Compared Efficacy of Adjuvant Intravesical BCG-TICE vs. BCG-RIVM for High-Risk Non-Muscle Invasive Bladder Cancer (NMIBC): A Propensity Score Matched Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Eligibility CRITERIA
2.2. BCG Protocol, Treatment Schedule, and Strains Administered
2.3. Statistical Analysis
3. Results
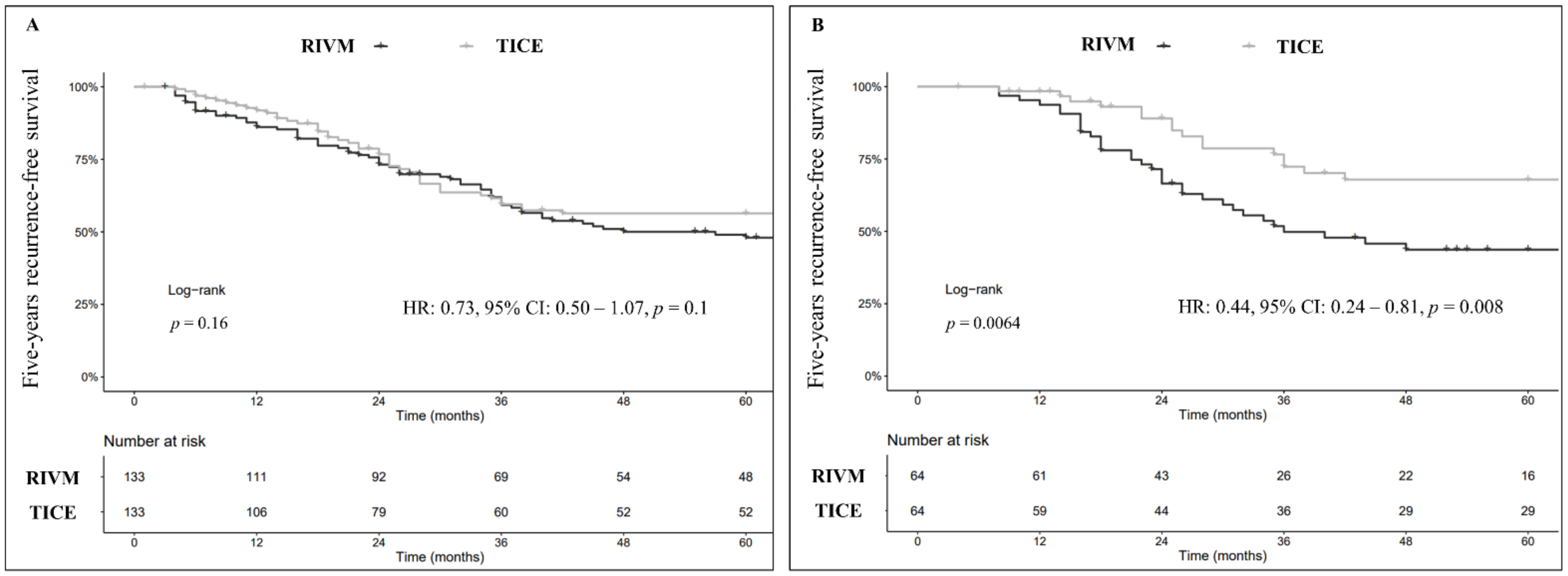
3.1. Recurrence-Free Survival (RFS)
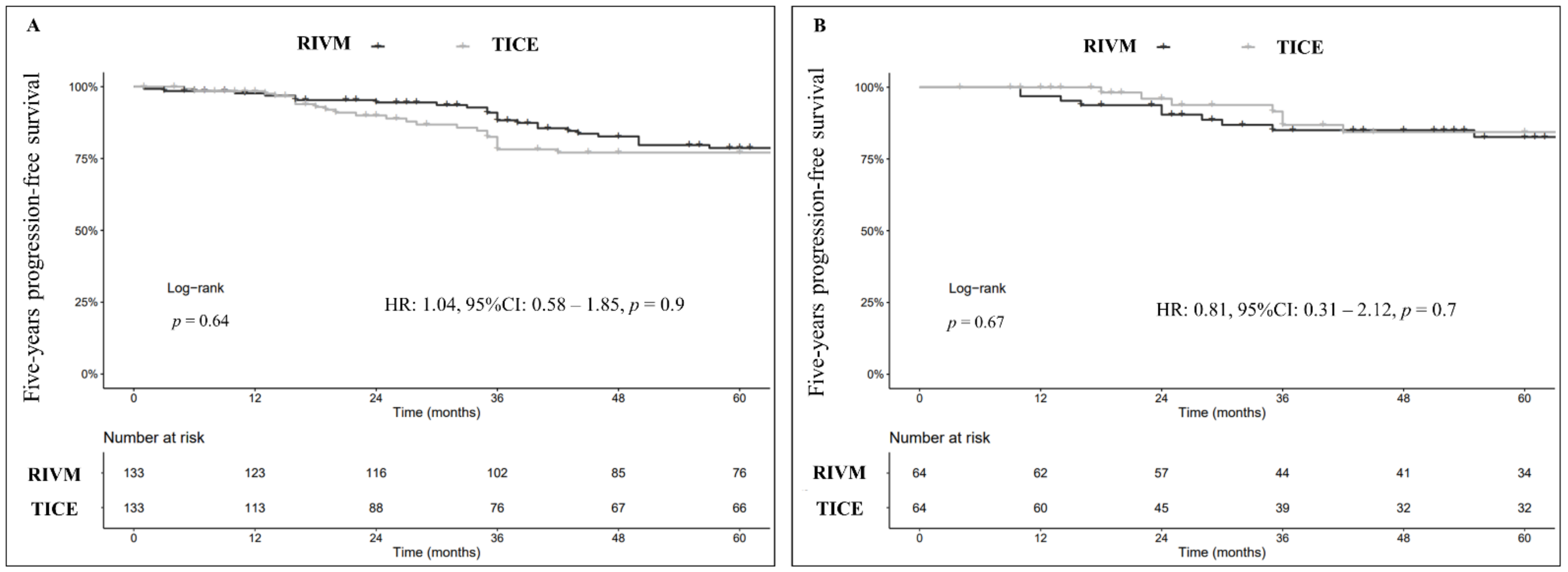
3.2. Progression-Free Survival (PFS)
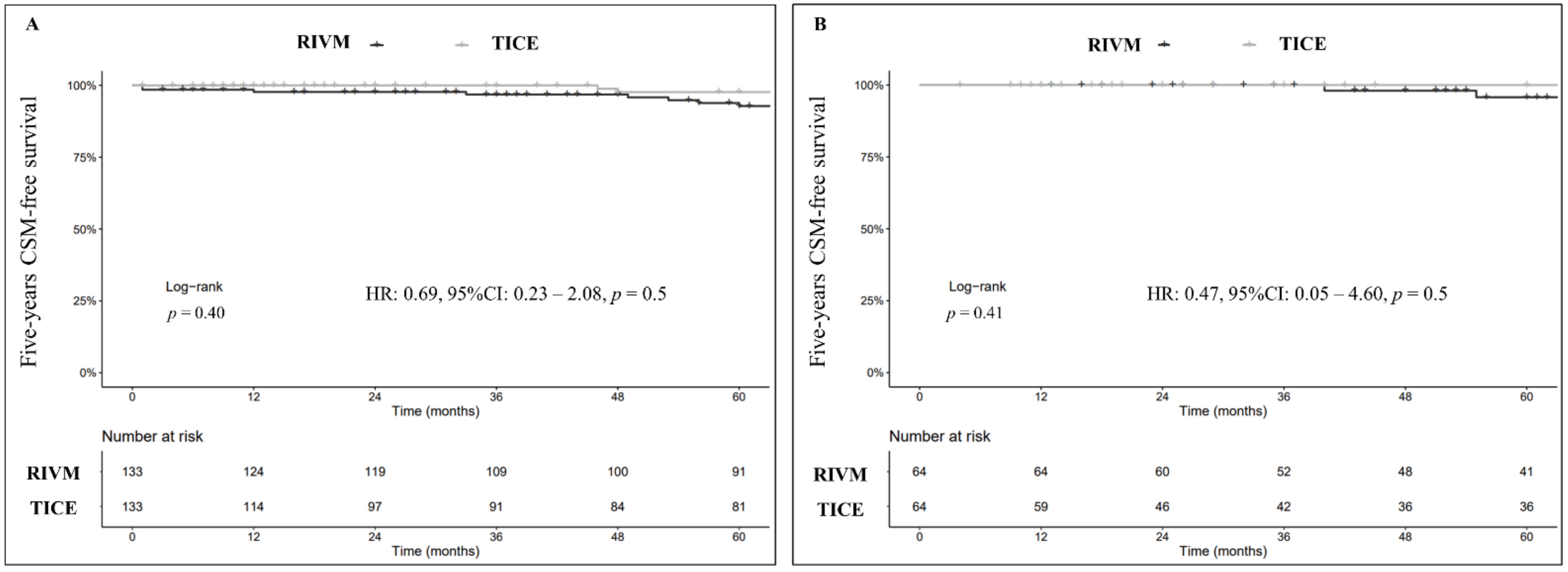
3.3. Cancer-Specific Survival (CSS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morales, A.; Eidinger, D.; Bruce, A.W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 1976, 116, 180–182. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-Muscle-Invasive Bladder Cancer (Ta, T1, and Carcinoma In Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-Invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network: Bladder Cancer (Version 5.2021). Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1440 (accessed on 28 January 2022).
- Lamm, D.L.; Blumenstein, B.A.; Crissman, J.D.; Montie, J.E.; Gottesman, J.E.; Lowe, B.A.; Sarosdy, M.F.; Bohl, R.D.; Grossman, H.B.; Beck, T.M.; et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent Ta, T1 and carcinoma in situ transitional cell carcinoma of the bladder: A randomized Southwest Oncology Group study. J. Urol. 2000, 163, 1124–1129. [Google Scholar] [CrossRef]
- Böhle, A.; Bock, P.R. Intravesical bacille Calmette-Guérin versus mitomycin C in superficial bladder cancer: Formal meta-analysis of comparative studies on tumor progression. Urology 2004, 63, 682–686. [Google Scholar] [CrossRef]
- Oddens, J.; Brausi, M.; Sylvester, R.; Bono, A.; Van De Beek, C.; Van Andel, G.; Gontero, P.; Hoeltl, W.; Turkeri, L.; Marreaud, S.; et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: One-third dose versus full dose and 1 year versus 3 years of maintenance. Eur. Urol. 2013, 63, 462–472. [Google Scholar] [CrossRef]
- Grimm, M.O.; van der Heijden, A.G.; Colombel, M.; Muilwijk, T.; Martínez-Piñeiro, L.; Babjuk, M.M.; Türkeri, L.N.; Palou, J.; Patel, A.; Bjartell, A.S.; et al. Treatment of High-Grade Non-Muscle-Invasive Bladder Carcinoma by Standard Number and Dose of BCG Instillations Versus Reduced Number and Standard Dose of BCG Instillations: Results of the European Association of Urology Research Foundation Randomised Phase III Clinical Trial ‘NIMBUS’. Eur. Urol. 2020, 78, 690–698. [Google Scholar] [CrossRef]
- Sylvester, R.J.; Van der Meijden, A.P.M.; Lamm, D.L. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: A meta-analysis of the published results of randomized clinical trials. J. Urol. 2002, 168, 1964–1970. [Google Scholar] [CrossRef]
- Unda-Urzaiz, M.; Cozar-Olmos, J.M.; Miñana-Lopez, B.; Camarero-Jimenez, J.; Brugarolas-Rossello, X.; Zubiaur-Libano, C.; Ribal-Caparros, M.J.; Suarez-Charneco, A.J.; Rodriguez-Tesedo, V.; Chantada-Abal, V.; et al. Safety and efficacy of various strains of bacille Calmette-Guérin in the treatment of bladder tumours in standard clinical practice. Actas Urol. Esp. 2018, 42, 238–248. [Google Scholar] [CrossRef]
- Steinberg, R.L.; Brooks, N.A.; Thomas, L.J.; Mott, S.L.; O’Donnell, M.A. Bacillus Calmette-Guerin strain may not effect recurrence-free survival when used intravesically with interferon-alpha2b for non-muscle-invasive bladder cancer. Urol. Oncol. 2017, 35, 201–207. [Google Scholar] [CrossRef]
- Del Giudice, F.; Busetto, G.M.; Gross, M.S.; Maggi, M.; Sciarra, A.; Salciccia, S.; Ferro, M.; Sperduti, I.; Flammia, S.; Canale, V.; et al. Efficacy of three BCG strains (Connaught, TICE and RIVM) with or without secondary resection (re-TUR) for intermediate/high-risk non-muscle-invasive bladder cancers: Results from a retrospective single-institution cohort analysis. J. Cancer Res. Clin. Oncol. 2021, 147, 3073–3080. [Google Scholar] [CrossRef]
- Lamm, D.L. Efficacy and safety of bacille Calmette-Guérin immunotherapy in superficial bladder cancer. Clin. Infect. Dis. 2000, 31 (Suppl. S3), S86–S90. [Google Scholar] [CrossRef]
- Gontero, P.; Sylvester, R.; Pisano, F.; Joniau, S.; Vander Eeckt, K.; Serretta, V.; Larré, S.; Di Stasi, S.; Van Rhijn, B.; Witjes, A.J.; et al. Prognostic factors and risk groups in T1G3 non-muscle-invasive bladder cancer patients initially treated with Bacillus Calmette-Guérin: Results of a retrospective multicenter study of 2451 patients. Eur. Urol. 2015, 67, 74–82. [Google Scholar] [CrossRef] [Green Version]
- van Puffelen, J.H.; Keating, S.T.; Oosterwijk, E.; van der Heijden, A.G.; Netea, M.G.; Joosten, L.A.B.; Vermeulen, S.H. Trained immunity as a molecular mechanism for BCG immunotherapy in bladder cancer. Nat. Rev. Urol. 2020, 17, 513–525. [Google Scholar] [CrossRef]
- Cernuschi, T.; Malvolti, S.; Nickels, E.; Friede, M. Bacillus Calmette-Guérin (BCG) vaccine: A global assessment of demand and supply balance. Vaccine 2018, 36, 498–506. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, H.; Wang, Y.; Zhang, C.; Xu, T. Determining optimal maintenance schedules for adjuvant intravesical bacillus Calmette-Guerin immunotherapy in non-muscle-invasive bladder cancer: A systematic review and network meta-analysis. Curr. Med. Res. Opin. 2017, 33, 1379–1387. [Google Scholar] [CrossRef]
- Boehm, B.E.; Cornell, J.E.; Wang, H.; Mukherjee, N.; Oppenheimer, J.S.; Svatek, R.S. Efficacy of bacillus Calmette-Guérin Strains for Treatment of Nonmuscle Invasive Bladder Cancer: A Systematic Review and Network Meta-Analysis. J. Urol. 2017, 198, 503–510. [Google Scholar] [CrossRef]
- Miyake, M.; Tatsumi, Y.; Matsumoto, H.; Nagao, K.; Matsuyama, H.; Inamoto, T.; Azuma, H.; Yasumoto, H.; Shiina, H.; Fujimoto, K. Outcomes of subsequent non-muscle-invasive bladder cancer treated with intravesical Bacillus Calmette-Guérin after radical nephroureterectomy for upper urinary tract urothelial carcinoma. BJU Int. 2018, 121, 764–773. [Google Scholar] [CrossRef] [Green Version]
- Rentsch, C.A.; Birkhäuser, F.D.; Biot, C.; Gsponer, J.R.; Bisiaux, A.; Wetterauer, C.; Lagranderie, M.; Marchal, G.; Orgeur, M.; Bouchier, C.; et al. Bacillus Calmette-Guérin strain differences have an impact on clinical outcome in bladder cancer immunotherapy. Eur. Urol. 2014, 66, 677–688. [Google Scholar] [CrossRef]
- Sengiku, A.; Ito, M.; Miyazaki, Y.; Sawazaki, H.; Takahashi, T.; Ogura, K. A prospective comparative study of intravesical bacillus Calmette-Guérin therapy with the Tokyo or Connaught strain for nonmuscle invasive bladder cancer. J. Urol. 2013, 190, 50–54. [Google Scholar] [CrossRef]
- Hofbauer, S.L.; Shariat, S.F.; Chade, D.C.; Sarkis, A.S.; Ribeiro-Filho, L.A.; Nahas, W.C.; Klatte, T. The Moreau Strain of Bacillus Calmette-Guerin (BCG) for High-Risk Non-Muscle Invasive Bladder Cancer: An Alternative during Worldwide BCG Shortage? Urol. Int. 2016, 96, 46–50. [Google Scholar] [CrossRef]
- Witjes, J.A.; Dalbagni, G.; Karnes, R.J.; Shariat, S.; Joniau, S.; Palou, J.; Serretta, V.; Larré, S.; di Stasi, S.; Colombo, R.; et al. The efficacy of BCG TICE and BCG Connaught in a cohort of 2099 patients with T1G3 non-muscle-invasive bladder cancer. Urol. Oncol. 2016, 34, 484.e19–484.e25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krajewski, W.; Matuszewski, M.; Poletajew, S.; Grzegrzółka, J.; Zdrojowy, R.; Kołodziej, A. Are There Differences in Toxicity and Efficacy between Various Bacillus Calmette-Guerin Strains in Bladder Cancer Patients? Analysis of 844 Patients. Urol. Int. 2018, 101, 277–284. [Google Scholar] [CrossRef]
- Nowak, Ł.; Krajewski, W.; Moschini, M.; Chorbińska, J.; Poletajew, S.; Tukiendorf, A.; Muilwijk, T.; Joniau, S.; Tafuri, A.; Antonelli, A.; et al. Assessment of the oncological outcomes of three different bacillus Calmette-Guérin strains in patients with high-grade T1 non-muscle-invasive bladder cancer. Arab. J. Urol. 2021, 19, 78–85. [Google Scholar] [CrossRef]
- D’Andrea, D.; Soria, F.; Abufaraj, M.; Pones, M.; Gontero, P.; Machado, A.T.; Waksman, R.; Enikeev, D.V.; Glybochko, P.V.; Adonias, S.P.; et al. Comparative Effectiveness of Intravesical BCG-Tice and BCG-Moreau in Patients with Non-Muscle-Invasive Bladder Cancer. Clin. Genitourin. Cancer 2020, 18, 20e.2–25.e2. [Google Scholar] [CrossRef] [Green Version]
| Characteristic | Overall, n = 266 1 | RIVM, n = 133 (50%) 1 | TICE, n = 133 (50%) 1 | p-Value 2 |
|---|---|---|---|---|
| Age, years (IQR) | 68 (64–74) | 68 (64–75) | 68 (62–73) | 0.2 |
| Gender | 0.7 | |||
| male | 201 (75.6%) | 99 (74.4%) | 102 (76.7%) | |
| female | 65 (24.4%) | 34 (25.6%) | 31 (23.3%) | |
| Smoking Status | 0.5 | |||
| No smoker | 105 (39.5%) | 57 (42.9%) | 48 (36.1%) | |
| Former smoker | 73 (27.4%) | 33 (24.8%) | 40 (30.1%) | |
| Active smoker | 88 (33.1%) | 43 (32.3%) | 45 (33.8%) | |
| Tumor Size | >0.9 | |||
| <3 cm | 161 (60.5%) | 80 (60.2%) | 81 (60.9%) | |
| ≥3 cm | 105 (39.5%) | 53 (39.8%) | 52 (39.1%) | |
| Tumor focality | 0.5 | |||
| Unifocal | 101 (38.0%) | 53 (38.8%) | 48 (36.1%) | |
| Multifocal | 165 (62.0%) | 80 (60.2%) | 85 (63.9%) | |
| T stage | 0.9 | |||
| Ta | 73 (27.4%) | 36 (27.1%) | 37 (27.8%) | |
| T1 | 193 (72.6%) | 97 (72.9%) | 96 (72.2%) | |
| Tumor Grade | >0.9 | |||
| LG | 20 (7.5%) | 10 (7.5%) | 10 (7.5%) | |
| HG | 246 (92.5%) | 123 (92.5%) | 123 (92.5%) | |
| Concomitant Cis | 25 (9.4%) | 10 (7.5%) | 15 (11.3%) | 0.3 |
| Previous TURBT | 147 (55.3%) | 75 (56.4%) | 72 (54.1%) | 0.7 |
| Re-TUR | 138 (51.9%) | 63 (47.4%) | 75 (56.4%) | 0.14 |
| BCG schedule | 0.19 | |||
| Only induction | 34 (12.8%) | 21 (15.8%) | 13 (9.8%) | |
| Maintenance | 232 (87.2%) | 112 (84.2%) | 120 (90%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Giudice, F.; Flammia, R.S.; Chung, B.I.; Moschini, M.; Pradere, B.; Mari, A.; Soria, F.; Albisinni, S.; Krajewski, W.; Szydełko, T.; et al. Compared Efficacy of Adjuvant Intravesical BCG-TICE vs. BCG-RIVM for High-Risk Non-Muscle Invasive Bladder Cancer (NMIBC): A Propensity Score Matched Analysis. Cancers 2022, 14, 887. https://doi.org/10.3390/cancers14040887
Del Giudice F, Flammia RS, Chung BI, Moschini M, Pradere B, Mari A, Soria F, Albisinni S, Krajewski W, Szydełko T, et al. Compared Efficacy of Adjuvant Intravesical BCG-TICE vs. BCG-RIVM for High-Risk Non-Muscle Invasive Bladder Cancer (NMIBC): A Propensity Score Matched Analysis. Cancers. 2022; 14(4):887. https://doi.org/10.3390/cancers14040887
Chicago/Turabian StyleDel Giudice, Francesco, Rocco Simone Flammia, Benjamin I. Chung, Marco Moschini, Benjamin Pradere, Andrea Mari, Francesco Soria, Simone Albisinni, Wojciech Krajewski, Tomasz Szydełko, and et al. 2022. "Compared Efficacy of Adjuvant Intravesical BCG-TICE vs. BCG-RIVM for High-Risk Non-Muscle Invasive Bladder Cancer (NMIBC): A Propensity Score Matched Analysis" Cancers 14, no. 4: 887. https://doi.org/10.3390/cancers14040887
APA StyleDel Giudice, F., Flammia, R. S., Chung, B. I., Moschini, M., Pradere, B., Mari, A., Soria, F., Albisinni, S., Krajewski, W., Szydełko, T., Laukhtina, E., D’Andrea, D., Gallioli, A., Mertens, L. S., Maggi, M., Sciarra, A., Salciccia, S., Ferro, M., Scornajenghi, C. M., ... on behalf of European Association of Urology (EAU)—Young Academic Urologists (YAU) Urothelial Cancer Working Party, on behalf of European Association of Urology (EAU)—Young Academic Urologists (YAU) Urothelial Cancer Working Party. (2022). Compared Efficacy of Adjuvant Intravesical BCG-TICE vs. BCG-RIVM for High-Risk Non-Muscle Invasive Bladder Cancer (NMIBC): A Propensity Score Matched Analysis. Cancers, 14(4), 887. https://doi.org/10.3390/cancers14040887
















