Risk of Second Primary Thyroid Cancer in Women with Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Laboratory Analyses
2.3. Statistical Analysis
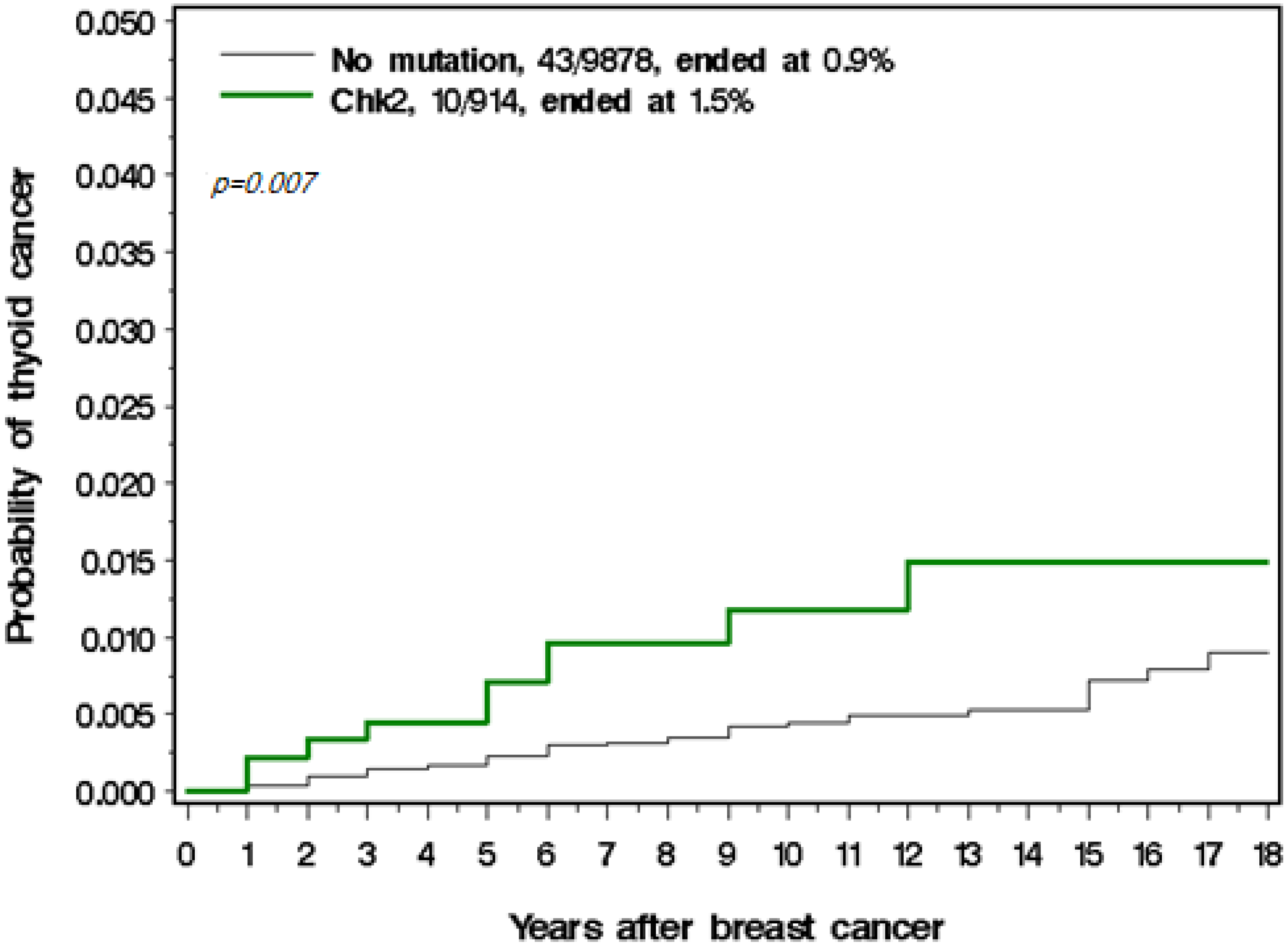
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breast Cancer: Statistics. Available online: https://www.cancer.net/cancer-types/breast-cancer/statistics (accessed on 23 October 2021).
- Joseph, K.R.; Edirimanne, S.; Eslick, G.D. The association between breast cancer and thyroid cancer: A meta-analysis. Breast Cancer Res. Treat. 2015, 152, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.M.; White, M.; Hong, S.; Aschebrook-Kilfoy, B.; Kaplan, E.L.; Angelos, P.; Kulkarni, S.A.; Olopade, O.I.; Grogan, R.H. The Breast–Thyroid Cancer Link: A Systematic Review and Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2016, 25, 231–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.I.; Rossing, M.A.; Voigt, L.F.; Daling, J.R. Multiple primary breast and thyroid cancers: Role of age at diagnosis and cancer treatments (United States). Cancer Causes Control 2000, 11, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Zafon, C.; Obiols, G.; Mesa, J. Second primary cancer in patients with papillary thyroid carcinoma. Anticancer Res. 2013, 33, 337–340. [Google Scholar] [PubMed]
- Grantzau, T.; Overgaard, J. Risk of second non-breast cancer after radiotherapy for breast cancer: A systematic review and meta-analysis of 762,468 patients. Radiother. Oncol. 2015, 114, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Walker, R.; Groome, P.G.; Shelley, W.; MacKillop, W.J. Risk of thyroid carcinoma in a female population after radiotherapy for breast carcinoma. Cancer 2001, 92, 1411–1418. [Google Scholar] [CrossRef]
- Sun, L.-M.; Lin, C.-L.; Liang, J.-A.; Huang, W.-S.; Kao, C.-H. Radiotherapy did not increase thyroid cancer risk among women with breast cancer: A nationwide population-based cohort study. Int. J. Cancer 2015, 137, 2896–2903. [Google Scholar] [CrossRef] [Green Version]
- Yehia, L.; Keel, E.; Eng, C. The Clinical Spectrum of PTEN Mutations. Annu. Rev. Med. 2020, 71, 103–116. [Google Scholar] [CrossRef] [Green Version]
- Cybulski, C.; Górski, B.; Huzarski, T.; Masojć, B.; Mierzejewski, M.; Dębniak, T.; Teodorczyk, U.; Byrski, T.; Gronwald, J.; Matyjasik, J.; et al. CHEK2 Is a Multiorgan Cancer Susceptibility Gene. Am. J. Hum. Genet. 2004, 75, 1131–1135. [Google Scholar] [CrossRef] [Green Version]
- Teodorczyk, U.; Cybulski, C.; Wokołorczyk, D.; Jakubowska, A.; Starzyńska, T.; Ławniczak, M.; Domagala, P.; Ferenc, K.; Marlicz, K.; Banaszkiewicz, Z.; et al. The risk of gastric cancer in carriers of CHEK2 mutations. Fam. Cancer 2013, 12, 473–478. [Google Scholar] [CrossRef]
- Cybulski, C.; Wokołorczyk, D.; Kładny, J.; Kurzawski, G.; Suchy, J.; Grabowska, E.; Gronwald, J.; Huzarski, T.; Byrski, T.; Górski, B.; et al. Germline CHEK2 mutations and colorectal cancer risk: Different effects of a missense and truncating mutations? Eur. J. Hum. Genet. 2007, 15, 237–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zlowocka-Perlowska, E.; Narod, S.A.; Cybulski, C. CHEK2 Alleles Predispose to Renal Cancer in Poland. JAMA Oncol. 2019, 5, 576. [Google Scholar] [CrossRef] [PubMed]
- Siołek, M.; Cybulski, C.; Gąsior-Perczak, D.; Kowalik, A.; Kozak-Klonowska, B.; Kowalska, A.; Chłopek, M.; Kluźniak, W.; Wokołorczyk, D.; Palyga, I.; et al. CHEK2 mutations and the risk of papillary thyroid cancer. Int. J. Cancer 2015, 137, 548–552. [Google Scholar] [CrossRef]
- Cybulski, C.; Lubiński, J.; Wokołorczyk, D.; Kuźniak, W.; Kashyap, A.; Sopik, V.; Huzarski, T.; Gronwald, J.; Byrski, T.; Szwiec, M.; et al. Mutations predisposing to breast cancer in 12 candidate genes in breast cancer patients from Poland. Clin. Genet. 2014, 88, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Cybulski, C.; Kluźniak, W.; Huzarski, T.; Wokołorczyk, D.; Kashyap, A.; Rusak, B.; Stempa, K.; Gronwald, J.; Szymiczek, A.; Bagherzadeh, M.; et al. The spectrum of mutations predisposing to familial breast cancer in Poland. Int. J. Cancer 2019, 145, 3311–3320. [Google Scholar] [CrossRef] [PubMed]
- Wokołorczyk, D.; Kluźniak, W.; Huzarski, T.; Gronwald, J.; Szymiczek, A.; Rusak, B.; Stempa, K.; Gliniewicz, K.; Kashyap, A.; Morawska, S.; et al. Mutations in ATM, NBN and BRCA2 predispose to aggressive prostate cancer in Poland. Int. J. Cancer 2020, 147, 2793–2800. [Google Scholar] [CrossRef]
- National Cancer Registry—Reports [Online]. Available online: http://onkologia.org.pl/raporty/ (accessed on 18 May 2021).
- Zhang, L.; Wu, Y.; Liu, F.; Fu, L.; Tong, Z. Characteristics and survival of patients with metachronous or synchronous double primary malignancies: Breast and thyroid cancer. Oncotarget 2016, 7, 52450–52459. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Tsukuma, H.; Koyama, H.; Kinoshita, Y.; Kinoshita, N.; Oshima, A. Second Primary Cancers Following Breast Cancer in the Japanese Female Population. Jpn. J. Cancer Res. 2001, 92, 1–8. [Google Scholar] [CrossRef]
- Bolf, E.L.; Sprague, B.L.; Carr, F.E. A Linkage Between Thyroid and Breast Cancer: A Common Etiology? Cancer Epidemiol. Biomark. Prev. 2019, 28, 643–649. [Google Scholar] [CrossRef]
- Park, J.S.; Oh, K.K.; Kim, E.-K.; Chang, H.-S.; Hong, S. Sonographic Screening for Thyroid Cancer in Females Undergoing Breast Sonography. Am. J. Roentgenol. 2006, 186, 1025–1028. [Google Scholar] [CrossRef]
- Park, C.M.; Lee, Y.D.; Oh, E.M.; Kim, K.-I.; Park, H.K.; Ko, K.-P.; Chung, Y.S. The prognosis and treatment of primary thyroid cancer occurred in breast cancer patients: Comparison with ordinary thyroid cancer. Ann. Surg. Treat. Res. 2014, 86, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variables | No Thyroid Cancer n = 10,739 | Had Thyroid Cancer n = 53 | p-Value |
|---|---|---|---|
| Year of birth | 1955.6 (1918–1993) | 1956.6 (1933–78) | 0.48 |
| Age at diagnosis Year of diagnosis | 51.2 (18–92) 2006.9 (1988–2014) | 49.1 (31–75) 2005.7 (1996–2012) | 0.17 0.05 |
| Age at thyroid cancer Years from breast cancer to thyroid cancer | 55.5 (35–84) 6.3 (1–17) | ||
| ER status positive negative missing | 5878 (67.5) 2825 (32.5) 1672 | 33 (70.2) 14 (29.8) 7 | 0.70 |
| Her2 status Positive Negative Missing | 1239 (17.3) 5929 (82.7) 3571 | 4 (11.1) 32 (88.9) 17 | 0.75 |
| Tumor Size <2 cm >2 cm | 4251 (50.6) 4143 (49.4) | 30 (66.7) 15 (33.3) | 0.03 |
| Nodes Positive Negative | 3899 (44.6) 4842 (55.4) | 10 (21.7) 36 (78.3) | 0.002 |
| Chemotherapy yes no | 5953 (62.2) 3611 (37.8) | 27 (56.3) 21 (43.8) | 0.39 |
| Radiotherapy yes no | 4574 (59.2) 3158 (40.8) | 25 (53.2) 22 (46.8) | 0.41 |
| Tamoxifen yes no | 6175 (68.1) 2892 (31.9) | 37 (77.1) 11 (22.9) | 0.18 |
| CHEK2 mutation No Yes | 9835 (91.6) 904 (8.4) | 43 (81.1) 10 (18.9) | 0.006 |
| BRCA1 mutation No Yes | 10,220 (95.3) 502 (4.7) | 50 (98.0) 1 (2.0) | 0.36 |
| Thyroid Cancer Patients | Poland General Population (Female) Per 100,000 Per Year | ||||
|---|---|---|---|---|---|
| Age Group | Cases Observed | Person Years | Annual Risk Per 100,000 Per Year | Annual Risk (Poland) | Cases Expected |
| 35–40 | 4 | 3531 | 113.2 | 9.8 | 0.34 |
| 40–44 | 3 | 8461 | 35.4 | 8.6 | 0.72 |
| 45–49 | 5 | 17,812 | 39.2 | 12.5 | 2.22 |
| 50–54 | 15 | 24,196 | 70.2 | 12.5 | 3.02 |
| 55–59 | 8 | 20,175 | 39.6 | 14.3 | 2.88 |
| 60–64 | 9 | 12,389 | 72.6 | 17.8 | 2.20 |
| 65–70 | 6 | 6067 | 98.9 | 16.8 | 1.02 |
| Thyroid Cancer Patients | Poland general Population (Female) Per 100,000 Per Year | ||||
|---|---|---|---|---|---|
| Age Group | Number of Events | Person Years | Annual Risk Per 100,000 Per Year | Annual Risk (Poland) | Cases Expected |
| 35–40 | 1 | 298 | 335 | 9.8 | 0.03 |
| 40–44 | 0 | 735 | 0 | 8.6 | 0.06 |
| 45–49 | 1 | 1538 | 65.0 | 12.5 | 0.19 |
| 50–54 | 2 | 2067 | 96.8 | 12.5 | 0.26 |
| 55–59 | 2 | 1692 | 118 | 14.3 | 0.24 |
| 60–64 | 1 | 1034 | 96.7 | 17.8 | 0.18 |
| 65–70 | 2 | 489 | 409 | 16.8 | 0.08 |
| Variables | Control/Case | Univariate HR(95%CI)P | Multivariate HR(95%CI)P |
|---|---|---|---|
| Age at breast cancer diagnosis <45 45–50 50–65 >65 | 3419/17 3253/22 2687/11 1308/3 | 1 1.32 (0.70–2.49) 0.38 1.31 (0.60–2.88) 0.50 0.77 (0.22–2.68) 0.68 | 1 1.24 (0.66–2.35) 0.51 1.00 (0.44–2.27) 1.00 0.50 (0.14–1.81) 0.29 |
| ER-negative ER-positive | 2825/14 5878/33 | 1 1.18(0.63–2.20) 0.61 | |
| Her2 negative Her2 positive | 5929/32 1239/4 | 1 0.58(0.18–1.92) 0.37 | |
| BRCA1 No Yes | 10,220/50 502/1 | 1 0.38 (0.05–2.72) 0.33 | 1 0.39 (0.05–2.99) 0.37 |
| CHEK2 No Yes Yes, truncating Yes, missense | 9835/43 904/10 242/2 662/8 | 1 2.52 (1.27–5.01) 0.009 1.89 (0.46–7.79) 0.38 2.75 (1.29–5.85) 0.009 | 1 2.17 (1.08–4.37) 0.03 1.68 (0.40–7.04) 0.48 2.24 (1.09–5.01) 0.03 |
| Chemotherapy No Yes | 3611/21 5953/27 | 1 0.70 (0.40–1.25) 0.23 | 1 0.52 (0.28–0.97) 0.04 |
| Radiotherapy No Yes | 3158/22 4572/25 | 1 0.91 (0.50–1.62) 0.76 | 1 0.96 (0.53–1.72) 0.88 |
| Tamoxifen No Yes | 2897/11 6175/37 | 1 1.60 (0.82–3.15) 0.17 | 1 1.40 (0.70–2.79) 0.34 |
| Oophorectomy No Yes | 7539/37 1162/10 | 1 1.40 (0.48–1.65) 0.71 | 1 1.33 (0.61–2.93) 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cieszyńska, M.; Kluźniak, W.; Wokołorczyk, D.; Cybulski, C.; Huzarski, T.; Gronwald, J.; Falco, M.; Dębniak, T.; Jakubowska, A.; Derkacz, R.; et al. Risk of Second Primary Thyroid Cancer in Women with Breast Cancer. Cancers 2022, 14, 957. https://doi.org/10.3390/cancers14040957
Cieszyńska M, Kluźniak W, Wokołorczyk D, Cybulski C, Huzarski T, Gronwald J, Falco M, Dębniak T, Jakubowska A, Derkacz R, et al. Risk of Second Primary Thyroid Cancer in Women with Breast Cancer. Cancers. 2022; 14(4):957. https://doi.org/10.3390/cancers14040957
Chicago/Turabian StyleCieszyńska, Monika, Wojciech Kluźniak, Dominika Wokołorczyk, Cezary Cybulski, Tomasz Huzarski, Jacek Gronwald, Michał Falco, Tadeusz Dębniak, Anna Jakubowska, Róża Derkacz, and et al. 2022. "Risk of Second Primary Thyroid Cancer in Women with Breast Cancer" Cancers 14, no. 4: 957. https://doi.org/10.3390/cancers14040957
APA StyleCieszyńska, M., Kluźniak, W., Wokołorczyk, D., Cybulski, C., Huzarski, T., Gronwald, J., Falco, M., Dębniak, T., Jakubowska, A., Derkacz, R., Marciniak, W., Lener, M., Woronko, K., Mocarz, D., Baszuk, P., Bryśkiewicz, M., Narod, S. A., & Lubiński, J. (2022). Risk of Second Primary Thyroid Cancer in Women with Breast Cancer. Cancers, 14(4), 957. https://doi.org/10.3390/cancers14040957







