Integration of Baseline Metabolic Parameters and Mutational Profiles Predicts Long-Term Response to First-Line Therapy in DLBCL Patients: A Post Hoc Analysis of the SAKK38/07 Study †
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Mutational Profile Evaluation
2.3. PET/CT Images Analysis
2.4. Statistical Analysis
3. Results
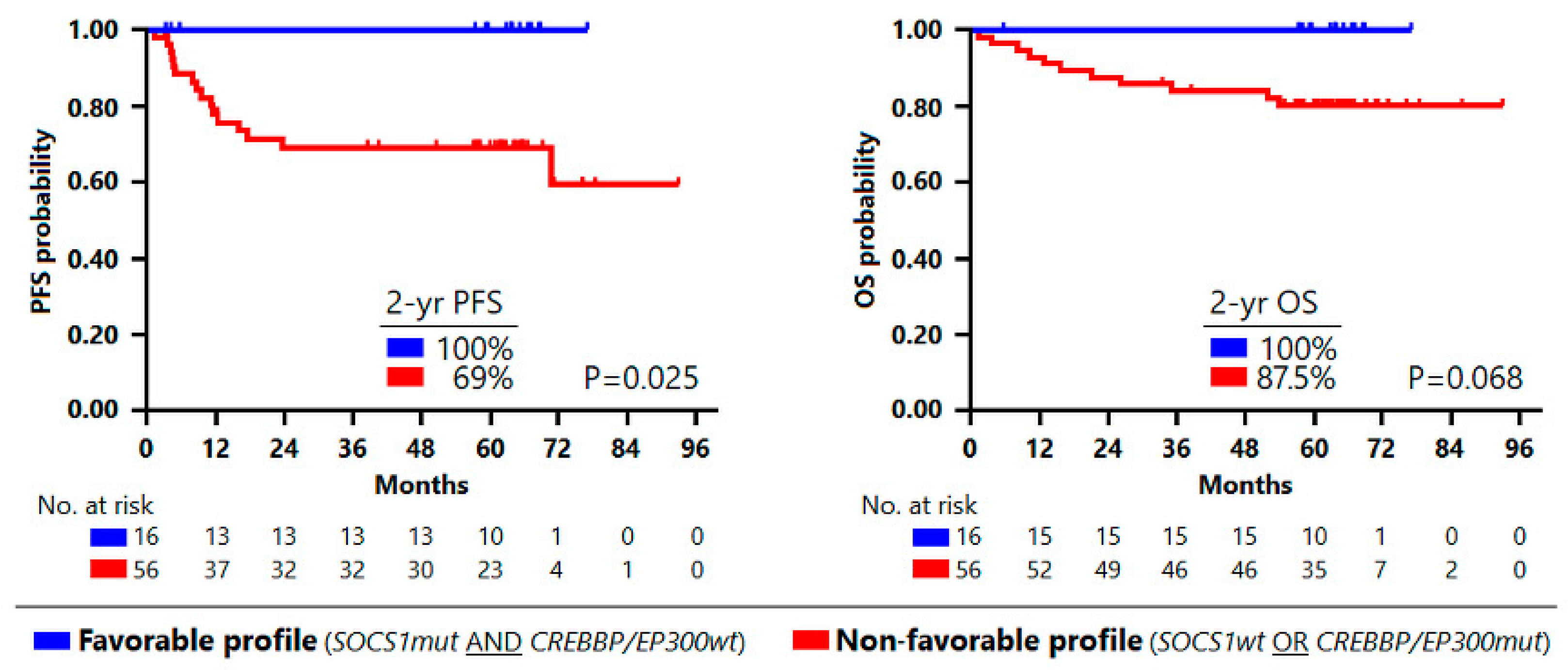
3.1. Mutational Profile Impact on Outcome
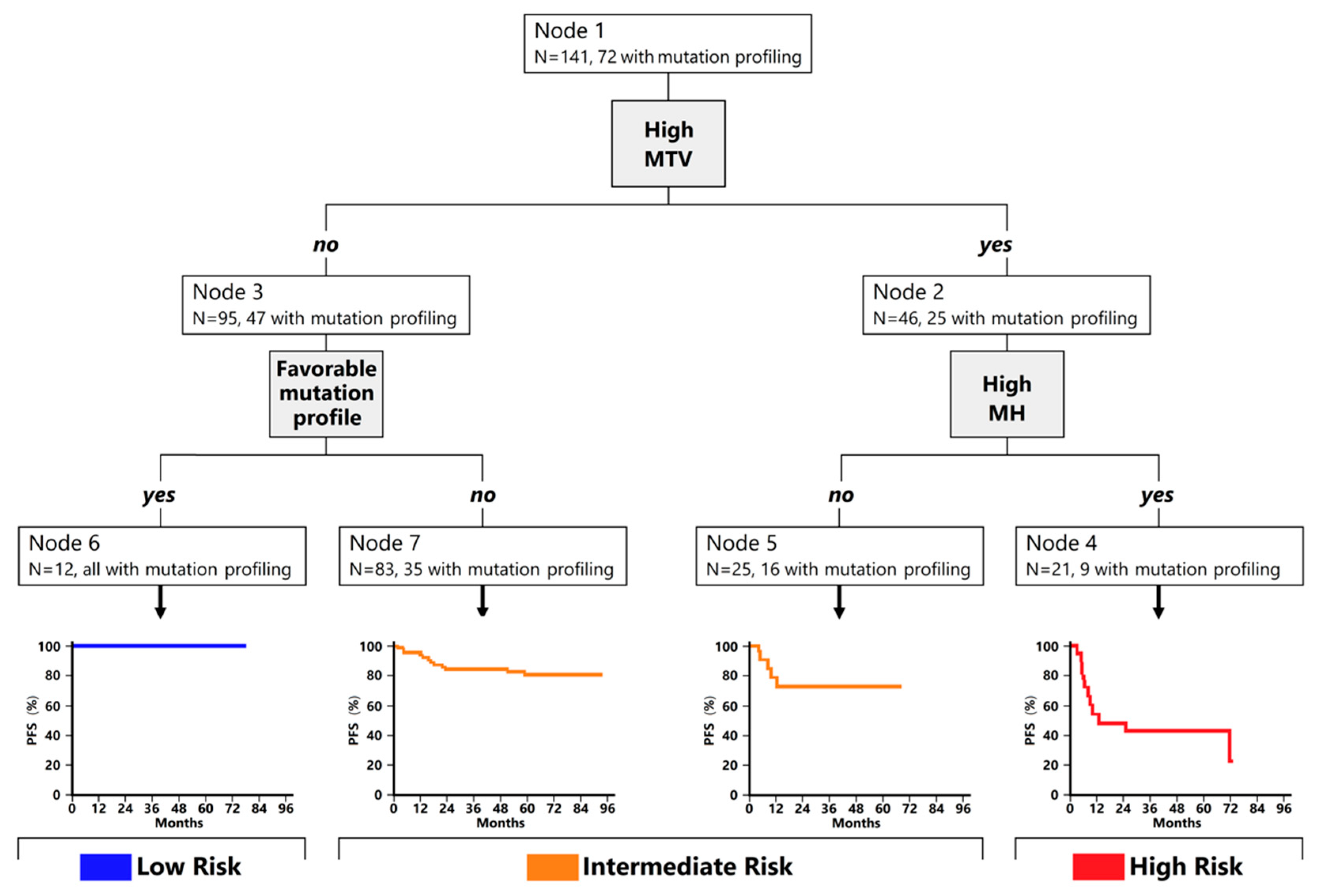
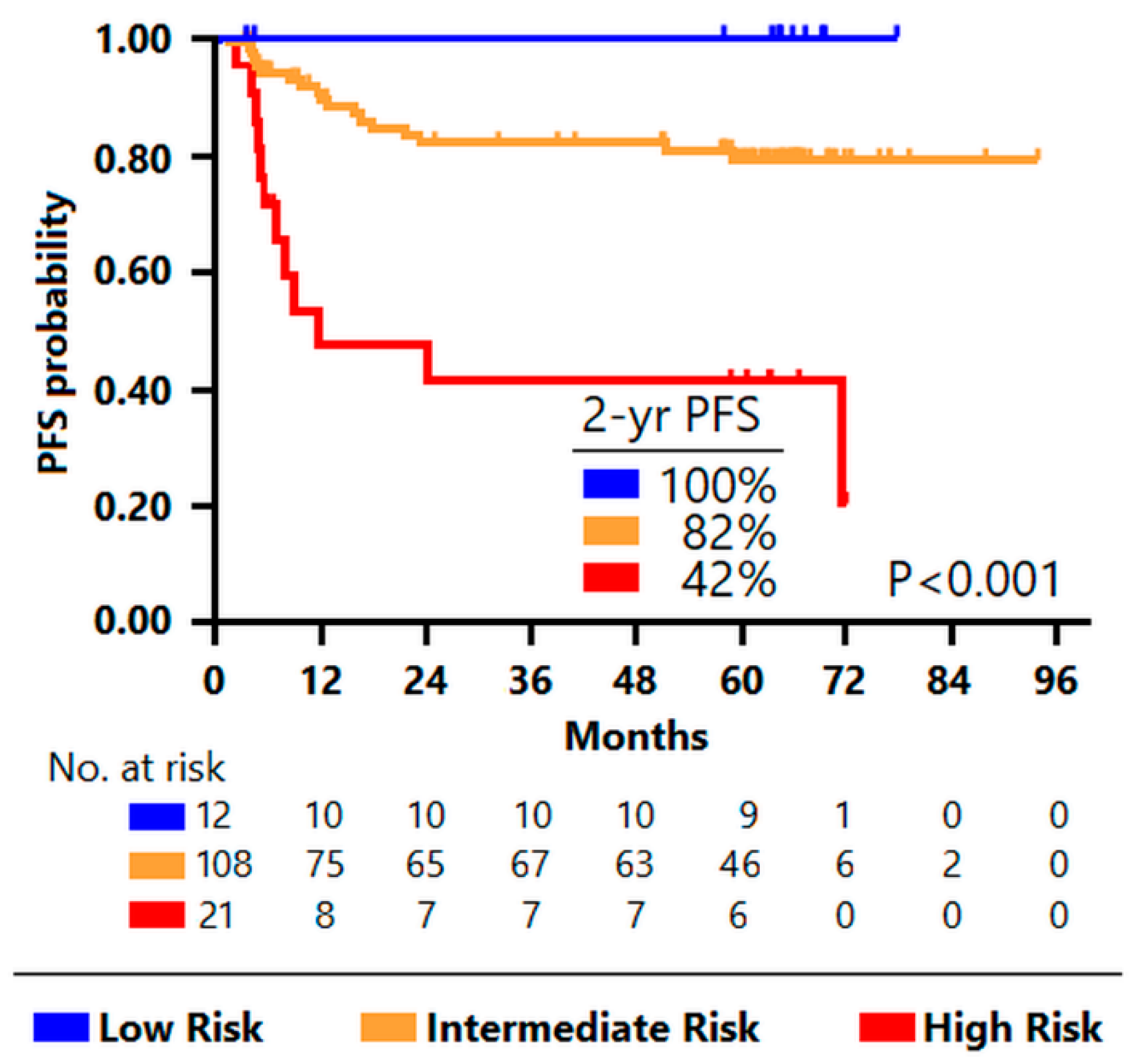
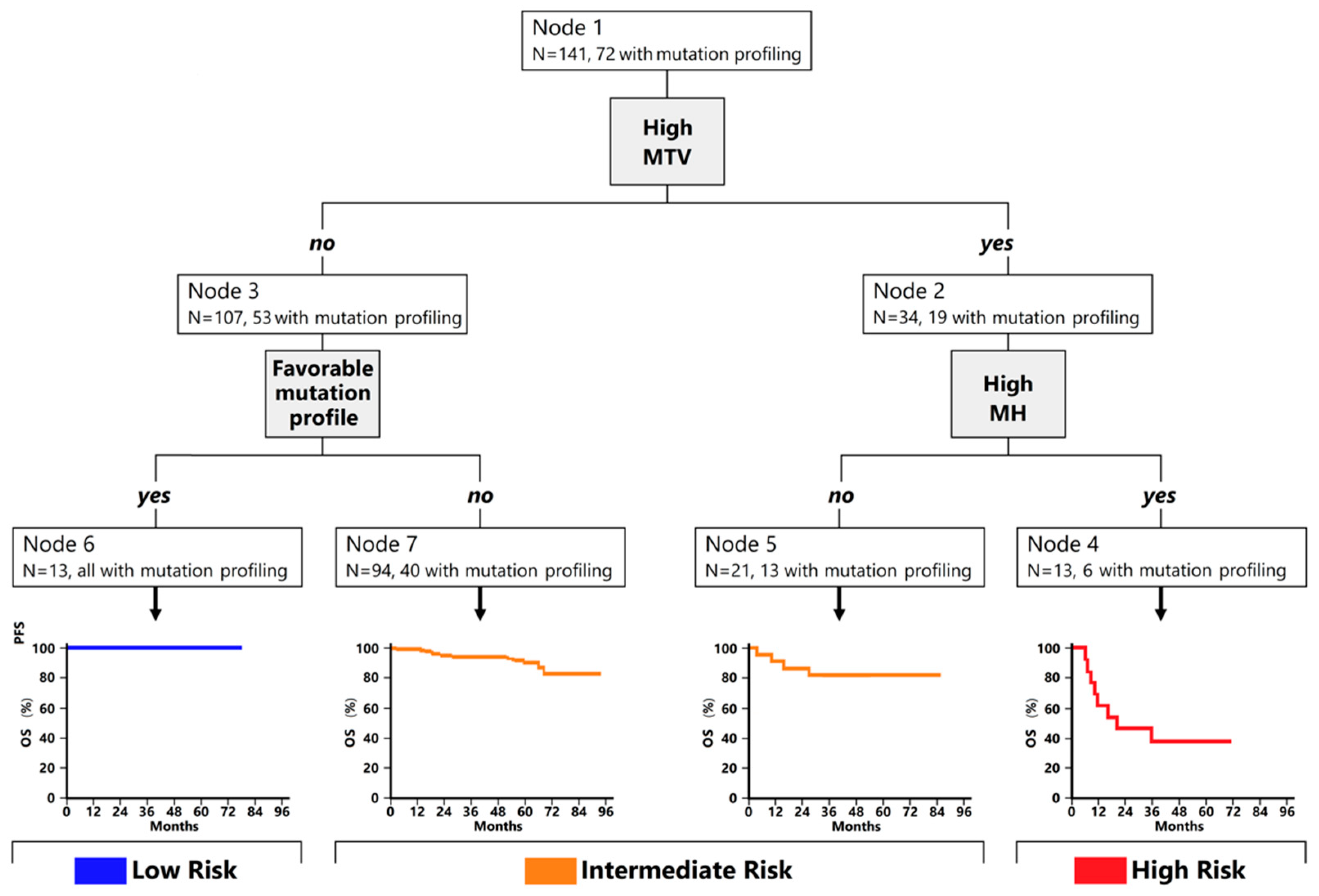
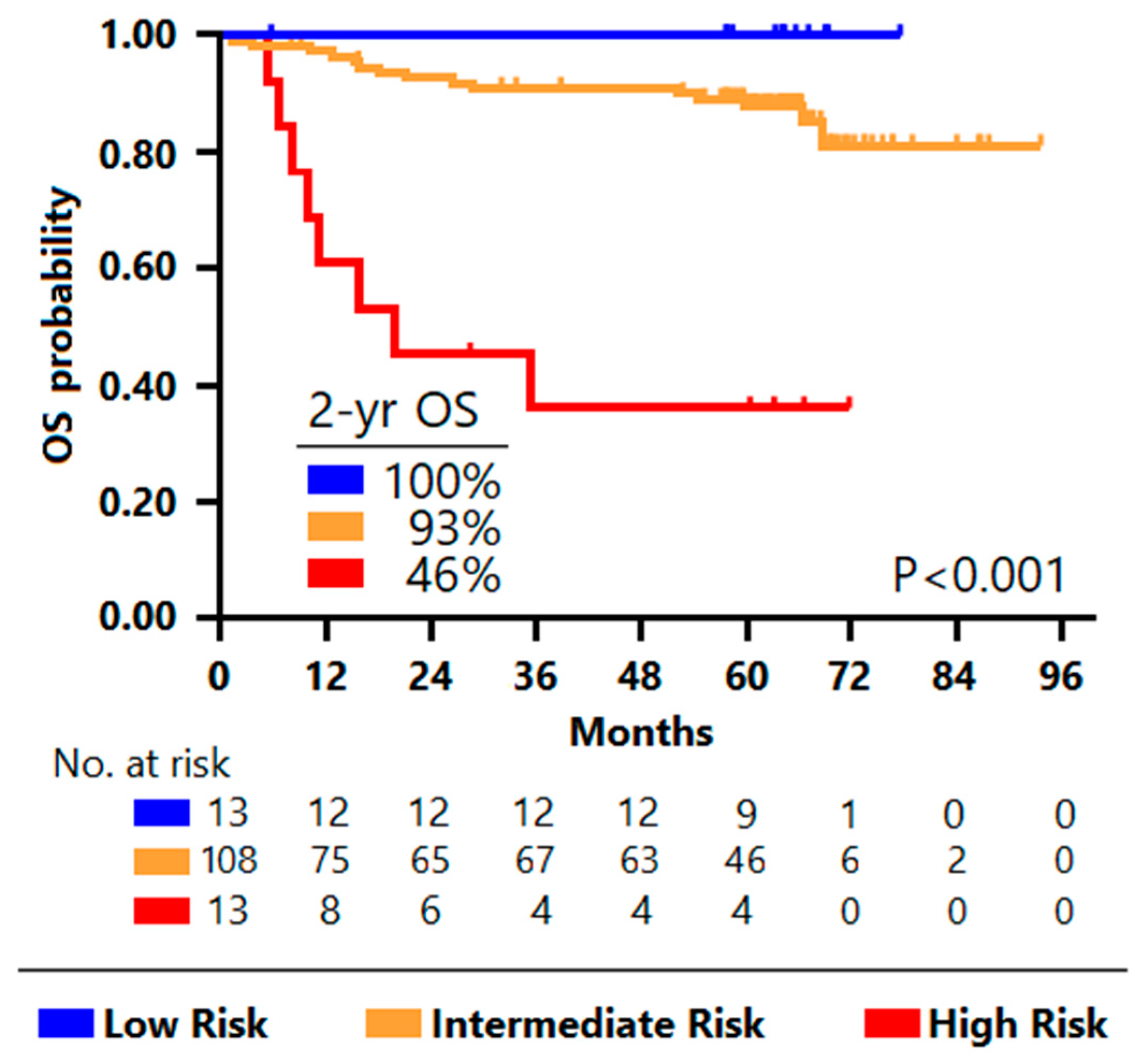
3.2. Classification Trees for Outcome Prediction
3.3. Comparison between Prognostic Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Hamadani, M.; Habermann, T.M.; Cerhan, J.R.; Macon, W.R.; Maurer, M.J.; Go, R.S. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: A longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am. J. Hematol. 2015, 90, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Coiffier, B.; Thieblemont, C.; Van Den Neste, E.; Lepeu, G.; Plantier, I.; Castaigne, S.; Lefort, S.; Marit, G.; Macro, M.; Sebban, C.; et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: A study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood 2010, 116, 2040–2045. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Barta, S.K. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am. J. Hematol. 2019, 94, 604–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, U.; Maurer, M.J.; Thompson, C.A.; Thanarajasingam, G.; Inwards, D.J.; Micallef, I.; Macon, W.; Syrbu, S.; Lin, T.; Lin, Y.; et al. Clinical heterogeneity of diffuse large B cell lymphoma following failure of front-line immunochemotherapy. Br. J. Haematol. 2017, 179, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Crump, M.; Neelapu, S.S.; Farooq, U.; Van Den Neste, E.; Kuruvilla, J.; Westin, J.; Link, B.K.; Hay, A.; Cerhan, J.R.; Zhu, L.; et al. Outcomes in refractory diffuse large B-cell lymphoma: Results from the international SCHOLAR-1 study. Blood 2017, 130, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Sehn, L.H.; Herrera, A.F.; Flowers, C.R.; Kamdar, M.K.; McMillan, A.; Hertzberg, M.; Assouline, S.; Kim, T.M.; Kim, W.S.; Ozcan, M.; et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2020, 38, 155–165. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bartlett, N.L.; Assouline, S.; Yoon, S.S.; Bosch, F.; Sehn, L.H.; Cheah, C.Y.; Shadman, M.; Gregory, G.P.; Ku, M.; et al. Mosunetuzumab induces complete remissions in poor prognosis non-Hodgkin lymphoma patients, including those who are resistant to or relapsing after chimeric antigen receptor T-cell (CAR-T) therapies, and Is active in treatment through multiple lines. Blood 2019, 134, 6. [Google Scholar] [CrossRef]
- Hutchings, M.; Carlo-Stella, C.; Bachy, E.; Offner, F.C.; Morschhauser, F.; Crump, M.; Iacoboni, G.; Balari, A.S.; Martinez-Lopez, J.; Lundberg, L.; et al. Glofitamab Step-up Dosing Induces High Response Rates in Patients with Hard-to-Treat Refractory or Relapsed Non-Hodgkin Lymphoma. Blood 2020, 136, 46–48. [Google Scholar] [CrossRef]
- International Non-Hodgkin’s Lymphoma Prognostic Factors Project a Predictive Model for Aggressive Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 1993, 329, 987–994. [CrossRef] [PubMed]
- Sehn, L.H.; Berry, B.; Chhanabhai, M.; Fitzgerald, C.; Gill, K.; Hoskins, P.; Klasa, R.; Savage, K.J.; Shenkier, T.; Sutherland, J.; et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 2007, 109, 1857–1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Sehn, L.H.; Rademaker, A.W.; Gordon, L.; LaCasce, A.S.; Crosby-Thompson, A.; Vanderplas, A.; Zelenetz, A.; Abel, G.A.; Rodriguez, M.A.; et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014, 123, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Biccler, J.; Eloranta, S.; de Nully Brown, P.; Frederiksen, H.; Jerkeman, M.; Smedby, K.E.; Bøgsted, M.; El-Galaly, T.C. Simplicity at the cost of predictive accuracy in diffuse large B-cell lymphoma: A critical assessment of the R-IPI, IPI, and NCCN-IPI. Cancer Med. 2018, 7, 114–122. [Google Scholar] [CrossRef]
- Shagera, Q.A.; Cheon, G.J.; Koh, Y.; Yoo, M.Y.; Kang, K.W.; Lee, D.S.; Kim, E.E.; Yoon, S.-S.; Chung, J.-K. Prognostic value of metabolic tumour volume on baseline 18F-FDG PET/CT in addition to NCCN-IPI in patients with diffuse large B-cell lymphoma: Further stratification of the group with a high-risk NCCN-IPI. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1417–1427. [Google Scholar] [CrossRef]
- Zhao, P.; Yu, T.; Pan, Z. Prognostic value of the baseline 18F-FDG PET/CT metabolic tumour volume (MTV) and further stratification in low-intermediate (L-I) and high-intermediate (H-I) risk NCCNIPI subgroup by MTV in DLBCL MTV predict prognosis in DLBCL. Ann. Nucl. Med. 2021, 35, 24–30. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A.; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef]
- Chang, C.-C.; Cho, S.-F.; Chuang, Y.-W.; Lin, C.-Y.; Chang, S.-M.; Hsu, W.-L.; Huang, Y.-F. Prognostic significance of total metabolic tumor volume on 18F-fluorodeoxyglucose positron emission tomography/ computed tomography in patients with diffuse large B-cell lymphoma receiving rituximab-containing chemotherapy. Oncotarget 2017, 8, 99587–99600. [Google Scholar] [CrossRef] [Green Version]
- Mikhaeel, N.G.; Smith, D.; Dunn, J.T.; Phillips, M.; Møller, H.; Fields, P.A.; Wrench, D.; Barrington, S.F. Combination of baseline metabolic tumour volume and early response on PET/CT improves progression-free survival prediction in DLBCL. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1209–1219. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Chen, Y.; Huang, H.; Zhou, X.; Liu, J.; Huang, G. Prognostic value of total lesion glycolysis of baseline 18F-fluorodeoxyglucose positron emission tomography/computed tomography in diffuse large B-cell lymphoma. Oncotarget 2016, 7, 83544–83553. [Google Scholar] [CrossRef] [Green Version]
- Sasanelli, M.; Meignan, M.; Haioun, C.; Berriolo-Riedinger, A.; Casasnovas, R.-O.; Biggi, A.; Gallamini, A.; Siegel, B.A.; Cashen, A.F.; Véra, P.; et al. Pretherapy metabolic tumour volume is an independent predictor of outcome in patients with diffuse large B-cell lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2017–2022. [Google Scholar] [CrossRef] [PubMed]
- Vercellino, L.; Cottereau, A.-S.; Casasnovas, R.-O.; Tilly, H.; Feugier, P.; Chartier, L.; Fruchart, C.; Roulin, L.; Oberic, L.; Pica, G.M.; et al. High total metabolic tumor volume at baseline predicts survival independent of response to therapy. Blood 2020, 135, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Ceriani, L.; Gritti, G.; Cascione, L.; Pirosa, M.C.; Polino, A.; Ruberto, T.; Stathis, A.; Bruno, A.; Moccia, A.A.; Giovanella, L.; et al. SAKK38/07 study: Integration of baseline metabolic heterogeneity and metabolic tumor volume in DLBCL prognostic model. Blood Adv. 2020, 4, 1082–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zucca, E.; Cascione, L.; Ruberto, T.; Facchinelli, D.; Schär, S.; Hayoz, S.; Dirnhofer, S.; Giovanella, L.; Bargetzi, M.; Mamot, C.; et al. Prognostic models integrating quantitative parameters from baseline and interim positron emission computed tomography in patients with diffuse large B-cell lymphoma: Post-hoc analysis from the SAKK38/07 clinical trial. Hematol. Oncol. 2020, 38, 715–725. [Google Scholar] [CrossRef]
- Cottereau, A.-S.; Nioche, C.; Dirand, A.-S.; Clerc, J.; Morschhauser, F.; Casasnovas, R.-O.; Meignan, M.A.; Buvat, I. 18F-FDG PET Dissemination Features in Diffuse Large B-Cell Lymphoma Are Predictive of Outcome. J. Nucl. Med. 2020, 61, 40–45. [Google Scholar] [CrossRef]
- Chapuy, B.; Stewart, C.; Dunford, A.J.; Kim, J.; Kamburov, A.; Redd, R.A.; Lawrence, M.S.; Roemer, M.G.M.; Li, A.J.; Ziepert, M.; et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 2018, 24, 679–690. [Google Scholar] [CrossRef]
- Schmitz, R.; Wright, G.W.; Huang, D.W.; Johnson, C.A.; Phelan, J.D.; Wang, J.Q.; Roulland, S.; Kasbekar, M.; Young, R.M.; Shaffer, A.L.; et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018, 378, 1396–1407. [Google Scholar] [CrossRef]
- El Hussein, S.; Shaw, K.R.M.; Vega, F. Evolving insights into the genomic complexity and immune landscape of diffuse large B-cell lymphoma: Opportunities for novel biomarkers. Mod. Pathol. 2020, 33, 2422–2436. [Google Scholar] [CrossRef]
- Lacy, S.E.; Barrans, S.L.; Beer, P.A.; Painter, D.; Smith, A.G.; Roman, E.; Cooke, S.L.; Ruiz, C.; Glover, P.; Van Hoppe, S.J.L.; et al. Targeted sequencing in DLBCL, molecular subtypes, and outcomes: A Haematological Malignancy Research Network report. Blood 2020, 135, 1759–1771. [Google Scholar] [CrossRef]
- Wright, G.W.; Huang, D.W.; Phelan, J.D.; Coulibaly, Z.A.; Roulland, S.; Young, R.M.; Wang, J.Q.; Schmitz, R.; Morin, R.; Tang, J.; et al. A Probabilistic Classification Tool for Genetic Subtypes of Diffuse Large B Cell Lymphoma with Therapeutic Implications. Cancer Cell 2020, 37, 551–568.e14. [Google Scholar] [CrossRef]
- Cottereau, A.-S.; Lanic, H.; Mareschal, S.; Meignan, M.; Vera, P.; Tilly, H.; Jardin, F.; Becker, S. Molecular Profile and FDG-PET/CT Total Metabolic Tumor Volume Improve Risk Classification at Diagnosis for Patients with Diffuse Large B-Cell Lymphoma. Clin. Cancer Res. 2016, 22, 3801–3809. [Google Scholar] [CrossRef] [Green Version]
- Toledano, M.N.; Desbordes, P.; Banjar, A.; Gardin, I.; Vera, P.; Ruminy, P.; Jardin, F.; Tilly, H.; Becker, S. Combination of baseline FDG PET/CT total metabolic tumour volume and gene expression profile have a robust predictive value in patients with diffuse large B-cell lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 680–688. [Google Scholar] [CrossRef]
- Mamot, C.; Klingbiel, D.; Hitz, F.; Renner, C.; Pabst, T.; Driessen, C.; Mey, U.; Pless, M.; Bargetzi, M.; Krasniqi, F.; et al. Final Results of a Prospective Evaluation of the Predictive Value of Interim Positron Emission Tomography in Patients with Diffuse Large B-Cell Lymphoma Treated With R-CHOP-14 (SAKK 38/07). J. Clin. Oncol. 2015, 33, 2523–2529. [Google Scholar] [CrossRef]
- Tzankov, A.; Leu, N.; Muenst, S.; Juskevicius, D.; Klingbiel, D.; Mamot, C.; Dirnhofer, S. Multiparameter analysis of homogeneously R-CHOP-treated diffuse large B cell lymphomas identifies CD5 and FOXP1 as relevant prognostic biomarkers: Report of the prospective SAKK 38/07 study. J. Hematol. Oncol. 2015, 8, 70. [Google Scholar] [CrossRef] [Green Version]
- Juskevicius, D.; Jucker, D.; Klingbiel, D.; Mamot, C.; Dirnhofer, S.; Tzankov, A. Mutations of CREBBP and SOCS1 are independent prognostic factors in diffuse large B cell lymphoma: Mutational analysis of the SAKK 38/07 prospective clinical trial cohort. J. Hematol. Oncol. 2017, 10, 70. [Google Scholar] [CrossRef] [Green Version]
- Hans, C.P.; Weisenburger, D.D.; Greiner, T.C.; Gascoyne, R.D.; Delabie, J.; Ott, G.; Müller-Hermelink, H.K.; Campo, E.; Braziel, R.M.; Jaffe, E.S.; et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004, 103, 275–282. [Google Scholar] [CrossRef]
- Ceriani, L.; Milan, L.; Martelli, M.; Ferreri, A.J.M.; Cascione, L.; Zinzani, P.L.; Di Rocco, A.; Conconi, A.; Stathis, A.; Cavalli, F.; et al. Metabolic heterogeneity on baseline 18FDG-PET/CT scan is a predictor of outcome in primary mediastinal B-cell lymphoma. Blood 2018, 132, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased Recursive Partitioning: A Conditional Inference Framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef] [Green Version]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Posada, D.; Buckley, T.R. Model Selection and Model Averaging in Phylogenetics: Advantages of Akaike Information Criterion and Bayesian Approaches Over Likelihood Ratio Tests. Syst. Biol. 2004, 53, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Pasqualucci, L.; Dominguez-Sola, D.; Chiarenza, A.; Fabbri, G.; Grunn, A.; Trifonov, V.; Kasper, L.H.; Lerach, S.; Tang, H.; Ma, J.; et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature 2011, 471, 189–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrington, S.F.; Meignan, M.A. Time to Prepare for Risk Adaptation in Lymphoma by Standardizing Measurement of Metabolic Tumor Burden. J. Nucl. Med. 2019, 60, 1096–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meignan, M.; Itti, E.; Gallamini, A.; Younes, A. FDG PET/CT imaging as a biomarker in lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 623–633. [Google Scholar] [CrossRef]
- Cottereau, A.-S.; Lanic, H.; Mareschal, S.; Meignan, M.; Vera, P.; Tilly, H.; Jardin, F.; Becker, S. Molecular Profile and FDG-PET Metabolic Volume at Staging in DLBCL—Response. Clin. Cancer Res. 2016, 22, 3414–3415. [Google Scholar] [CrossRef] [Green Version]
- Juskevicius, D.; Lorberl, T.; Gsponer, J.; Perrina, V.; Ruiz, C.; Stenner-Liewen, F.; Dirnhofer, S.; Tzankov, A. Distinct genetic evolution patterns of relapsing diffuse large B-cell lymphoma revealed by genome-wide copy number aberration and targeted sequencing analysis. Leukemia 2016, 30, 2385–2395. [Google Scholar] [CrossRef]
- Sun, P.; Chen, C.; Xia, Y.; Wang, Y.; Liu, P.-P.; Bi, X.-W.; Shao, Y.W.; Ou, Q.-X.; Wu, X.; Yang, H.; et al. Mutation Profiling of Malignant Lymphoma by Next-Generation Sequencing of Circulating Cell-Free DNA. J. Cancer 2019, 10, 323–331. [Google Scholar] [CrossRef]
- Lenz, G.; Davis, R.E.; Ngo, V.N.; Lam, L.; George, T.C.; Wright, G.W.; Dave, S.S.; Zhao, H.; Xu, W.; Rosenwald, A.; et al. Oncogenic CARD11 Mutations in Human Diffuse Large B Cell Lymphoma. Science 2008, 319, 1676–1679. [Google Scholar] [CrossRef]
- Lee, B.; Lee, H.; Cho, J.; Yoon, S.E.; Kim, S.J.; Park, W.-Y.; Kim, W.S.; Ko, Y.H. Mutational Profile and Clonal Evolution of Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Front. Oncol. 2021, 11, 628807. [Google Scholar] [CrossRef]
- Morin, R.D.; Arthur, S.E.; Hodson, D.J. Molecular profiling in diffuse large B-cell lymphoma: Why so many types of subtypes? Br. J. Haematol. 2021, 196, 814–829. [Google Scholar] [CrossRef]
- Eude, F.; Toledano, M.; Vera, P.; Tilly, H.; Mihailescu, S.-D.; Becker, S. Reproducibility of Baseline Tumour Metabolic Volume Measurements in Diffuse Large B-Cell LymphomA: Is There a Superior Method? Metabolites 2021, 11, 72. [Google Scholar] [CrossRef]
- Cottereau, A.-S.; Buvat, I.; Kanoun, S.; Versari, A.; Casasnovas, R.-O.; Chauvie, S.; Clerc, J.; Gallamini, A.; Meignan, M. Is there an optimal method for measuring baseline metabolic tumor volume in diffuse large B cell lymphoma? Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1463–1464. [Google Scholar] [CrossRef]
- Ilyas, H.; Mikhaeel, N.G.; Dunn, J.T.; Rahman, F.; Moller, H.; Smith, D.; Barrington, S.F. Defining the optimal method for measuring baseline metabolic tumour volume in diffuse large B cell lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1142–1154. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Number | % | |
|---|---|---|---|
| Sex, male | 73 | 51.8 | |
| Age ≥ 60 years | 68 | 48.2 | |
| LDH elevated | 68 | 48.2 | |
| Extranodal sites > 1 | 34 | 24.1 | |
| ECOG PS ≥ 2 | 10 | 7.1 | |
| Stage III-IV | 80 | 56.7 | |
| High-intermediate or high-risk IPI | 44 | 31.2 | |
| High-risk R-IPI | 44 | 31.2 | |
| High-intermediate or high-risk NCCN-IPI | 52 | 36.9 | |
| Germinal center B-like subtype (COO tested in 113) | 84 | 74.3 | |
| cMYC and BCL-2 double expression (tested in 87) | 12 | 13.8 | |
| MTV | ≥931 mL (cut-off point for PFS) | 46 34 | 32.6 24.1 |
| ≥1149 mL (cut-off point for OS) | |||
| MH ≥ 0.43 AUC-CSH | 55 | 39.0 | |
| Mutated Gene | Frequency N = 72 (100%) | No Progression or Death N = 56 (77.8%) | Progression or Death N = 16 (22.2%) | p-Value |
|---|---|---|---|---|
| ATM | 14 (19.4%) | 11 (19.6%) | 3 (18.8%) | 0.937 |
| B2M | 11 (15.3%) | 9 (16.1%) | 2 (12.5%) | 0.726 |
| BCL2 | 4 (3.6%) | 3 (5.4%) | 1 (6.2%) | 0.891 |
| BCL6 | 2 (2.8%) | 2 (3.6%) | 0 (0%) | 0.443 |
| BCL10 | 3 (4.2%) | 3 (5.4%) | 0 (0%) | 0.344 |
| BTG1 | 8 (11.1%) | 8 (14.3%) | 0 (0%) | 0.109 |
| CARD11 | 9 (12.5%) | 5 (8.9%) | 4 (25.0%) | 0.086 |
| CD79B | 4 (3.6%) | 2 (3.6%) | 2 (12.5%) | 0.169 |
| CREBBP_EP300 | 14 (19.4%) | 7 (12.5%) | 7 (43.8%) | 0.005 |
| EBF1 | 3 (4.2%) | 3 (5.4%) | 0 (0%) | 0.344 |
| EZH2 | 10 (13.9%) | 8 (14.3%) | 2 (12.5%) | 0.855 |
| FOXO1 | 3 (4.2%) | 3 (5.4%) | 0 (0%) | 0.344 |
| GNA13 | 13 (18.1%) | 10 (17.9%) | 3 (18.8%) | 0.935 |
| HIST1H1C | 7 (9.7%) | 6 (10.7%) | 1 (6.2%) | 0.595 |
| IDH1 | 1 (1.4%) | 1 (1.8%) | 0 (0%) | 0.590 |
| IKZF1 | 2 (2.8%) | 2 (3.6%) | 0 (0%) | 0.443 |
| IRF4 | 1 (1.4%) | 1 (1.8%) | 0 (0%) | 0.590 |
| JAK2 | 1 (1.4%) | 1 (1.8%) | 0 (0%) | 0.590 |
| KLHL6 | 1 (1.4%) | 1 (1.8%) | 0 (0%) | 0.590 |
| KMT2D | 25 (34.7%) | 20 (35.7%) | 5 (31.2%) | 0.741 |
| KMT2C | 2 (2.8%) | 2 (3.6%) | 0 (0%) | 0.443 |
| KRAS | 1 (1.4%) | 1 (1.8%) | 0 (0%) | 0.590 |
| MCL1 | 3 (4.2%) | 3 (5.4%) | 0 (0%) | 0.344 |
| MEF2B | 6 (8.3%) | 5 (8.9%) | 1 (6.2%) | 0.732 |
| MYC | 5 (6.9%) | 4 (7.1%) | 1 (6.2%) | 0.901 |
| MYD88 | 5 (6.9%) | 3 (5.4%) | 2 (12.5%) | 0.322 |
| NOTCH1 | 1 (1.4%) | 1 (1.8%) | 0 (0%) | 0.590 |
| NOTCH2 | 3 (4.2%) | 2 (3.6%) | 1 (6.2%) | 0.636 |
| PAX5 | 2 (2.8%) | 2 (3.6%) | 0 (0%) | 0.443 |
| PIK3CD | 2 (2.8%) | 2 (3.6%) | 0 (0%) | 0.443 |
| PIK3R1 | 1 (1.4%) | 1 (1.8%) | 0 (0%) | 0.590 |
| PIM1 | 7 (9.7%) | 6 (10.7%) | 1 (6.2%) | 0.595 |
| PRDM1 | 2 (2.8%) | 1 (1.8%) | 1 (6.2%) | 0.338 |
| PTEN | 5 (6.9%) | 4 (7.1%) | 1 (6.2%) | 0.901 |
| PTPN1 | 2 (2.8%) | 2 (3.6%) | 0 (0%) | 0.443 |
| RELN | 1 (1.4%) | 1 (1.8%) | 0 (0%) | 0.590 |
| RHOA | 1 (1.4%) | 1 (1.8%) | 0 (0%) | 0.590 |
| SGK1 | 4 (3.6%) | 3 (5.4%) | 1 (6.2%) | 0.891 |
| SOCS1 | 19 (26.4%) | 18 (32.1%) | 1 (6.2%) | 0.038 |
| STAT6 | 6 (8.3%) | 5 (8.9%) | 1 (6.2%) | 0.732 |
| TET2 | 3 (4.2%) | 1 (1.8%) | 2 (12.5%) | 0.122 |
| TNFAIP3 | 11 (15.3%) | 10 (17.9%) | 1 (6.2%) | 0.255 |
| TP53 | 8 (11.1%) | 6 (10.7%) | 2 (12.5%) | 0.841 |
| U2AF1 | 1 (1.4%) | 1 (1.8%) | 0 (0%) | 0.590 |
| XPO1 | 2 (2.8%) | 2 (3.6%) | 0 (0%) | 0.443 |
| Characteristics | Low Risk (N = 12) | Intermediate Risk (N = 108) | High Risk (N = 21) | p-Value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Age | 0.061 | |||
| ≥60 years | 2 (16.7) | 54 (50.0) | 12 (57.1) | |
| LDH | <0.001 | |||
| Elevated | 8 (66.7) | 42 (38.9) | 18 (85.7) | |
| Extranodal sites | 0.159 | |||
| >1 | 1 (8.3) | 25 (23.1) | 8 (38.7) | |
| ECOG PS | 0.269 | |||
| 0–1 | 12 (100.0) | 101 (93.5) | 18 (85.7) | |
| Ann Arbor stage | 0.019 | |||
| I-II | 8 (66.7) | 49 (45.4) | 4 (19.0) | |
| III-IV | 4 (33.3) | 59 (54.6) | 17 (81.0) | |
| IPI risk group | 54 (50.0) | 0.012 | ||
| Low risk | 8 (66.7) | 23 (21.3) | 3 (14.3) | |
| Low-intermediate risk | 3 (25.0) | 20 (18.5) | 6 (28.6) | |
| High-intermediate risk | 0 (0.0) | 11 (10.2) | 7 (33.3) | |
| High risk | 1 (8.3) | 5 (23.8) | ||
| COO (n = 113) | 0.669 | |||
| GCB | 4 (33.3) | 22 (25.9) | 3 (18.8) | |
| non-GCB | 8 (66.7) | 63 (74.1) | 13 (81.2) | |
| Mutational profile (n = 72) | <0.001 | |||
| Favorable (SOCS1mut and CREBBP/EP300wt) | 12 (100.0) | 3 (5.9) | 1 (11.1) | |
| Unfavorable (SOCS1wt and/or CREBBP/EP300mut) | 0 | 48 (94.1) | 8 (88.9) |
| Prognostic Indices | PFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| PET/mutational Model | 4.42 | 2.07 to 9.45 | <0.001 | 5.96 | 2.41 to 14.73 | <0.001 |
| IPI | 0.93 | 0.47 to 1.85 | 0.839 | 0.75 | 0.34 to 1.64 | 0.470 |
| R-IPI | 1.33 | 0.43 to 4.13 | 0.623 | 1.54 | 0.35 to 6.73 | 0.563 |
| NCCN_IPI | 1.04 | 0.54 to 1.99 | 0.906 | 1.56 | 0.70 to 3.48 | 0.278 |
| Prognostic Indices | PFS | OS | ||
|---|---|---|---|---|
| AIC | CPE | AIC | CPE | |
| PET/mutational Model | 257 | 0.67 | 199 | 0.69 |
| IPI | 273 | 0.59 | 215 | 0.61 |
| R-IPI | 272 | 0.59 | 214 | 0.62 |
| NCCN-IPI | 272 | 0.58 | 211 | 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genta, S.; Ghilardi, G.; Cascione, L.; Juskevicius, D.; Tzankov, A.; Schär, S.; Milan, L.; Pirosa, M.C.; Esposito, F.; Ruberto, T.; et al. Integration of Baseline Metabolic Parameters and Mutational Profiles Predicts Long-Term Response to First-Line Therapy in DLBCL Patients: A Post Hoc Analysis of the SAKK38/07 Study. Cancers 2022, 14, 1018. https://doi.org/10.3390/cancers14041018
Genta S, Ghilardi G, Cascione L, Juskevicius D, Tzankov A, Schär S, Milan L, Pirosa MC, Esposito F, Ruberto T, et al. Integration of Baseline Metabolic Parameters and Mutational Profiles Predicts Long-Term Response to First-Line Therapy in DLBCL Patients: A Post Hoc Analysis of the SAKK38/07 Study. Cancers. 2022; 14(4):1018. https://doi.org/10.3390/cancers14041018
Chicago/Turabian StyleGenta, Sofia, Guido Ghilardi, Luciano Cascione, Darius Juskevicius, Alexandar Tzankov, Sämi Schär, Lisa Milan, Maria Cristina Pirosa, Fabiana Esposito, Teresa Ruberto, and et al. 2022. "Integration of Baseline Metabolic Parameters and Mutational Profiles Predicts Long-Term Response to First-Line Therapy in DLBCL Patients: A Post Hoc Analysis of the SAKK38/07 Study" Cancers 14, no. 4: 1018. https://doi.org/10.3390/cancers14041018
APA StyleGenta, S., Ghilardi, G., Cascione, L., Juskevicius, D., Tzankov, A., Schär, S., Milan, L., Pirosa, M. C., Esposito, F., Ruberto, T., Giovanella, L., Hayoz, S., Mamot, C., Dirnhofer, S., Zucca, E., & Ceriani, L. (2022). Integration of Baseline Metabolic Parameters and Mutational Profiles Predicts Long-Term Response to First-Line Therapy in DLBCL Patients: A Post Hoc Analysis of the SAKK38/07 Study. Cancers, 14(4), 1018. https://doi.org/10.3390/cancers14041018










