The Role of Simultaneous Integrated Boost in Locally Advanced Rectal Cancer Patients with Positive Lateral Pelvic Lymph Nodes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Imaging
2.3. Neoadjuvant Treatment
2.4. Surgery
Clinical Workflow
- oxaliplatin iv 50 mg/m2/day 1, 8, 21, 28, and 5-Fluorouracil 250 mg/m2/day days 1–7 during the 1st–2nd and 4th–5th week of radiotherapy
- oxaliplatin iv 60 mg/m2/day g1 q7 and capecitabine per os 1300 mg/m2/day 1–7 days
- 5-fluorouracil iv 225 mg/m2/day in continuous infusion days 1–7 q7
- capecitabine per os 1650 mg/m2/day 1–7 q7
- capecitabine per os 1650 mg/m2/day days 1–5 q7 and avelumab iv 10 mg/kg/dieg1 q14
2.5. Follow-Up
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aly, E. Time for a Renewed Strategy in the Management of Rectal Cancer: Critical Reflection on the Surgical Management of Rectal Cancer over 100 Years. Dis. Colon Rectum 2014, 57, 399–402. [Google Scholar] [CrossRef] [PubMed]
- On, J.; Aly, E.H. “Watch and Wait” in Rectal Cancer: Summary of the Current Evidence. Int. J. Colorectal Dis. 2018, 33, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.; Petrelli, N.; Carlin, A.; Couture, J.; Fleshman, J.; Guillem, J.; Miedema, B.; Ota, D.; Sargent, D. Guidelines 2000 for Colon and Rectal Cancer Surgery. J. Natl. Cancer Inst. 2001, 93, 583–596. [Google Scholar] [CrossRef]
- Koukourakis, G.V. Role of Radiation Therapy in Neoadjuvant Era in Patients with Locally Advanced Rectal Cancer. World J. Gastrointest. Oncol. 2012, 4, 230. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, H.; Kawai, K.; Hata, K.; Tanaka, T.; Nishikawa, T.; Sasaki, K.; Kaneko, M.; Murono, K.; Emoto, S.; Morikawa, T.; et al. Correlations between the Recurrence Patterns and Sizes of Lateral Pelvic Lymph Nodes before and after Chemoradiotherapy in Patients with Lower Rectal Cancer. Oncology 2019, 96, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, T.H.; Kim, D.Y.; Kim, S.Y.; Baek, J.Y.; Chang, H.J.; Park, S.C.; Park, J.W.; Oh, J.H. Can Chemoradiation Allow for Omission of Lateral Pelvic Node Dissection for Locally Advanced Rectal Cancer? J. Surg. Oncol. 2015, 111, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Ogura, A.; Konishi, T.; Cunningham, C.; Garcia-Aguilar, J.; Iversen, H.; Toda, S.; Lee, I.K.; Lee, H.X.; Uehara, K.; Lee, P.; et al. Neoadjuvant (Chemo)Radiotherapy With Total Mesorectal Excision Only Is Not Sufficient to Prevent Lateral Local Recurrence in Enlarged Nodes: Results of the Multicenter Lateral Node Study of Patients With Low CT3/4 Rectal Cancer. J. Clin. Oncol. 2019, 37, 33. [Google Scholar] [CrossRef]
- Hida, J.; Yasutomi, M.; Fujimoto, K.; Maruyama, T.; Okuno, K.; Shindo, K. Does Lateral Lymph Node Dissection Improve Survival in Rectal Carcinoma? Examination of Node Metastases by the Clearing Method. J. Am. Coll. Surg. 1997, 184, 475–480. [Google Scholar] [PubMed]
- Yagi, R.; Shimada, Y.; Kameyama, H.; Tajima, Y.; Okamura, T.; Sakata, J.; Kobayashi, T.; Kosugi, S.; Wakai, T.; Nogami, H.; et al. Clinical Significance of Extramural Tumor Deposits in the Lateral Pelvic Lymph Node Area in Low Rectal Cancer: A Retrospective Study at Two Institutions. Ann. Surg. Oncol. 2016, 23, 552. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, K.; Kobayashi, H.; Kato, T.; Mori, T.; Mochizuki, H.; Kameoka, S.; Shirouzu, K.; Muto, T. Indication and Benefit of Pelvic Sidewall Dissection for Rectal Cancer. Dis. Colon Rectum 2006, 49, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Akasu, T.; Mizusawa, J.; Saito, N.; Kinugasa, Y.; Kanemitsu, Y.; Ohue, M.; Fujii, S.; Shiozawa, M.; Yamaguchi, T.; et al. Postoperative Morbidity and Mortality after Mesorectal Excision with and without Lateral Lymph Node Dissection for Clinical Stage II or Stage III Lower Rectal Cancer (JCOG0212): Results from a Multicentre, Randomised Controlled, Non-Inferiority Trial. Lancet Oncol. 2012, 13, 616–621. [Google Scholar] [CrossRef]
- Georgiou, P.; Tan, E.; Gouvas, N.; Antoniou, A.; Brown, G.; Nicholls, R.J.; Tekkis, P. Extended Lymphadenectomy versus Conventional Surgery for Rectal Cancer: A Meta-Analysis. Lancet Oncol. 2009, 10, 1053–1062. [Google Scholar] [CrossRef]
- Saito, S.; Fujita, S.; Mizusawa, J.; Kanemitsu, Y.; Saito, N.; Kinugasa, Y.; Akazai, Y.; Ota, M.; Ohue, M.; Komori, K.; et al. Male Sexual Dysfunction after Rectal Cancer Surgery: Results of a Randomized Trial Comparing Mesorectal Excision with and without Lateral Lymph Node Dissection for Patients with Lower Rectal Cancer: Japan Clinical Oncology Group Study JCOG0212. Eur. J. Surg. Oncol. 2016, 42, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Kusters, M.; Beets, G.; van de Velde, C.J.H.; Beets-Tan, R.G.H.; Marijnen, C.; Rutten, H.J.T.; Putter, H.; Moriya, Y. A Comparison between the Treatment of Low Rectal Cancer in Japan and the Netherlands, Focusing on the Patterns of Local Recurrence. Ann. Surg. 2009, 249, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Hida, J.-I.; Ike, H.; Kinugasa, T.; Ota, M.; Shinto, E.; Itabashi, M.; Kameoka, S.; Sugihara, K. Selection of Lymph Node-Positive Cases Based on Perirectal and Lateral Pelvic Lymph Nodes Using Magnetic Resonance Imaging: Study of the Japanese Society for Cancer of the Colon and Rectum. Ann. Surg. Oncol. 2016, 23, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, T.; Matsueda, K.; Hiratsuka, M.; Unno, T.; Nagata, J.; Nagasaki, T.; Konishi, T.; Fujimoto, Y.; Nagayama, S.; Fukunaga, Y.; et al. Indications for Lateral Pelvic Lymph Node Dissection Based on Magnetic Resonance Imaging Before and After Preoperative Chemoradiotherapy in Patients with Advanced Low-Rectal Cancer. Ann. Surg. Oncol. 2015, 22 (Suppl. S3), 614–620. [Google Scholar] [CrossRef] [PubMed]
- Beets-Tan, R.G.H.; Lambregts, D.M.J.; Maas, M.; Bipat, S.; Barbaro, B.; Curvo-Semedo, L.; Fenlon, H.M.; Gollub, M.J.; Gourtsoyianni, S.; Halligan, S.; et al. Magnetic Resonance Imaging for Clinical Management of Rectal Cancer: Updated Recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) Consensus Meeting. Eur. Radiol. 2018, 28, 1465–1475. [Google Scholar] [CrossRef]
- Han, D.; Qin, Q.; Hao, S.; Huang, W.; Wei, Y.; Zhang, Z.; Wang, Z.; Li, B. Feasibility and Efficacy of Simultaneous Integrated Boost Intensity-Modulated Radiation Therapy in Patients with Limited-Disease Small Cell Lung Cancer. Radiat. Oncol. 2014, 9, 280. [Google Scholar] [CrossRef][Green Version]
- Jeter, M.D.; Gomez, D.; Nguyen, Q.N.; Komaki, R.; Zhang, X.; Zhu, X.; O’Reilly, M.; Fossella, F.V.; Xu, T.; Wei, X.; et al. Simultaneous Integrated Boost for Radiation Dose Escalation to the Gross Tumor Volume with Intensity-Modulated (Photon) Radiation Therapy or Intensity-Modulated Proton Therapy and Concurrent Chemotherapy for Stage II–III Non-Small Cell Lung Cancer: A Phase I Study. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 730. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Meng, L.; Zhu, F.; Ren, G. Dosimetric Feasibility on Hypofractionated Intensity-Modulated Radiotherapy and Simultaneous Integrated Boost for Locally Advanced Unresectable Pancreatic Cancer with Helical Tomotherapy. J. Gastrointest. Oncol. 2021, 12, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Nakamura, A.; Masaki, T.; Sugiyama, M.; Nitatori, T.; Ohkura, Y.; Sakamoto, A.; Atomi, Y. Optimal Diagnostic Criteria for Lateral Pelvic Lymph Node Metastasis in Rectal Carcinoma. Anticancer Res. 2007, 27, 3529–3533. [Google Scholar]
- Benson, A.B., III; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; et al. Rectal Cancer, Version 2.2018 Clinical Practice Guidelines in Oncology. JNCCN J. Natl. Compr. Cancer Netw. 2018, 16, 874–901. [Google Scholar] [CrossRef] [PubMed]
- Emile, S.H.; Elfeki, H.; Shalaby, M.; Sakr, A.; Kim, N.K. Outcome of Lateral Pelvic Lymph Node Dissection with Total Mesorectal Excision in Treatment of Rectal Cancer: A Systematic Review and Meta-Analysis. Surgery 2021, 169, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Santiago, I.; Pares, O.; Carvalho, C.; Figueiredo, N.; Heald, R.J. The Perfect Total Mesorectal Excision Obviates the Need for Anything Else in the Management of Most Rectal Cancers. Clin. Colon Rectal Surg. 2017, 30, 324–332. [Google Scholar] [CrossRef]
- Inoue, Y.; Saigusa, S.; Hiro, J.; Toiyama, Y.; Araki, T.; Tanaka, K.; Mohri, Y.; Kusunoki, M. Clinical Significance of Enlarged Lateral Pelvic Lymph Nodes before and after Preoperative Chemoradiotherapy for Rectal Cancer. Mol. Clin. Oncol. 2016, 4, 994–1002. [Google Scholar] [CrossRef]
- Petersen, S.H.; Harling, H.; Kirkeby, L.T.; Wille-Jørgensen, P.; Mocellin, S. Postoperative Adjuvant Chemotherapy in Rectal Cancer Operated for Cure. Cochrane Database Syst. Rev. 2012, 2012, CD004078. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Muro, K.; Ajioka, Y.; Hashiguchi, Y.; Ito, Y.; Saito, Y.; Hamaguchi, T.; Ishida, H.; Ishiguro, M.; Ishihara, S.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2016 for the Treatment of Colorectal Cancer. Int. J. Clin. Oncol. 2018, 23, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Burton, S.; Brown, G.; Daniels, I.; Norman, A.; Swift, I.; Abulafi, M.; Wotherspoon, A.; Tait, D. MRI Identified Prognostic Features of Tumors in Distal Sigmoid, Rectosigmoid, and Upper Rectum: Treatment with Radiotherapy and Chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Beets-Tan, R.G.H. Pretreatment MRI of Lymph Nodes in Rectal Cancer: An Opinion-Based Review. Colorectal Dis. 2013, 15, 781–784. [Google Scholar] [CrossRef]
- Dayal, S.; Moran, B. LOREC: The English Low Rectal Cancer National Development Programme. Br. J. Hosp. Med. 2013, 74, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Arbea, L.; Ramos, L.I.; Martínez-Monge, R.; Moreno, M.; Aristu, J. Intensity-Modulated Radiation Therapy (IMRT) vs. 3D Conformal Radiotherapy (3DCRT) in Locally Advanced Rectal Cancer (LARC): Dosimetric Comparison and Clinical Implications. Radiat. Oncol. 2010, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Mok, H.; Crane, C.H.; Palmer, M.B.; Briere, T.M.; Beddar, S.; Delclos, M.E.; Krishnan, S.; Das, P. Intensity Modulated Radiation Therapy (IMRT): Differences in Target Volumes and Improvement in Clinically Relevant Doses to Small Bowel in Rectal Carcinoma. Radiat. Oncol. 2011, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Engels, B.; Platteaux, N.; Van Den Begin, R.; Gevaert, T.; Sermeus, A.; Storme, G.; Verellen, D.; De Ridder, M. Preoperative Intensity-Modulated and Image-Guided Radiotherapy with a Simultaneous Integrated Boost in Locally Advanced Rectal Cancer: Report on Late Toxicity and Outcome. Radiother. Oncol. 2014, 110, 155–159. [Google Scholar] [CrossRef] [PubMed]
- But-Hadzic, J.; Velenik, V. Preoperative Intensity-Modulated Chemoradiation Therapy with Simultaneous Integrated Boost in Rectal Cancer: 2-Year Follow-up Results of Phase II Study. Radiol. Oncol. 2018, 52, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Geng, J.; Wang, L.; Teng, H.; Wang, Z.; Zhu, X.; Zhang, Y.; Wang, H.; Li, Y.; Cai, Y.; et al. Effect of Simultaneous Integrated Boost Intensity Modulated Radiation Therapy (SIB-IMRT) and Non-Operative Strategy on Outcomes of Distal Rectal Cancer Patients with Clinically Positive Lateral Pelvic Lymph Node. Cancer Manag. Res. 2021, 13, 537–546. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Yu, Y.; Zhu, X.; Geng, J.; Teng, H.; Wang, Z.; Sun, T.; Wang, L.; Wang, H.; et al. Simultaneous Integrated Boost Intensity-Modulated Radiation Therapy Can Benefit the Locally Advanced Rectal Cancer Patients With Clinically Positive Lateral Pelvic Lymph Node. Front. Oncol. 2021, 10, 3429. [Google Scholar] [CrossRef] [PubMed]
- Bujko, K.; Nowacki, M.P.; Nasierowska-Guttmejer, A.; Michalski, W.; Bebenek, M.; Kryj, M. Long-Term Results of a Randomized Trial Comparing Preoperative Short-Course Radiotherapy with Preoperative Conventionally Fractionated Chemoradiation for Rectal Cancer. Br. J. Surg. 2006, 93, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Ciseł, B.; Pietrzak, L.; Michalski, W.; Wyrwicz, L.; Rutkowski, A.; Kosakowska, E.; Cencelewicz, A.; Spałek, M.; Polkowski, W.; Jankiewicz, M.; et al. Long-Course Preoperative Chemoradiation versus 5 × 5 Gy and Consolidation Chemotherapy for Clinical T4 and Fixed Clinical T3 Rectal Cancer: Long-Term Results of the Randomized Polish II Study. Ann. Oncol. 2019, 30, 1298–1303. [Google Scholar] [CrossRef]
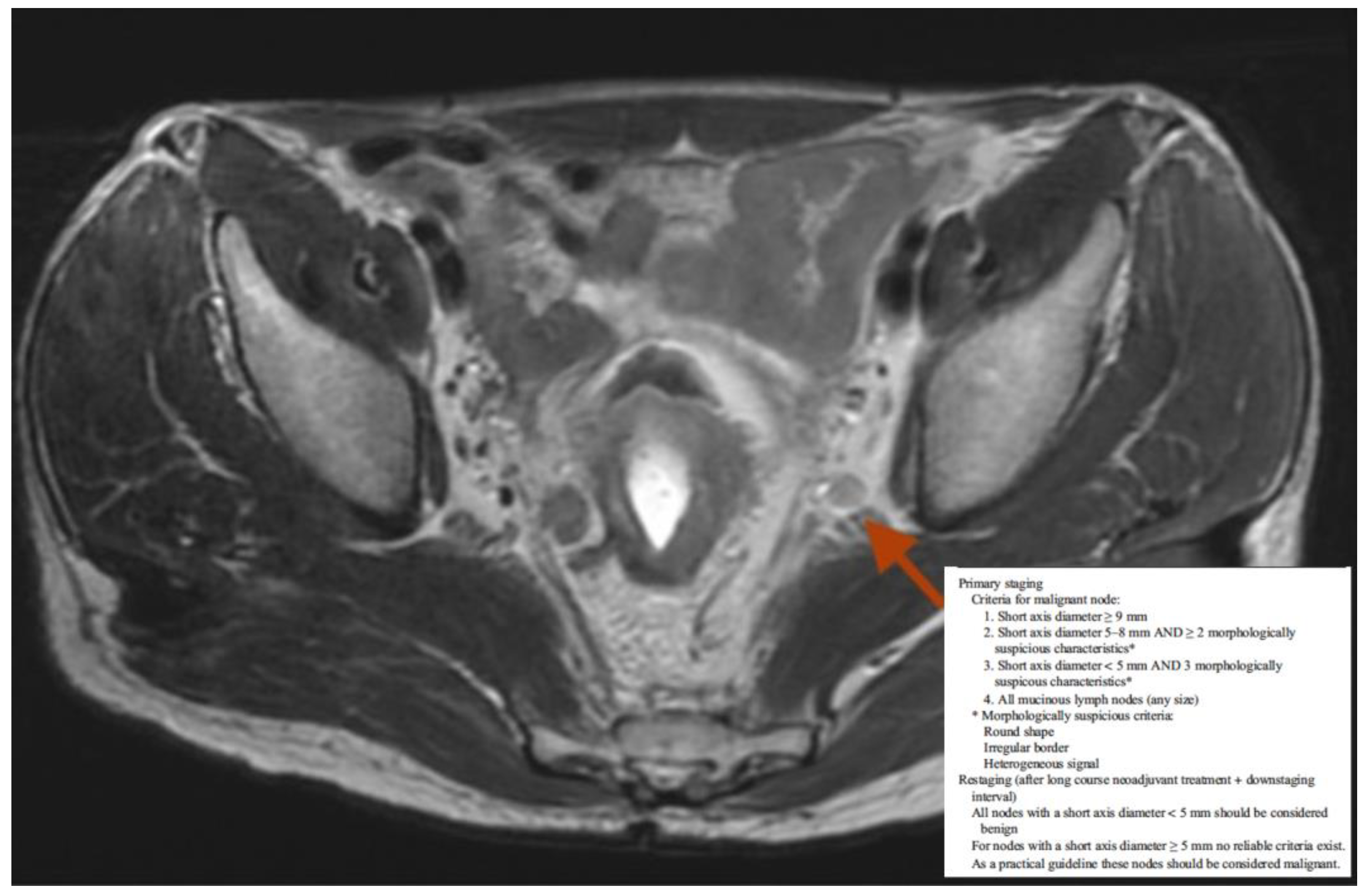


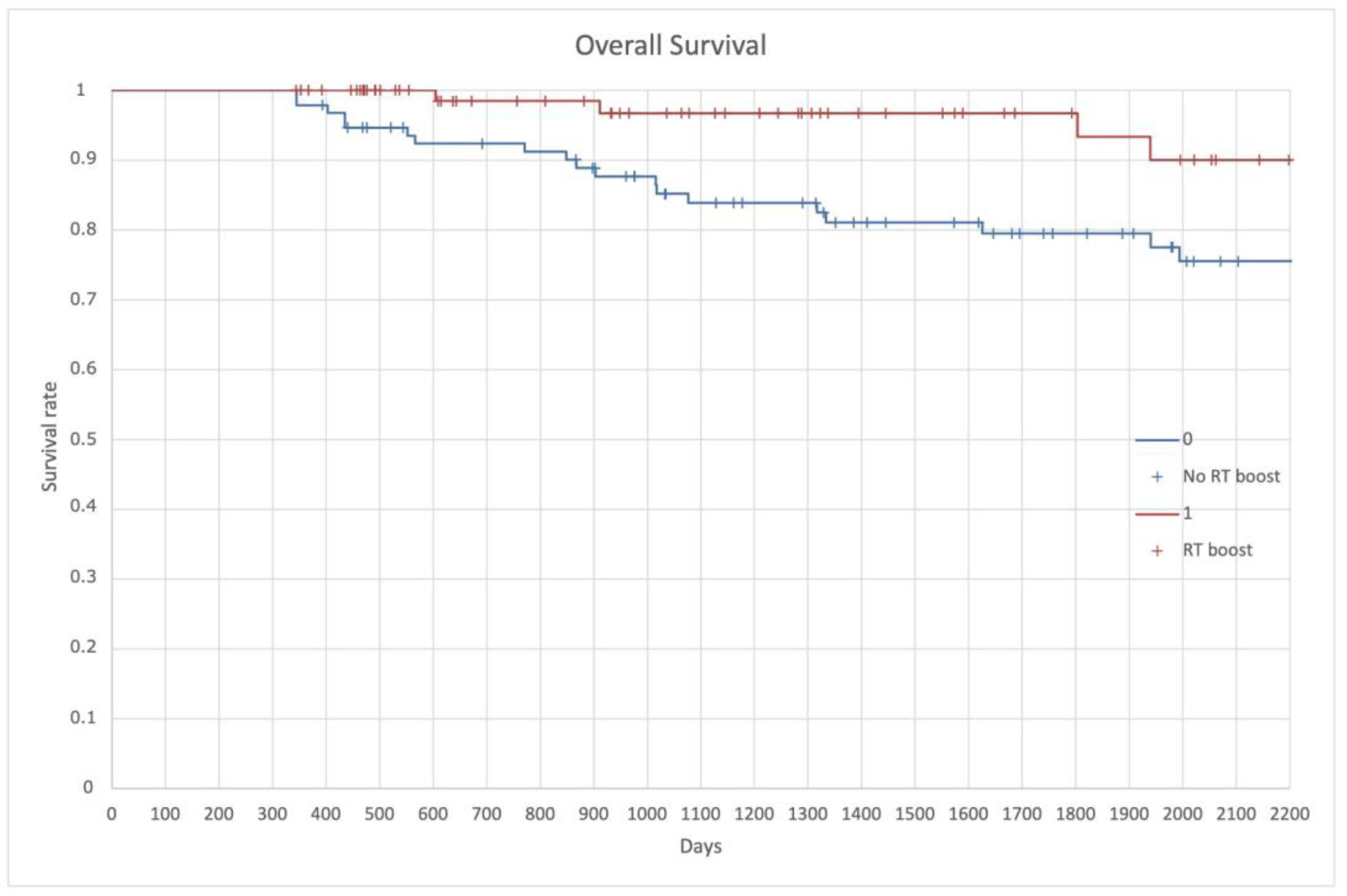
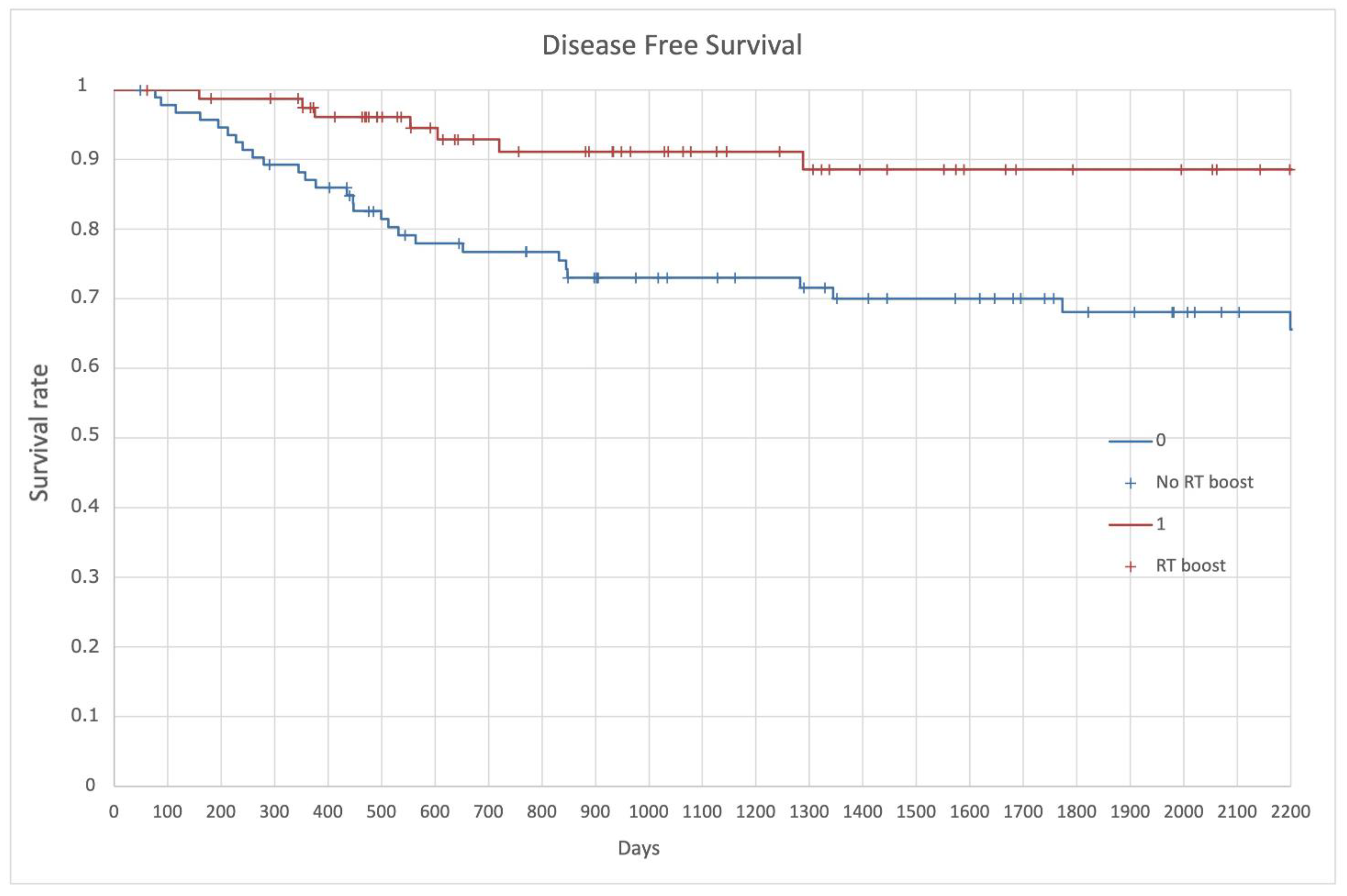

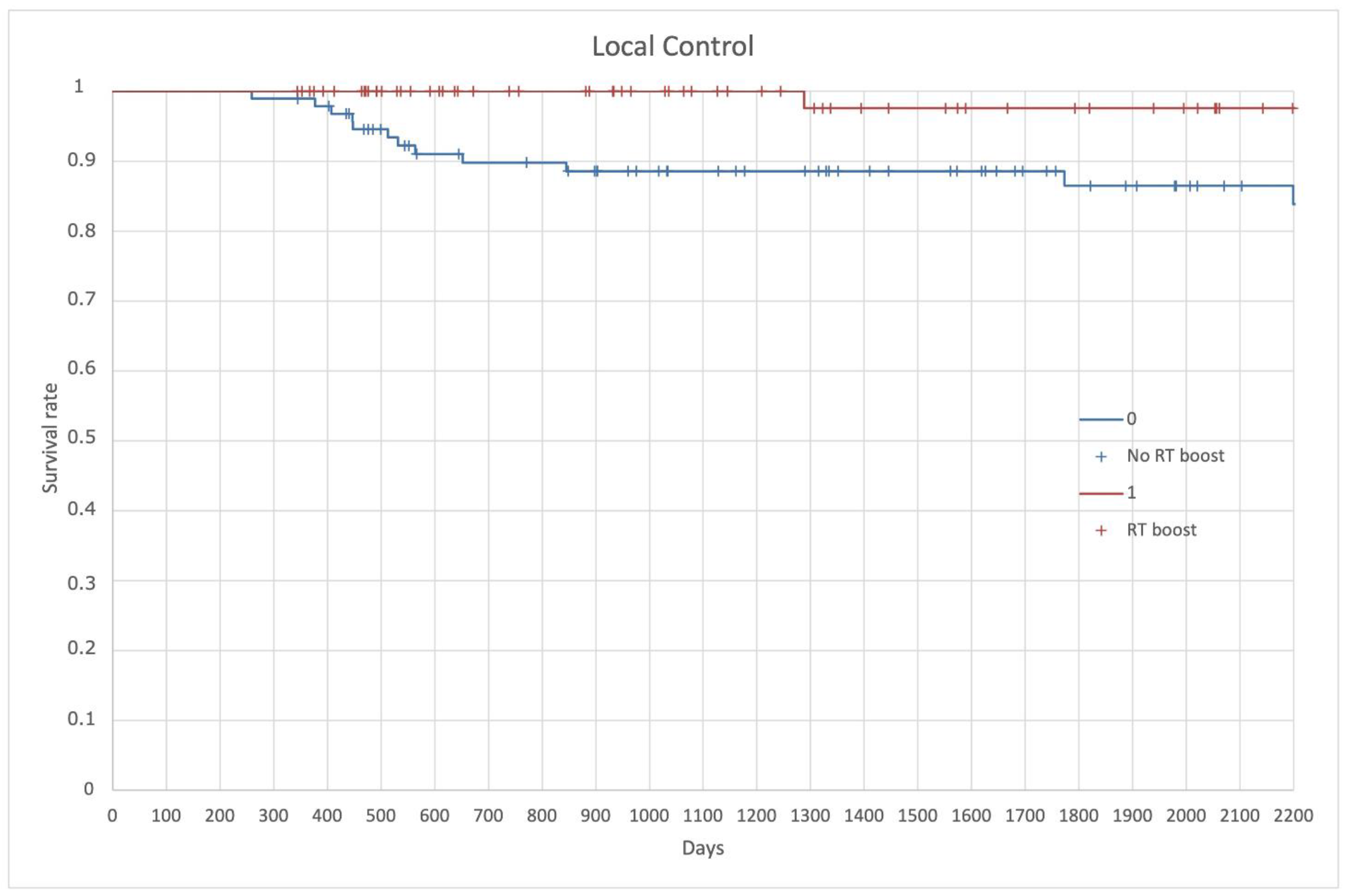
| Inclusion Criteria |
| • Histologically documented adenocarcinoma of the rectum; • Minimum age of 18; • Locally advanced non-metastatic rectal cancer; • Undergoing neoadjuvant CRT treatment followed or not by TME surgery; • MRI pelvis staging and restaging available; • Follow-up of at least one year; • Signature of informed consent to the processing of their data, if applicable. |
| Exclusion Criteria |
| • Patients treated for palliative purposes; • Patients with metastatic disease. |
| Patient’s Characteristics | Boost Yes | Boost No | p Value § |
|---|---|---|---|
| Sex | 0.368 | ||
| Male | 46 (56.1%) | 59 (62.8%) | |
| Female | 36 (43.9%) | 35 (37.2%) | |
| Age | 0.760 | ||
| ≤50 years | 9 (11%) | 9 (9.6%) | |
| <50 years | 73 (89%) | 85 (90.4%) | |
| cT | 0.644 | ||
| 2 | 1 (1.2%) | 2 (2.1%) | |
| 3 | 49 (59.8%) | 50 (53.2%) | |
| 4 | 32 (39%) | 42 (44.7%) | |
| cN | 0.577 | ||
| 1 | 19 (23.2%) | 21 (22.3%) | |
| 2 | 63 (76.8%) | 73 (77.7%) | |
| EMVI | 0.001 | ||
| + | 9 (11%) | 5 (5.3%) | |
| − | 71 (86.6%) | 69 (73.4%) | |
| N/A | 2 (2.4%) | 20 (21.3%) | |
| Mucinous | 0.003 | ||
| Yes | 4 (4.9%) | 6 (6.4%) | |
| No | 76 (92.7%) | 71 (75.5%) | |
| N/A | 2 (2.4%) | 17 (18.1%) | |
| MRF | 0.834 | ||
| + | 40 (48.8%) | 48 (51.1%) | |
| − | 38 (46.3%) | 43 (45.7%) | |
| N/A | 4 (4.9%) | 3 (3.2%) | |
| Surgery Type | 0.101 | ||
| Anterior Resection (AR) | 53 (64.6%) | 56 (59.6%) | |
| Abdominoperineal Resection (APR) | 20 (24.4%) | 20 (21.3%) | |
| Hartmann | 1 (1.2%) | 5 (5.3%) | |
| Local Excision | 7 (8.5%) | 5 (5.3%) | |
| Colostomy | 1 (1.2%) | 8 (8.5%) | |
| RT dose on T | <0.001 | ||
| 55 Gy | 78 (95.1%) | 71 (75.5%) | |
| <55 Gy | 4 (4.9%) | 23 (24.5%) | |
| RT dose on LPLN | 8.45 | ||
| 55 Gy | 78 (95.1%) | 0 (0%) | |
| <55 Gy | 4 (4.9%) | 94 (100%) |
| Patient’s Characteristics | OS | DFS | MFS | LC | ||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |
| p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | |
| Gender | 0.09 | 0.29 | 0.42 | 0.44 | ||||
| Age | 0.50 | 0.25 | 0.24 | 0.27 | ||||
| cT | 0.04 | 0.01 | 0.03 | 0.05 | 0.04 | 0.02 | 0.22 | 0.16 |
| cN | 0.74 | 0.37 | 0.07 | 0.58 | ||||
| EMVI | 0.46 | 0.98 | 0.99 | 0.99 | ||||
| Mucinous | 0.63 | 0.98 | 0.99 | 0.99 | ||||
| Mesorectal Fascia (MRF) | 0.63 | 0.61 | 0.37 | 0.15 | ||||
| Surgery Type | 0.17 | 0.02 | 0.07 | 0.29 | <0.001 | 0.01 | ||
| RT dose on T | 0.04 | 0.06 | 0.49 | 0.77 | 0.15 | |||
| RT dose on LPLN | <0.01 | 0.03 | <0.01 | 0.01 | 0.04 | 0.04 | 0.04 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meldolesi, E.; Chiloiro, G.; Giannini, R.; Menghi, R.; Persiani, R.; Corvari, B.; Coco, C.; Manfrida, S.; Ratto, C.; De Luca, V.; et al. The Role of Simultaneous Integrated Boost in Locally Advanced Rectal Cancer Patients with Positive Lateral Pelvic Lymph Nodes. Cancers 2022, 14, 1643. https://doi.org/10.3390/cancers14071643
Meldolesi E, Chiloiro G, Giannini R, Menghi R, Persiani R, Corvari B, Coco C, Manfrida S, Ratto C, De Luca V, et al. The Role of Simultaneous Integrated Boost in Locally Advanced Rectal Cancer Patients with Positive Lateral Pelvic Lymph Nodes. Cancers. 2022; 14(7):1643. https://doi.org/10.3390/cancers14071643
Chicago/Turabian StyleMeldolesi, Elisa, Giuditta Chiloiro, Roberta Giannini, Roberta Menghi, Roberto Persiani, Barbara Corvari, Claudio Coco, Stefania Manfrida, Carlo Ratto, Viola De Luca, and et al. 2022. "The Role of Simultaneous Integrated Boost in Locally Advanced Rectal Cancer Patients with Positive Lateral Pelvic Lymph Nodes" Cancers 14, no. 7: 1643. https://doi.org/10.3390/cancers14071643
APA StyleMeldolesi, E., Chiloiro, G., Giannini, R., Menghi, R., Persiani, R., Corvari, B., Coco, C., Manfrida, S., Ratto, C., De Luca, V., Sofo, L., Reina, S., Crucitti, A., Masiello, V., Dinapoli, N., Valentini, V., & Gambacorta, M. A. (2022). The Role of Simultaneous Integrated Boost in Locally Advanced Rectal Cancer Patients with Positive Lateral Pelvic Lymph Nodes. Cancers, 14(7), 1643. https://doi.org/10.3390/cancers14071643








