Cutaneous Melanoma Systematic Diagnostic Workflows and Integrated Reflectance Confocal Microscopy Assessed with a Retrospective, Comparative Longitudinal (2009–2018) Study
Abstract
:Simple Summary
Abstract
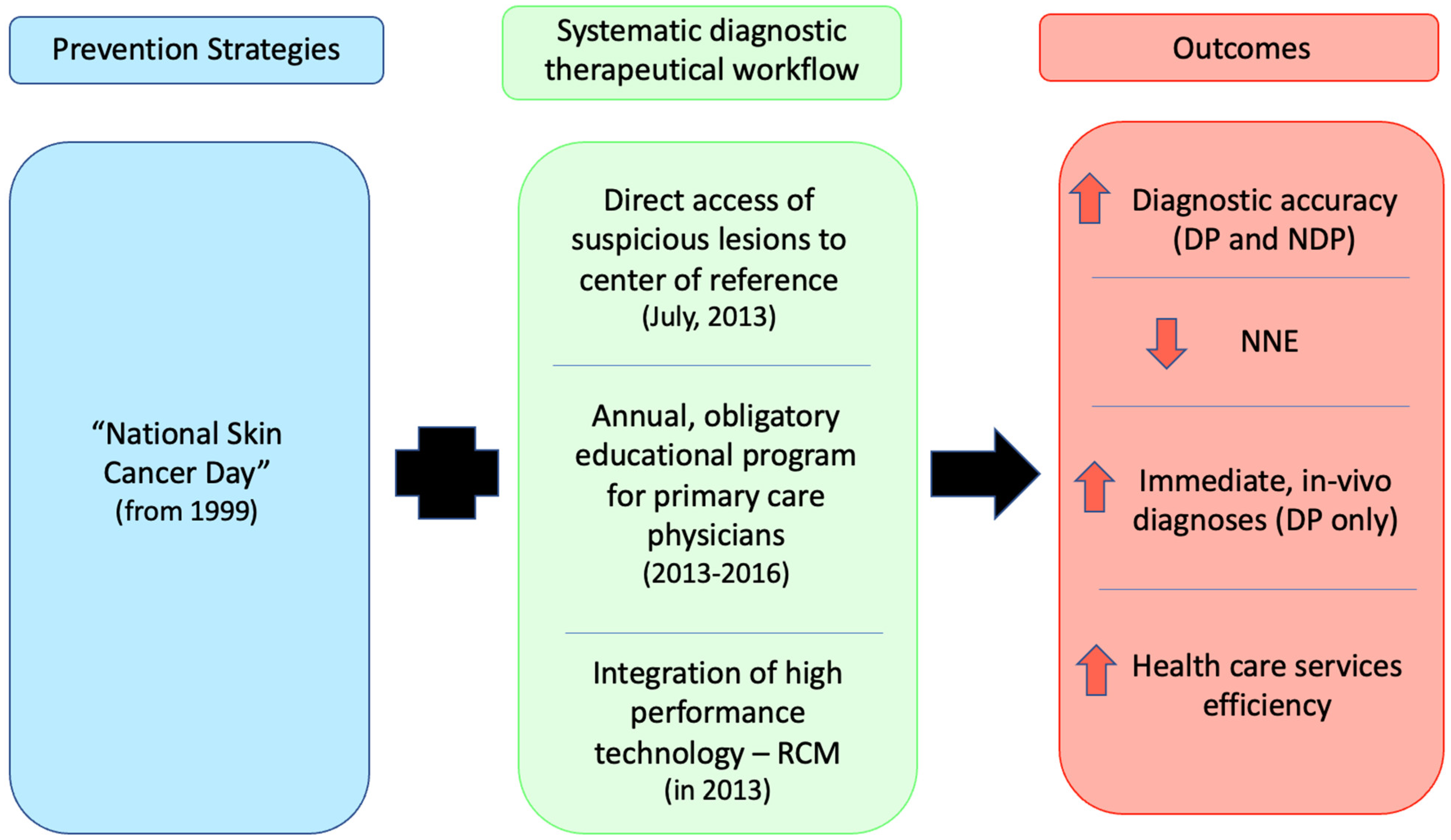
1. Introduction
2. Methods
3. Statistical Analysis
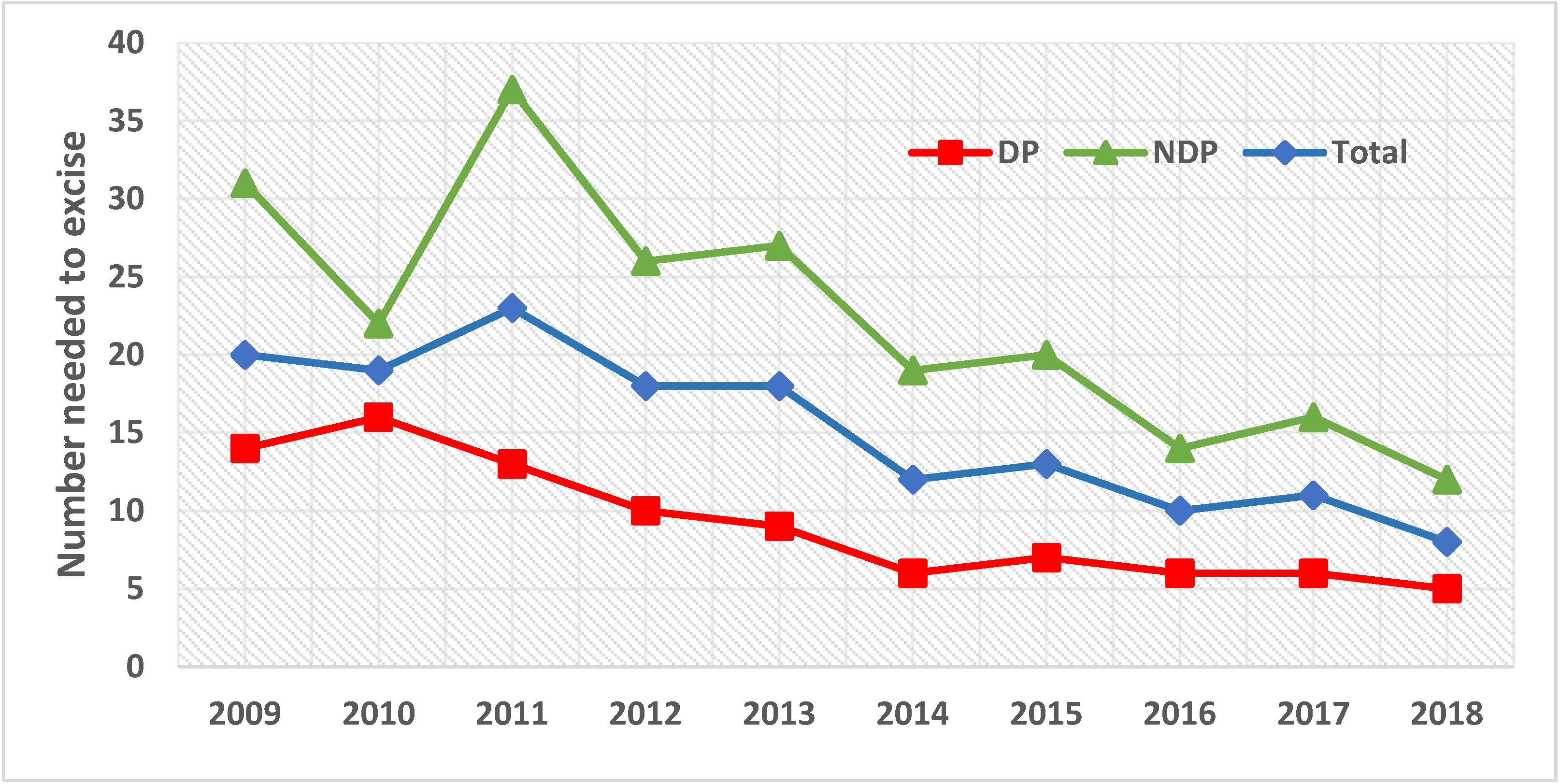
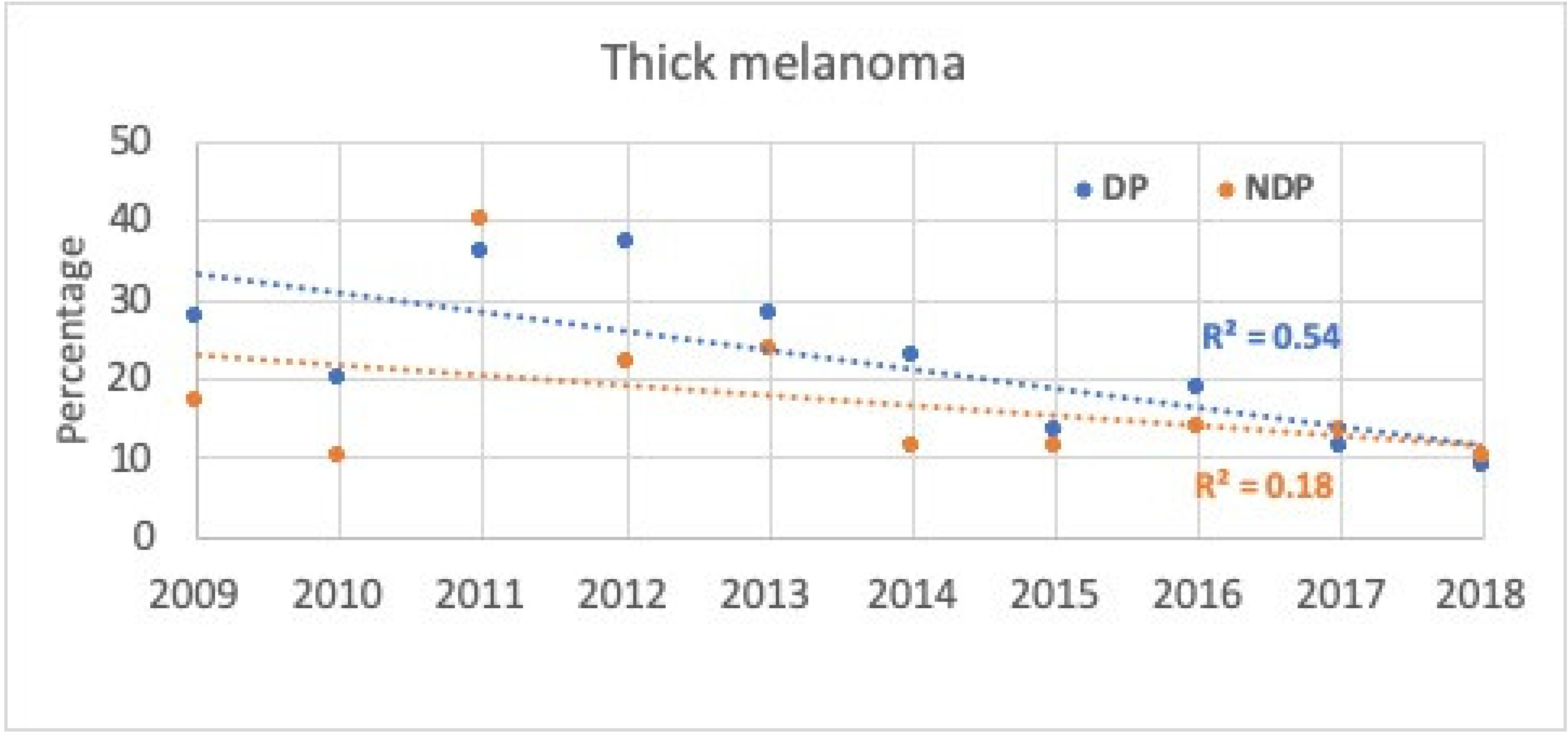
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017, 7, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-González, E.; López-Abente, G.; Aragones, N.; Pollán, M.; Pastor-Barriuso, R.; Sánchez, M.; Pérez-Gómez, B. Trends in mortality from cutaneous malignant melanoma in Spain (1982–2016): Sex-specific age-cohort-period effects. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J.; Mihm, M.C. Melanoma. N. Engl. J. Med. 2006, 355, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trakatelli, M.; Siskou, S.; Proby, C.; Tiplica, G.S.; Hinrichs, B.; Altsitsiadis, E.; Kitsou, A.; Ferrandiz, L.; Aquilina, S.; Apap, C.; et al. The patient journey: A report of skin cancer care across Europe. Br. J. Dermatol. 2012, 167, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Leiter, U.; Garbe, C. Epidemiology of Melanoma and Nonmelanoma Skin Cancer—The Role of Sunlight. Adv. Exp. Med. Biol. 2008, 624, 89–103. [Google Scholar] [CrossRef]
- Whiteman, D.C.; Green, A.C.; Olsen, C.M. The growing burden of invasive melanoma: Projections of incidence rates and numbers of new cases in six susceptible populations to 2031. J. Investig. Dermatol. 2016, 136, 1161–1171. [Google Scholar] [CrossRef] [Green Version]
- Garbe, C.; Peris, K.; Hauschild, A.; Saiag, P.; Middleton, M.; Bastholt, L.; Grob, J.-J.; Malvehy, J.; Newton-Bishop, J.; Stratigos, A.J.; et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline – Update 2016. Eur. J. Cancer 2016, 63, 201–217. [Google Scholar] [CrossRef]
- Gilmore, S. Melanoma screening: Informing public health policy with quantitative modelling. PLoS ONE 2017, 12, e0182349. [Google Scholar] [CrossRef]
- Robinson, J.K.; Mallett, K.A. The duty to inspect the skin and counsel those at risk to develop melanoma. JAMA 2009, 301, 1702–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EADO: European Association of Dermato Oncology. Euro Melanoma. Available online: www.euromelanoma.org (accessed on 7 December 2020).
- Carli, P.; De Giorgi, V.; Giannotti, B.; Seidenari, S.; Pellacani, G.; Peris, K.; Piccolo, D.; Rubegni, P.; Andreassi, L. Skin cancer day in Italy: Method of referral to open access clinics and tumor prevalence in the examined population. Eur. J. Dermatol. 2003, 13, 76–79. [Google Scholar] [PubMed]
- Nørgaard, C.; Glud, M.; Gniadecki, R. Are All Melanomas Dangerous? Acta Derm. Venereol. 2011, 91, 499–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welch, H.G.; Woloshin, S.; Schwartz, L.M. Skin biopsy rates and incidence of melanoma: Population based ecological study. BMJ 2005, 331, 481. [Google Scholar] [CrossRef] [Green Version]
- Hansen, C.; Wilkinson, D.; Hansen, M.; Argenziano, G. How good are skin cancer clinics at melanoma detection? Number needed to treat variability across a national clinic group in Australia. J. Am. Acad. Dermatol. 2009, 61, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Petty, A.J.; Ackerson, B.; Garza, R.; Peterson, M.; Liu, B.; Green, C.; Pavlis, M. Meta-analysis of number needed to treat for diagnosis of melanoma by clinical setting. J. Am. Acad. Dermatol. 2020, 82, 1158–1165. [Google Scholar] [CrossRef]
- Kittler, H.; Pehamberger, H.; Wolff, K.; Binder, M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002, 3, 159–165. [Google Scholar] [CrossRef]
- Dinnes, J.; Deeks, J.J.; Saleh, D.; Chuchu, N.; Bayliss, S.E.; Patel, L.; Davenport, C.; Takwoingi, Y.; Godfrey, K.; Matin, R.N.; et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst. Rev. 2018, 12, CD013190. [Google Scholar] [CrossRef]
- Sommariva, A.; Forsea, A.-M.; Agius, D.; Ascierto, P.A.; Bastiaannet, E.; Borgognoni, L.; Demetriou, A.; Garbe, C.; Gavric, Z.; Hocevar, M.; et al. Quality assurance in melanoma care: The EU-MELACARE study. Eur. J. Surg. Oncol. 2018, 44, 1773–1778. [Google Scholar] [CrossRef]
- Pezzini, C.; Kaleci, S.; Chester, J.; Farnetani, F.; Longo, C.; Pellacani, G. Reflectance confocal microscopy diagnostic accuracy for malignant melanoma in different clinical settings: Systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2268–2279. [Google Scholar] [CrossRef]
- Guida, S.; Longo, C.; Casari, A.; Ciardo, S.; Manfredini, M.; Reggiani, C.; Pellacani, G.; Farnetani, F. Update on the use of confocal microscopy in melanoma and non-melanoma skin cancer. G. Ital. di Dermatol. e Venereol. 2015, 150, 547–563. [Google Scholar]
- Longo, C.; Farnetani, F.; Moscarella, E.; de Pace, B.; Ciardo, S.; Ponti, G.; Piana, S.; Cesinaro, A.M.; Cota, C.; Argenziano, G.; et al. Can noninvasive imaging tools potentially predict the risk of ulceration in invasive melanomas showing blue and black colors? Melanoma Res. 2013, 23, 125–131. [Google Scholar] [CrossRef]
- Pellacani, G.; Witkowski, A.; Cesinaro, A.M.; Losi, A.; Colombo, G.L.; Campagna, A.; Longo, C.; Piana, S.; De Carvalho, N.; Giusti, F.; et al. Cost-benefit of reflectance confocal microscopy in the diagnostic performance of melanoma. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Regione Emilia Romagna Statistics. Available online: https://statistica.regione.emilia-romagna.it/servizi-online/statistica-self-service/popolazione/popolazione-per-eta-e-sesso/pop_eta_ammontare (accessed on 10 December 2021).
- Pellacani, G.; Lo Scocco, G.; Vinceti, M.; Albertini, G.; Raccagni, A.A.; Baldassari, L.; Catrani, S.; Donelli, S.; Ghetti, P.; Lanzoni, A.; et al. Melanoma epidemic across the millennium: Time trends of cutaneous melanoma in Emilia-Romagna (Italy) from 1997 to 2004. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.G.; Rowell, D. Health system costs of skin cancer and cost-effectiveness of skin cancer prevention and screening: A systematic review. Eur. J. Cancer Prev. 2015, 24, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Siesling, S.; Louwman, W.; Kwast, A.; Hurk, C.V.D.; O’Callaghan, M.; Rosso, S.; Zanetti, R.; Storm, H.; Comber, H.; Steliarova-Foucher, E.; et al. Uses of cancer registries for public health and clinical research in Europe: Results of the European Network of Cancer Registries survey among 161 population-based cancer registries during 2010–2012. Eur. J. Cancer 2015, 51, 1039–1049. [Google Scholar] [CrossRef]
- Shaikh, W.R.; Dusza, S.W.; Weinstock, M.A.; Oliveria, S.A.; Geller, A.C.; Halpern, A.C. Melanoma thickness and survival trends in the United States, 1989 to 2009. J. Natl. Cancer Inst. 2015, 108, djv294. [Google Scholar] [CrossRef] [Green Version]
- Privalle, A.; Havighurst, T.; Kim, K.; Bennett, D.D.; Xu, Y.G. Number of skin biopsies needed per malignancy: Comparing the use of skin biopsies among dermatologists and nondermatologist clinicians. J. Am. Acad. Dermatol. 2020, 82, 110–116. [Google Scholar] [CrossRef]
- Marushchak, O.; Hazan, E.; Kriegel, D.A. Analyzing Controversies in Management and Surveillance of Early-Stage Melanoma. Oncol. Ther. 2020, 8, 191–196. [Google Scholar] [CrossRef]
- Johnson-Obaseki, S.E.; Labajian, V.; Corsten, M.J.; McDonald, J.T. Incidence of cutaneous malignant melanoma by socioeconomic status in Canada: 1992–2006. J. Otolaryngol. Head Neck Surg. 2015, 44, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Vainio, H.; Miller, A.B.; Bianchini, F. An international evaluation of the cancer-preventive potential of sunscreens. Int. J. Cancer 2000, 88, 838–842. [Google Scholar] [CrossRef]
- Naert, K.A.; Trotter, M.J. Utilization and Utility of Immunohistochemistry in Dermatopathology. Am. J. Dermatopathol. 2013, 35, 74–77. [Google Scholar] [CrossRef] [PubMed]

| All lesions | Melanoma Thickness | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Total | Melanoma | Nevi | NNE | Thin | Thick | |||||
| n | % (total) | n | % * | n | % * | n | % ^ | n | % ^ | ||
| 2009 | 4013 | 9.8 | 203 | 5.1 | 3810 | 94.9 | 20 | 154 | 75.9 | 49 | 24.1 |
| 2010 | 4300 | 10.5 | 225 | 5.2 | 4075 | 94.8 | 19 | 192 | 85.3 | 33 | 14.7 |
| 2011 | 4133 | 10.1 | 183 | 4.4 | 3950 | 95.6 | 23 | 114 | 62.3 | 69 | 37.7 |
| 2012 | 3718 | 9.1 | 201 | 5.4 | 3517 | 94.6 | 18 | 143 | 71.1 | 58 | 28.9 |
| 2013 | 3422 | 8.4 | 189 | 5.5 | 3233 | 94.5 | 18 | 140 | 74.1 | 49 | 25.9 |
| 2014 | 3653 | 8.9 | 309 | 8.5 | 3344 | 91.5 | 12 | 255 | 82.5 | 54 | 17.5 |
| 2015 | 3865 | 9.5 | 306 | 7.9 | 3559 | 92.1 | 13 | 267 | 87.3 | 39 | 12.7 |
| 2016 | 4267 | 10.5 | 429 | 10.1 | 3838 | 89.9 | 10 | 359 | 83.7 | 70 | 16.3 |
| 2017 | 4749 | 11.6 | 442 | 9.3 | 4307 | 90.7 | 11 | 387 | 87.6 | 55 | 12.4 |
| 2018 | 4712 | 11.5 | 567 | 12.0 | 4145 | 88.0 | 8 | 513 | 90.5 | 54 | 9.5 |
| Total | 40,832 | 100 | 3054 | 7.5 | 37,778 | 92.5 | 13 | 2524 | 82.6 | 530 | 17.4 |
| DP | NDP | |||
|---|---|---|---|---|
| No. Lesions | % | No. Lesions | % | |
| No. all lesions: | 12,977 | 31.8 | 27,855 | 68.2 |
| Melanoma | 1628 | 12.5 | 1426 | 5.1 |
| Thin | 1315 | 80.8 | 1209 | 84.8 |
| Thick | 313 | 19.2 | 217 | 15.2 |
| Nevi | 11,349 | 87.5 | 26,429 | 94.9 |
| Ratio | Ratio | |||
| NNE | 8:1 | 20:1 | ||
| NNE, DP:NDP * | 1:2.5 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellacani, G.; Farnetani, F.; Chester, J.; Kaleci, S.; Ciardo, S.; Bassoli, S.; Casari, A.; Longo, C.; Manfredini, M.; Cesinaro, A.M.; et al. Cutaneous Melanoma Systematic Diagnostic Workflows and Integrated Reflectance Confocal Microscopy Assessed with a Retrospective, Comparative Longitudinal (2009–2018) Study. Cancers 2022, 14, 838. https://doi.org/10.3390/cancers14030838
Pellacani G, Farnetani F, Chester J, Kaleci S, Ciardo S, Bassoli S, Casari A, Longo C, Manfredini M, Cesinaro AM, et al. Cutaneous Melanoma Systematic Diagnostic Workflows and Integrated Reflectance Confocal Microscopy Assessed with a Retrospective, Comparative Longitudinal (2009–2018) Study. Cancers. 2022; 14(3):838. https://doi.org/10.3390/cancers14030838
Chicago/Turabian StylePellacani, Giovanni, Francesca Farnetani, Johanna Chester, Shaniko Kaleci, Silvana Ciardo, Sara Bassoli, Alice Casari, Caterina Longo, Marco Manfredini, Anna Maria Cesinaro, and et al. 2022. "Cutaneous Melanoma Systematic Diagnostic Workflows and Integrated Reflectance Confocal Microscopy Assessed with a Retrospective, Comparative Longitudinal (2009–2018) Study" Cancers 14, no. 3: 838. https://doi.org/10.3390/cancers14030838
APA StylePellacani, G., Farnetani, F., Chester, J., Kaleci, S., Ciardo, S., Bassoli, S., Casari, A., Longo, C., Manfredini, M., Cesinaro, A. M., Giusti, F., Iacuzio, A., & Migaldi, M. (2022). Cutaneous Melanoma Systematic Diagnostic Workflows and Integrated Reflectance Confocal Microscopy Assessed with a Retrospective, Comparative Longitudinal (2009–2018) Study. Cancers, 14(3), 838. https://doi.org/10.3390/cancers14030838






