New Frontiers in Management of Early and Advanced Rectal Cancer
Abstract
Simple Summary
Abstract
1. Early Rectal Cancer (Pre-Malignant Polyps, Carcinoma In-Situ, and T1 Carcinoma)
1.1. Transanal Endoscopic Microsurgery (TEM) and Transanal Minimally Invasive Surgery (TAMIS)
1.2. Robotic Transanal Minimally Invasive Surgery
2. Locally Advanced Rectal Cancer (Stage 2–3 Disease)
2.1. Adjunctive Chemoradiation Therapy
2.2. Short- vs. Long-Course Radiation Therapy
2.3. Total Neoadjuvant Chemotherapy
2.4. Adjuvant Chemotherapy after Neoadjuvant Chemoradiation
2.5. Watch and Wait Protocol
3. Surgical Management of Rectal Cancer
3.1. Robotic Total Mesorectal Excision
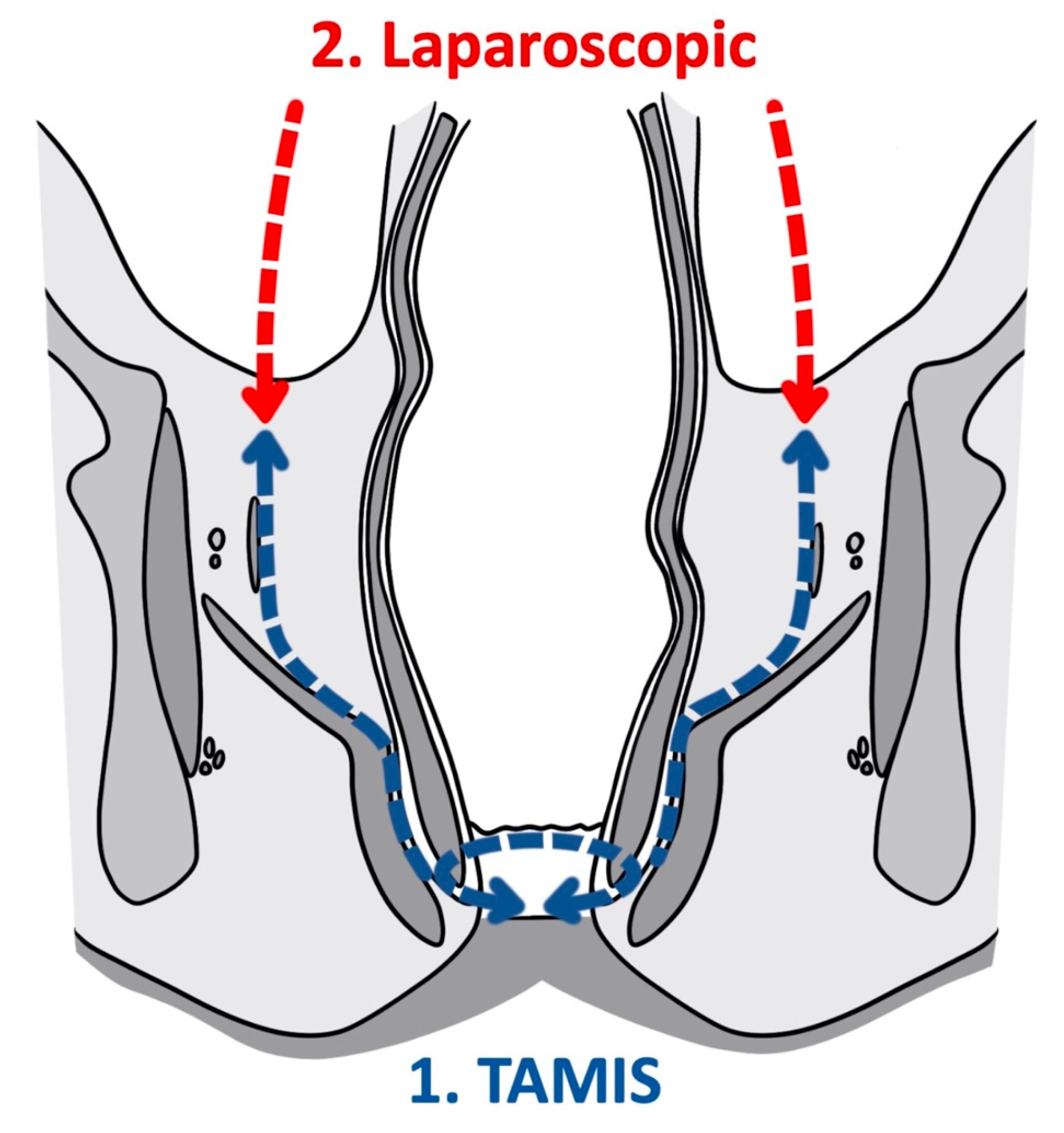
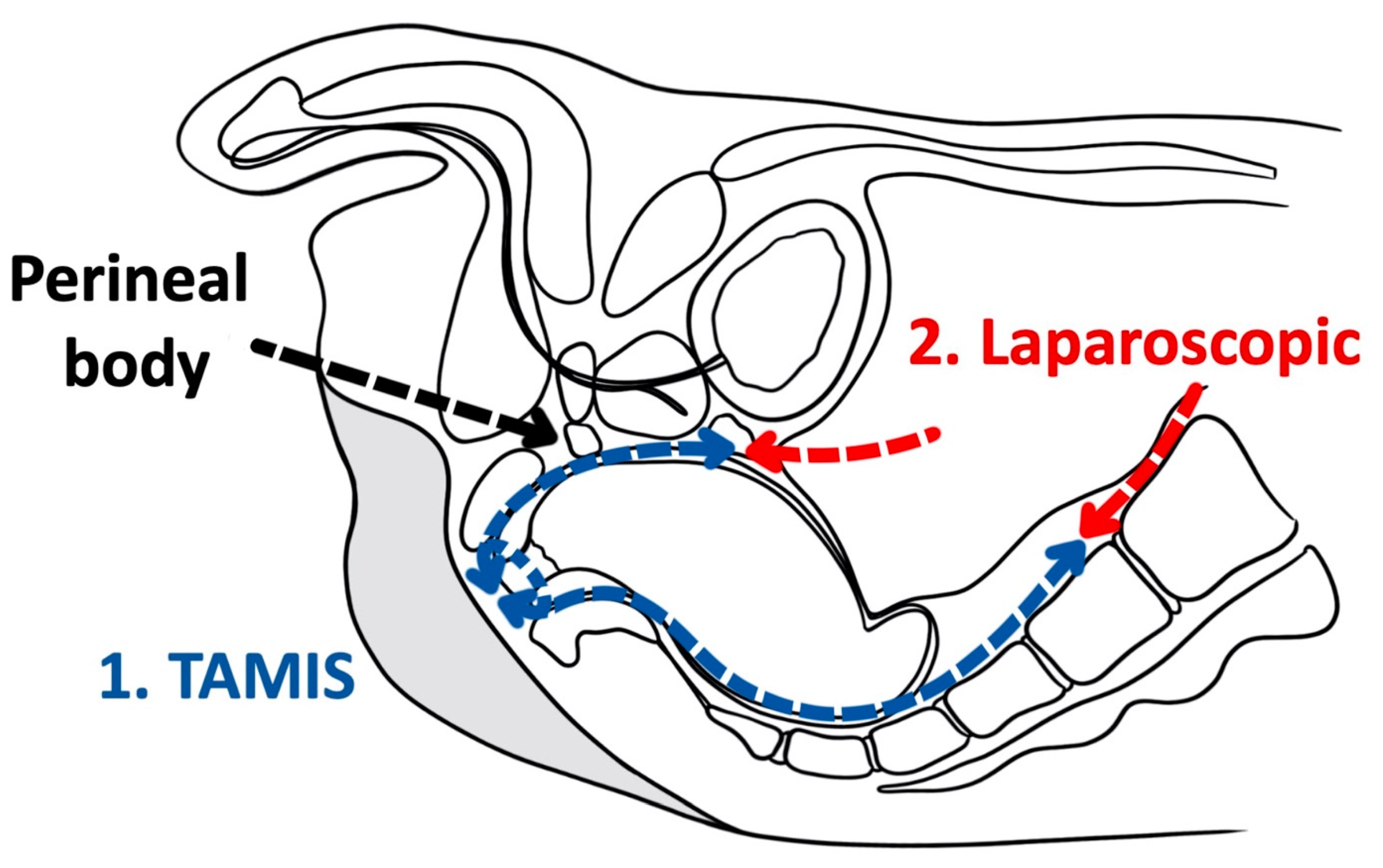
3.2. Transanal Total Mesorectal Excision
4. Distant Solid Organ Metastasis and Peritoneal Carcinomatosis in Rectal Cancer (Stage 4 Disease)
4.1. Metastasectomy as a Treatment of Isolated Hepatic Solid Organ Metastasis
4.2. Metastasectomy as a Treatment of Isolated Pulmonary Solid Organ Metastasis
4.3. Intraperitoneal Chemotherapy and Cytoreductive Surgery
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Buess, G.; Theiss, R.; Gunther, M.; Hutterer, F.; Pichlmaier, H. Transanal endoscopic microsurgery. Leber Magen Darm 1985, 15, 271–279. [Google Scholar] [PubMed]
- Buess, G.; Kipfmuller, K.; Naruhn, M.; Braunstein, S.; Junginger, T. Endoscopic microsurgery of rectal tumors. Endoscopy 1987, 19, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.S.; Cataldo, P.A.; Osler, T.; Hyman, N.H. Transanal endoscopic microsurgery is more effective than traditional transanal excision for resection of rectal masses. Dis. Colon Rectum 2008, 51, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Khoo, R.E.H. Transanal excision of a rectal adenoma using single-access laparoscopic port. Dis. Colon Rectum 2010, 53, 1078–1079. [Google Scholar] [CrossRef]
- Dardamanis, D. Transanal polypectomy using single incision laparoscopic instruments. World J. Gastrointest. Surg. 2011, 3, 56. [Google Scholar] [CrossRef]
- Canda, A.E.; Terzi, C.; Sagol, O.; Sarioglu, S.; Obuz, F.; Fuzun, M. Transanal single-port access microsurgery (TSPAM). Surg. Laparosc. Endosc. Percutaneous Tech. 2012, 22, 349–353. [Google Scholar] [CrossRef]
- Schiphorst, A.H.W.; Langenhoff, B.S.; Maring, J.; Pronk, A.; Zimmerman, D.D.E. Transanal minimally invasive surgery: Initial experience and short-term functional results. Dis. Colon Rectum 2014, 57, 927–932. [Google Scholar] [CrossRef]
- Sevá-Pereira, G.; Trombeta, V.L.; Capochim Romagnolo, L.G. Transanal minimally invasive surgery (TAMIS) using a new disposable device: Our initial experience. Tech. Coloproctol. 2014, 18, 393–397. [Google Scholar] [CrossRef]
- McLemore, E.C.; Leland, H.; Devaraj, B.; Pola, S.; Docherty, M.J.; Patel, D.R.; Levesque, B.G.; Sandborn, W.J.; Talamini, M.A.; Ramamoorthy, S.L. McLemore Transanal Endoscopic Surgical Proctectomy for Proctitis Case Series Report: Diversion, Radiation, Ulcerative Colitis, and Crohn’s Disease. Glob. J. Gastroenterol. Hepatol. 2013, 1, 51–57. [Google Scholar] [CrossRef]
- Benson, A.B.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Deming, D.; Farkas, L.; Garrido-Laguna, I.; Grem, J.L.; et al. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Canc. Netw. 2020, 18, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Barel, F.; Cariou, M.; Saliou, P.; Kermarrec, T.; Auffret, A.; Samaison, L.; Bourhis, A.; Badic, B.; Jézéquel, J.; Cholet, F.; et al. Histopathological factors help to predict lymph node metastases more efficiently than extra-nodal recurrences in submucosa invading pT1 colorectal cancer. Sci. Rep. 2019, 9, 8342. [Google Scholar] [CrossRef] [PubMed]
- Lezoche, G.; Baldarelli, M.; Mario; Paganini, A.M.; De Sanctis, A.; Bartolacci, S.; Lezoche, E. A prospective randomized study with a 5-year minimum follow-up evaluation of transanal endoscopic microsurgery versus laparoscopic total mesorectal excision after neoadjuvant therapy. Surg. Endosc. Other Interv. Tech. 2008, 22, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Halverson, A.L.; Morris, A.M.; Cleary, R.K.; Chang, G.J. For Patients with Early Rectal Cancer, Does Local Excision Have an Impact on Recurrence, Survival, and Quality of Life Relative to Radical Resection? Ann. Surg. Oncol. 2019, 26, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- You, Y.N.; Hardiman, K.M.; Bafford, A.; Poylin, V.; Francone, T.D.; Davis, K.; Paquette, I.M.; Steele, S.R.; Feingold, D.L. The american society of colon and rectal surgeons clinical practice guidelines for the management of rectal cancer. Dis. Colon Rectum 2020, 63, 1191–1222. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Suzuki, T.; Murray, B.W.; Parry, L.; Johnson, C.S.; Horgan, S.; Ramamoorthy, S.; Eisenstein, S. Robotic transanal minimally invasive surgery (TAMIS) with the newest robotic surgical platform: A multi-institutional North American experience. Surg. Endosc. 2019, 33, 543–548. [Google Scholar] [CrossRef]
- Atallah, S. Robotic transanal minimally invasive surgery for local excision of rectal neoplasms. Br. J. Surg. 2014, 101, 578–581. [Google Scholar] [CrossRef]
- Draganov, P.V.; Wang, A.Y.; Othman, M.O.; Fukami, N. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin. Gastroenterol. Hepatol. 2019, 17, 16–25.e1. [Google Scholar] [CrossRef]
- You, Y.N.; Baxter, N.N.; Stewart, A.; Nelson, H. Is the increasing rate of local excision for stage I rectal cancer in the United States justified? A nationwide cohort study from the National Cancer Database. Ann. Surg. 2007, 245, 726–733. [Google Scholar] [CrossRef]
- Kidane, B.; Chadi, S.A.; Kanters, S.; Colquhoun, P.H.; Ott, M.C. Local resection compared with radical resection in the treatment of T1N0M0 rectal adenocarcinoma: A systematic review and meta-analysis. Dis. Colon Rectum 2015, 58, 122–140. [Google Scholar] [CrossRef]
- Douglass, H.O., Jr.; Moertel, C.G.; Mayer, R.J.; Thomas, P.R.; Lindblad, A.S.; Mittleman, A.; Stablein, D.M.; Bruckner, H.W.; Gastrointestinal Tumor Study Group. Survival after Postoperative Combination Treatment of Rectal Cancer. N. Engl. J. Med. 1986, 315, 1294–1295. [Google Scholar] [CrossRef] [PubMed]
- Prolongation of the Disease-Free Interval in Surgically Treated Rectal Carcinoma. N. Engl. J. Med. 1985, 312, 1465–1472. [CrossRef] [PubMed]
- Thomas, P.R.M.; Lindblad, A.S. Adjuvant postoperative radiotherapy and chemotherapy in rectal carcinoma: A review of the gastrointestinal tumor study group experience. Radiother. Oncol. 1988, 13, 245–252. [Google Scholar] [CrossRef]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rödel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus Postoperative Chemoradiotherapy for Rectal Cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef]
- Bujko, K.; Nowacki, M.P.; Nasierowska-Guttmejer, A.; Michalski, W.; Bebenek, M.; Kryj, M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br. J. Surg. 2006, 93, 1215–1223. [Google Scholar] [CrossRef]
- Ansari, N.; Solomon, M.J.; Fisher, R.J.; MacKay, J.; Burmeister, B.; Ackland, S.; Heriot, A.; Joseph, D.; McLachlan, S.A.; McClure, B.; et al. Acute Adverse Events and Postoperative Complications in a Randomized Trial of Preoperative Short-course Radiotherapy Versus Long-course Chemoradiotherapy for T3 Adenocarcinoma of the Rectum: Trans-Tasman Radiation Oncology Group Trial (TROG 01.04). Ann. Surg. 2017, 265, 882–888. [Google Scholar] [CrossRef]
- Erlandsson, J.; Holm, T.; Pettersson, D.; Berglund, Å.; Cedermark, B.; Radu, C.; Johansson, H.; Machado, M.; Hjern, F.; Hallböök, O.; et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): A multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017, 18, 336–346. [Google Scholar] [CrossRef]
- Pettersson, D.; Lörinc, E.; Holm, T.; Iversen, H.; Cedermark, B.; Glimelius, B.; Martling, A. Tumour regression in the randomized Stockholm III Trial of radiotherapy regimens for rectal cancer. Br. J. Surg. 2015, 102, 972–978. [Google Scholar] [CrossRef]
- De Caluwé, L.; Van Nieuwenhove, Y.; Ceelen, W.P. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst. Rev. 2013, 2013, CD006041. [Google Scholar] [CrossRef]
- Bosset, J.F.; Calais, G.; Mineur, L.; Maingon, P.; Stojanovic-Rundic, S.; Bensadoun, R.J.; Bardet, E.; Beny, A.; Ollier, J.C.; Bolla, M.; et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: Long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014, 15, 184–190. [Google Scholar] [CrossRef]
- Cercek, A.; Roxburgh, C.S.D.; Strombom, P.; Smith, J.J.; Temple, L.K.F.; Nash, G.M.; Guillem, J.G.; Paty, P.B.; Yaeger, R.; Stadler, Z.K.; et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. 2018, 4, e180071. [Google Scholar] [CrossRef]
- Conroy, T.; Lamfichekh, N.; Etienne, P.-L.; Rio, E.; FRANCOIS, E.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: Final results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J. Clin. Oncol. 2020, 38, 4007. [Google Scholar] [CrossRef]
- Van der Valk, M.J.M.; Marijnen, C.A.M.; van Etten, B.; Dijkstra, E.A.; Hilling, D.E.; Kranenbarg, E.M.K.; Putter, H.; Roodvoets, A.G.H.; Bahadoer, R.R.; Fokstuen, T.; et al. Compliance and tolerability of short-course radiotherapy followed by preoperative chemotherapy and surgery for high-risk rectal cancer—Results of the international randomized RAPIDO-trial. Radiother. Oncol. 2020, 147, 75–83. [Google Scholar] [CrossRef]
- Hospers, G.; Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.; Putter, H.; Meershoek-Klein Kranenbarg, E.; Roodvoets, A.G.; Nagtegaal, I.D.; Beets-Tan, R.G.; et al. Short-course radiotherapy followed by chemotherapy before TME in locally advanced rectal cancer: The randomized RAPIDO trial. J. Clin. Oncol. 2020, 38, 4006. [Google Scholar] [CrossRef]
- Fokas, E.; Allgäuer, M.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.L.; et al. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J. Clin. Oncol. 2019, 37, 3212–3222. [Google Scholar] [CrossRef]
- Abdalla, A.; Aref, A. Upfront Chemotherapy Followed by Chemoradiation Remains the Sequence of Choice for Total Neoadjuvant Chemotherapy for Locally Advanced Rectal Cancer. J. Clin. Oncol. 2019, 37, 3561–3563. [Google Scholar] [CrossRef]
- Kasi, A.; Abbasi, S.; Handa, S.; Al-Rajabi, R.; Saeed, A.; Baranda, J.; Sun, W. Total Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2030097. [Google Scholar] [CrossRef]
- Sainato, A.; Cernusco Luna Nunzia, V.; Valentini, V.; De Paoli, A.; Maurizi, E.R.; Lupattelli, M.; Aristei, C.; Vidali, C.; Conti, M.; Galardi, A.; et al. No benefit of adjuvant Fluorouracil Leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): Long term results of a randomized trial (I-CNR-RT). Radiother. Oncol. 2014, 113, 223–229. [Google Scholar] [CrossRef]
- Breugom, A.J.; Van Gijn, W.; Muller, E.W.; Berglund, A.; van den Broek, C.B.M.; Fokstuen, T.; Gelderblom, H.; Kapiteijn, E.; Leer, J.W.H.; Marijnen, C.A.M.; et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: A Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 696–701. [Google Scholar] [CrossRef]
- Anderson, H.; Palmer, M.K. Measuring quality of life: Impact of chemotherapy for advanced colorectal cancer. Experience from two recent large phase III trials. Br. J. Cancer 1998, 77, 9–14. [Google Scholar] [CrossRef][Green Version]
- Xu, Z.; Mohile, S.G.; Tejani, M.A.; Becerra, A.; Aquina, C.T.; Hensley, B.J.; Arsalanizadeh, R.; Noyes, K.; Monson, J.R.; Fleming, F.J. Association of poor compliance with adjuvant chemotherapy with poorer survival in patients with rectal cancer: A NCDB analysis (N = 14,742). J. Clin. Oncol. 2016, 34, 3569. [Google Scholar] [CrossRef]
- Paun, B.C.; Cassie, S.; MacLean, A.R.; Dixon, E.; Buie, W.D. Postoperative complications following surgery for rectal cancer. Ann. Surg. 2010, 251, 807–818. [Google Scholar] [CrossRef]
- Habr-Gama, A.; Perez, R.O.; Nadalin, W.; Sabbaga, J.; Ribeiro, U., Jr.; Sousa, A.H.S.E.; Campos, F.G.; Kiss, D.R.; Gama-Rodrigues, J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann. Surg. 2004, 240, 711–718. [Google Scholar] [CrossRef]
- Dossa, F.; Chesney, T.R.; Acuna, S.A.; Baxter, N.N. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 501–513. [Google Scholar] [CrossRef]
- Rullier, E.; Rouanet, P.; Tuech, J.J.; Valverde, A.; Lelong, B.; Rivoire, M.; Faucheron, J.L.; Jafari, M.; Portier, G.; Meunier, B.; et al. Organ preservation for rectal cancer (GRECCAR 2): A prospective, randomised, open-label, multicentre, phase 3 trial. Lancet 2017, 390, 469–479. [Google Scholar] [CrossRef]
- Zhao, G.H.; Deng, L.; Ye, D.M.; Wang, W.H.; Yan, Y.; Yu, T. Efficacy and safety of wait and see strategy versus radical surgery and local excision for rectal cancer with cCR response after neoadjuvant chemoradiotherapy: A meta-analysis. World J. Surg. Oncol. 2020, 18, 232. [Google Scholar] [CrossRef]
- Ault, G.T.; Cologne, K.G. Colorectal Cancer: Management of Stage IV Disease. In The ASCRS Textbook of Colon and Rectal Surgery; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 589–616. [Google Scholar]
- Topor, B.; Acland, R.; Kolodko, V.; Galandiuk, S. Mesorectal lymph nodes: Their location and distribution within the mesorectum. Dis. Colon Rectum 2003, 46, 779–785. [Google Scholar] [CrossRef]
- Heald, R.J.; Ryall, R.D.H. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986, 327, 1479–1482. [Google Scholar] [CrossRef]
- Kusters, M.; Marijnen, C.A.M.; van de Velde, C.J.H.; Rutten, H.J.T.; Lahaye, M.J.; Kim, J.H.; Beets-Tan, R.G.H.; Beets, G.L. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur. J. Surg. Oncol. 2010, 36, 470–476. [Google Scholar] [CrossRef]
- MacFarlane, J.K.; Ryall, R.D.H.; Heald, R.J. Mesorectal excision for rectal cancer. Lancet 1993, 341, 457–460. [Google Scholar] [CrossRef]
- Cecil, T.D.; Sexton, R.; Moran, B.J.; Heald, R.J. Total mesorectal excision results in low local recurrence rates in lymph node-positive rectal cancer. Dis. Colon Rectum 2004, 47, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, J.R.; Driman, D.K. The total mesorectal excision specimen for rectal cancer: A review of its pathological assessment. J. Clin. Pathol. 2007, 60, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Quirke, P.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Couture, J.; O’Callaghan, C.; Myint, A.S.; Bessell, E.; Thompson, L.C.; et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: A prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 2009, 373, 821–828. [Google Scholar] [CrossRef]
- Bujko, K.; Rutkowski, A.; Chang, G.J.; Michalski, W.; Chmielik, E.; Kusnierz, J. Is the 1-cm rule of distal bowel resection margin in rectal cancer based on clinical evidence? A systematic review. Ann. Surg. Oncol. 2012, 19, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Kuvshinoff, B.; Maghfoor, I.; Miedema, B.; Bryer, M.; Westgate, S.; Wilkes, J.; Ota, D. Distal margin requirements after preoperative chemoradiotherapy for distal rectal carcinomas: Are ≤ 1 cm Distal Margins Sufficient. Ann. Surg. Oncol. 2001, 8, 163–169. [Google Scholar] [CrossRef]
- Andreola, S.; Leo, E.; Belli, F.; Lavarino, C.; Bufalino, R.; Tomasic, G.; Baldini, M.T.; Valvo, F.; Navarria, P.; Lombardi, F. Distal intramural spread in adenocarcinoma of the lower third of the rectum treated with total rectal resection and coloanal anastomosis. Dis. Colon Rectum 1997, 40, 25–29. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Chokshi, R.V. Low Anterior Resection Syndrome. Curr. Gastroenterol. Rep. 2020, 22, 48. [Google Scholar] [CrossRef]
- Juul, T.; Ahlberg, M.; Biondo, S.; Espin, E.; Jimenez, L.M.; Matzel, K.E.; Palmer, G.J.; Sauermann, A.; Trenti, L.; Zhang, W.; et al. Low anterior resection syndrome and quality of life: An international multicenter study. Dis. Colon Rectum 2014, 57, 585–591. [Google Scholar] [CrossRef]
- Abraham, N.S.; Young, J.M.; Solomon, M.J. Meta-analysis of short-term outcomes after laparoscopic resection for colorectal cancer. Br. J. Surg. 2004, 91, 1111–1124. [Google Scholar] [CrossRef]
- Heikkinen, T.; Msika, S.; Desvignes, G.; Schwandner, O.; Schiedeck, T.H.; Shekarriz, H.; Bloechle, C.H.; Baca, I.; Weiss, O.; Morino, M.; et al. Laparoscopic surgery versus open surgery for colon cancer: Short-term outcomes of a randomised trial. Lancet Oncol. 2005, 6, 477–484. [Google Scholar] [CrossRef]
- Fleshman, J.; Sargent, D.J.; Green, E.; Anvari, M.; Stryker, S.J.; Beart, R.W.; Hellinger, M.; Flanagan, R.; Peters, W.; Nelson, H. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann. Surg. 2007, 246, 655–662. [Google Scholar] [CrossRef]
- Jayne, D.G.; Guillou, P.J.; Thorpe, H.; Quirke, P.; Copeland, J.; Smith, A.M.H.; Heath, R.M.; Brown, J.M. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-Year results of the UK MRC CLASICC trial group. J. Clin. Oncol. 2007, 25, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D.; Pigazzi, A.; Marshall, H.; Croft, J.; Corrigan, N.; Copeland, J.; Quirke, P.; West, N.; Rautio, T.; Thomassen, N.; et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer the rolarr randomized clinical trial. JAMA J. Am. Med. Assoc. 2017, 318, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Rouanet, P.; Bertrand, M.M.; Jarlier, M.; Mourregot, A.; Traore, D.; Taoum, C.; de Forges, H.; Colombo, P.E. Robotic Versus Laparoscopic Total Mesorectal Excision for Sphincter-Saving Surgery: Results of a Single-Center Series of 400 Consecutive Patients and Perspectives. Ann. Surg. Oncol. 2018, 25, 3572–3579. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Park, S.C.; Park, J.W.; Chang, H.J.; Kim, D.Y.; Nam, B.H.; Sohn, D.K.; Oh, J.H. Robot-assisted Versus Laparoscopic Surgery for Rectal Cancer: A Phase II Open Label Prospective Randomized Controlled Trial. Ann. Surg. 2018, 267, 243–251. [Google Scholar] [CrossRef]
- Gavriilidis, P.; Wheeler, J.; Spinelli, A.; de‘Angelis, N.; Simopoulos, C.; Di Saverio, S. Robotic vs laparoscopic total mesorectal excision for rectal cancers: Has a paradigm change occurred? A systematic review by updated meta-analysis. Colorectal Dis. 2020, 22, 1506–1517. [Google Scholar] [CrossRef]
- Jeon, Y.; Park, E.J.; Baik, S.H. Robotic Surgery for Rectal Cancer and Cost-Effectiveness. J. Minim. Invasive Surg. 2019, 22, 139–149. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Yamaguchi, T.; Kinugasa, Y.; Shiomi, A.; Kagawa, H.; Yamakawa, Y.; Furutani, A.; Manabe, S.; Torii, K.; Koido, K.; et al. Mesorectal fat area as a useful predictor of the difficulty of robotic-assisted laparoscopic total mesorectal excision for rectal cancer. Surg. Endosc. 2019, 33, 557–566. [Google Scholar] [CrossRef]
- Escal, L.; Nougaret, S.; Guiu, B.; Bertrand, M.M.; de Forges, H.; Tetreau, R.; Thézenas, S.; Rouanet, P. MRI-based score to predict surgical difficulty in patients with rectal cancer. Br. J. Surg. 2018, 105, 140–146. [Google Scholar] [CrossRef]
- Cavaliere, R.; Tedesco, M.; Giannarelli, D.; Aloe, L.; Perri, P.; Di Filippo, F.; Crecco, M.; Gabrielli, F.; Cosimelli, M.; Stipa, S. Radical surgery in rectal cancer patients: What does it mean today? J. Surg. Oncol. 1991, 48, 24–31. [Google Scholar] [CrossRef]
- Sylla, P.; Rattner, D.W.; Delgado, S.; Lacy, A.M. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg. Endosc. 2010, 24, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- De Lacy, A.M.; Rattner, D.W.; Adelsdorfer, C.; Tasende, M.M.; Fernández, M.; Delgado, S.; Sylla, P.; Martínez-Palli, G. Transanal natural orifice transluminal endoscopic surgery (NOTES) rectal resection: “Down-to-up” total mesorectal excision (TME)—Short-term outcomes in the first 20 cases. Surg. Endosc. 2013, 27, 3165–3172. [Google Scholar] [CrossRef] [PubMed]
- Veltcamp Helbach, M.; Deijen, C.L.; Velthuis, S.; Bonjer, H.J.; Tuynman, J.B.; Sietses, C. Transanal total mesorectal excision for rectal carcinoma: Short-term outcomes and experience after 80 cases. Surg. Endosc. 2016, 30, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Tuech, J.J.; Karoui, M.; Lelong, B.; De Chaisemartin, C.; Bridoux, V.; Manceau, G.; Delpero, J.R.; Hanoun, L.; Michot, F. A step toward notes total mesorectal excision for rectal cancer endoscopic transanal proctectomy. Ann. Surg. 2015, 261, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Lacy, A.M.; Tasende, M.M.; Delgado, S.; Fernandez-Hevia, M.; Jimenez, M.; De Lacy, B.; Castells, A.; Bravo, R.; Wexner, S.D.; Heald, R.J. Transanal Total Mesorectal Excision for Rectal Cancer: Outcomes after 140 Patients. J. Am. Coll. Surg. 2015, 221, 415–423. [Google Scholar] [CrossRef]
- Denost, Q.; Adam, J.P.; Rullier, A.; Buscail, E.; Laurent, C.; Rullier, E. Perineal transanal approach: A new standard for laparoscopic sphincter-saving resection in low rectal cancer, a randomized trial. Ann. Surg. 2014, 260, 993–999. [Google Scholar] [CrossRef]
- Deijen, C.L.; Velthuis, S.; Tsai, A.; Mavroveli, S.; de Lange-de Klerk, E.S.M.; Sietses, C.; Tuynman, J.B.; Lacy, A.M.; Hanna, G.B.; Bonjer, H.J. COLOR III: A multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg. Endosc. 2016, 30, 3210–3215. [Google Scholar] [CrossRef]
- Nadler, A.; McCart, J.A.; Govindarajan, A. Peritoneal Carcinomatosis from Colon Cancer: A Systematic Review of the Data for Cytoreduction and Intraperitoneal Chemotherapy. Clin. Colon Rectal Surg. 2015, 28, 234–246. [Google Scholar] [CrossRef]
- Tomlinson, J.S.; Jarnagin, W.R.; DeMatteo, R.P.; Fong, Y.; Kornprat, P.; Gonen, M.; Kemeny, N.; Brennan, M.F.; Blumgart, L.H.; D’Angelica, M. Actual 10-year survival after resection of colorectal liver metastases defines cure. J. Clin. Oncol. 2007, 25, 4575–4580. [Google Scholar] [CrossRef]
- Tsoulfas, G.; Pramateftakis, M.G. Management of rectal cancer and liver metastatic disease: Which comes first? Int. J. Surg. Oncol. 2012, 2012, 196908. [Google Scholar] [CrossRef]
- O’Connell, J.B.; Maggard, M.A.; Ko, C.Y. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J. Natl. Cancer Inst. 2004, 96, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, W.; Rosen, H.; Kornek, G.V.; Sebesta, C.; Depisch, D. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. Br. Med. J. 1993, 306, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.C.; Primrose, J.N.; Colquitt, J.L.; Garden, O.J.; Poston, G.J.; Rees, M. Surgical resection of hepatic metastases from colorectal cancer: A systematic review of published studies. Br. J. Cancer 2006, 94, 982–999. [Google Scholar] [CrossRef] [PubMed]
- Neeff, H.; Hörth, W.; Makowiec, F.; Fischer, E.; Imdahl, A.; Hopt, U.T.; Passlick, B. Outcome after resection of hepatic and pulmonary metastases of colorectal cancer. J. Gastrointest. Surg. 2009, 13, 1813–1820. [Google Scholar] [CrossRef]
- Scheele, J.; Stangl, R.; Altendorf-Hofmann, A. Hepatic metastases from colorectal carcinoma: Impact of surgical resection on the natural history. Br. J. Surg. 1990, 77, 1241–1246. [Google Scholar] [CrossRef]
- Yoshidome, H.; Kimura, F.; Shimizu, H.; Ohtsuka, M.; Kato, A.; Yoshitomi, H.; Furukawa, K.; Mitsuhashi, N.; Takeuchi, D.; Iida, A.; et al. Interval period tumor progression: Does delayed hepatectomy detect occult metastases in synchronous colorectal liver metastases? J. Gastrointest. Surg. 2008, 12, 1391–1398. [Google Scholar] [CrossRef]
- Scheele, J. Hepatectomy for liver metastases. Br. J. Surg. 1993, 80, 274–276. [Google Scholar] [CrossRef]
- Lambert, L.A.; Colacchio, T.A.; Barth, R.J. Interval hepatic resection of colorectal metastases improves patient selection. Arch. Surg. 2000, 135, 473–480. [Google Scholar] [CrossRef]
- Shimizu, Y.; Yasui, K.; Sano, T.; Hirai, T.; Kanemitsu, Y.; Komori, K.; Kato, T. Validity of observation interval for synchronous hepatic metastases of colorectal cancer: Changes in hepatic and extrahepatic metastatic foci. Langenbeck’s Arch. Surg. 2008, 393, 181–184. [Google Scholar] [CrossRef]
- Lee, W.S.; Kim, M.J.; Yun, S.H.; Chun, H.K.; Lee, W.Y.; Kim, S.J.; Choi, S.H.; Heo, J.S.; Joh, J.W.; Kim, Y. Il Risk factor stratification after simultaneous liver and colorectal resection for synchronous colorectal metastasis. Langenbeck’s Arch. Surg. 2008, 393, 13–19. [Google Scholar] [CrossRef]
- Capussotti, L.; Vigano, L.; Ferrero, A.; Lo Tesoriere, R.; Ribero, D.; Polastri, R. Timing of resection of liver metastases synchronous to colorectal tumor: Proposal of prognosis-based decisional model. Ann. Surg. Oncol. 2007, 14, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Boostrom, S.Y.; Vassiliki, L.T.; Nagorney, D.M.; Wolff, B.G.; Chua, H.K.; Harmsen, S.; Larson, D.W. Synchronous Rectal and Hepatic Resection of Rectal Metastatic Disease. J. Gastrointest. Surg. 2011, 15, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, A.; Gomez, D.; Brooks, A.; Cameron, I.C. “liver-first” approach for synchronous colorectal liver metastases: Is this a justifiable approach? J. Hepatobiliary Pancreat. Sci. 2013, 20, 263–270. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.C.; Van Dam, R.M.; Maas, M.; Bemelmans, M.H.A.; Olde Damink, S.W.M.; Beets, G.L.; Dejong, C.H.C. The liver-first approach for synchronous colorectal liver metastasis: A 5-year single-centre experience. HPB 2011, 13, 745–752. [Google Scholar] [CrossRef]
- Verhoef, C.; Van Der Pool, A.E.M.; Nuyttens, J.J.; Planting, A.S.T.; Eggermont, A.M.M.; De Wilt, J.H.W. The “liver-first approach” for patients with locally advanced rectal cancer and synchronous liver metastases. Dis. Colon Rectum 2009, 52, 23–30. [Google Scholar] [CrossRef]
- Blalock, A. Recent Advances in Surgery. N. Engl. J. Med. 1944, 231, 293–300. [Google Scholar] [CrossRef]
- McCormack, P.M.; Attiyeh, F.F. Resected pulmonary metastases from colorectal cancer. Dis. Colon Rectum 1979, 22, 553–556. [Google Scholar] [CrossRef]
- Dahabre, J.; Vasilaki, M.; Stathopoulos, G.P.; Kondaxis, A.; Iliadis, K. Surgical management in lung metastases from colorectal cancer. Anticancer Res. 2007, 27, 4387–4390. [Google Scholar]
- Milosevic, M.; Edwards, J.; Tsang, D.; Dunning, J.; Shackcloth, M.; Batchelor, T.; Coonar, A.; Hasan, J.; Davidson, B.; Marchbank, A.; et al. Pulmonary Metastasectomy in Colorectal Cancer: Updated analysis of 93 randomized patients—Control survival is much better than previously assumed. Colorectal Dis. 2020, 22, 1314–1324. [Google Scholar] [CrossRef]
- Pastorino, U.; Buyse, M.; Friedel, G.; Ginsberg, R.J.; Girard, P.; Goldstraw, P.; Johnston, M.; McCormack, P.; Pass, H.; Putnam, J.; et al. Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J. Thorac. Cardiovasc. Surg. 1997, 113, 37–49. [Google Scholar] [CrossRef]
- Zink, S.; Kayser, G.; Gabius, H.J.; Kayser, K. Survival, disease-free interval, and associated tumor features in patients with colon/rectal carcinomas and their resected intra-pulmonary metastases. Eur. J. Cardio-Thorac. Surg. 2001, 19, 908–913. [Google Scholar] [CrossRef][Green Version]
- Okumura, S.; Kondo, H.; Tsuboi, M.; Nakayama, H.; Asamura, H.; Tsuchiya, R.; Naruke, T. Pulmonary resection for metastatic colorectal cancer: Experiences with 159 patients. J. Thorac. Cardiovasc. Surg. 1996, 112, 867–874. [Google Scholar] [CrossRef][Green Version]
- Gonzalez, M.; Poncet, A.; Combescure, C.; Robert, J.; Ris, H.B.; Gervaz, P. Risk factors for survival after lung metastasectomy in colorectal cancer patients: A systematic review and meta-analysis. Ann. Surg. Oncol. 2013, 20, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Salah, S.; Watanabe, K.; Welter, S.; Park, J.S.; Park, J.W.; Zabaleta, J.; Ardissone, F.; Kim, J.; Riquet, M.; Nojiri, K.; et al. Colorectal cancer pulmonary oligometastases: Pooled analysis and construction of a clinical lung metastasectomy prognostic model. Ann. Oncol. 2012, 23, 2649–2655. [Google Scholar] [CrossRef]
- Pfannschmidt, J.; Dienemann, H.; Hoffmann, H. Surgical Resection of Pulmonary Metastases From Colorectal Cancer: A Systematic Review of Published Series. Ann. Thorac. Surg. 2007, 84, 324–338. [Google Scholar] [CrossRef]
- Girard, P.; Ducreux, M.; Baldeyrou, P.; Rougier, P.; Le Chevalier, T.; Bougaran, J.; Lasser, P.; Gayet, B.; Ruffié, P.; Grunenwald, D. Surgery for lung metastases from colorectal cancer: Analysis of prognostic factors. J. Clin. Oncol. 1996, 14, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Shirouzu, K.; Isomoto, H.; Hayashi, A.; Nagamatsu, Y.; Kakegawa, T. Surgical treatment for patients with pulmonary metastases after resection of primary colorectal carcinoma. Cancer 1995, 76, 393–398. [Google Scholar] [CrossRef]
- Van Halteren, H.K.; Van Geel, A.N.; Hart, A.A.M.; Zoetmulder, F.A.N. Pulmonary resection for metastases of colorectal origin. Chest 1995, 107, 1526–1531. [Google Scholar] [CrossRef]
- McAfee, M.K.; Allen, M.S.; Trastek, V.F.; Ilstrup, D.M.; Deschamps, C.; Pairolero, P.C. Colorectal lung metastases: Results of surgical excision. Ann. Thorac. Surg. 1992, 53, 780–786. [Google Scholar] [CrossRef]
- McCormack, P.M.; Burt, M.E.; Bains, M.S.; Martini, N.; Rusch, V.W.; Ginsberg, R.J. Lung Resection for Colorectal Metastases: 10-Year Results. Arch. Surg. 1992, 127, 1403–1406. [Google Scholar] [CrossRef]
- Mori, M.; Tomoda, H.; Ishida, T.; Kido, A.; Shimono, R.; Matsushima, T.; Kuwano, H.; Sugimachi, K. Surgical Resection of Pulmonary Metastases from Colorectal Adenocarcinoma: Special Reference to Repeated Pulmonary Resections. Arch. Surg. 1991, 126, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, H.K.; Cho, J.H.; Choi, Y.S.; Kim, K.; Kim, J.; Zo, J.I.; Shim, Y.M.; Heo, J.S.; Lee, W.Y.; et al. Prognostic factors after pulmonary metastasectomy of colorectal cancers: Influence of liver metastasis. World J. Surg. Oncol. 2016, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, F.; Hunt, I.; Teoh, K.; Treasure, T.; Utley, M. Pulmonary metastasectomy in colorectal cancer: A systematic review and quantitative synthesis. J. R. Soc. Med. 2010, 103, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Nagakura, S.; Shirai, Y.; Yamato, Y.; Yokoyama, N.; Suda, T.; Hatakeyama, K. Simultaneous detection of colorectal carcinoma liver and lung metastases does not warrant resection. J. Am. Coll. Surg. 2001, 193, 153–160. [Google Scholar] [CrossRef]
- Cho, J.H.; Hamaji, M.; Allen, M.S.; Cassivi, S.D.; Nichols, F.C.; Wigle, D.A.; Shen, K.R.; Deschamps, C. The prognosis of pulmonary metastasectomy depends on the location of the primary colorectal cancer. Ann. Thorac. Surg. 2014, 98, 1231–1237. [Google Scholar] [CrossRef]
- Glehen, O.; Osinsky, D.; Beaujard, A.C.; Gilly, F.N. Natural history of peritoneal carcinomatosis from nongynecologic malignancies. Surg. Oncol. Clin. N. Am. 2003, 12, 729–739. [Google Scholar] [CrossRef]
- Minsky, B.D.; Mies, C.; Rich, T.A.; Recht, A.; Chaffey, J.T. Potentially curative surgery of colon cancer: Patterns of failure and survival. J. Clin. Oncol. 1988, 6, 106–118. [Google Scholar] [CrossRef]
- Jayne, D.G.; Fook, S.; Loi, C.; Seow-Choen, F. Peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 2002, 89, 1545–1550. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Peritonectomy procedures. Ann. Surg. 1995, 221, 29–42. [Google Scholar] [CrossRef]
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Slooten, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A.N. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Dohan, A.; Hoeffel, C.; Soyer, P.; Jannot, A.S.; Valette, P.J.; Thivolet, A.; Passot, G.; Glehen, O.; Rousset, P. Evaluation of the peritoneal carcinomatosis index with CT and MRI. Br. J. Surg. 2017, 104, 1244–1249. [Google Scholar] [CrossRef]
- Faron, M.; Macovei, R.; Goéré, D.; Honoré, C.; Benhaim, L.; Elias, D. Linear Relationship of Peritoneal Cancer Index and Survival in Patients with Peritoneal Metastases from Colorectal Cancer. Ann. Surg. Oncol. 2016, 23, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Goéré, D.; Glehen, O.; Quenet, F.; Guilloit, J.M.; Bereder, J.M.; Lorimier, G.; Thibaudeau, E.; Ghouti, L.; Pinto, A.; Tuech, J.J.; et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP–PRODIGE 15): A randomised, phase 3 study. Lancet Oncol. 2020, 21, 1147–1154. [Google Scholar] [CrossRef]
- De Cuba, E.M.V.; Kwakman, R.; Knol, D.L.; Bonjer, H.J.; Meijer, G.A.; te Velde, E.A. Cytoreductive surgery and HIPEC for peritoneal metastases combined with curative treatment of colorectal liver metastases. Systematic review of all literature and meta-analysis of observational studies. Cancer Treat. Rev. 2013, 39, 321–327. [Google Scholar] [CrossRef] [PubMed]
- El-Nakeep, S.; Rashad, N.; Oweira, H.; Schmidt, J.; Helbling, D.; Giryes, A.; Petrausch, U.; Mehrabi, A.; Decker, M.; Abdel-Rahman, O. Intraperitoneal chemotherapy and cytoreductive surgery for peritoneal metastases coupled with curative treatment of colorectal liver metastases: An updated systematic review. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 249–258. [Google Scholar] [CrossRef]
- Weber, T.; Roitman, M.; Link, K.H. Current status of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis from colorectal cancer. Clin. Colorectal Cancer 2012, 11, 167–176. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wlodarczyk, J.R.; Lee, S.W. New Frontiers in Management of Early and Advanced Rectal Cancer. Cancers 2022, 14, 938. https://doi.org/10.3390/cancers14040938
Wlodarczyk JR, Lee SW. New Frontiers in Management of Early and Advanced Rectal Cancer. Cancers. 2022; 14(4):938. https://doi.org/10.3390/cancers14040938
Chicago/Turabian StyleWlodarczyk, Jordan R., and Sang W. Lee. 2022. "New Frontiers in Management of Early and Advanced Rectal Cancer" Cancers 14, no. 4: 938. https://doi.org/10.3390/cancers14040938
APA StyleWlodarczyk, J. R., & Lee, S. W. (2022). New Frontiers in Management of Early and Advanced Rectal Cancer. Cancers, 14(4), 938. https://doi.org/10.3390/cancers14040938




