Cardiac Myxomas Resembling Malignant Neoplasia: Incidentally Diagnosed vs. Cerebral Embolized Myxomas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Diagnostics
2.3. Surgical Techniques
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reynen, K. Frequency of primary tumors of the heart. Am. J. Cardiol. 1996, 77, 107. [Google Scholar] [CrossRef]
- Pinede, L.; Duhaut, P.; Loire, R. Clinical Presentation of Left Atrial Cardiac Myxoma. Medicine 2001, 80, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Schiele, S.; Maurer, S.J.; Pujol Salvador, C.; Vitanova, K.; Weirich, G.; Meierhofer, C.; Voss, B.; Ewert, P.; Tutarel, O. Left Atrial Myxoma: When Big Is Too Big. Circ. Cardiovasc. Imaging 2019, 12, e008820. [Google Scholar] [CrossRef] [PubMed]
- Roeltgen, D.; Kidwell, C.S. Neurologic complications of cardiac tumors. Handb. Clin. Neurol. 2014, 119, 209–222. [Google Scholar] [PubMed]
- Cetin, G.; Gursoy, M.; Ugurlucan, M.; Uzunhasan, I.; Hatemi, A.C.; Tireli, E.; Kucukoglu, S.; Kansiz, E. Single-institutional 22 years experience on cardiac myxomas. Angiology 2010, 6, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Grothusen, C.; Schöttler, J.; Cremer, J.; Frey, N.; Langer, C. Very large left atrial myxoma: An unusual differential diagnosis of bronchial hyper-reactivity. Cardiovasc. Diagn. Ther. 2014, 4, 50–51. [Google Scholar] [PubMed]
- MacGowan, S.W.; Sidhy, P.; Aherne, T.; Luke, D.; Wood, A.E.; Neligan, M.C.; McGovern, E. Atrial myxoma: National incidence, diagnosis and surgical management. Ir. J. Med. Sci. 1993, 162, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Tasoglu, I.; Tutun, U.; Lafci, G.; Hijaazi, A.; Yener, U.; Yalcinkaya, A.; Ulus, T.; Aksoyek, A.; Saritas, A.; Birincioglu, L.; et al. Primary Cardiac Myxomas: Clinical Experience and Surgical Results in 67 Patients. J. Card. Surg. 2009, 24, 256–259. [Google Scholar] [CrossRef] [PubMed]
- García-Quintana, A.; Martín-Lorenzo, P.; Suárez de Lezo, J.; Díaz-Escofet, M.; Llorens, R.; Medina, A. Mixoma auricular izquierdo infectado [Infected left atrial myxoma]. Rev. Esp. Cardiol. 2005, 58, 1358–1360. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Stefanou, M.I.; Rath, D.; Stadler, V.; Richter, H.; Hennersdorf, F.; Lausberg, H.F.; Lescan, M.; Greulich, S.; Poli, S.; Gawaz, M.P.; et al. Cardiac Myxoma and Cerebrovscular Events: A Retrospective Cohort Study. Front. Neurol. 2018, 9, 823. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, J.H.; Na, C.Y.; Oh, S.S. Eleven Year’s Experience with Korean Cardiac Myxoma Patients: Focus on Embolic Complications. Cerebrovasc. Dis. 2012, 33, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Keeling, I.M.; Oberwalder, P.; Anelli-Monti, M.; Schuchlenz, H.; Demel, U.; Tilz, G.P.; Rehak, P.; Rigler, B. Cardiac myxomas: 24 years of experience in 49 patients. Eur. J. Cardiothorac. Surg. 2002, 22, 971–977. [Google Scholar] [CrossRef] [Green Version]
- Swarzt, M.F.; Lutz, C.J.; Chandan, V.S.; Landas, S.; Fink, G.W. Atrial Myxomas: Pathologic Types, Tumor Location, and Presenting Symptoms. J. Cardiac. Surg. 2006, 21, 435–440. [Google Scholar]
- Garatti, A.; Nano, G.; Canziani, A.; Gagliardotto, P.; Mossuto, E.; Frigiola, A.; Menicanti, L. Surgical Excision of Cardiac Myxomas: Twenty Years Experience at a Single Institution. Ann. Thorac. Surg. 2012, 93, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Sanki, P.; Hossain, M.; Charles, A.; Bhattacharya, S.; Sarkar, U. Cardiac myxoma: A surgical experience of 38 patients over 9 years, at SSKM hospital Kolkata, India. South Asian J. Cancer 2013, 2, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L.; Kumar, S.K.; Okuno, S.H.; Schaff, H.V.; Porrata, L.F.; Buckner, J.C.; Moynihan, T.J. Malignant primary cardiac tumors: Review of a single institution experience. Cancer 2008, 112, 2440–2446. [Google Scholar] [CrossRef] [PubMed]

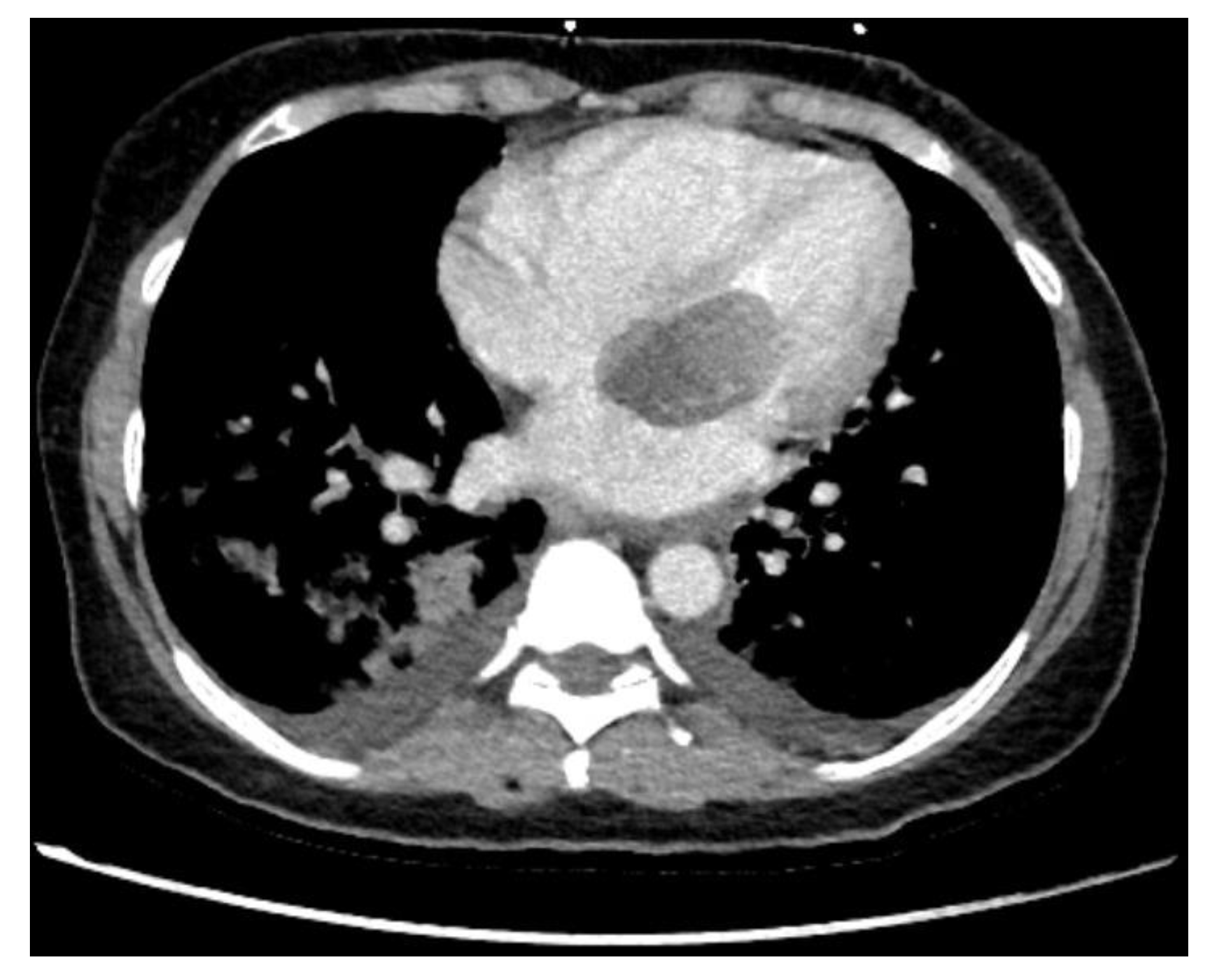
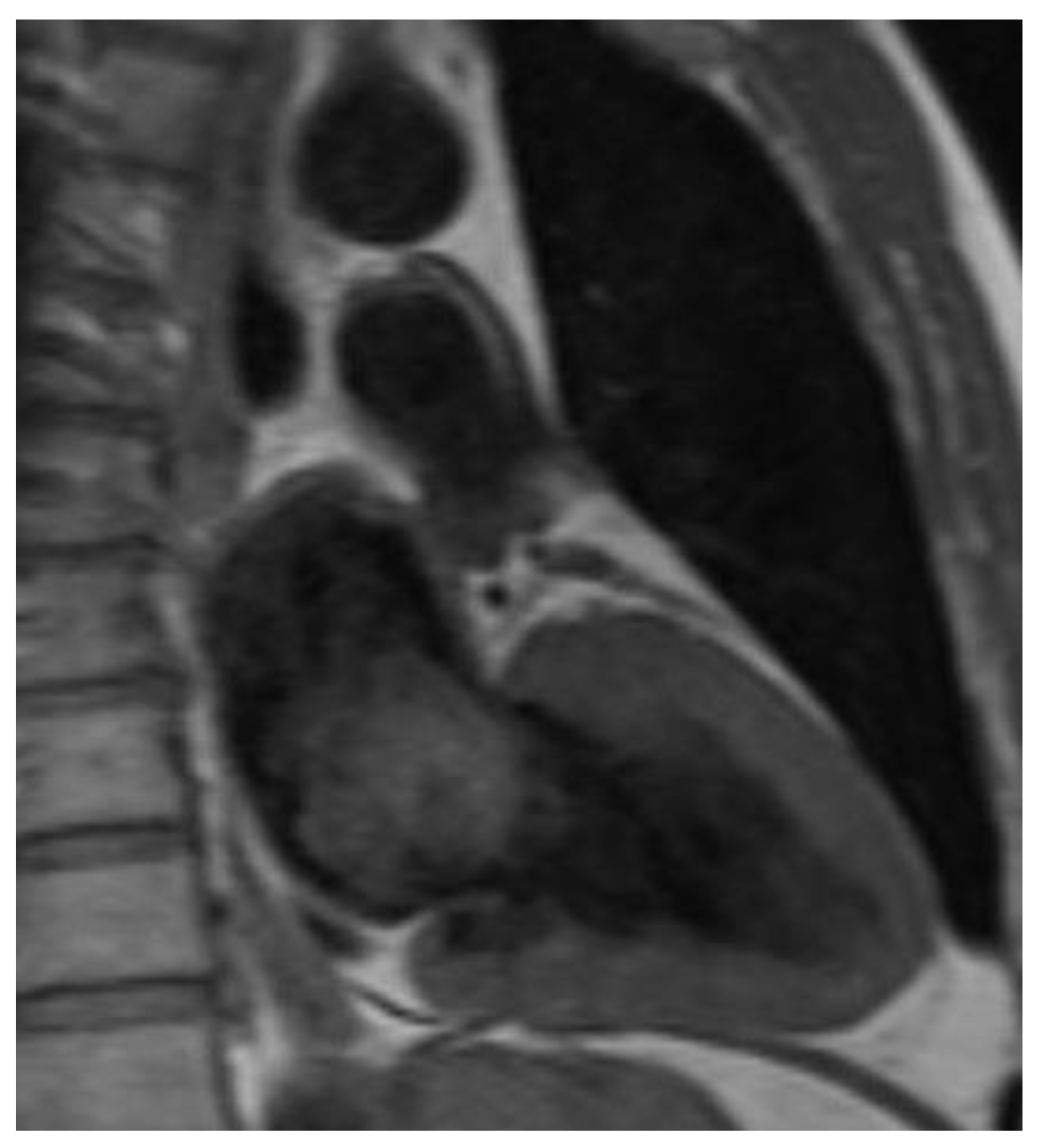

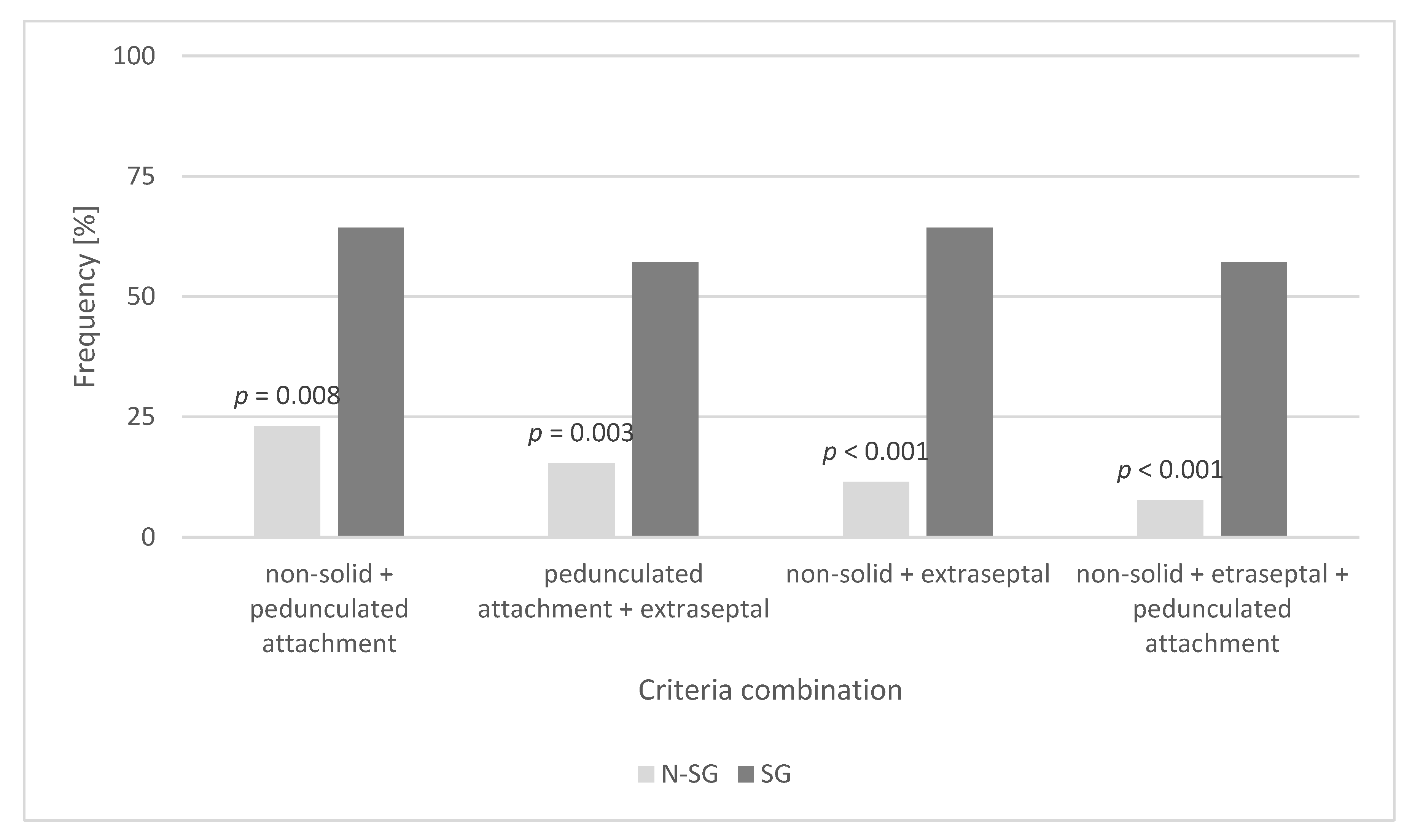
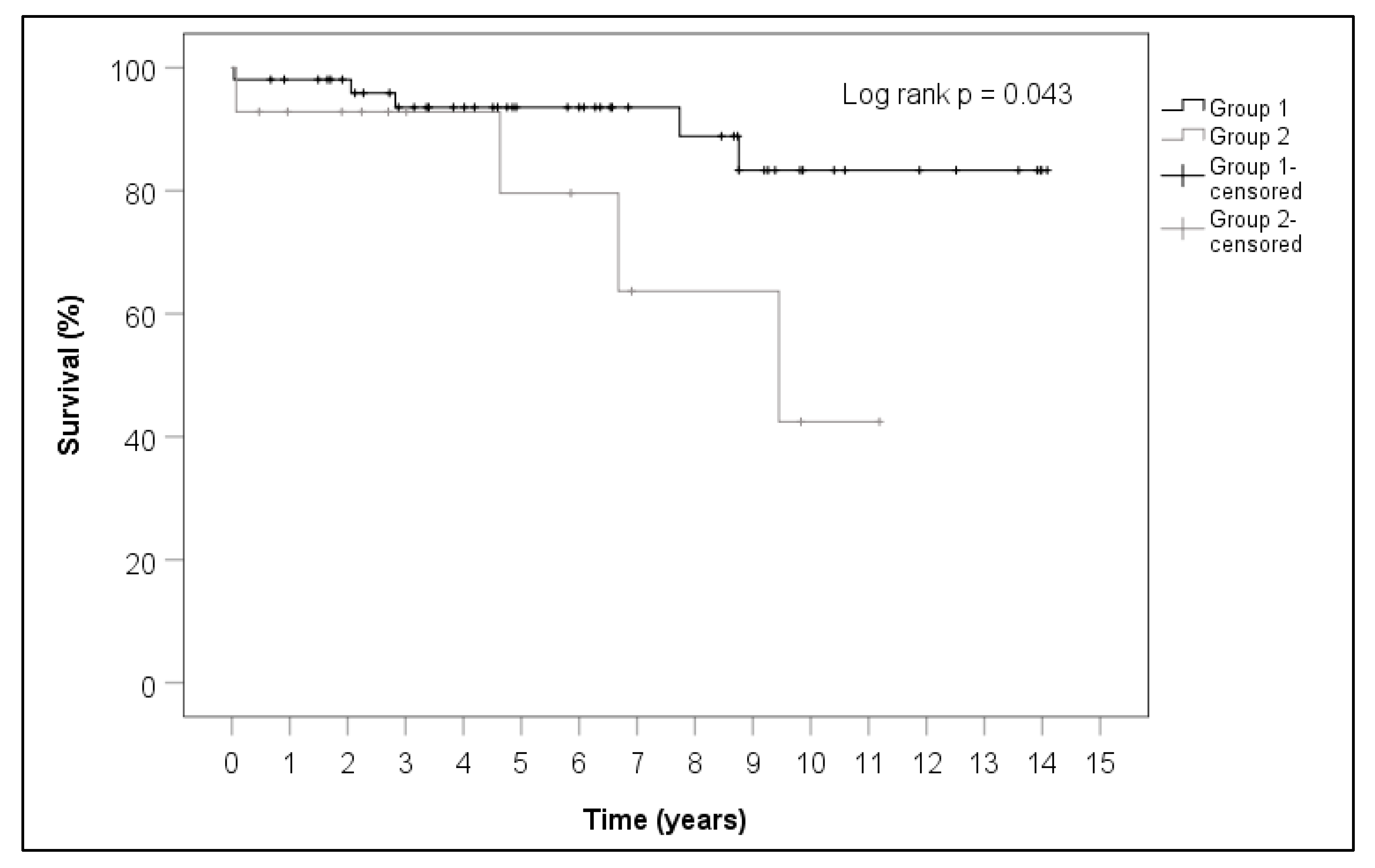
| Preoperative Patient Characteristics | SG | N-SG | p-Value |
|---|---|---|---|
| Mean age (years) | 58.4 ± 12.7 | 62.8 ± 11.7 | 0.226 |
| Female gender (%) | 35.7 | 61.5 | 0.084 |
| Mean body mass index | 27.2 ± 4.4 | 26.1 ± 4.3 | 0.408 |
| Mean left ventricle ejection fraction (%) | 70 (63–70) | 65 (60–70) | 0.113 |
| Preoperative atrial fibrillation (%) | 14.3 | 17.3 | 1.000 |
| Pulmonary hypertension *(%) | 14.3 | 32.7 | 0.318 |
| Arterial hypertension (%) | 50.0 | 53.8 | 0.798 |
| Diabetes mellitus (%) | 14.3 | 11.5 | 0.674 |
| Hyperlipoproteinemia ** | 57.1 | 30.8 | 0.069 |
| History of smoking (%) | 42.9 | 55.8 | 0.390 |
| Chronic obstructive lung disease (%) | 7.1 | 7.7 | 1.000 |
| Peripheral artery disease (%) | 7.1 | 3.8 | 0.517 |
| Oncologic disease (%) | 7.1 | 3.8 | 0.517 |
| Concomitant Cardiac Diseases | SG | N-SG | p-Value |
|---|---|---|---|
| Coronary artery disease (%) | 42.9 | 36.5 | 0.665 |
| Mitral valve insufficiency (%) | 35.7 | 57.7 | 0.144 |
| Persistent foramen ovale (%) | 7.1 | 5.8 | 1.000 |
| Atrial septal defect (%) | 0 | 1.9 | 1.000 |
| Diagnostic Methods | SG | N-SG | p-Value |
|---|---|---|---|
| Transthoracal echocardiography (%) | 57.1 | 73.1 | 0.328 |
| Transesophageal echocardiography (%) | 85.7 | 69.2 | 0.318 |
| Magnetic resonance imaging (%) | 7.1 | 13.5 | 1.000 |
| Computertomography (%) | 7.1 | 15.4 | 0.671 |
| Left heart catheterization (%) | 92.9 | 88.5 | 1.000 |
| Tumor-Associated Symptoms | SG | N-SG | p-Value |
|---|---|---|---|
| Chest pain (%) | 7.1 | 11.5 | 1.000 |
| Palpitation (%) | 0 | 9.6 | 0.576 |
| Dyspnea (%) | 0 | 34.6 | 0.007 |
| Syncope (%) | 7.1 | 9.6 | 1.000 |
| Fatigue (%) | 0 | 9.6 | 0.576 |
| Neurological dysfunction (%) | 100 | 0 | <0.001 |
| Asymptomatic (%) | 0 | 42.3 | 0.003 |
| Intraoperative Data | SG | N-SG | p-Value |
|---|---|---|---|
| Time from diagnosis to surgery (days) | 7 (3;24) | 23 (5;55) | 0.120 |
| Minimal invasive surgery (%) | 28.6 | 42.3 | 0.350 |
| Mean operating time (min) | 192.5 | 215.5 | 0.046 |
| Mean bypass time (min) | 102.5 ± 45.7 | 133.1 ± 54.3 | 0.058 |
| Mean aortic-cross-clamptime (min) | 54.5 | 78.5 | 0.035 |
| Endocardial reconstruction (%) | 0 | 51.9 | <0.001 |
| Concomitant cardiac surgery (%) | 21.4 | 34.6 | 0.520 |
| Details of Extirpated Cardiac Myxomas | SG | N-SG | p-Value |
|---|---|---|---|
| Location in the left ventricle (%) | 4.5 | 3.8 | 0.517 |
| Location in the left atrium (%) | 92.9 | 78.8 | 1.000 |
| Location in the right atrium (%) | 0 | 17.3 | 0.186 |
| Atrial septum attachment (%) | 21.4 | 50.0 | 0.056 |
| Pedunculated tumor base (%) | 51.5 | 46.2 | 0.093 |
| Maximal tumor size (cm) | 3.4 ± 1.5 | 3.8 ± 2.1 | 0.538 |
| Non-solid tumor type (%) | 78.6 | 50.0 | 0.103 |
| Calcification of the tumor | 7.1 | 13.5 | 1.000 |
| Postoperative Clinical Data | SG | N-SG | p-Value |
|---|---|---|---|
| Stay on intensive care unit (d) | 3 | 2 | 0.020 |
| Ventilation time (h) | 10.5 | 10.5 | 0.783 |
| Tracheotomy (%) | 7.1 | 5.8 | 1.000 |
| Rethoracotomy (%) | 0 | 3.8 | 1.000 |
| Postoperative atrial fibrillation (%) | 7.1 | 26.9 | 0.161 |
| Postoperative stroke (%) | 14.3 | 1.9 | 0.111 |
| Postoperative dialysis (%) | 7.1 | 1.9 | 0.382 |
| Hospital stay (d) | 11.0 | 12.5 | 0.776 |
| Thirty-day mortality (%) | 7.1 | 1.9 | 0.382 |
| Follow-Up | SG | N-SG | p-Value |
|---|---|---|---|
| Rate of response (%) | 42.9 | 55.8 | 0.390 |
| Mortality (%) | 28.6 | 9.6 | 0.087 |
| Stroke after hospital discharge (%) | 0 | 0 | 1.000 |
| Recurrence of myxoma (%) | 0 | 3.4 | 1.000 |
| Cardiac redo surgery (%) | 0 | 3.4 | 1.000 |
| NYHA I-II (%) | 50.0 | 27.6 | 0.352 |
| Neurological limitations (%) | 66.7 | 0 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salem, M.; Hillmer, J.; Friedrich, C.; Panholzer, B.; Saad, M.; Salem, M.; Frank, D.; Ernst, M.; Maetzler, W.; Puehler, T.; et al. Cardiac Myxomas Resembling Malignant Neoplasia: Incidentally Diagnosed vs. Cerebral Embolized Myxomas. Cancers 2022, 14, 1111. https://doi.org/10.3390/cancers14051111
Salem M, Hillmer J, Friedrich C, Panholzer B, Saad M, Salem M, Frank D, Ernst M, Maetzler W, Puehler T, et al. Cardiac Myxomas Resembling Malignant Neoplasia: Incidentally Diagnosed vs. Cerebral Embolized Myxomas. Cancers. 2022; 14(5):1111. https://doi.org/10.3390/cancers14051111
Chicago/Turabian StyleSalem, Mohamed, Jonas Hillmer, Christine Friedrich, Bernd Panholzer, Mohammed Saad, Mostafa Salem, Derk Frank, Markus Ernst, Walter Maetzler, Thomas Puehler, and et al. 2022. "Cardiac Myxomas Resembling Malignant Neoplasia: Incidentally Diagnosed vs. Cerebral Embolized Myxomas" Cancers 14, no. 5: 1111. https://doi.org/10.3390/cancers14051111
APA StyleSalem, M., Hillmer, J., Friedrich, C., Panholzer, B., Saad, M., Salem, M., Frank, D., Ernst, M., Maetzler, W., Puehler, T., Lutter, G., Schoeneich, F., Haneya, A., Cremer, J., & Schoettler, J. (2022). Cardiac Myxomas Resembling Malignant Neoplasia: Incidentally Diagnosed vs. Cerebral Embolized Myxomas. Cancers, 14(5), 1111. https://doi.org/10.3390/cancers14051111







