Safety and Efficacy of Indocyanine Green in Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis of 11,047 Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Outcomes
2.5. Risk of Bias Assessment
2.6. Statistical Analysis
3. Results
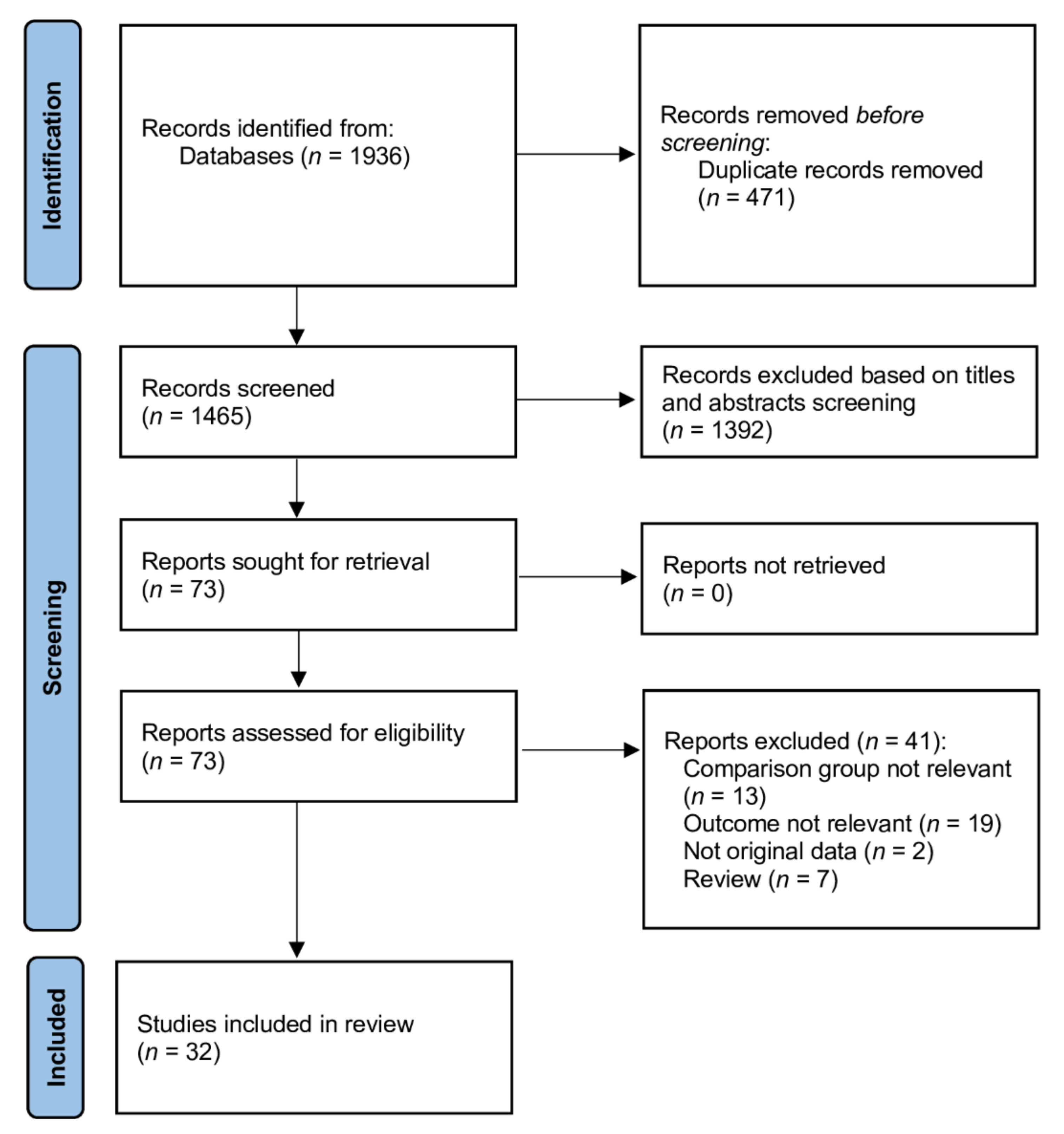
3.1. Literature Search and Study Characteristics
3.2. Intraoperative Evaluation
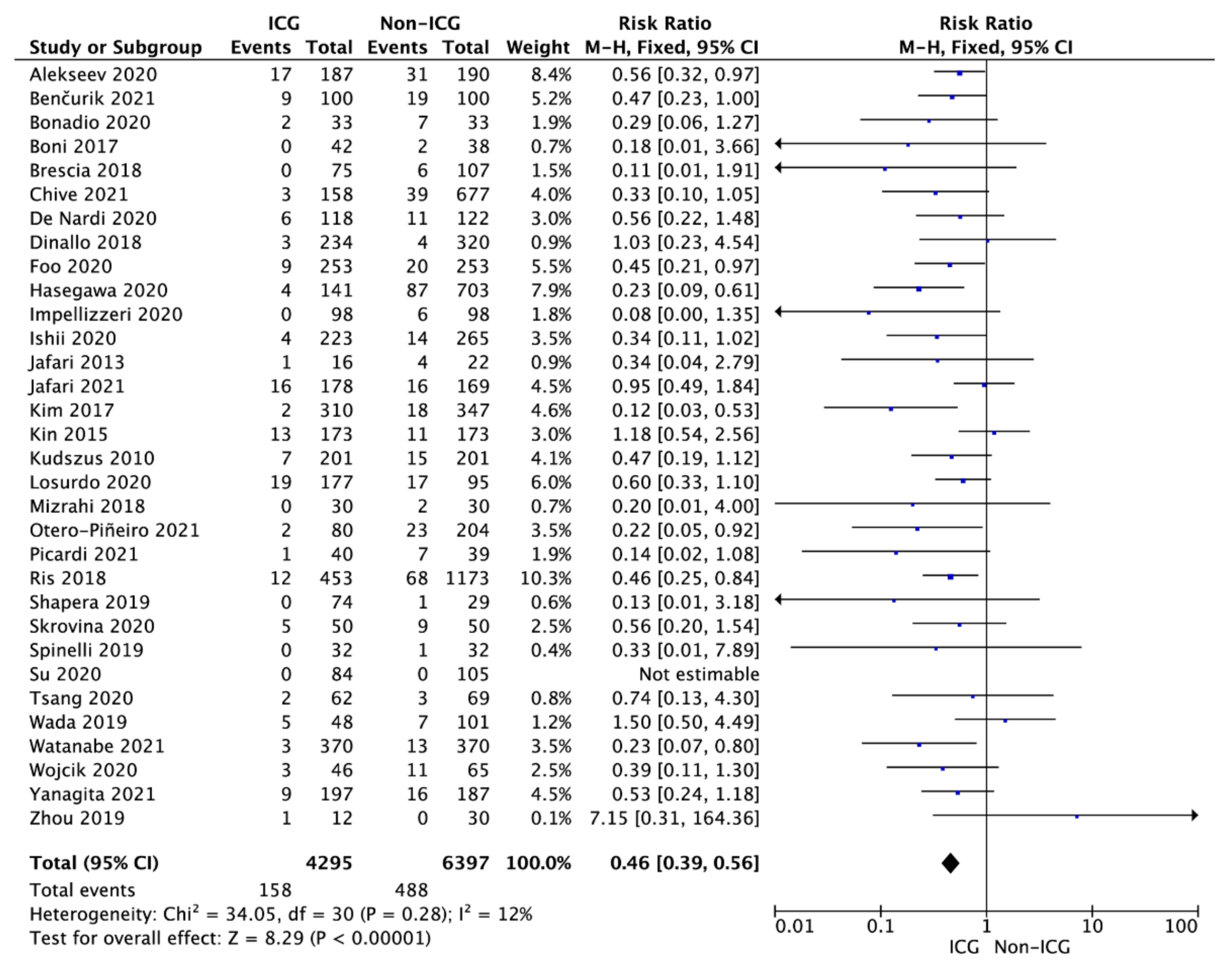
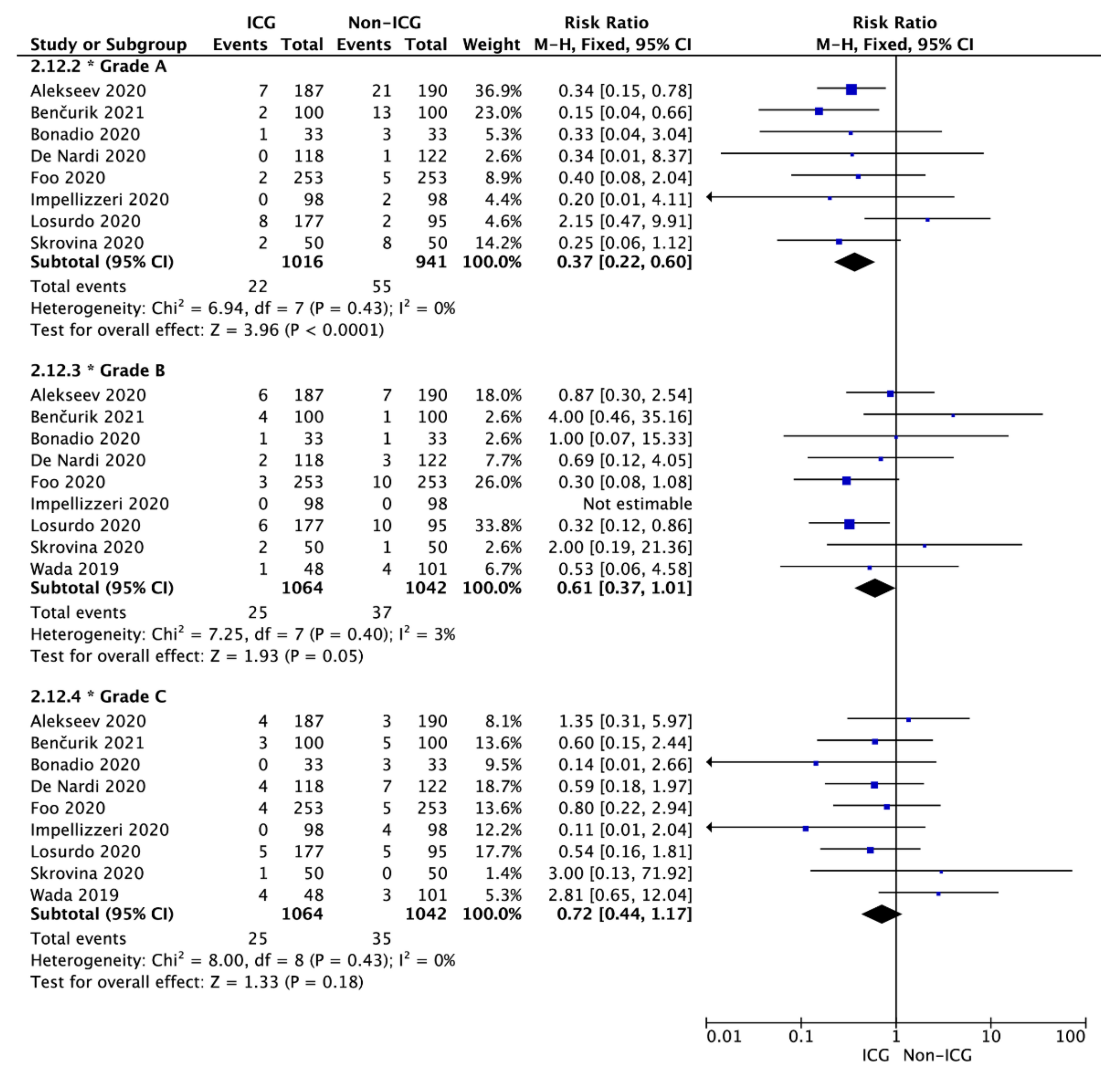
3.3. Anastomotic Leak
3.4. Postoperative Period Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Landsman, M.L.; Kwant, G.; Mook, G.A.; Zijlstra, W.G. Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J. Appl. Physiol. 1976, 40, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Arezzo, A.; Bonino, M.A.; Ris, F.; Boni, L.; Cassinotti, E.; Foo, D.C.C.; Shum, N.F.; Brolese, A.; Ciarleglio, F.; Keller, D.S.; et al. Intraoperative use of fluorescence with indocyanine green reduces anastomotic leak rates in rectal cancer surgery: An individual participant data analysis. Surg. Endosc. 2020, 34, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Baiocchi, G.L.; Guercioni, G.; Vettoretto, N.; Scabini, S.; Millo, P.; Muratore, A.; Clementi, M.; Sica, G.; Delrio, P.; Longo, G.; et al. ICG fluorescence imaging in colorectal surgery: A snapshot from the ICRAL study group. BMC Surg. 2021, 21, 190. [Google Scholar] [CrossRef]
- Vallance, A.; Wexner, S.; Berho, M.; Cahill, R.; Coleman, M.; Haboubi, N.; Heald, R.J.; Kennedy, R.H.; Moran, B.; Mortensen, N.; et al. A collaborative review of the current concepts and challenges of anastomotic leaks in colorectal surgery. Colorectal Dis. 2017, 19, O1–O12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDermott, F.D.; Heeney, A.; Kelly, M.E.; Steele, R.J.; Carlson, G.L.; Winter, D.C. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br. J. Surg. 2015, 102, 462–479. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, H.; Biondo, S.; Codina, A.; Ciga, M.Á.; Enríquez-Navascués, J.; Espín, E.; García-Granero, E.; Roig, J.V. Hospital variation in anastomotic leakage after rectal cancer surgery in the Spanish Association of Surgeons project: The contribution of hospital volume. Cir. Esp. 2016, 94, 213–220. [Google Scholar]
- Chadi, S.A.; Fingerhut, A.; Berho, M.; DeMeester, S.R.; Fleshman, J.W.; Hyman, N.H.; Margolin, D.A.; Martz, J.E.; McLemore, E.C.; Molena, D.; et al. Emerging trends in the etiology, prevention, and treatment of gastrointestinal anastomotic leakage. J. Gastrointest. Surg. 2016, 20, 2035–2051. [Google Scholar] [CrossRef]
- Sparreboom, C.L.; Wu, Z.-Q.; Ji, J.-F.; Lange, J.F. Integrated approach to colorectal anastomotic leakage: Communication, infection and healing disturbances. World J. Gastroenterol. 2016, 22, 7226–7235. [Google Scholar]
- Rutegård, M.; Rutegård, J. Anastomotic leakage in rectal cancer surgery: The role of blood perfusion. World J. Gastrointest. Surg. 2015, 7, 289–292. [Google Scholar] [CrossRef]
- Blanco-Colino, R.; Espin-Basany, E. Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: A systematic review and meta-analysis. Tech. Coloproctol. 2018, 22, 15–23. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; version 6.2 (Updated February 2021), Cochrane. 2021. Available online: www.training.cochrane.org/handbook (accessed on 10 November 2021).
- Alekseev, M.; Rybakov, E.; Shelygin, Y.; Chernyshov, S.; Zarodnyuk, I. A study investigating the perfusion of colorectal anastomoses using fluorescence angiography: Results of the FLAG randomized trial. Colorectal Dis. 2020, 22, 1147–1153. [Google Scholar] [CrossRef]
- Benčurik, V.; Škrovina, M.; Martínek, L.; Bartoš, J.; Macháčková, M.; Dosoudil, M.; Štěpánová, E.; Přibylová, L.; Briš, R.; Vomáčková, K. Intraoperative fluorescence angiography and risk factors of anastomotic leakage in mini-invasive low rectal resections. Surg. Endosc. 2021, 35, 5015–5023. [Google Scholar] [CrossRef]
- Bonadio, L.; Iacuzzo, C.; Cosola, D.; Cipolat Mis, T.; Giudici, F.; Casagranda, B.; Biloslavo, A.; de Manzini, N. Indocyanine green-enhanced fluorangiography (ICGf) in laparoscopic extraperitoneal rectal cancer resection. Updates Surg. 2020, 72, 477–482. [Google Scholar] [CrossRef]
- Boni, L.; Fingerhut, A.; Marzorati, A.; Rausei, S.; Dionigi, G.; Cassinotti, E. Indocyanine green fluorescence angiography during laparoscopic low anterior resection: Results of a case-matched study. Surg Endosc. 2017, 31, 1836–1840. [Google Scholar] [CrossRef]
- Brescia, A.; Pezzatini, M.; Romeo, G.; Cinquepalmi, M.; Pindozzi, F.; Dall’Oglio, A.; Gasparrini, M.; Lazar, F. Indocyanine green fluorescence angiography: A new ERAS item. Updates Surg. 2018, 70, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Chivé, E.; Sabbagh, C.; Guérin, O.; Pellegrin, A.; Dembinski, J.; Regimbeau, J.-M. Is intraoperative fluorescence imaging with indocyanine green associated with a lower incidence of anastomotic leakage after colorectal surgery? A propensity score matching study. Surg. Open Dig. Adv. 2021, 2, 100014. [Google Scholar] [CrossRef]
- De Nardi, P.; Elmore, U.; Maggi, G.; Maggiore, R.; Boni, L.; Cassinotti, E.; Fumagalli, U.; Gardani, M.; De Pascale, S.; Parise, P.; et al. Intraoperative angiography with indocyanine green to assess anastomosis perfusion in patients undergoing laparoscopic colorectal resection: Results of a multicenter randomized controlled trial. Surg Endosc. 2020, 34, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Dinallo, A.M.; Kolarsick, P.; Boyan, W.P.; Protyniak, B.; James, A.; Dressner, R.M.; Arvanitis, M.L. Does routine use of indocyanine green fluorescence angiography prevent anastomotic leaks? A retrospective cohort analysis. Am. J. Surg. 2019, 218, 136–139. [Google Scholar] [CrossRef]
- Foo, C.C.; Ng, K.K.; Tsang, J.; Wei, R.; Chow, F.; Chan, T.Y.; Lo, O.; Law, W.L. Colonic perfusion assessment with indocyanine-green fluorescence imaging in anterior resections: A propensity score-matched analysis. Tech. Coloproctol. 2020, 24, 935–942. [Google Scholar] [CrossRef]
- Hasegawa, H.; Tsukada, Y.; Wakabayashi, M.; Nomura, S.; Sasaki, T.; Nishizawa, Y.; Ikeda, K.; Akimoto, T.; Ito, M. Impact of intraoperative indocyanine green fluorescence angiography on anastomotic leakage after laparoscopic sphincter-sparing surgery for malignant rectal tumors. Int. J. Colorectal Dis. 2020, 35, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, H.G.; Pulvirenti, A.; Inama, M.; Bacchion, M.; Marrano, E.; Creciun, M.; Casaril, A.; Moretto, G. Near-infrared fluorescence angiography for colorectal surgery is associated with a reduction of anastomotic leak rate. Updates Surg. 2020, 72, 991–998. [Google Scholar] [CrossRef]
- Ishii, M.; Hamabe, A.; Okita, K.; Nishidate, T.; Okuya, K.; Usui, A.; Akizuki, E.; Satoyoshi, T.; Takemasa, I. Efficacy of indocyanine green fluorescence angiography in preventing anastomotic leakage after laparoscopic colorectal cancer surgery. Int. J. Colorectal Dis. 2020, 35, 269–275. [Google Scholar] [CrossRef]
- Jafari, M.D.; Lee, K.H.; Halabi, W.J.; Mills, S.D.; Carmichael, J.C.; Stamos, M.J.; Pigazzi, A. The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg. Endosc. 2013, 27, 3003–3008. [Google Scholar] [CrossRef]
- Jafari, M.D.; Pigazzi, A.; McLemore, E.C.; Mutch, M.G.; Haas, E.; Rasheid, S.H.; Wait, A.D.; Paquette, I.M.; Bardakcioglu, O.; Safar, B.; et al. Perfusion Assessment in Left-Sided/Low Anterior Resection (PILLAR III): A randomized, controlled, parallel, multicenter study assessing perfusion outcomes with PINPOINT near-infrared fluorescence imaging in low anterior resection. Dis. Colon Rectum 2021, 64, 995–1002. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, J.L.; Park, S.H. Interpretative guidelines and possible indications for indocyanine green gluorescence imaging in robot-assisted sphincter-saving operations. Dis. Colon Rectum 2017, 60, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Kin, C.; Vo, H.; Welton, L.; Welton, M. Equivocal effect of intraoperative fluorescence angiography on colorectal anastomotic leaks. Dis. Colon Rectum 2015, 58, 582–587. [Google Scholar] [CrossRef]
- Kudszus, S.; Roesel, C.; Schachtrupp, A.; Höer, J.J. Intraoperative laser fluorescence angiography in colorectal surgery: A noninvasive analysis to reduce the rate of anastomotic leakage. Langenbecks Arch. Surg. 2010, 395, 1025–1030. [Google Scholar] [CrossRef]
- Losurdo, P.; Cipolat Mis, T.; Cosola, D.; Bonadio, L.; Giudici, F.; Casagranda, B.; Bortul, M.; de Manzini, N. Anastomosis leak: Is there still a place for indocyanine green fluorescence imaging in colon-rectal surgery? A retrospective, propensity score-matched cohort study. Surg. Innov. 2020, 1553350620975258. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, I.; Abu-Gazala, M.; Rickles, A.S.; Fernandez, L.M.; Petrucci, A.; Wolf, J.; Sands, D.R.; Wexner, S.D. Indocyanine green fluorescence angiography during low anterior resection for low rectal cancer: Results of a comparative cohort study. Tech. Coloproctol. 2018, 22, 535–540. [Google Scholar] [CrossRef]
- Otero-Piñeiro, A.M.; de Lacy, F.B.; Van Laarhoven, J.J.; Martín-Perez, B.; Valverde, S.; Bravo, R.; Lacy, A.M. The impact of fluorescence angiography on anastomotic leak rate following transanal total mesorectal excision for rectal cancer: A comparative study. Surg. Endosc. 2021, 35, 754–762. [Google Scholar] [CrossRef]
- Picardi, B.; Rossi, S.; Del Monte, S.R.; Cortese, F.; Muttillo, E.M.; Mazzarella, G.; Puccioni, C.; Muttillo, I.A. The use of indocyanine green fluorescence in the assessment of bowel perfusion in emergency and elective colorectal surgery. Res. Sq. 2021. [Google Scholar]
- Ris, F.; Liot, E.; Buchs, N.C.; Kraus, R.; Ismael, G.; Belfontali, V.; Douissard, J.; Cunningham, C.; Lindsey, I.; Guy, R.; et al. Multicentre phase II trial of near-infrared imaging in elective colorectal surgery. Br. J. Surg. 2018, 105, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Shapera, E.; Hsiung, R.W. Assessment of anastomotic perfusion in left-sided robotic assisted colorectal resection by indocyanine green fluorescence angiography. Minim. Invasive Surg. 2019, 2019, 3267217. [Google Scholar] [CrossRef] [Green Version]
- Skrovina, M.; Bencurik, V.; Martinek, L.; Machackova, M.; Bartos, J.; Andel, P.; Stepanova, E.; Bunakova, M.; Vomackova, K. The significance of intraoperative fluorescence angiography in miniinvasive low rectal resections. Videosurg. Miniinv. 2020, 15, 43–48. [Google Scholar] [CrossRef]
- Spinelli, A.; Carvello, M.; Kotze, P.G.; Maroli, A.; Montroni, I.; Montorsi, M.; Buchs, N.C.; Ris, F. Ileal pouch-anal anastomosis with fluorescence angiography: A case-matched study. Colorectal Dis. 2019, 21, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Wu, H.; Bao, M.; Luo, S.; Wang, X.; Zhao, C.; Liu, Q.; Wang, X.; Zhou, Z.; Zhou, H. Indocyanine green fluorescence imaging to assess bowel perfusion during totally laparoscopic surgery for colon cancer. BMC Surg. 2020, 20, 102. [Google Scholar] [CrossRef]
- Tsang, Y.-P.; Leung, L.-H.A.; Lau, C.-W.; Tang, C.-N. Indocyanine green fluorescence angiography to evaluate anastomotic perfusion in colorectal surgery. Int. J. Colorectal Dis. 2020, 35, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Kawada, K.; Hoshino, N.; Inamoto, S.; Yoshitomi, M.; Hida, K.; Sakai, Y. The effects of intraoperative ICG fluorescence angiography in laparoscopic low anterior resection: A propensity score-matched study. Int. J. Clin. Oncol. 2019, 24, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Ishibe, A.; Ohya, H.; Suwa, Y.; Suwa, H.; Kunisaki, C.; Endo, I. Evaluating the effect of intraoperative near-infrared observation on anastomotic leakage after stapled side-to-side anastomosis in colon cancer surgery using propensity score matching. Dis. Colon Rectum 2021, 64, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, M.; Doussot, A.; Manfredelli, S.; Duclos, C.; Paquette, B.; Turco, C.; Heyd, B.; Lakkis, Z. Intra-operative fluorescence angiography is reproducible and reduces the rate of anastomotic leak after colorectal resection for cancer: A prospective case-matched study. Colorectal Dis. 2020, 22, 1263–1270. [Google Scholar] [CrossRef]
- Yanagita, T.; Hara, M.; Osaga, S.; Nakai, N.; Maeda, Y.; Shiga, K.; Hirokawa, T.; Matsuo, Y.; Takahashi, H.; Takiguchi, S. Efficacy of intraoperative ICG fluorescence imaging evaluation for preventing anastomotic leakage after left-sided colon or rectal cancer surgery: A propensity score-matched analysis. Surg. Endosc. 2021, 35, 2373–2385. [Google Scholar] [CrossRef]
- Zhou, S.-C.; Tian, Y.-T.; Wang, X.-W.; Zhao, C.-D.; Ma, S.; Jiang, J.; Li, E.-N.; Zhou, H.-T.; Liu, Q.; Liang, J.-W.; et al. Application of indocyanine green-enhanced near-infrared fluorescence-guided imaging in laparoscopic lateral pelvic lymph node dissection for middle-low rectal cancer. World J. Gastroenterol. 2019, 25, 4502–4511. [Google Scholar] [CrossRef]
- Jayne, D.; Pigazzi, A.; Marshall, H.; Croft, J.; Corrigan, N.; Copeland, J.; Quirke, P.; West, N.; Rautio, T.; Thomassen, N.; et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: The ROLARR randomized clinical trial. JAMA 2017, 318, 1569–1580. [Google Scholar] [CrossRef]
- Senagore, A.; Lane, F.R.; Lee, E.; Wexner, S.; Dujovny, N.; Sklow, B.; Rider, P.; Bonello, J.; Bioabsorbable Staple Line Reinforcement Study Group. Bioabsorbable staple line reinforcement in restorative proctectomy and anterior resection: A randomized study. Dis. Colon Rectum 2014, 57, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Pigazzi, A.; Luca, F.; Patriti, A.; Valvo, M.; Ceccarelli, G.; Casciola, L.; Biffi, R.; Garcia-Aguilar, J.; Baek, J.-H. Multicentric study on robotic tumour-specific mesorectal excision for the treatment of rectal cancer. Ann. Surg. Oncol. 2010, 17, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-K.; Law, W.-L.; Ho, J.W. Leakage after resection and intraperitoneal anastomosis for colorectal malignancy: Analysis of risk factors. Dis. Colon Rectum 2006, 49, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.D.; Wexner, S.D.; Martz, J.E.; McLemore, E.C.; Margolin, D.A.; Sherwinter, D.A.; Lee, S.W.; Senagore, A.J.; Phelan, M.J.; Stamos, M.J. Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): A multi-institutional study. J. Am. Coll. Surg. 2015, 220, 82–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vignali, A.; Gianotti, L.; Braga, M.; Radaelli, G.; Malvezzi, L.; Di Carlo, V. Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis. Colon Rectum 2000, 43, 76–82. [Google Scholar] [CrossRef]
| Study | Country | Study Design | Operation Method | ICG Group | Non-ICG Group | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age (Years) | Sex, Male | AL Rate | No. | Age (Years) | Sex, Male | AL Rate | ||||
| Alekseev et al., 2020 [18] | Russia | RCT | LAR, AR, LC | 187 | 63 (21–86) | 92 (49.2%) | 17/187 | 190 | 63 (66–85) | 92 (48.4%) | 31 (16.3%) |
| Benčurik et al., 2021 [19] | Czech Republic | Retrospective | LAR with TME | 100 | 62.6 ± 9.7 | 66 (66.0%) | 9 (9.0%) | 100 | 64.4 ± 9.2 | 64 (64.0%) | 19 (19.0%) |
| Bonadio et al., 2020 [20] | Italy | Retrospective | RAR | 33 | 71.85 ± 11.1 | 21 (63.6%) | 2 (6.06%) | 33 | 63.03 ± 11.3 | 15 (45.5%) | 7 (21.21%) |
| Boni et al., 2017 [21] | Italy | Retrospective | LAR with TME | 42 | 69 ± 8 | 28 (66.7%) | 0 (0.0%) | 38 | 67 ± 7 | 22 (57.9%) | 2 (5.3%) |
| Brescia et al., 2018 [22] | Italy | Retrospective | CL, LAR, ACR | 75 | 67.1 ± 6 | 43 (57.3%) | 0 (0.0%) | 107 | 65.7 ± 7 | 63 (58.9%) | 6 (5.6%) |
| Chivé et al., 2021 [23] | France | Retrospective | CL, PR | 158 | 64 ± 15 | 95 (60.1%) | 3 (1.9%) | 677 | 62 ± 16 | 374 (55.2%) | 39 (5.8%) |
| De Nardi et al., 2020 [24] | Italy | RCT | LAR, CL | 118 | 66.1 | 60 (50.8%) | 6 (5.1%) | 122 | 65.1 | 66 (54.1%) | 11 (9.0%) |
| Dinallo et al., 2019 [25] | USA | Retrospective | LAR | 234 | 61.5 (34.6–88.4) | 108 (46.2%) | 3 (1.3%) | 320 | 62.5 (35.3–89.7) | 138 (43.1%) | 4 (1.3%) |
| Foo et al., 2020 [26] | China | Retrospective | TME | 253 | 66.6 ± 10.6 | (65.6%) | 3.6% | 253 | 67.2 ± 11.0 | 64.4% | 7.9% |
| Hasegawa et al., 2020 [27] | Japan | Retrospective | LAR, ISR | 141 | 63 (51–69) | 99 (70.2%) | 4 (2.8%) | 703 | 62 (55–68) | 450 (0%) | 87 (12.4%) |
| Impellizzeri et al., 2020 [28] | Italy | Retrospective | LAR, LSH, SR | 98 | 66 (59–74) | 53 (54.1%) | 0 (0.0%) | 98 | 71 (58–79) | 57 (58.2%) | 6 (6.1%) |
| Ishii et al., 2020 [29] | Japan | Retrospective | Mixed | 233 | 67 (30–90) | 126 (43.1%) | 4 (1.8%) | 265 | 69 (27–93) | 136 (51.3%) | 14 (5.3%) |
| Jafari et al., 2013 [30] | USA | Retrospective | LAR, ISR | 16 | 58 | 12 (75.0%) | 1 (6.3%) | 22 | 63 | 16 (73%) | 4 (18.2%) |
| Jafari et al., 2021 [31] | USA | RCT | LAR | 178 | 57.2 ± 11.4 | 109 (61.2%) | 16 (9.0%) | 169 | 57.0 ± 11.4 | 99 (58.6%) | 16 (9.6%) |
| Kim et al., 2017 [32] | Korea | Case cohort | LAR | 310 | 58 ± 11 | 182 (58.9%) | 2 (0.6%) | 347 | 57 ± 11 | 216 (62.2%) | 18 (5.2%) |
| Kin et al., 2015 [33] | USA | Retrospective | CL, PR | 173 | 58.2 ± 13.2 | 54 (31.2%) | 13 (7.5%) | 173 | 58.1 ± 13.2 | 54 (31.2%) | 11 (6.4%) |
| Kudszus et al., 2010 [34] | Germany | Retrospective | HC | 201 | 67.8 ± 25.2 | 85 (42.2%) | 7 (3.5%) | 201 | 69.0 ± 21.9 | 85 (42.2%) | 15 (7.5%) |
| Losurdo et al., 2020 [35] | France | Retrospective | CR | 177 | 69.9 ± 11.2 | 109 (61.4%) | 19 (10.8%) | 95 | 67.9 ± 10.0 | 37 (38.6%) | 17 (17.8%) |
| Mizrahi et al., 2018 [36] | USA | Retrospective | LAR | 30 | 58 ± 12 | 16 (53.3%) | 0 (0.0%) | 30 | 58 ± 13 | 18 (60.0%) | 2 (6.7%) |
| Otero-Piñeiro et al., 2021 [37] | Spain | Retrospective analysis of prospectively collected data | TaTME | 80 | 68.0 ± 11.4 | 51 (63.7%) | 2 (2.5%) | 204 | 66.6 ± 12.3 | 123 (60.3%) | 23 (11.3%) |
| Picardi et al., 2021 [38] | Italy | Retrospective | Mixed | 40 | 62.6 ± 10.5 | 17 (42.5%) | 1 (2.5%) | 39 | 67.74 ± 13.4 | 19 (48.7%) | 7 (17.9%) |
| Ris et al., 2018 [39] | Multicenter | Prospective open-label clinical study | Mixed | 504 | 64 (18–88) | 279 (55.4%) | 0 (0.0%) | 1173 | NS | NS | 68 (5.8%) |
| Shapera et al., 2019 [40] | USA | Prospectively maintained database | LAR, HC, SI | 74 | 58 | 42 (56.8%) | 0 (0.0%) | 29 | 60 | 16 (55.2%) | 1 (3.4%) |
| Skrovina et al., 2020 [41] | Czech Republic | Retrospective | TME | 50 | 62.4 ± 9.0 | 34 (68.0%) | 5 (10.0%) | 50 | 65.0 ± 9.4 | 29 (58.0%) | 9 (18.0%) |
| Spinelli et al., 2019 [42] | Italy | Retrospective | IPAA | 32 | 39.41 ± 14.09 | 21 (65.6%) | 0 (0.0%) | 32 | 45.75 ± 15.9 | 17 (53.1%) | 1 (3.12%) |
| Su et al., 2020 [43] | China | Retrospective | CL | 84 | 59.1 ± 11.1 | 48 (57.1%) | 0 (0.0%) | 105 | 60.2 ± 9.8 | 55 (52.4%) | 0 (0.0%) |
| Tsang et al., 2020 [44] | China | Prospective | LAR, HC, AR | 62 | 69.82 ± 9.89 | 39 (62.9%) | 2 (3.2%) | 69 | 67.71 ± 11.65 | 47 (68.1%) | 3 (4.3%) |
| Wada et al., 2019 [45] | Japan | Retrospective | LAR | 48 | 66 | 31 (64.6%) | 5 (10.4%) | 101 | 67 | 70 (69.3%) | 7 (6.9%) |
| Watanabe et al., 2021 [46] | Japan | Retrospective | SSSA | 532 | 74 (68–80) | 273 (51.3%) | 2/260 (0.8%) | 502 | 73 (66–79) | 268 (44.4%) | 7/274 (2.6%) |
| Wojcik et al., 2020 [47] | France | Prospective | CL, AR | 46 | 65.7 ± 11.1 | 30 (65.2%) | 3 (6.5%) | 65 | 68.6 ± 12 | 40 (61.5%) | 11 (16.9%) |
| Yanagita et al., 2021 [48] | Japan | Retrospective analysis of prospectively collected data | Mixed | 197 | 70 (34–93) | 116 (58.9%) | 9 (4.6%) | 187 | 69 (38–94) | 115 (61.5%) | 16 (8.6%) |
| Zhou et al., 2019 [49] | China | Retrospective | TME | 12 | 60.3 ± 9.6 | 5 (41.7%) | 1 (8.3%) | 30 | 58.5 ± 9.5 | 19 (63.3%) | 0 (0.0%) |
| Adverse Event Type | No. of Studies | Events/Participants | Events | Heterogeneity between Trials | p Value for Differences across Groups | |||
|---|---|---|---|---|---|---|---|---|
| ICG | Non-ICG | RR | 95% CI | p Value | I2 Statistic | |||
| No. of patients with adverse events | 12 | 218/1129 (19.3%) | 376/1358 (27.7%) | 0.80 | 0.70–0.92 | 0.11 | 36% | 0.002 |
| Wound infection | 13 | 34/1401 (2.4%) | 53/1615 (3.3%) | 0.72 | 0.47–1.09 | 0.83 | 0% | 0.12 |
| Ileus | 12 | 64/1381 (4.6%) | 91/1624 (5.6%) | 0.90 | 0.67–1.23 | 0.06 | 43% | 0.51 |
| Abdominal bleeding | 4 | 6/401 (1.5%) | 11/538 (2.0%) | 1.02 | 0.38–2.79 | 1.00 | 0% | 0.96 |
| Abdominal abscess | 4 | 7/266 (2.6%) | 18/442 (4.1%) | 0.83 | 0.36–1.92 | 0.60 | 0% | 0.66 |
| Bowel obstruction | 2 | 4/182 (2.2%) | 1/203 (0.5%) | 3.32 | 0.50–21.85 | 0.39 | 0% | 0.21 |
| Urinary retention | 10 | 23/829 (2.8%) | 34/1112 (3.1%) | 0.88 | 0.51–1.50 | 0.90 | 0% | 0.63 |
| Urinary tract infections | 6 | 17/774 (2.2%) | 125/301 (41.5%) | 0.77 | 0.43–1.37 | 0.56 | 0% | 0.37 |
| Urinary injury | 2 | 1/70 (1.4%) | 1/69 (1.4%) | 0.99 | 0.14–6.83 | 0.33 | 0% | 0.99 |
| Pulmonary complications | 7 | 26/678 (3.8%) | 34/760 (4.5%) | 0.86 | 0.53–1.38 | 0.31 | 15% | 0.53 |
| Cardiovascular complications | 2 | 2/128 (1.6%) | 1/128 (0.8%) | 1.00 | 0.18–5.62 | 0.37 | 0% | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safiejko, K.; Tarkowski, R.; Kozlowski, T.P.; Koselak, M.; Jachimiuk, M.; Tarasik, A.; Pruc, M.; Smereka, J.; Szarpak, L. Safety and Efficacy of Indocyanine Green in Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis of 11,047 Patients. Cancers 2022, 14, 1036. https://doi.org/10.3390/cancers14041036
Safiejko K, Tarkowski R, Kozlowski TP, Koselak M, Jachimiuk M, Tarasik A, Pruc M, Smereka J, Szarpak L. Safety and Efficacy of Indocyanine Green in Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis of 11,047 Patients. Cancers. 2022; 14(4):1036. https://doi.org/10.3390/cancers14041036
Chicago/Turabian StyleSafiejko, Kamil, Radoslaw Tarkowski, Tomasz Piotr Kozlowski, Maciej Koselak, Marcin Jachimiuk, Aleksander Tarasik, Michal Pruc, Jacek Smereka, and Lukasz Szarpak. 2022. "Safety and Efficacy of Indocyanine Green in Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis of 11,047 Patients" Cancers 14, no. 4: 1036. https://doi.org/10.3390/cancers14041036
APA StyleSafiejko, K., Tarkowski, R., Kozlowski, T. P., Koselak, M., Jachimiuk, M., Tarasik, A., Pruc, M., Smereka, J., & Szarpak, L. (2022). Safety and Efficacy of Indocyanine Green in Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis of 11,047 Patients. Cancers, 14(4), 1036. https://doi.org/10.3390/cancers14041036








