Oncogenic Alterations in Histologically Negative Lymph Nodes Are Associated with Prognosis of Patients with Stage I Lung Adenocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Specimen Processing and DNA/RNA Extraction
2.3. Detection of Oncogenic Gene Alterations in Lung Cancer Specimens
2.4. Detection of Oncogenic Gene Alterations in Lymph Nodes
2.5. Immunohistochemical (IHC) Detection of Occult Lymph Node Metastases
2.6. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Oncogenic Alternations in Primary Lung Adenocarcinoma Specimens
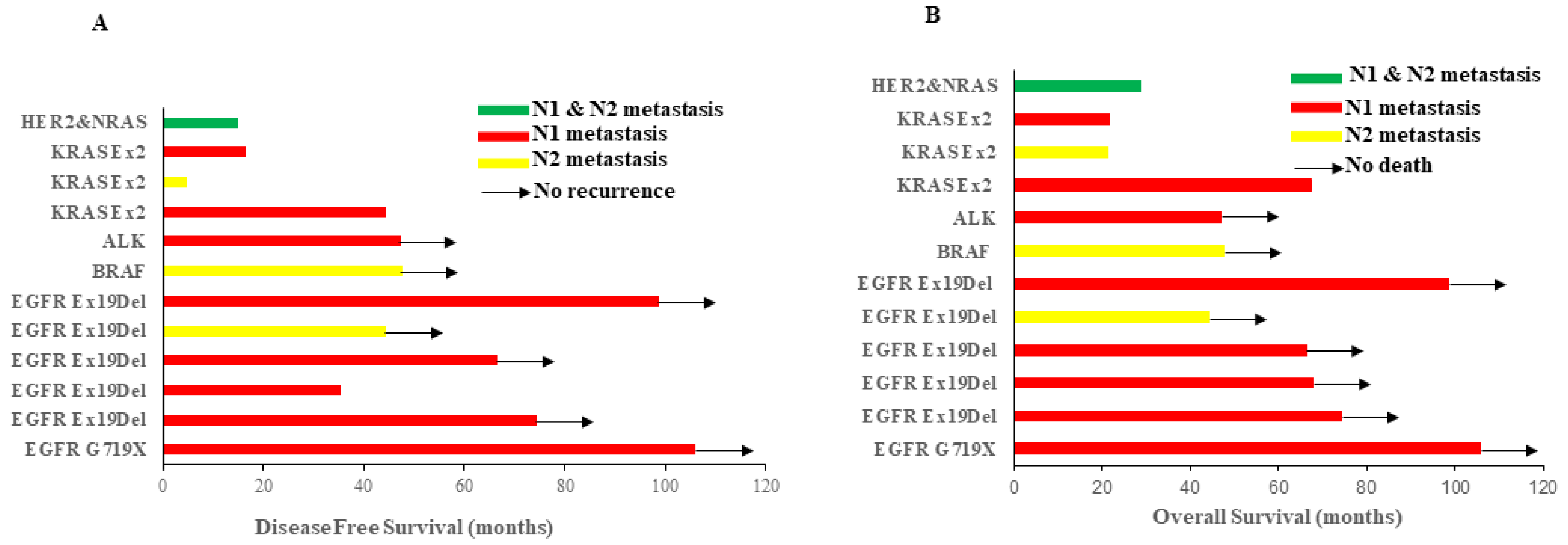
3.3. Oncogenic Alternations Were Present in Histologically Negative Lymph Nodes
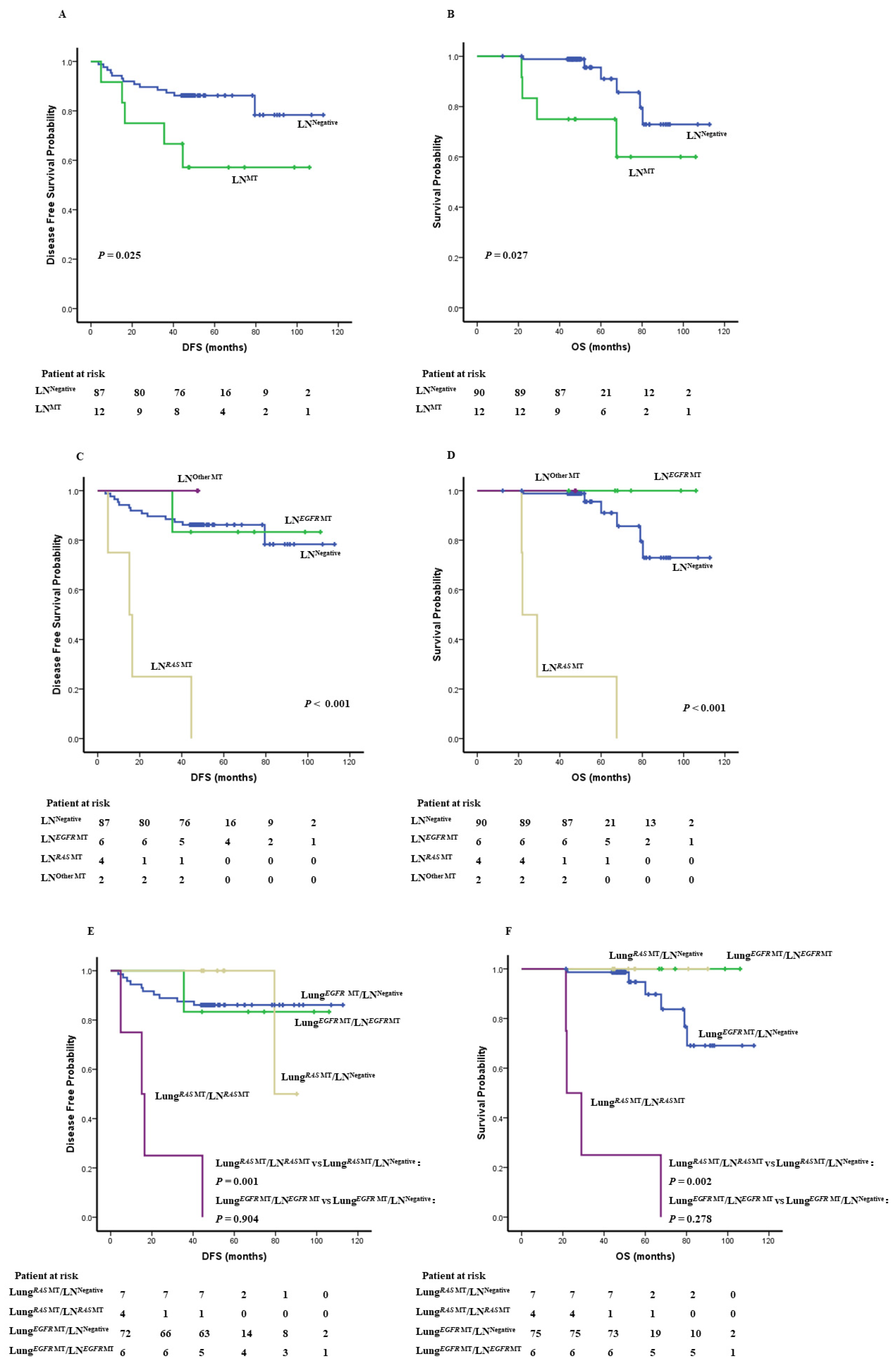
3.4. Oncogenic Alternations in LN Impacts Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benlloch, S.; Galbis-Caravajal, J.M.; Alenda, C.; Peiro, F.M.; Sanchez-Ronco, M.; Rodriguez-Paniagua, J.M.; Baschwitz, B.; Rojas, E.; Massuti, B. Expression of molecular markers in mediastinal nodes from resected stage I non-small-cell lung cancer (NSCLC): Prognostic impact and potential role as markers of occult micrometastases. Ann. Oncol. 2009, 20, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Howington, J.A.; Blum, M.G.; Chang, A.C.; Balekian, A.A.; Murthy, S.C. Treatment of Stage I and II Non-Small Cell Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e278S–e313S. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.W.; D–Cunha, J.; Wang, X.; Herzan, D.; Gu, L.; Abraham, N.; Demmy, T.L.; Detterbeck, F.C.; Groth, S.S.; Harpole, D.H.; et al. Detection of Occult Micrometastases in Patients with Clinical Stage I Non-Small-Cell Lung Cancer: A Prospective Analysis of Mature Results of CALGB 9761 (Alliance). J. Clin. Oncol. 2016, 34, 1484–1491. [Google Scholar] [CrossRef]
- Erhunmwunsee, L.; D–Amico, T.A. Detection of occult N2 disease with molecular techniques. Thorac Surg Clin. 2008, 18, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Kubuschok, B.; Passlick, B.; Izbicki, J.R.; Thetter, O.; Pantel, K. Disseminated tumor cells in lymph nodes as a determinant for survival in surgically resected non-small-cell lung cancer. J. Clin. Oncol. 1999, 17, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.D.; Osaki, T.; Oyama, T.; Inoue, M.; Kodate, M.; Dobashi, K.; Oka, T.; Yasumoto, K. Detection of micrometastatic tumor cells in pN0 lymph nodes of patients with completely resected nonsmall cell lung cancer: Impact on recurrence and Survival. Ann. Surg. 2002, 235, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, A.; Mitsudomi, T.; Sugio, K.; Tsuda, T.; Oyama, T.; Nishida, K.; Osaki, T.; Yasumoto, K. Micrometastatic tumor cells in the bone marrow of patients with non-small cell lung cancer. Annals Thorac. Surg. 1997, 64, 363–367. [Google Scholar] [CrossRef]
- Saintigny, P.; Coulon, S.; Kambouchner, M.; Ricci, S.; Martinot, E.; Danel, C.; Breau, J.L.; Bernaudin, J.F. Real-time RT-PCR detection of CK19, CK7 and MUC1 mRNA for diagnosis of lymph node micrometastases in non small cell lung carcinoma. Int. J. Cancer 2005, 115, 777–782. [Google Scholar] [CrossRef]
- Dobashi, K.; Sugio, K.; Osaki, T.; Oka, T.; Yasumoto, K. Micrometastatic P53-positive cells in the lymph nodes of non-small-cell lung cancer: Prognostic significance. J. Thorac. Cardiovasc. Surg. 1997, 114, 339–346. [Google Scholar] [CrossRef]
- Nicholson, A.G.; Graham, A.N.; Pezzella, F.; Agneta, G.; Goldstraw, P.; Pastorino, U. Does the use of immunohistochemistry to identify micrometastases provide useful information in the staging of node-negative non-small cell lung carcinomas? Lung Cancer 1997, 18, 231–240. [Google Scholar] [CrossRef]
- Recondo, G.; Facchinetti, F.; Olaussen, K.A.; Besse, B.; Friboulet, L. Making the first move in EGFR-driven or ALK-driven NSCLC: First-generation or next-generation TKI? Nat. Rev. Clin. Oncol. 2018, 15, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.; Giordano, M.; Giannini, R.; Ali, G.; Puppo, G.; Ribechini, A.; Chella, A.; Fontanini, G. Aberrant expression of anaplastic lymphoma kinase in lung adenocarcinoma: Analysis of circulating free tumor RNA using one-step reverse transcription-polymerase chain reaction. Mol. Med. Rep. 2016, 14, 2238–2242. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Baidoo, A.A.H.; Su, S.; Ye, J.; Chen, C.; Xie, Y.; Bertolaccini, L.; Ismail, M.; Ricciuti, B.; Ng, C.S.H.; et al. A comparison of EGFR mutation status in tissue and plasma cell-free DNA detected by ADx-ARMS in advanced lung adenocarcinoma patients. Transl. Lung Cancer Res. 2019, 8, 135–143. [Google Scholar] [CrossRef]
- Shaozhang, Z.; Ming, Z.; Haiyan, P.; Aiping, Z.; Qitao, Y.; Xiangqun, S. Comparison of ARMS and direct sequencing for detection of EGFR mutation and prediction of EGFR-TKI efficacy between surgery and biopsy tumor tissues in NSCLC patients. Med. Oncol. 2014, 31, 926. [Google Scholar] [CrossRef]
- Herpel, E.; Muley, T.; Schneider, T.; Palm, E.; Kieslich de Hol, D.; Warth, A.; Meister, M.; Storz, K.; Schnabel, P.A.; Schirmacher, P.; et al. A pragmatic approach to the diagnosis of nodal micrometastases in early stage non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 1206–1212. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, X.; Cheng, G.; Ji, E.; Yang, S.; Feng, J.; Zheng, L. The association of CMTM6 expression with prognosis and PD-L1 expression in triple-negative breast cancer. Ann. Transl. Med. 2021, 9, 131. [Google Scholar] [CrossRef]
- Detterbeck, F.C. Pushing forward into the darkness, leaping, and landing securely: Prognostication and adjuvant chemotherapy for lung cancer. Chest 2011, 140, 1398–1400. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Xiang, J.; Zhang, Y.; Hu, H.; Chen, H. A clinicopathologic prediction model for postoperative recurrence in stage Ia non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2014, 148, 1193–1199. [Google Scholar] [CrossRef]
- Ou, S.H.; Zell, J.A.; Ziogas, A.; Anton-Culver, H. Prognostic factors for survival of stage I nonsmall cell lung cancer patients: A population-based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer 2007, 110, 1532–1541. [Google Scholar] [CrossRef]
- Shimada, Y.; Saji, H.; Yoshida, K.; Kakihana, M.; Honda, H.; Nomura, M.; Usuda, J.; Kajiwara, N.; Ohira, T.; Ikeda, N. Prognostic factors and the significance of treatment after recurrence in completely resected stage I non-small cell lung cancer. Chest 2013, 143, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Dong, W.; Su, B.; Liu, Q.; Du, J. The prognostic value of ratio-based lymph node staging in resected non-small-cell lung cancer. J. Thorac. Oncol. 2013, 8, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Peng, A.; Wang, B.; Gusdon, A.M.; Sun, X.; Jiang, G.; Zhang, P. The prognostic impact of lymph node metastasis in patients with non-small cell lung cancer and distant organ metastasis. Clin. Exp. Metastasis 2019, 36, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.Z.; Tanabe, K.K.; Luo, S.; Muzikansky, A.; Sober, A.J.; Tsao, H.; Cosimi, A.B.; Duncan, L.M. The distribution of microscopic melanoma metastases in sentinel lymph nodes: Implications for pathology protocols. Am. J. Surg Pathol. 2012, 36, 1841–1848. [Google Scholar] [CrossRef]
- Lee, W.C.; Diao, L.; Wang, J.; Zhang, J.; Roarty, E.B.; Varghese, S.; Chow, C.W.; Fujimoto, J.; Behrens, C.; Cascone, T.; et al. Multiregion gene expression profiling reveals heterogeneity in molecular subtypes and immunotherapy response signatures in lung cancer. Mod. Pathol. 2018, 31, 947–955. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Aubry, M.C.; Moser, J.C.; Harrington, S.M.; Dronca, R.S.; Park, S.S.; Dong, H. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann. Oncol. 2016, 27, 1953–1958. [Google Scholar] [CrossRef]
- Aramaki, N.; Ishii, G.; Yamada, E.; Morise, M.; Aokage, K.; Kojima, M.; Hishida, T.; Yoshida, J.; Ikeda, N.; Tsuboi, M.; et al. Drastic morphological and molecular differences between lymph node micrometastatic tumors and macrometastatic tumors of lung adenocarcinoma. J. Cancer Res. Clin. Oncol. 2016, 142, 37–46. [Google Scholar] [CrossRef]
- Zhang, J.; Fujimoto, J.; Zhang, J.; Wedge, D.C.; Song, X.; Zhang, J.; Seth, S.; Chow, C.W.; Cao, Y.; Gumbs, C.; et al. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science 2014, 346, 256–259. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Li, L.; Yin, G.; Zhang, J.; Zheng, S.; Cheung, H.; Wu, N.; Lu, N.; Mao, X.; et al. Genomic heterogeneity of multiple synchronous lung cancer. Nat. Commun. 2016, 7, 13200. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, H.; Lin, J.; Cai, X.; Pan, Z.; Liu, J.; Xie, X.; Li, C.; Cheng, B.; Zhao, Y.; et al. The incidence of lymph node metastasis in patients with different oncogenic driver mutations among T1 non-small-cell lung cancer. Lung Cancer 2019, 134, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, M.; Gadgeel, S.M. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev. Anticancer Ther. 2018, 18, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kris, M.G.; Gaspar, L.E.; Chaft, J.E.; Kennedy, E.B.; Azzoli, C.G.; Ellis, P.M.; Lin, S.H.; Pass, H.I.; Seth, R.; Shepherd, F.A.; et al. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA Completely Resected Non-Small-Cell Lung Cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 2960–2974. [Google Scholar] [CrossRef] [PubMed]
- Osmani, L.; Askin, F.; Gabrielson, E.; Li, Q.K. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018, 52, 103–109. [Google Scholar] [CrossRef]
- Vansteenkiste, J.; Wauters, E.; Reymen, B.; Ackermann, C.J.; Peters, S.; De Ruysscher, D. Current status of immune checkpoint inhibition in early-stage NSCLC. Ann. Oncol 2019, 30, 1244–1253. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Connolly, J.L.; Edge, S.B.; Mittendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Hortobagyi, G.N. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 290–303. [Google Scholar] [CrossRef]
- Reed, J.; Rosman, M.; Verbanac, K.M.; Mannie, A.; Cheng, Z.; Tafra, L. Prognostic implications of isolated tumor cells and micrometastases in sentinel nodes of patients with invasive breast cancer: 10-year analysis of patients enrolled in the prospective East Carolina University/Anne Arundel Medical Center Sentinel Node Multicenter Study. J. Am. Coll Surg. 2009, 208, 333–340. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Hawes, D.; Ballman, K.V.; Whitworth, P.W.; Blumencranz, P.W.; Reintgen, D.S.; Morrow, M.; Leitch, A.M.; Hunt, K.K.; McCall, L.M.; et al. Association of occult metastases in sentinel lymph nodes and bone marrow with survival among women with early-stage invasive breast cancer. JAMA 2011, 306, 385–393. [Google Scholar] [CrossRef]
- Abbosh, C.; Birkbak, N.J.; Wilson, G.A.; Jamal-Hanjani, M.; Constantin, T.; Salari, R.; Le Quesne, J.; Moore, D.A.; Veeriah, S.; Rosenthal, R.; et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 2017, 545, 446–451. [Google Scholar] [CrossRef]
- Poulet, G.; Massias, J.; Taly, V. Liquid Biopsy: General Concepts. Acta Cytol. 2019, 63, 449–455. [Google Scholar] [CrossRef]
- Li, Y.S.; Jiang, B.Y.; Yang, J.J.; Zhang, X.C.; Zhang, Z.; Ye, J.Y.; Zhong, W.Z.; Tu, H.Y.; Chen, H.J.; Wang, Z.; et al. Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: A new medium of liquid biopsy. Ann. Oncol. 2018, 29, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Pollard, J.W. Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018, 17, 887–904. [Google Scholar] [CrossRef] [PubMed]
- Noy, R.; Pollard, J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Total (N = 123) | Without Genetic Alterations in Tumor (N = 21) | With Genetic Alterations in Tumor (N = 102) | p-Value | |
|---|---|---|---|---|---|
| Age | ≤60 | 55 (44.7%) | 12 (21.8%) | 43 (78.2%) | |
| >60 | 68 (55.3%) | 9 (13.2%) | 59 (86.6%) | 0.208 | |
| Sex | Male | 78 (63.4%) | 16 (20.5%) | 62 (79.5%) | |
| Female | 45 (36.6%) | 5 (11.1%) | 40 (88.9%) | 0.182 | |
| Smoke | No | 69 (56.1%) | 6 (8.7%) | 63 (91.3%) | |
| Yes | 54 (43.9%) | 15 (27.8%) | 39 (72.2%) | 0.005 | |
| Family history | No | 82 (66.7%) | 11 (13.4%) | 71 (86.6%) | |
| Yes | 41 (33.3%) | 10 (24.4%) | 31 (75.6%) | 0.127 | |
| Tumor Size | ≤3 cm | 107 (87.0%) | 18 (16.8%) | 89 (83.2%) | |
| >3 cm | 16 (13.0%) | 3 (18.8%) | 13 (81.3%) | 0.736 | |
| Surgery type | Lobectomies | 113 (91.9%) | 18 (15.9%) | 95 (84.1%) | |
| Segmentectomies | 10 (8.1%) | 3 (30.0%) | 7 (70.0%) | 0.372 | |
| Gene Type | Mutations in Primary Lung Cancer Tissues | |
|---|---|---|
| No. | % | |
| Wild Type | 21 | 17.07 |
| EGFR mutation | 81 | 65.85 |
| KRAS mutation | 9 | 7.32 |
| NRAS mutation | 1 | 0.81 |
| HER2 mutation | 2 | 1.63 |
| ALK fusion | 3 | 2.44 |
| RET fusion | 2 | 1.63 |
| ROS1 fusion | 1 | 0.81 |
| BRAF mutation | 1 | 0.81 |
| PIK3CA&EGFR mutations | 1 | 0.81 |
| HER2&NRAS mutations | 1 | 0.81 |
| Molecular Alteration Type | Number (Percentage) | Lymph Node Station | Average Age (Year) |
|---|---|---|---|
| Total | 12 | 56.4 | |
| EGFR mutation | 6 (50%) | N1; N2 | 63.5 |
| ALK fusion | 1 (8.3%) | N1 | 58 |
| KRAS mutation | 3 (25%) | N1; N2 | 59.6 |
| Her2&NRAS mutaions | 1 (8.3%) | N1 & N2 | 51 |
| BRAF mutation | 1 (8.3%) | N2 | 71 |
| No LN Molecular Alterations | LN Molecular Alterations | p | |
|---|---|---|---|
| EGFR mutation | 75 (92.59%) | 6 (7.41%) | 0.017 |
| RAS mutation | 7 (63.64%) | 4 (36.36%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Lai, Q.; Zheng, Y.; Ying, L.; Wang, C.; Jin, J.; Huang, M.; Wu, Y.; Li, H.; Zhang, J.; et al. Oncogenic Alterations in Histologically Negative Lymph Nodes Are Associated with Prognosis of Patients with Stage I Lung Adenocarcinoma. Cancers 2022, 14, 824. https://doi.org/10.3390/cancers14030824
Tian Y, Lai Q, Zheng Y, Ying L, Wang C, Jin J, Huang M, Wu Y, Li H, Zhang J, et al. Oncogenic Alterations in Histologically Negative Lymph Nodes Are Associated with Prognosis of Patients with Stage I Lung Adenocarcinoma. Cancers. 2022; 14(3):824. https://doi.org/10.3390/cancers14030824
Chicago/Turabian StyleTian, Yiping, Qian Lai, Yuansi Zheng, Lisha Ying, Canming Wang, Jiaoyue Jin, Minran Huang, Yingxue Wu, Huizhang Li, Jianjun Zhang, and et al. 2022. "Oncogenic Alterations in Histologically Negative Lymph Nodes Are Associated with Prognosis of Patients with Stage I Lung Adenocarcinoma" Cancers 14, no. 3: 824. https://doi.org/10.3390/cancers14030824
APA StyleTian, Y., Lai, Q., Zheng, Y., Ying, L., Wang, C., Jin, J., Huang, M., Wu, Y., Li, H., Zhang, J., & Su, D. (2022). Oncogenic Alterations in Histologically Negative Lymph Nodes Are Associated with Prognosis of Patients with Stage I Lung Adenocarcinoma. Cancers, 14(3), 824. https://doi.org/10.3390/cancers14030824






