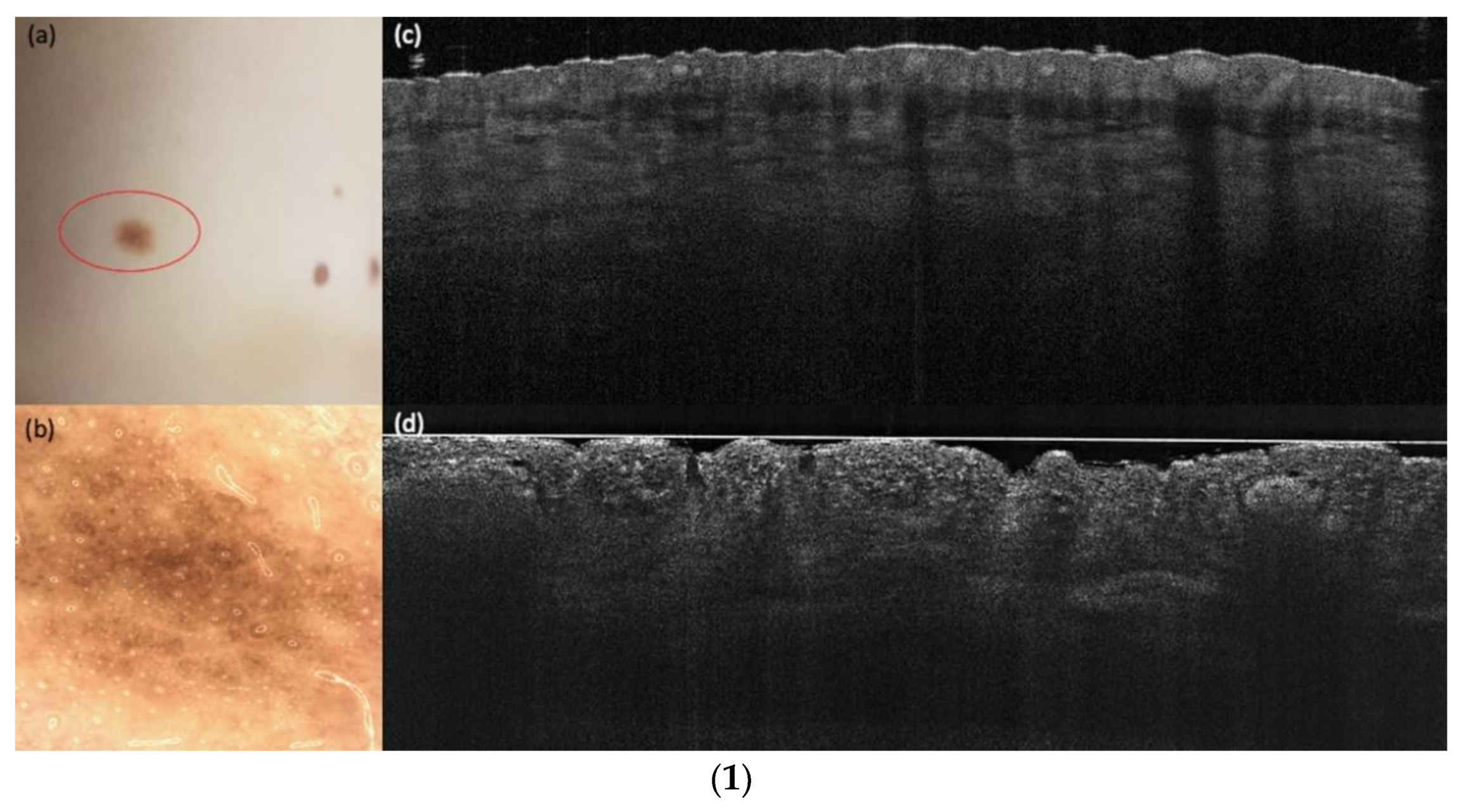
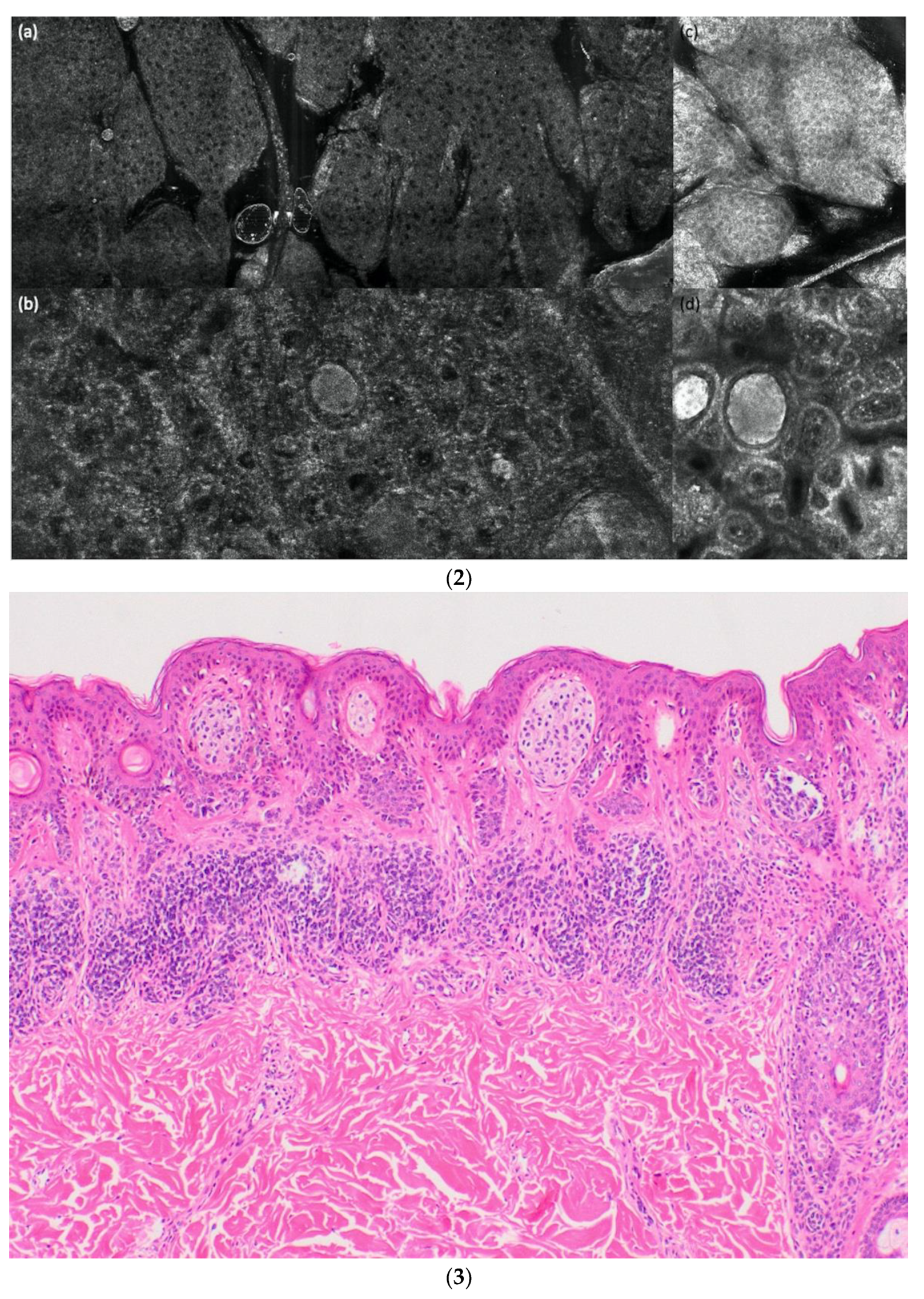
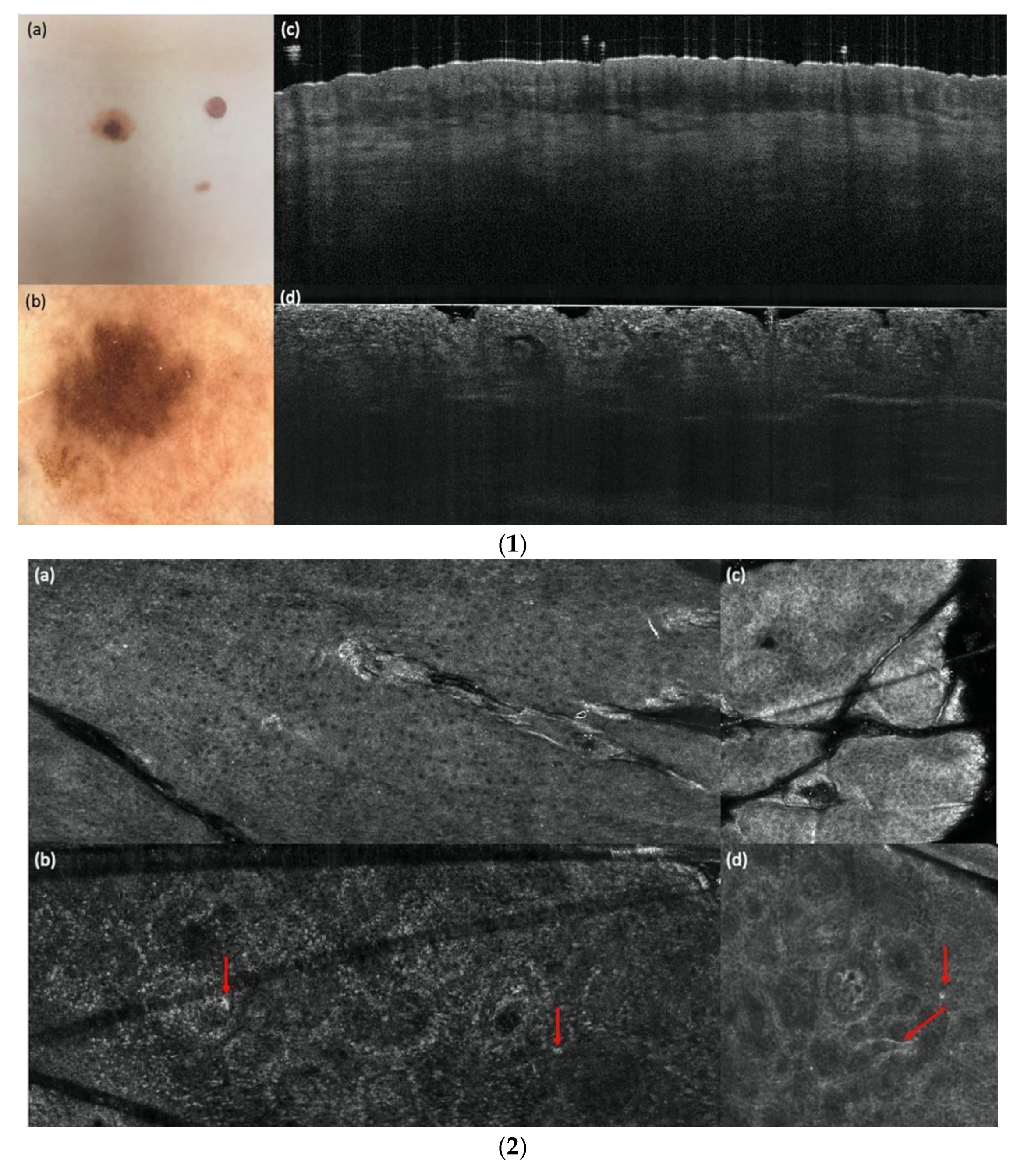
3.3. LC-OCT and RCM Performance for Diagnosing a Melanoma vs. a Nevus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welzel, J.; Schuh, S. Noninvasive diagnosis in dermatology. J. Dtsch. Dermtol. Ges. 2017, 15, 999–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, J.; von Braunmühl, T.; Berking, C.; Sattler, E.C.; Ulrich, M.; Reinhold, U.; Kurzen, H.; Dirschka, T.; Kellner, C.; Schuh, S.; et al. Optical coherence tomography of basal cell carcinoma: Influence of location, subtype, observer variability and image quality on diagnostic performance. Br. J. Dermatol. 2018, 178, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- di Ruffano, L.F.; Dinnes, J.; Deeks, J.J.; Chuchu, N.; Bayliss, S.E.; Davenport, C.; Takwoingi, Y.; Godfrey, K.; O’Sullivan, C.; Matin, R.N.; et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst. Rev. 2018, 12, CD013189. [Google Scholar] [CrossRef]
- Dinnes, J.; Deeks, J.J.; Saleh, D.; Chuchu, N.; Bayliss, S.E.; Patel, L.; Davenport, C.; Takwoingi, Y.; Godfrey, K.; Matin, R.N.; et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst. Rev. 2018, 12, CD013190. [Google Scholar] [CrossRef]
- Que, S.K.T.; Grant-Kels, J.M.; Longo, C.; Pellacani, G. Basics of confocal microscopy and the complexity of diagnosing skin tumors: New imaging tools in clinical practice, diagnostic workflows, cost-estimate, and new trends. Dermatol. Clin. 2016, 34, 367–375. [Google Scholar] [CrossRef]
- Ogien, J.; Levecq, O.; Azimani, H.; Dubois, A. Dual-mode line-field confocal optical coherence tomography for ultrahigh-resolution vertical and horizontal section imaging of human skin in vivo. Biomed. Opt. Express 2020, 11, 1327–1335. [Google Scholar] [CrossRef]
- Davis, A.; Levecq, O.; Azimani, H.; Siret, D.; Dubois, A. Simultaneous dual-band line-field confocal optical coherence tomography: Application to skin imaging. Biomed. Opt. Express 2019, 10, 694–706. [Google Scholar] [CrossRef]
- Dubois, A.; Levecq, O.; Azimani, H.; Siret, D.; Barut, A.; Suppa, M.; Del Marmol, V.; Malvehy, J.; Cinotti, E.; Rubegni, P.; et al. Line-field confocal optical coherence tomography for high-resolution noninvasive imaging of skin tumors. J. Biomed. Opt. 2018, 23, 106007. [Google Scholar] [CrossRef] [Green Version]
- Dubois, A.; Levecq, O.; Azimani, H.; Davis, A.; Ogien, J.; Siret, D.; Barut, A. Line-field confocal time-domain optical coherence tomography with dynamic focusing. Opt. Express 2018, 26, 33534–33542. [Google Scholar] [CrossRef]
- Ogien, J.; Daures, A.; Cazalas, M.; Perrot, J.L.; Dubois, A. Line-field confocal optical coherence tomography for three-dimensional skin imaging. Front. Optoelectron. 2020, 13, 381–392. [Google Scholar] [CrossRef]
- Pedrazzani, M.; Breugnot, J.; Rouaud-Tinguely, P.; Cazalas, M.; Davis, A.; Bordes, S.; Dubois, A.; Closs, B. Comparison of line-field confocal optical coherence tomography images with histological sections: Validation of a new method for in vivo and non-invasive quantification of superficial dermis thickness. Skin Res. Technol. 2020, 26, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Rajadhyaksha, M.; Grossman, M.; Esterowitz, D.; Webb, R.H.; Anderson, R.R. In vivo confocal scanning laser microscopy of human skin: Melanin provides strong contrast. J. Invest Dermatol. 1995, 104, 946–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambichler, T.; Plura, I.; Schmid-Wendtner, M.; Valavanis, K.; Kulichova, D.; Stücker, M.; Pljakic, A.; Berking, C.; Maier, T. High-definition optical coherence tomography of melanocytic skin lesions. J. Biophotonics 2015, 8, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Schmid-Wendtner, M.H.; Plura, I.; Kampilafkos, P.; Stücker, M.; Berking, C.; Maier, T. A multicentre pilot study investigating high-definition optical coherence tomography in the differentiation of cutaneous melanoma and melanocytic naevi. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 537–541. [Google Scholar] [CrossRef]
- Monnier, J.; Tognetti, L.; Miyamoto, M.; Suppa, M.; Cinotti, E.; Fontaine, M.; Perez, J.; Cano, C.O.; Yélamos, O.; Puig, S.; et al. In vivo characterization of healthy human skin with a novel, non-invasive imaging technique: Line-field confocal optical coherence tomography. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2914–2921. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Sattler, E.; Welzel, J. Line-field confocal optical coherence tomography—Practical applications in dermatology and comparison with established imaging methods. Skin Res. Technol. 2021, 27, 340–352. [Google Scholar] [CrossRef]
- Schuh, S.; Ruini, C.; Sattler, E.; Welzel, J. Confocal line-field OCT. Hautarzt 2021, 72, 1039–1047. [Google Scholar] [CrossRef]
- Gust, C.; Schuh, S.; Welzel, J.; Daxenberger, F.; Hartmann, D.; French, L.E.; Ruini, C.; Sattler, E. Line-field confocal optical coherence tomography increases the diagnostic accuracy and confidence for basal cell carcinoma in equivocal lesions: A prospective study. Cancers 2022, 14, 1082. [Google Scholar] [CrossRef]
- Suppa, M.; Fontaine, M.; Dejonckheere, G.; Cinotti, E.; Yélamos, O.; Diet, G.; Tognetti, L.; Miyamoto, M.; Cano, C.O.; Perez-Anker, J.; et al. Line-field confocal optical coherence tomography of basal cell carcinoma: A descriptive study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1099–1110. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Gust, C.; Kendziora, B.; Frommherz, L.; French, L.E.; Hartmann, D.; Welzel, J.; Sattler, E.C. Line-field optical coherence tomography: In vivo diagnosis of basal cell carcinoma subtypes compared to histopathology. Clin. Exp. Dermatol. 2021, 46, 1471–1481. [Google Scholar] [CrossRef]
- Lenoir, C.; Diet, G.; Cinotti, E.; Tognetti, L.; Cano, C.O.; Rocq, L.; Trepant, A.L.; Monnier, J.; Perez-Anker, J.; Rubegni, P.; et al. Line-field confocal optical coherence tomography of sebaceous hyperplasia: A case series. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e509–e511. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Gust, C.; Hartmann, D.; French, L.E.; Sattler, E.C.; Welzel, J. In-vivo LC-OCT evaluation of the downward proliferation pattern of keratinocytes in actinic keratosis in comparison with histology: First impressions from a pilot study. Cancers 2021, 13, 2856. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; Tognetti, L.; Cartocci, A.; Lamberti, A.; Gherbassi, S.; Cano, C.O.; Lenoir, C.; Dejonckheere, G.; Diet, G.; Fontaine, M.; et al. Line-field confocal optical coherence tomography for actinic keratosis and squamous cell carcinoma: A descriptive study. Clin. Exp. Dermatol. 2021, 46, 1530–1541. [Google Scholar] [CrossRef] [PubMed]
- Ruini, C.; Schuh, S.; Gust, C.; Kendziora, B.; Frommherz, L.; French, L.E.; Hartmann, D.; Welzel, J.; Sattler, E.C. Line-field confocal optical coherence tomography for the in-vivo real-time diagnosis of different stages of keratinocyte skin cancer: A preliminary study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2388–2397. [Google Scholar] [CrossRef] [PubMed]
- Carrera, C.; Marghoob, A.A. Discriminating nevi from melanomas: Clues and pitfalls. Dermatol. Clin. 2016, 34, 395–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, D.; Ruini, C.; Mathemeier, L.; Bachmann, M.R.; Dietrich, A.; Ruzicka, T.; von Braunmühl, T. Identification of ex-vivo confocal laser scanning microscopic features of melanocytic lesions and their histological correlates. J. Biophotonics 2017, 10, 128–142. [Google Scholar] [CrossRef]
- Hartmann, D.; Krammer, S.; Ruini, C.; Ruzicka, T.; von Braunmühl, T. Correlation of histological and ex-vivo confocal tumor thickness in malignant melanoma. Lasers Med. Sci. 2016, 31, 921–927. [Google Scholar] [CrossRef]
- Lenoir, C.; Perez-Anker, J.; Diet, G.; Tognetti, L.; Cinotti, E.; Trepant, A.L.; Rubegni, P.; Puig, S.; Perrot, J.L.; Malvehy, J.; et al. Line-field confocal optical coherence tomography of benign dermal melanocytic proliferations: A case series. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e399–e401. [Google Scholar] [CrossRef]
- Fraga-Braghiroli, N.; Grant-Kels, J.M.; Oliviero, M.; Rabinovitz, H.; Ferenci, K.; Scope, A. The role of reflectance confocal microscopy in differentiating melanoma in situ from dysplastic nevi with severe atypia: A cross-sectional study. J. Am. Acad. Dermatol. 2020, 83, 1035–1043. [Google Scholar] [CrossRef]
- Carrera, C.; Puig, S.; Malvehy, J. In vivo confocal reflectance microscopy in melanoma. Dermatol. Ther. 2012, 25, 410–422. [Google Scholar] [CrossRef]
- Pellacani, G.; Cesinaro, A.M.; Seidenari, S. Reflectance-mode confocal microscopy of pigmented skin lesions—Improvement in melanoma diagnostic specificity. J. Am. Acad. Dermatol. 2005, 53, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Kardynal, A.; Olszewska, M.; de Carvalho, N.; Walecka, I.; Pellacani, G.; Rudnicka, L. Reflectance confocal microscopy features of thin versus thick melanomas. G. Ital. Dermatol. Venereol. 2019, 154, 379–385. [Google Scholar] [CrossRef] [PubMed]
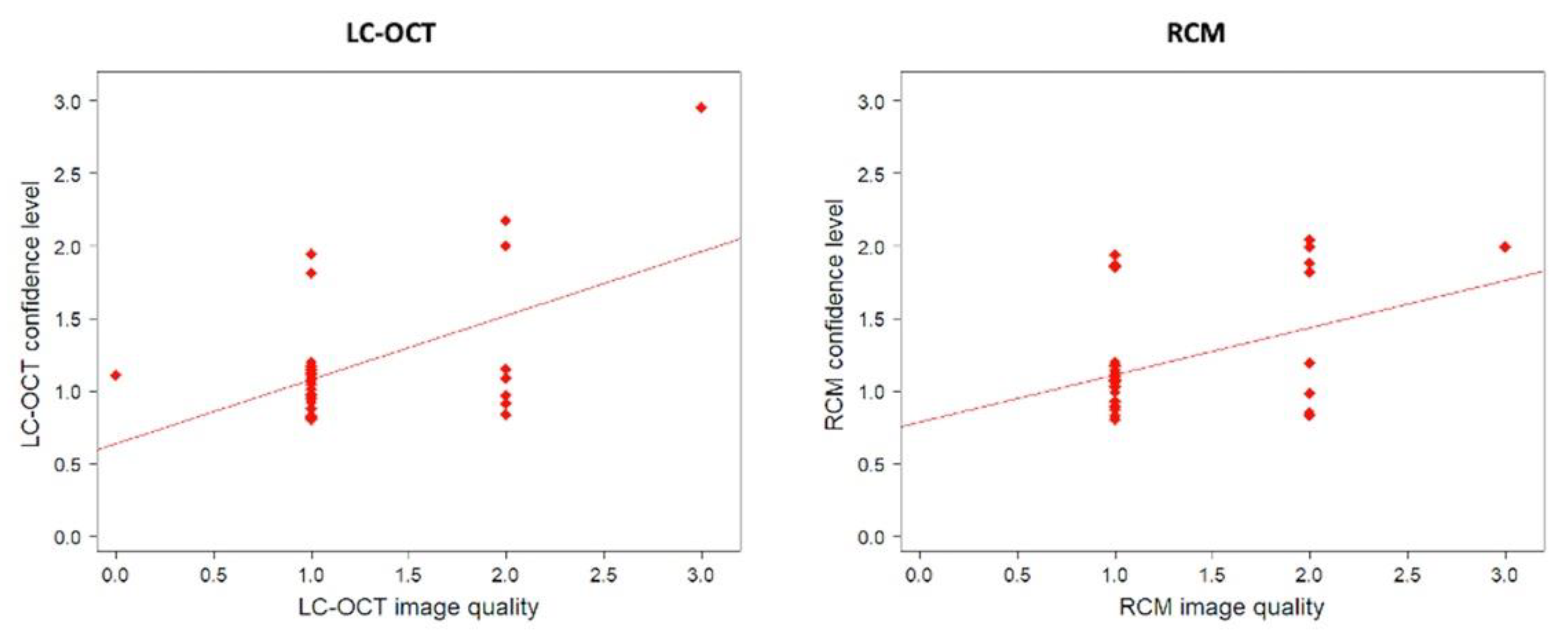

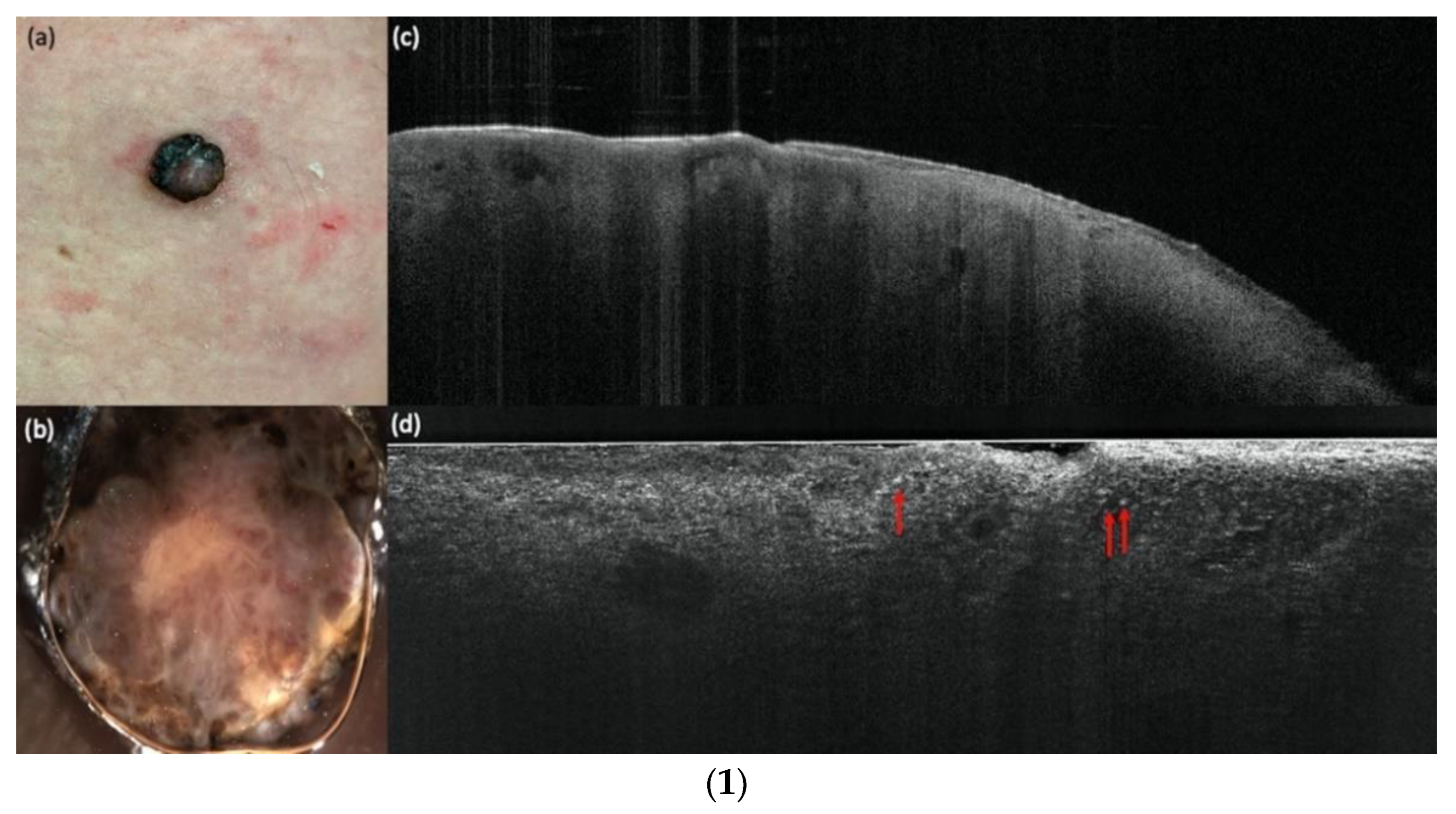
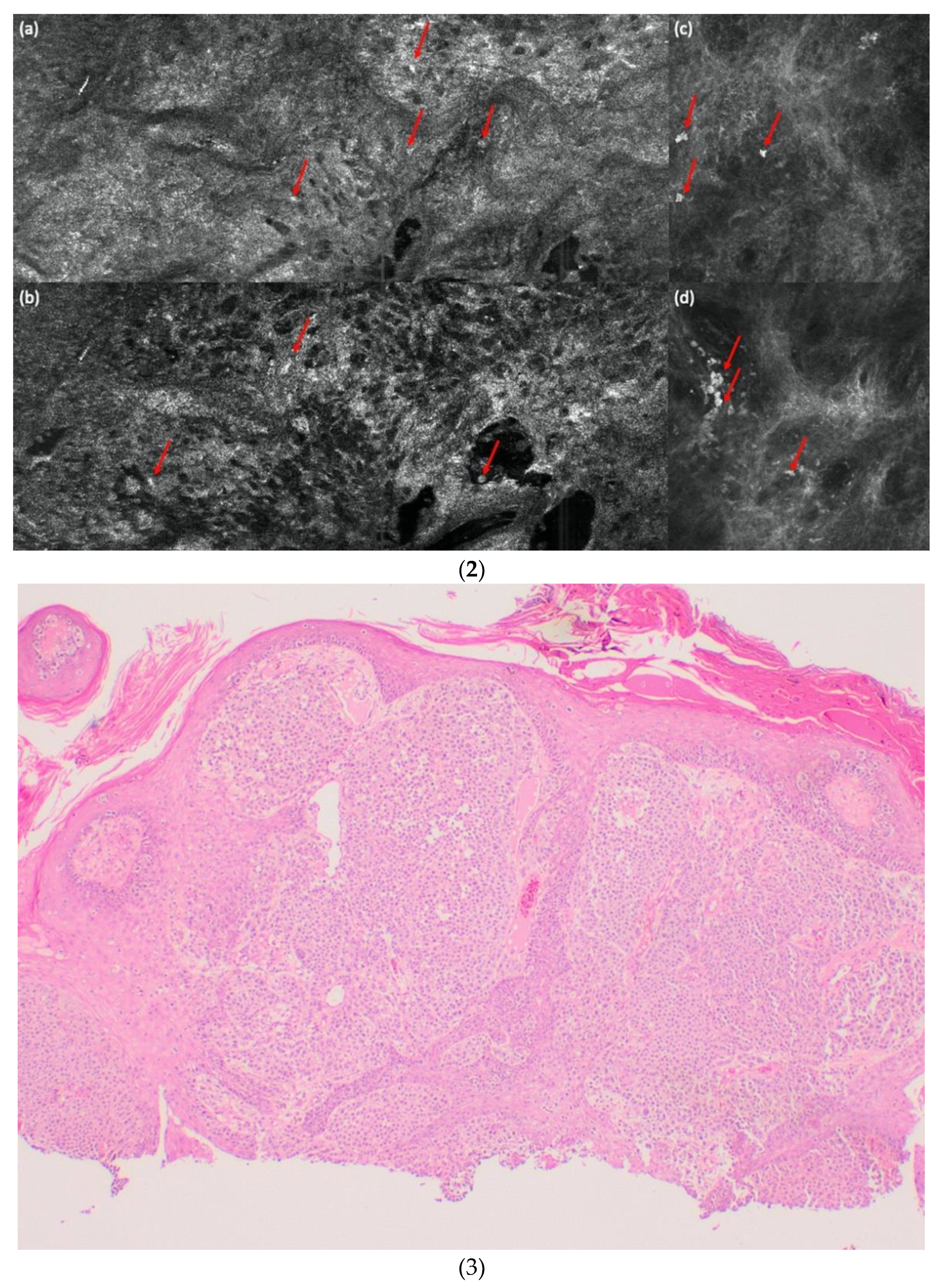
| LC-OCT | ||||
|---|---|---|---|---|
| For all lesions (n = 84) | Quality = 0 (n = 4) | Quality = 1 (n = 58) | Quality = 2 (n = 18) | Quality = 3 (n = 4) |
| Average confidence level | 0.8 | 1.2 | 1.4 | 2.3 |
| For lesions also imaged with RCM (n = 36) | Quality = 0 (n = 1) | Quality = 1 (n = 27) | Quality = 2 (n = 7) | Quality = 3 (n = 1) |
| Average confidence level | 1 | 1.1 | 1.3 | 3 |
| RCM | ||||
| n = 36 | Quality = 0 (n = 0) | Quality = 1 (n = 26) | Quality = 2 (n = 9) | Quality = 3 (n = 1) |
| Average confidence level | - | 1.1 | 1.4 | 2 |
| LC-OCT | RCM | |
|---|---|---|
| Image quality | n (%) | n (%) |
| Score 0 | 1 (2.8) | 0 (0) |
| Score 1 | 27 (75.0) | 26 (72.2) |
| Score 2 | 7 (19.4) | 9 (25.0) |
| Score 3 | 1 (2.8) | 1 (2.8) |
| Confidence level | n (%) | n (%) |
| Score 0 | 0 (0) | 0 (0) |
| Score 1 | 31 (86.1) | 28 (77.8) |
| Score 2 | 4 (11.1) | 8 (22.2) |
| Score 3 | 1 (2.8) | 0 (0) |
| All Lesions | Histology | ||||
|---|---|---|---|---|---|
| Melanoma | Nevus | Other | Total | ||
| LC-OCT | Melanoma | 26 | 0 | 0 | 26 |
| Nevus | 2 | 55 | 0 | 57 | |
| Other | 0 | 0 | 1 | 1 | |
| Total | 28 | 55 | 1 | 84 | |
| All Lesions | Histology | |||||
|---|---|---|---|---|---|---|
| Nevus | Melanoma | Dysplastic Nevus | Others | Total | ||
| LC-OCT | Nevus | 40 | 1 | 8 | 0 | 49 |
| Melanoma | 0 | 26 | 0 | 0 | 26 | |
| Dysplastic nevus | 2 | 1 | 5 | 0 | 8 | |
| Others | 0 | 0 | 0 | 1 | 1 | |
| Total | 42 | 28 | 13 | 1 | 84 | |
| n | Global Accuracy | Nevus (n = 42) | Dysplastic Nevus (n = 13) | Melanoma (n = 28) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Accuracy | Sensitivity | Specificity | Accuracy | Sensitivity | Specificity | Accuracy | Sensitivity | Specificity | |||
| LC-OCT | 83 | 86% | 87% | 95% | 78% | 87% | 38% | 96% | 98% | 93% | 100% |
| n = 36 | Histology | ||||
|---|---|---|---|---|---|
| Melanoma | Nevus | Other | Total | ||
| LC-OCT | Melanoma | 13 | 0 | 0 | 13 |
| Nevus | 1 | 21 | 0 | 22 | |
| Other | 0 | 0 | 1 | 1 | |
| Total | 14 | 21 | 1 | 36 | |
| RCM | Melanoma | 13 | 1 | 1 | 15 |
| Nevus | 1 | 20 | 0 | 21 | |
| Other | 0 | 0 | 0 | 0 | |
| Total | 14 | 21 | 1 | 36 | |
| n = 36 | Histology | |||||
|---|---|---|---|---|---|---|
| Nevus | Melanoma | Dysplastic Nevus | Others | Total | ||
| LC-OCT | Nevus | 14 | 1 | 4 | 0 | 19 |
| Melanoma | 0 | 13 | 0 | 0 | 13 | |
| Dysplastic nevus | 0 | 0 | 3 | 0 | 3 | |
| Others | 0 | 0 | 0 | 1 | 1 | |
| Total | 14 | 14 | 7 | 1 | 36 | |
| RCM | Nevus | 13 | 0 | 3 | 0 | 16 |
| Melanoma | 0 | 13 | 1 | 1 | 15 | |
| Dysplastic nevus | 1 | 1 | 3 | 0 | 5 | |
| Others | 0 | 0 | 0 | 0 | 0 | |
| Total | 14 | 14 | 7 | 1 | 36 | |
| n | Global Accuracy | Nevus (n = 14) | Dysplastic Nevus (n = 7) | Melanoma (n = 14) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Accuracy | Sensitivity | Specificity | Accuracy | Sensitivity | Specificity | Accuracy | Sensitivity | Specificity | |||
| LC-OCT | 35 | 86% | 86% | 100% | 76% | 89% | 43% | 100% | 97% | 93% | 100% |
| RCM | 35 | 83% | 89% | 93% | 86% | 83% | 43% | 93% | 94% | 93% | 95% |
| LC-OCT Parameters | OR (Univariate) | p Value | OR (Multivariate) | p Value |
|---|---|---|---|---|
| Horizontal parameters | ||||
| Irregular honeycombed pattern | 43.64 (10.80–299.67) | <0.001 | 18.03 (1.50–547.32) | 0.039 |
| Pagetoid spread with atypical melanocytes in basal/suprabasal layers | 41.21 (11.28–206.45) | <0.001 | 16.56 (1.43–435.95) | 0.037 |
| Edged papillae | 0.22 (0.08–0.59) | 0.003 | 0.43 (0.02–9.05) | 0.57 |
| Basal nests | 0.23 (0.07–0.63) | 0.007 | 0.51 (0.02–12.06) | 0.67 |
| Nests in the upper dermis | 0.21 (0.06–0.61) | 0.007 | 0.23 (0.01–3.80) | 0.35 |
| Irregular bright cells/sheets of cells in the upper dermis | 5.75 (1.77–20.94) | 0.005 | 0.51 (0.02–10.73) | 0.67 |
| Vertical parameters | ||||
| Pagetoid spread of bright cells in suprabasal/basal layers | 20.36 (6.44–74.84) | <0.001 | 4.74 (0.35–94.88) | 0.25 |
| Junctional nests | 0.31 (0.11–0.83) | 0.02 | 1.85 (0.06–103.11) | 0.73 |
| Disturbed DEJ | 10.83 (3.73–35.72) | <0.001 | 4.85 (0.26-144.46) | 0.31 |
| Dermal nests | 0.21 (0.07–0.58) | 0.004 | 0.08 (0.00-1.29) | 0.12 |
| Sheets of atypical bright cells | 26.47 (4.50-506.78) | 0.003 | 0.69 (0.02-29.63) | 0.83 |
| RCM parameters | OR (univariate) | p value | OR (multivariate) | p value |
| Irregular honeycombed pattern | 69.67 (9.13–1553.6) | <0.001 | 123.91 (2.84–19309) | 0.030 |
| Pagetoid spread with atypical melanocytes in suprabasal layers | 34.00 (5.91–315.56) | <0.001 | 0.76 (0.01–23.85) | 0.88 |
| Edged papillae | 0.08 (0.01–0.44) | 0.007 | 0.05 (0.00–0.76) | 0.053 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuh, S.; Ruini, C.; Perwein, M.K.E.; Daxenberger, F.; Gust, C.; Sattler, E.C.; Welzel, J. Line-Field Confocal Optical Coherence Tomography: A New Tool for the Differentiation between Nevi and Melanomas? Cancers 2022, 14, 1140. https://doi.org/10.3390/cancers14051140
Schuh S, Ruini C, Perwein MKE, Daxenberger F, Gust C, Sattler EC, Welzel J. Line-Field Confocal Optical Coherence Tomography: A New Tool for the Differentiation between Nevi and Melanomas? Cancers. 2022; 14(5):1140. https://doi.org/10.3390/cancers14051140
Chicago/Turabian StyleSchuh, Sandra, Cristel Ruini, Maria Katharina Elisabeth Perwein, Fabia Daxenberger, Charlotte Gust, Elke Christina Sattler, and Julia Welzel. 2022. "Line-Field Confocal Optical Coherence Tomography: A New Tool for the Differentiation between Nevi and Melanomas?" Cancers 14, no. 5: 1140. https://doi.org/10.3390/cancers14051140
APA StyleSchuh, S., Ruini, C., Perwein, M. K. E., Daxenberger, F., Gust, C., Sattler, E. C., & Welzel, J. (2022). Line-Field Confocal Optical Coherence Tomography: A New Tool for the Differentiation between Nevi and Melanomas? Cancers, 14(5), 1140. https://doi.org/10.3390/cancers14051140






