Long-Term Results of Kyocera Modular Limb Salvage System after Resection of Tumors in the Distal Part of the Femur: Report from Japanese Musculoskeletal Oncology Group Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Procedure for Tumor Resection
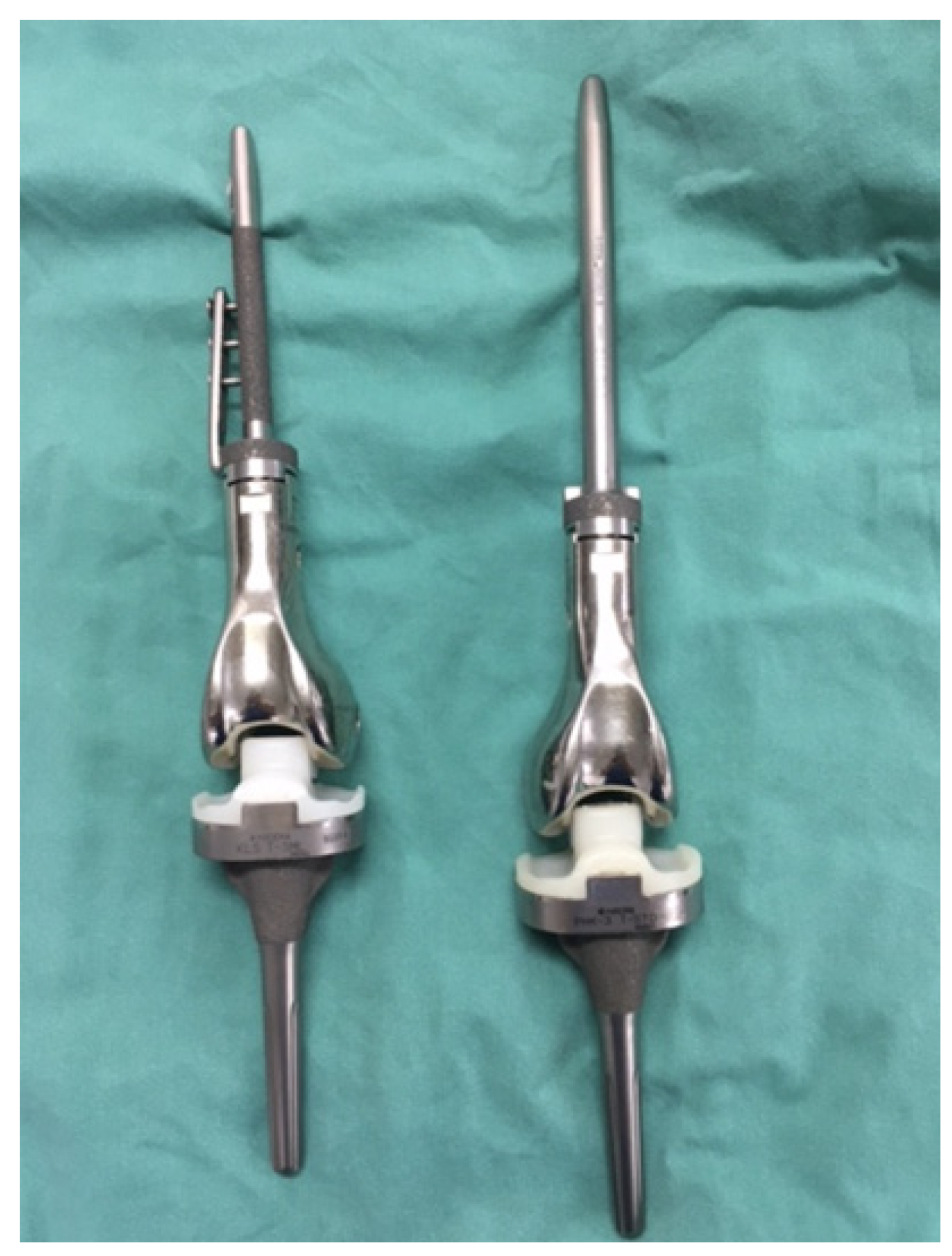
2.3. Prosthesis
2.4. Reconstruction
2.5. Statistical Analysis
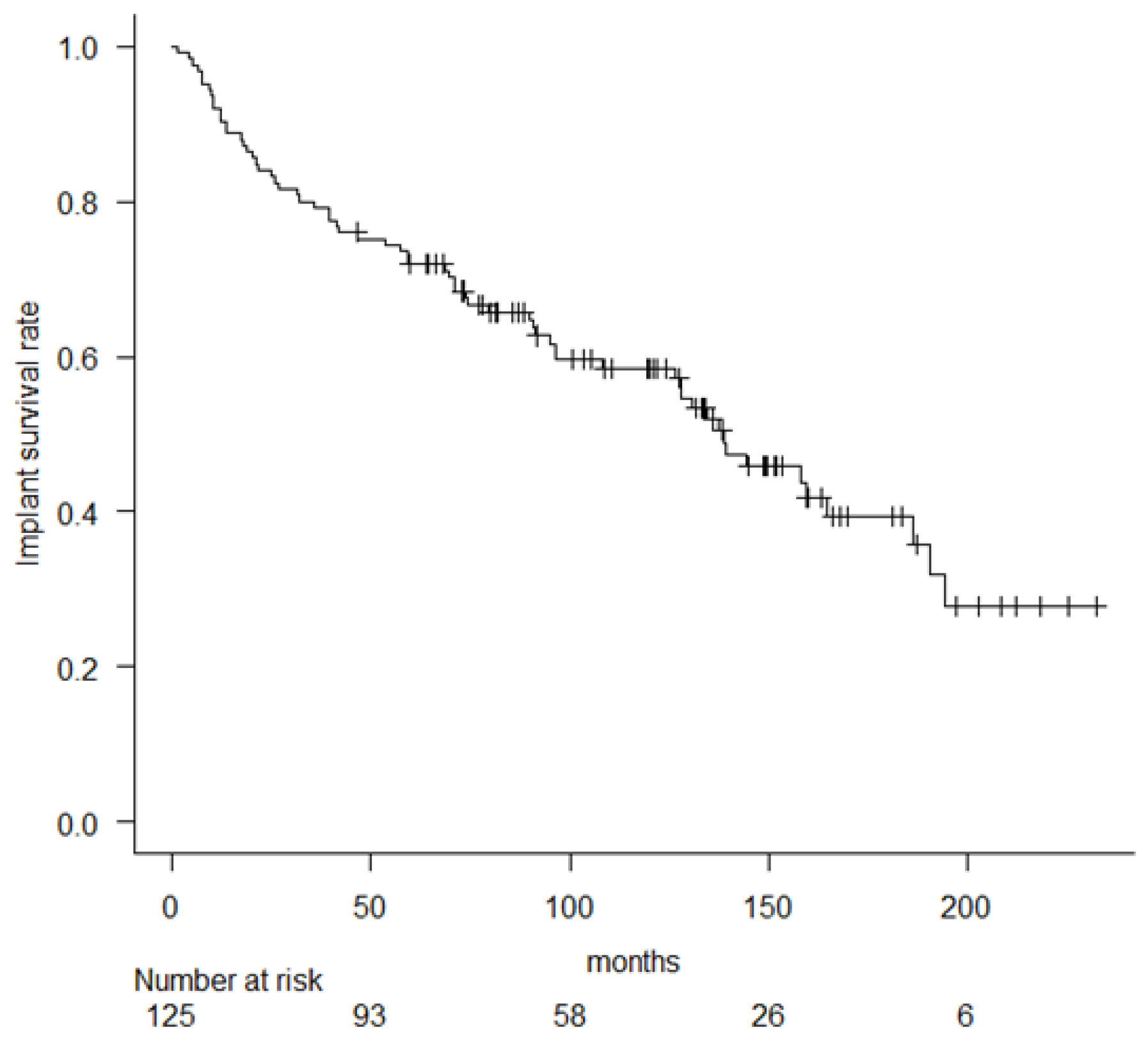
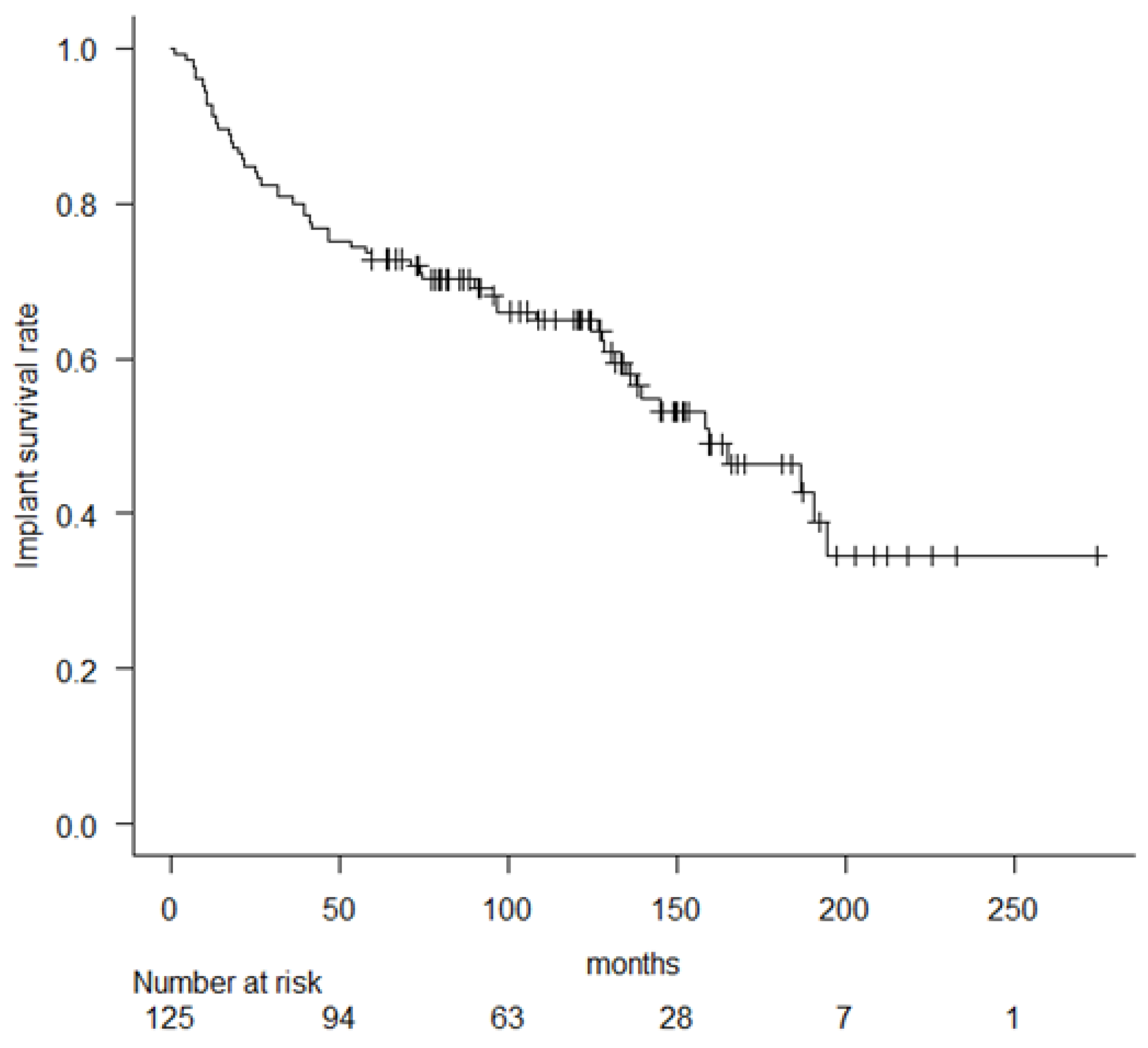
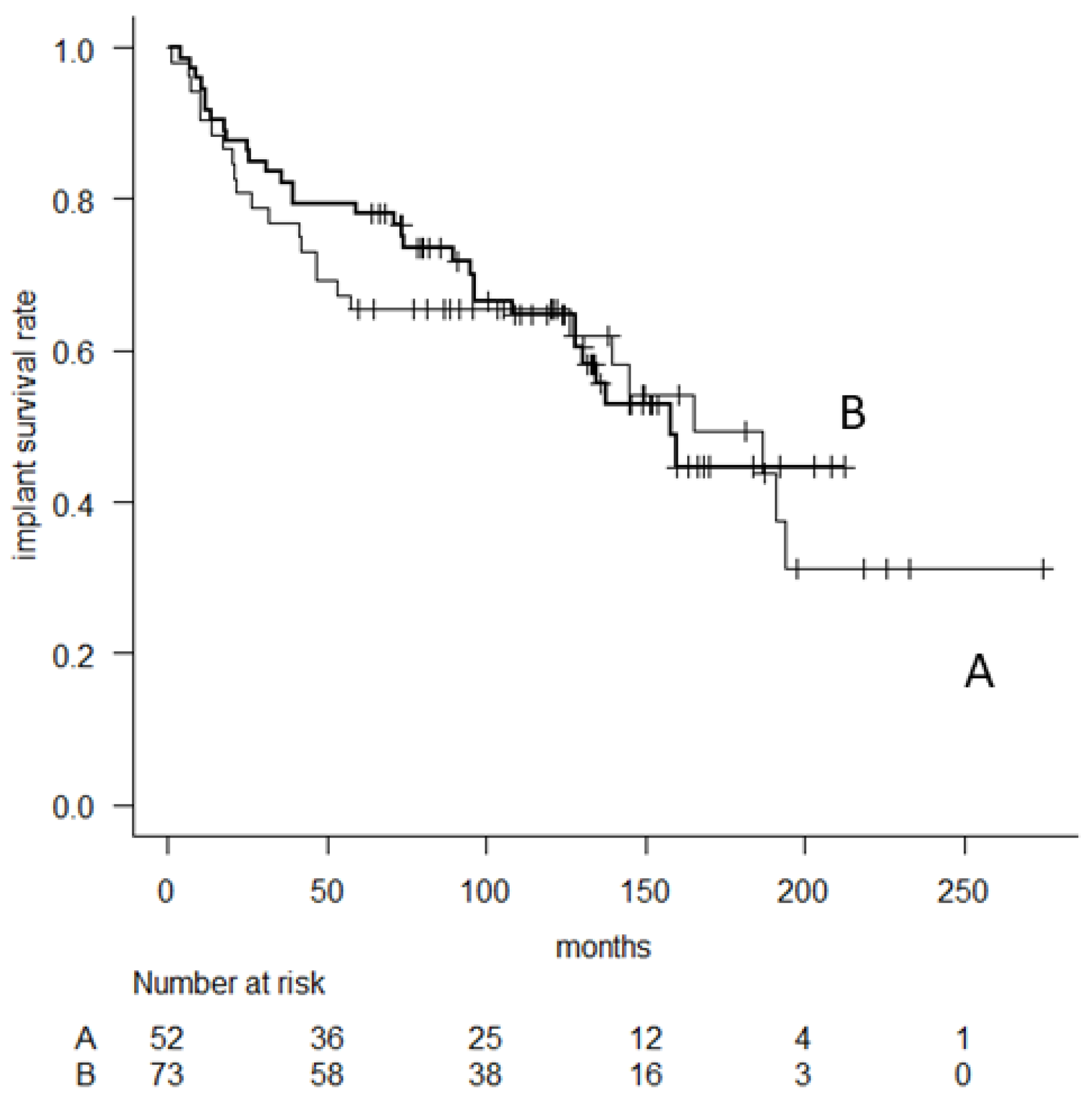
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goorin, A.M.; Schwartzentruber, D.J.; Devidas, M.; Gebhardt, M.C.; Ayala, A.G.; Harris, M.B.; Helman, L.J.; Grier, H.E.; Link, M.P. Presurgical Chemotherapy Compared With Immediate Surgery and Adjuvant Chemotherapy for Nonmetastatic Osteosarcoma: Pediatric Oncology Group Study POG-8651. J. Clin. Oncol. 2003, 21, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Sim, I.-W.; Tse, L.F.; Ek, E.T.; Powell, G.J.; Choong, P.F.M. Salvaging the limb salvage: Management of complications following endoprosthetic reconstruction for tumours around the knee. Eur. J. Surg. Oncol. 2007, 33, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.A.; Aschliman, M.A.; Thomas, N.; Mankin, H.J. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J. Bone Jt. Surg. 1986, 68, 1331–1337. [Google Scholar] [CrossRef]
- Ogura, K.; Higashi, T.; Kawai, A. Statistics of bone sarcoma in Japan: Report from the Bone and Soft Tissue Tumor Registry in Japan. J. Orthop. Sci. 2017, 22, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Matsumine, A.; Ueda, T.; Sugita, T.; Yazawa, Y.; Isu, K.; Kawai, A.; Abe, S.; Yakushiji, T.; Hiraga, H.; Sudo, A.; et al. Clinical outcomes of the KYOCERA Physio Hinge Total Knee System Type III after the resection of a bone and soft tissue tumor of the distal part of the femur. J. Surg. Oncol. 2011, 103, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Matsumine, A.; Uchida, A.; Kawai, A.; Nishida, Y.; Kunisada, T.; Araki, N.; Sugiura, H.; Tomita, M.; Yokouchi, M.; et al. Clinical outcomes of Kyocera Modular Limb Salvage system after resection of bone sarcoma of the distal part of the femur: The Japanese Musculoskeletal Oncology Group study. Int. Orthop. 2014, 38, 825–830. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Enneking, W.F.; Dunham, W.; Gebhardt, M.C.; Malawar, M.; Pritchard, D.J. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin. Orthop. Relat. Res. 1993, 286, 241–246. [Google Scholar] [CrossRef]
- Houdek, M.T.; Wagner, E.R.; Wilke, B.K.; Wyles, C.C.; Taunton, M.J.; Sim, F.H. Long term outcomes of cemented endoprosthetic reconstruction for periarticular tumors of the distal femur. Knee 2016, 23, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, P.; Mavrogenis, A.F.; Pala, E.; Abdel-Mota’Al, M.; Mercuri, M. Long term results of fixed-hinge megaprostheses in limb salvage for malignancy. Knee 2012, 19, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Batta, V.; Coathup, M.J.; Parratt, M.T.; Pollock, R.C.; Aston, W.J.; Cannon, S.R.; Skinner, J.; Briggs, T.W.; Blunn, G.W. Uncemented, custom-made, hydroxyapatite-coated collared distal femoral endoprostheses. Bone Joint. J. 2014, 96, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Myers, G.J.C.; Abudu, A.T.; Carter, S.R.; Tillman, R.M.; Grimer, R.J. Endoprosthetic replacement of the distal femur for bone tumours. J. Bone Jt. Surgery. Br. Vol. 2007, 89, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Bergin, P.F.; Noveau, J.B.; Jelinek, J.S.; Henshaw, R.M. Aseptic Loosening Rates in Distal Femoral Endoprostheses: Does Stem Size Matter? Clin. Orthop. Relat. Res. 2012, 470, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Griffin, A.M.; Parsons, J.A.; Davis, A.M.; Bell, R.S.; Wunder, J.S. Uncemented Tumor Endoprostheses at the Knee: Root causes of failure. Clin. Orthop. Relat. Res. 2005, &NA, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Capanna, R.; Morris, H.G.; Campanacci, D.; Del Ben, M.; Campanacci, M. Modular uncemented prosthetic reconstruction after resection of tumours of the distal femur. J. Bone Joint Surg. Br. 1994, 76, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Unwin, P.S.; Cannon, S.R.; Grimer, R.J.; Kemp, H.B.; Sneath, R.S.; Walker, P.S. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J. Bone Joint Surg. Br. 1996, 78, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Sanjay, B.K.S.; Moreau, P.G. Limb salvage surgery in bone tumour with modular endoprosthesis. Int. Orthop. 1999, 23, 41–46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bus, M.P.A.; van de Sande, M.A.J.; Fiocco, M.; Schaap, G.R.; Bramer, J.A.M.; Dijkstra, P.D.S. What Are the Long-term Results of MUTARS® Modular Endoprostheses for Reconstruction of Tumor Resection of the Distal Femur and Proximal Tibia? Clin. Orthop. Relat. Res. 2017, 475, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Henderson, E.R.; Groundland, J.S.; Pala, E.; Dennis, J.A.; Wooten, R.; Cheong, D.; Windhager, R.; Kotz, R.I.; Mercuri, M.; Funovics, P.T.; et al. Failure Mode Classification for Tumor Endoprostheses: Retrospective Review of Five Institutions and a Literature Review. J. Bone Joint Surg. Am. 2011, 93, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Henderson, E.R.; O’Connor, M.I.; Ruggieri, P.; Windhager, R.; Funovics, P.T.; Gibbons, C.L.; Guo, W.; Hornicek, F.J.; Temple, H.T.; Letson, G.D. Classification of failure of limb salvage after reconstructive surgery for bone tumours: A modified system including biological and expandable reconstructions. Bone Joint. J. 2014, 96-B, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
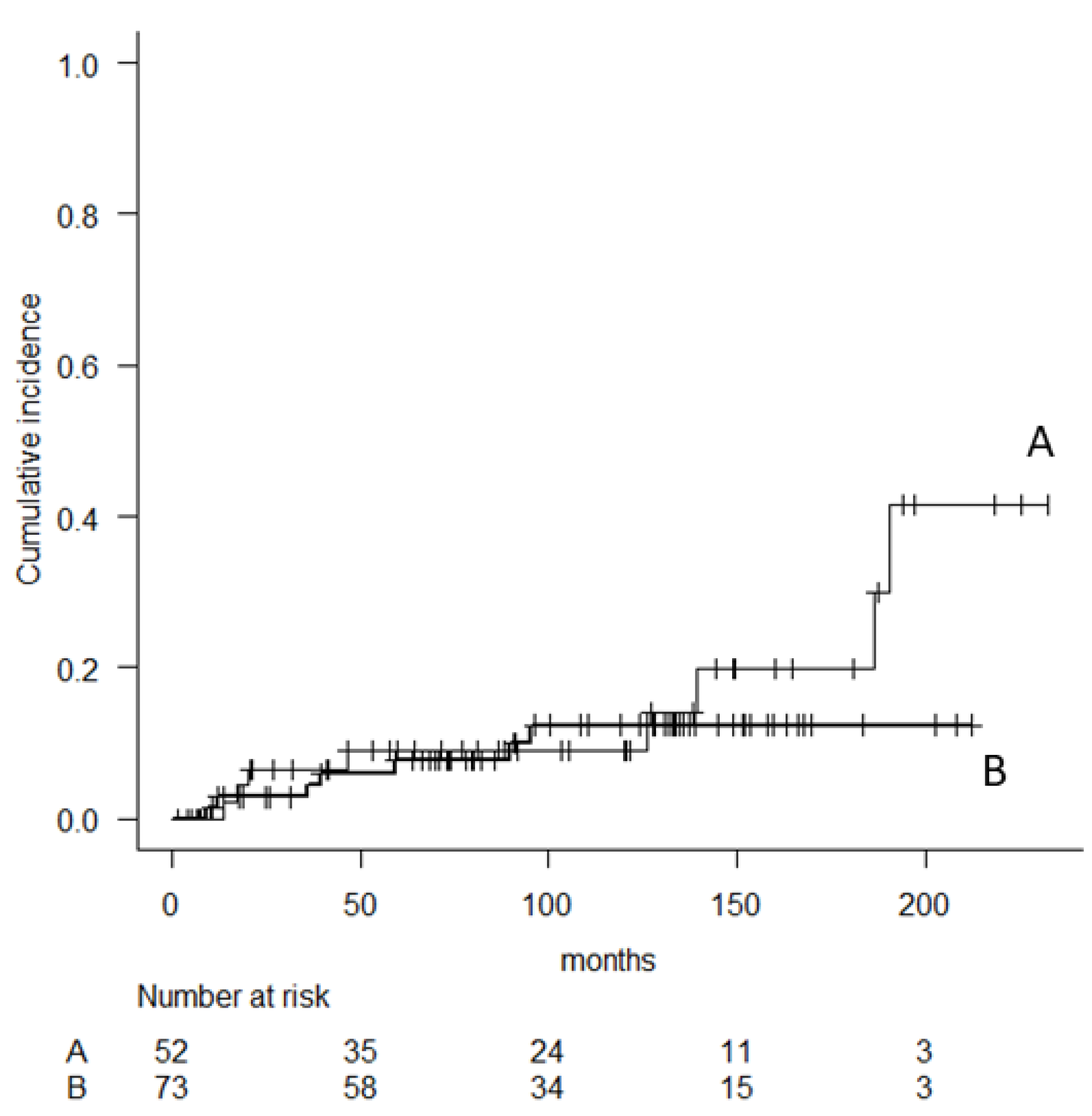
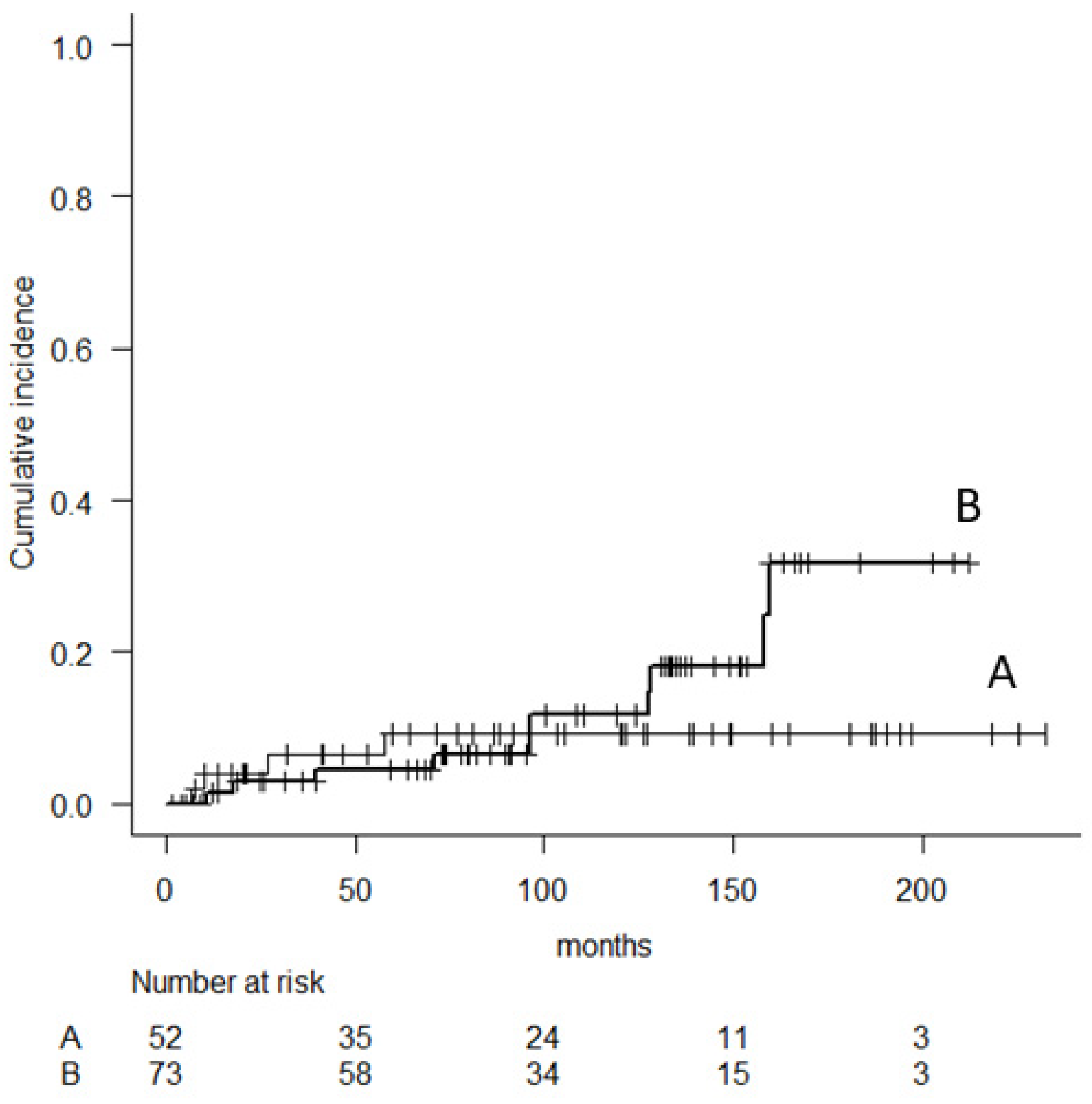
| Characteristics | Parameter | N |
|---|---|---|
| Age (years) | Mean | 35 |
| Range | 9–79 | |
| Sex (N) | Male | 59 |
| Female | 66 | |
| Perioperative cx (N) | Yes | 84 |
| No | 41 | |
| Femoral stem fixation (N) | cement-type | 52 |
| cementless-type | 73 | |
| Femoral component (N) | 90 mm | 7 |
| 110 mm | 19 | |
| 130 mm | 23 | |
| 150 mm | 30 | |
| 170 mm | 19 | |
| 190 mm | 8 | |
| >210 mm | 19 | |
| Stem length (N) | 130 mm | 120 |
| 170 mm | 5 | |
| TLP/SL (ratio) | 1.65–3.23 | |
| Stem diameter (N) | cement-type | |
| 10 mm | 4 | |
| 11 mm | 26 | |
| 12 mm | 12 | |
| 13 mm | 10 | |
| cementless-type | ||
| 10 mm | 1 | |
| 11 mm | 1 | |
| 12 mm | 46 | |
| 13 mm | 14 | |
| 14 mm | 7 | |
| 15 mm | 4 |
| Complications | N |
|---|---|
| Aseptic loosening | 15 |
| Stem breakage | 14 |
| Infection | 13 |
| Rotator-hinge bushing failure | 13 |
| Local recurrence | 5 |
| Fracture | 2 |
| Others | 3 |
| Variables | N | Implant Survival Rate (%) | ||||
|---|---|---|---|---|---|---|
| 5-Years | 10-Years | 15-Years | p Value | |||
| Age | <20 years | 52 | 71.2 | 60.9 | 37.3 | 0.96 |
| (56.8–81.5) | (46.1–72.8) | (22.1–52.6) | ||||
| >20 years | 73 | 72.5 | 56.2 | 42.9 | ||
| (60.7–81.3) | (43–67.5) | (27.8–57.2) | ||||
| BMI | >25 kg/m2 | 13 | 61.5 | 41 | 41 | 0.93 |
| (30.8–81.8) | (8.5–72.5) | (8.5–72.5) | ||||
| <25 kg/m2 | 83 | 71.4 | 58.1 | 35.6 | ||
| (60.4–79.8) | (46.2–68.3) | (21.8–49.7) | ||||
| Sex | Male | 59 | 71.1 | 57.5 | 41.2 | 0.73 |
| (57.7–80.9) | (43.5–69.3) | (25.7–56) | ||||
| Female | 66 | 72.7 | 59.3 | 37.5 | ||
| (60.3–81.9) | (45.7–70.6) | (22.1–52.8) | ||||
| Perioperative | Yes | 83 | 68.6 | 56 | 33.1 | 0.18 |
| (57.4–77.4) | (44.2–66.2) | (19.9–46.9) | ||||
| chemotherapy | No | 42 | 78.6 | 63.9 | 52.1 | |
| (62.9–88.2) | (46.4–77) | (33.7–67.7) | ||||
| Patella | Yes | 21 | 66.7 | 55.4 | 47.5 | 0.92 |
| (42.5–82.5) | (31.4–74) | (23.3–68.3) | ||||
| replacement | No | 104 | 73.1 | 59.3 | 37.2 | |
| (63.4–80.6) | (48.7–68.4) | (24.8–49.7) | ||||
| Resection of | Extra-capsule | 21 | 66.3 | 63.4 | 41.6 | 0.11 |
| (42–82.3) | (52.4–72.5) | (28.3–54.4) | ||||
| Joint | Intra-capsule | 94 | 72.3 | 38.3 | 38.3 | |
| (62.1–80.2) | (17.1–59.3) | (17.1–59.3) | ||||
| Resection | 3 to 4 | 12 | 66.7 | 57.1 | 28.6 | 0.83 |
| (33.7–86) | (25.4–79.6) | (4.9–59.4) | ||||
| of quadriceps femoris | 0 to 2 | 96 | 71.8 | 58.9 | 41.4 | |
| (61.7–79.7) | (47.8–68.4) | (28.4–53.8) | ||||
| Fixation of | Cementless | 73 | 76.7 | 55.7 | 35.3 | 0.82 |
| (65.2–84.8) | (42.9–66.8) | (21.7–49.3) | ||||
| femoral stem | Cement | 52 | 65.2 | 63.1 | 46.4 | |
| (50.6–76.5) | (48.3–74.6) | (28.8–62.4) | ||||
| Ref | N | Stem | Hinge | Implant survival | Complications | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fixation | Type | 5-years | 10-years | 15-years | Loosening | Infection | F-stem b | ||
| [8] | 152 | C | modular; RH | 74% * | 59% * | 50% * | 21% | 9.9% | 2% |
| custom; FH | |||||||||
| [9] | 669 # | C 9% | FH | 80% * | 5.7% | 8.2% | 4.4% | ||
| C-less 91% | |||||||||
| [10] | 69 | C-less | RH | 73% * | 65% * | 55% * | 13% | 7.2% | 4.3% |
| [11] | 335 | C 53% | FH; 48% | 83% * | 67% * | 51% * | 9.6% | 2.1% | |
| C-less 47% | RH; 52% | ||||||||
| [12] | 93 | C | RH | 73.3% * | 62.8% * | 4.9% | 11.7% | 5.8% | |
| Ours | 125 | C 42% | RH | 72.8% * | 64.9% * | 46.3% * | 12% | 10.4% | 11.2% |
| C-less 58% | (72% **) | (58.5% **) | (39.4% **) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, T.; Matsumine, A.; Toda, Y.; Takenaka, S.; Outani, H.; Fujiwara, T.; Nishida, Y.; Tsukushi, S.; Tome, Y.; Kawamoto, T.; et al. Long-Term Results of Kyocera Modular Limb Salvage System after Resection of Tumors in the Distal Part of the Femur: Report from Japanese Musculoskeletal Oncology Group Study. Cancers 2022, 14, 870. https://doi.org/10.3390/cancers14040870
Nakamura T, Matsumine A, Toda Y, Takenaka S, Outani H, Fujiwara T, Nishida Y, Tsukushi S, Tome Y, Kawamoto T, et al. Long-Term Results of Kyocera Modular Limb Salvage System after Resection of Tumors in the Distal Part of the Femur: Report from Japanese Musculoskeletal Oncology Group Study. Cancers. 2022; 14(4):870. https://doi.org/10.3390/cancers14040870
Chicago/Turabian StyleNakamura, Tomoki, Akihiko Matsumine, Yu Toda, Satoshi Takenaka, Hidetatsu Outani, Tomohiro Fujiwara, Yoshihiro Nishida, Satoshi Tsukushi, Yasunori Tome, Teruya Kawamoto, and et al. 2022. "Long-Term Results of Kyocera Modular Limb Salvage System after Resection of Tumors in the Distal Part of the Femur: Report from Japanese Musculoskeletal Oncology Group Study" Cancers 14, no. 4: 870. https://doi.org/10.3390/cancers14040870
APA StyleNakamura, T., Matsumine, A., Toda, Y., Takenaka, S., Outani, H., Fujiwara, T., Nishida, Y., Tsukushi, S., Tome, Y., Kawamoto, T., Kito, M., Shinohara, N., Tomita, M., Torigoe, T., Sudo, A., & Kawano, H. (2022). Long-Term Results of Kyocera Modular Limb Salvage System after Resection of Tumors in the Distal Part of the Femur: Report from Japanese Musculoskeletal Oncology Group Study. Cancers, 14(4), 870. https://doi.org/10.3390/cancers14040870






