Treatment Planning Study for Microbeam Radiotherapy Using Clinical Patient Data
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Data
2.2. Microbeam Treatment Planning
2.3. Microbeam Planning Parameters
2.4. Dosimetric Evaluation and Equivalent Uniform Dose
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slatkin, D.N.; Spanne, P.; Dilmanian, F.A.; Sandborg, M. Microbeam radiation therapy. Med. Phys. 1992, 19, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Bartzsch, S.; Corde, S.; Crosbie, J.C.; Day, L.; Donzelli, M.; Krisch, M.; Lerch, M.; Pellicioli, P.; Smyth, L.M.L.; Tehei, M. Technical advances in X-ray microbeam radiation therapy. Phys. Med. Biol. 2020, 65, 02TR01. [Google Scholar] [CrossRef]
- Emami, B.; Lyman, J.; Brown, A.; Coia, L.; Goitein, M.; Munzenrider, J.E.; Shank, B.; Solin, L.J.; Wesson, M. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 109–122. [Google Scholar] [CrossRef]
- Laissue, J.A.; Geiser, G.; Spanne, P.O.; Dilmanian, F.A.; Gebbers, J.O.; Geiser, M.; Wu, X.Y.; Makar, M.S.; Micca, P.L.; Nawrocky, M.M.; et al. Neuropathology of ablation of rat gliosarcomas and contiguous brain tissues using a microplanar beam of synchrotron-wiggler-generated X rays. Int. J. Cancer 1998, 78, 654–660. [Google Scholar] [CrossRef]
- Dilmanian, F.A.; Morris, G.M.; Zhong, N.; Bacarian, T.; Hainfeld, J.F.; Kalef-Ezra, J.; Brewington, L.J.; Tammam, J.; Rosen, E.M. Murine EMT-6 carcinoma: High therapeutic efficacy of microbeam radiation therapy. Radiat. Res. 2003, 159, 632–641. [Google Scholar] [CrossRef]
- Miura, M.; Blattmann, H.; Bräuer-Krisch, E.; Bravin, A.; Hanson, A.L.; Nawrocky, M.M.; Micca, P.L.; Slatkin, D.N.; Laissue, J.A. Radiosurgical palliation of aggressive murine SCCVII squamous cell carcinomas using synchrotron-generated X-ray microbeams. Br. J. Radiol. 2006, 79, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Blattmann, H.; Gebbers, J.-O.; Bräuer-Krisch, E.; Bravin, A.; Le Duc, G.; Burkard, W.; Di Michiel, M.; Djonov, V.; Slatkin, D.N.; Stepanek, J.; et al. Applications of synchrotron X-rays to radiotherapy. Nucl. Instrum. Methods Phys. Res. A 2005, 548, 17–22. [Google Scholar] [CrossRef]
- Laissue, J.A.; Blattmann, H.; Wagner, H.P.; Grotzer, M.A.; Slatkin, D.N. Prospects for microbeam radiation therapy of brain tumours in children to reduce neurological sequelae. Dev. Med. Child Neurol. 2007, 49, 577–581. [Google Scholar] [CrossRef]
- Smyth, L.M.; Rogers, P.A.W.; Crosbie, J.C.; Donoghue, J.F. Characterization of Diffuse Intrinsic Pontine Glioma Radiosensitivity using Synchrotron Microbeam Radiotherapy and Conventional Radiation Therapy In Vitro. Radiat. Res. 2018, 189, 146–155. [Google Scholar] [CrossRef]
- Yang, Y.; Crosbie, J.C.; Paiva, P.; Ibahim, M.; Stevenson, A.; Rogers, P.A.W. In vitro study of genes and molecular pathways differentially regulated by synchrotron microbeam radiotherapy. Radiat. Res. 2014, 182, 626–639. [Google Scholar] [CrossRef]
- Steel, H.; Brüningk, S.C.; Box, C.; Oelfke, U.; Bartzsch, S.H. Quantification of Differential Response of Tumour and Normal Cells to Microbeam Radiation in the Absence of FLASH Effects. Cancers 2021, 13, 3238. [Google Scholar] [CrossRef] [PubMed]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.-F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef] [PubMed]
- Schültke, E.; Bayat, S.; Bartzsch, S.; Bräuer-Krisch, E.; Djonov, V.; Fiedler, S.; Fernandez-Palomo, C.; Jaekel, F.; Pellicioli, P.; Trappetti, V.; et al. A Mouse Model for Microbeam Radiation Therapy of the Lung. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 521–525. [Google Scholar] [CrossRef]
- Eling, L.; Bouchet, A.; Ocadiz, A.; Adam, J.-F.; Kershmiri, S.; Elleaume, H.; Krisch, M.; Verry, C.; Laissue, J.A.; Balosso, J.; et al. Unexpected Benefits of Multiport Synchrotron Microbeam Radiation Therapy for Brain Tumors. Cancers 2021, 13, 936. [Google Scholar] [CrossRef]
- Crosbie, J.C.; Anderson, R.L.; Rothkamm, K.; Restall, C.M.; Cann, L.; Ruwanpura, S.; Meachem, S.; Yagi, N.; Svalbe, I.; Lewis, R.A.; et al. Tumor cell response to synchrotron microbeam radiation therapy differs markedly from cells in normal tissues. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Bouchet, A.; Lemasson, B.; Christen, T.; Potez, M.; Rome, C.; Coquery, N.; Clec’h, C.L.; Moisan, A.; Bräuer-Krisch, E.; Leduc, G.; et al. Synchrotron microbeam radiation therapy induces hypoxia in intracerebral gliosarcoma but not in the normal brain. Radiother. Oncol. 2013, 108, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Siegbahn, E.A.; Stepanek, J.; Bräuer-Krisch, E.; Bravin, A. Determination of dosimetrical quantities used in microbeam radiation therapy (MRT) with Monte Carlo simulations. Med. Phys. 2006, 33, 3248–3259. [Google Scholar] [CrossRef] [PubMed]
- Felici, M.D.; Felici, R.; del Rio, M.S.; Ferrero, C.; Bacarian, T.; Dilmanian, F.A. Dose distribution from X-ray microbeam arrays applied to radiation therapy: An EGS4 Monte Carlo study. Med. Phys. 2005, 32, 2455–2463. [Google Scholar] [CrossRef]
- Crosbie, J.C.; Svalbe, I.; Midgley, S.M.; Yagi, N.; Rogers, P.A.W.; Lewis, R.A. A method of dosimetry for synchrotron microbeam radiation therapy using radiochromic films of different sensitivity. Phys. Med. Biol. 2008, 53, 6861–6877. [Google Scholar] [CrossRef]
- Martínez-Rovira, I.; Sempau, J.; Fernández-Varea, J.M.; Bravin, A.; Prezado, Y. Monte Carlo dosimetry for forthcoming clinical trials in X-ray microbeam radiation therapy. Phys. Med. Biol. 2010, 55, 4375–4388. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Rovira, I.; Sempau, J.; Prezado, Y. Monte Carlo-based treatment planning system calculation engine for microbeam radiation therapy. Med. Phys. 2012, 39, 2829–2838. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.M.L.; Day, L.R.; Woodford, K.; Rogers, P.A.W.; Crosbie, J.C.; Senthi, S. Identifying optimal clinical scenarios for synchrotron microbeam radiation therapy: A treatment planning study. Phys. Med. 2019, 60, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.M.L.; Donoghue, J.F.; Ventura, J.A.; Livingstone, J.; Bailey, T.; Day, L.R.J.; Crosbie, J.C.; Rogers, P.A.W. Comparative toxicity of synchrotron and conventional radiation therapy based on total and partial body irradiation in a murine model. Sci. Rep. 2018, 8, 12044. [Google Scholar] [CrossRef] [PubMed]
- Serduc, R.; Bräuer-Krisch, E.; Siegbahn, E.A.; Bouchet, A.; Pouyatos, B.; Carron, R.; Pannetier, N.; Renaud, L.; Berruyer, G.; Nemoz, C.; et al. High-Precision Radiosurgical Dose Delivery by Interlaced Microbeam Arrays of High-Flux Low-Energy Synchrotron X-Rays. PLoS ONE 2010, 5, e9028. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, F.M.; Cornelius, I.; Blencowe, A.; Franich, R.D.; Geso, M. High resolution 3D imaging of synchrotron generated microbeams. Med. Phys. 2015, 42, 6973–6986. [Google Scholar] [CrossRef]
- Fernandez-Palomo, C.; Trappetti, V.; Potez, M.; Pellicioli, P.; Krisch, M.; Laissue, J.; Djonov, V. Complete Remission of Mouse Melanoma after Temporally Fractionated Microbeam Radiotherapy. Cancers 2020, 12, 2656. [Google Scholar] [CrossRef]
- Meyer, J.; Stewart, R.D.; Smith, D.; Eagle, J.; Lee, E.; Cao, N.; Ford, E.; Hashemian, R.; Schuemann, J.; Saini, J.; et al. Biological and dosimetric characterisation of spatially fractionated proton minibeams. Phys. Med. Biol. 2017, 62, 9260–9281. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.Z.; Li, X.A. Evaluation of external beam radiotherapy and brachytherapy for localized prostate cancer using equivalent uniform dose. Med. Phys. 2003, 30, 34–40. [Google Scholar] [CrossRef]
- Wu, Q.; Mohan, R.; Niemierko, A.; Schmidt-Ullrich, R. Optimization of intensity-modulated radiotherapy plans based on the equivalent uniform dose. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 224–235. [Google Scholar] [CrossRef]
- Wright, M.D.; Romanelli, P.; Bravin, A.; Duc, G.L.; Brauer-Krisch, E.; Requardt, H.; Bartzsch, S.; Hlushchuk, R.; Laissue, J.-A.; Djonov, V. Non-conventional Ultra-High Dose Rate (FLASH) Microbeam Radiotherapy Provides Superior Normal Tissue Sparing in Rat Lung Compared to Non-conventional Ultra-High Dose Rate (FLASH) Radiotherapy. Cureus 2021, 13, e19317. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Sanmamed, M.F.; Hastings, K.; Politi, K.; Rimm, D.L.; Chen, L.; Melero, I.; Schalper, K.A.; Herbst, R.S. Immunotherapy in Non-Small Cell Lung Cancer: Facts and Hopes. Clin. Cancer Res. 2019, 25, 4592–4602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deutsch, E.; Chargari, C.; Galluzzi, L.; Kroemer, G. Optimising efficacy and reducing toxicity of anticancer radioimmunotherapy. Lancet Oncol. 2019, 20, e452–e463. [Google Scholar] [CrossRef]
- Donzelli, M.; Bräuer-Krisch, E.; Oelfke, U.; Wilkens, J.J.; Bartzsch, S. Hybrid dose calculation: A dose calculation algorithm for microbeam radiation therapy. Phys. Med. Biol. 2018, 63, 045013. [Google Scholar] [CrossRef] [PubMed]
- 3D Slicer Image Computing Platform. Available online: https://slicer.org/ (accessed on 7 October 2021).
- Pinter, C.; Lasso, A.; Wang, A.; Jaffray, D.; Fichtinger, G. SlicerRT: Radiation therapy research toolkit for 3D Slicer. Med. Phys. 2012, 39, 6332–6338. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.J. The linear quadratic model: Usage, interpretation and challenges. Phys. Med. Biol. 2018, 64, 01TR01. [Google Scholar] [CrossRef] [PubMed]
- Kehwar, T.S. Analytical approach to estimate normal tissue complication probability using best fit of normal tissue tolerance doses into the NTCP equation of the linear quadratic model. J. Cancer Res. Ther. 2005, 1, 168–179. [Google Scholar] [CrossRef]
- van Leeuwen, C.M.; Oei, A.L.; Crezee, J.; Bel, A.; Franken, N.A.P.; Stalpers, L.J.A.; Kok, H.P. The alfa and beta of tumours: A review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Radiat. Oncol. Lond. Engl. 2018, 13, 96. [Google Scholar] [CrossRef]
- Marks, L.B.; Yorke, E.D.; Jackson, A.; Ten Haken, R.K.; Constine, L.S.; Eisbruch, A.; Bentzen, S.M.; Nam, J.; Deasy, J.O. The Use of Normal Tissue Complication Probability (NTCP) Models in the Clinic. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S10–S19. [Google Scholar] [CrossRef] [Green Version]
- Gerhard, S.G.; Palma, D.A.; Arifin, A.J.; Louie, A.V.; Li, G.J.; Al-Shafa, F.; Cheung, P.; Rodrigues, G.B.; Bassim, C.W.; Corkum, M.T. Organ at Risk Dose Constraints in SABR: A Systematic Review of Active Clinical Trials. Pract. Radiat. Oncol. 2021, 11, e355–e365. [Google Scholar] [CrossRef]
- Hanna, G.G.; Murray, L.; Patel, R.; Jain, S.; Aitken, K.L.; Franks, K.N.; van As, N.; Tree, A.; Hatfield, P.; Harrow, S.; et al. UK Consensus on Normal Tissue Dose Constraints for Stereotactic Radiotherapy. Clin. Oncol. (R. Coll. Radiol.) 2018, 30, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Rogers, C.L.; Won, M.; Vogelbaum, M.A.; Perry, A.; Ashby, L.S.; Modi, J.M.; Alleman, A.M.; Galvin, J.; Fogh, S.E.; Youssef, E.; et al. High-risk Meningioma: Initial Outcomes From NRG Oncology/RTOG 0539. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Emami, B. Tolerance of Normal Tissue to Therapeutic Radiation. Rep. Radiother. Oncol. 2013, 1, 123–127. [Google Scholar]
- Tommasino, F.; Scifoni, E.; Durante, M. New Ions for Therapy. Int. J. Part. Ther. 2016, 2, 428–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.-P.; Liu, F.; White, J.; Vicini, F.A.; Freedman, G.M.; Arthur, D.W.; Li, X.A. A planning comparison of seven irradiation options allowed in RTOG 1005 for early stage breast cancer. Med. Dosim. 2015, 40, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.-M.; Li, J. Dose specification for radiation therapy: Dose to water or dose to medium? Phys. Med. Biol. 2011, 56, 3073–3089. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.H. Dm rather than Dw should be used in Monte Carlo treatment planning. For the proposition. Med. Phys. 2002, 29, 922–923. [Google Scholar] [CrossRef]
- Dogan, N.; Siebers, J.V.; Keall, P.J. Clinical comparison of head and neck and prostate IMRT plans using absorbed dose to medium and absorbed dose to water. Phys. Med. Biol. 2006, 51, 4967–4980. [Google Scholar] [CrossRef]
- Siebers, J.V.; Keall, P.J.; Nahum, A.E.; Mohan, R. Converting absorbed dose to medium to absorbed dose to water for Monte Carlo based photon beam dose calculations. Phys. Med. Biol. 2000, 45, 983–995. [Google Scholar] [CrossRef]
- Hombrink, G.; Wilkens, J.J.; Combs, S.E.; Bartzsch, S. Simulation and measurement of microbeam dose distribution in lung tissue. Phys. Med. 2020, 75, 77–82. [Google Scholar] [CrossRef]
- Duncan, M.; Donzelli, M.; Pellicioli, P.; Brauer-Krisch, E.; Davis, J.A.; Lerch, M.L.F.; Rosenfeld, A.B.; Petasecca, M. First experimental measurement of the effect of cardio-synchronous brain motion on the dose distribution during microbeam radiation therapy. Med. Phys. 2020, 47, 213–222. [Google Scholar] [CrossRef]
- Peng, V.; Suchowerska, N.; Rogers, L.; Claridge Mackonis, E.; Oakes, S.; McKenzie, D.R. Grid therapy using high definition multileaf collimators: Realizing benefits of the bystander effect. Acta Oncol. 2017, 56, 1048–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkpatrick, J.P.; Meyer, J.J.; Marks, L.B. The Linear-Quadratic Model Is Inappropriate to Model High Dose per Fraction Effects in Radiosurgery. Semin. Radiat. Oncol. 2008, 18, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J. The Linear-Quadratic Model Is an Appropriate Methodology for Determining Isoeffective Doses at Large Doses Per Fraction. Semin. Radiat. Oncol. 2008, 18, 234–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, L.M.; Leblanc, J.; Wilkins, D.; Raaphorst, G.P. Fitting the linear–quadratic model to detailed data sets for different dose ranges. Phys. Med. Biol. 2006, 51, 2813–2823. [Google Scholar] [CrossRef] [PubMed]
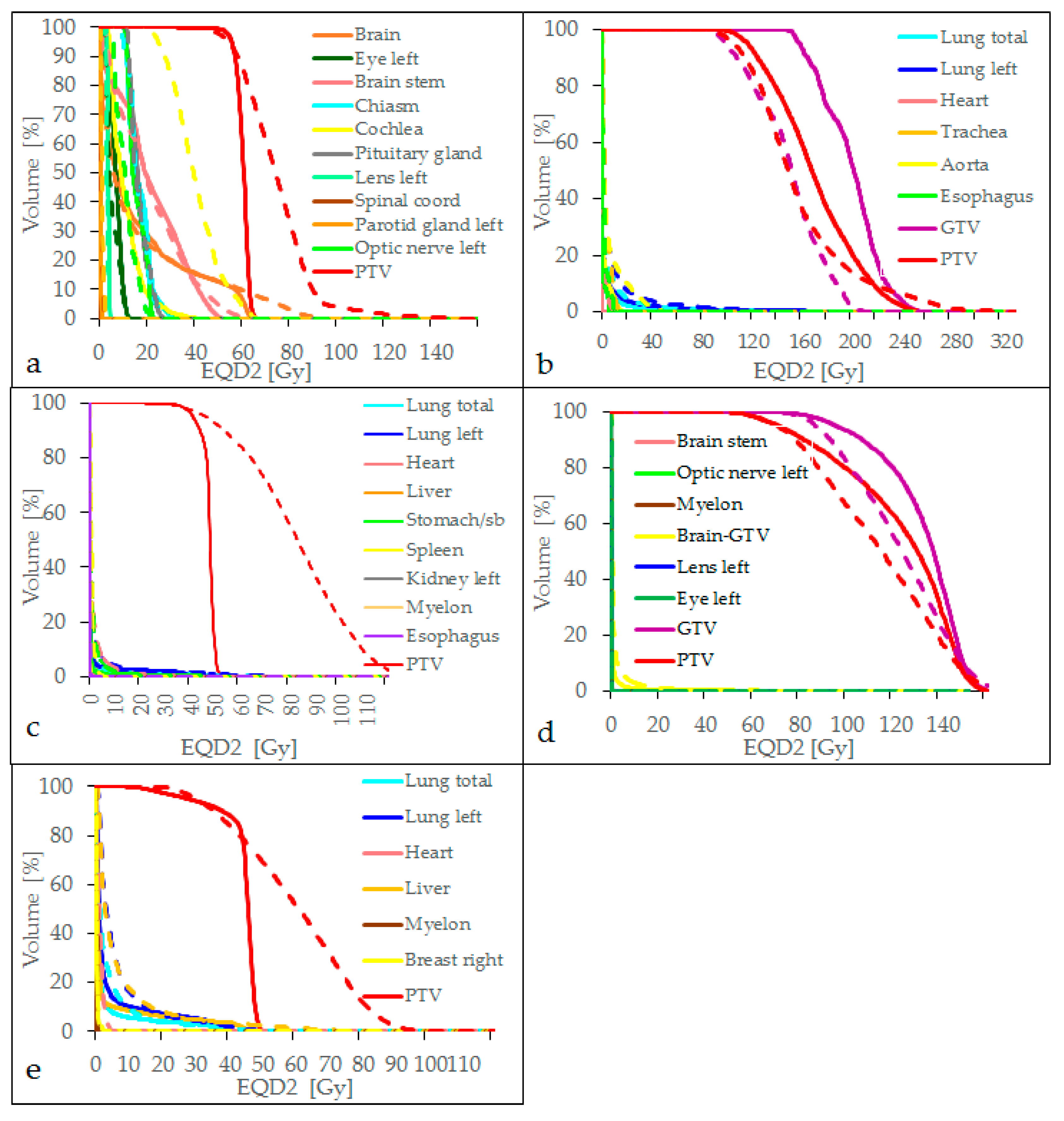
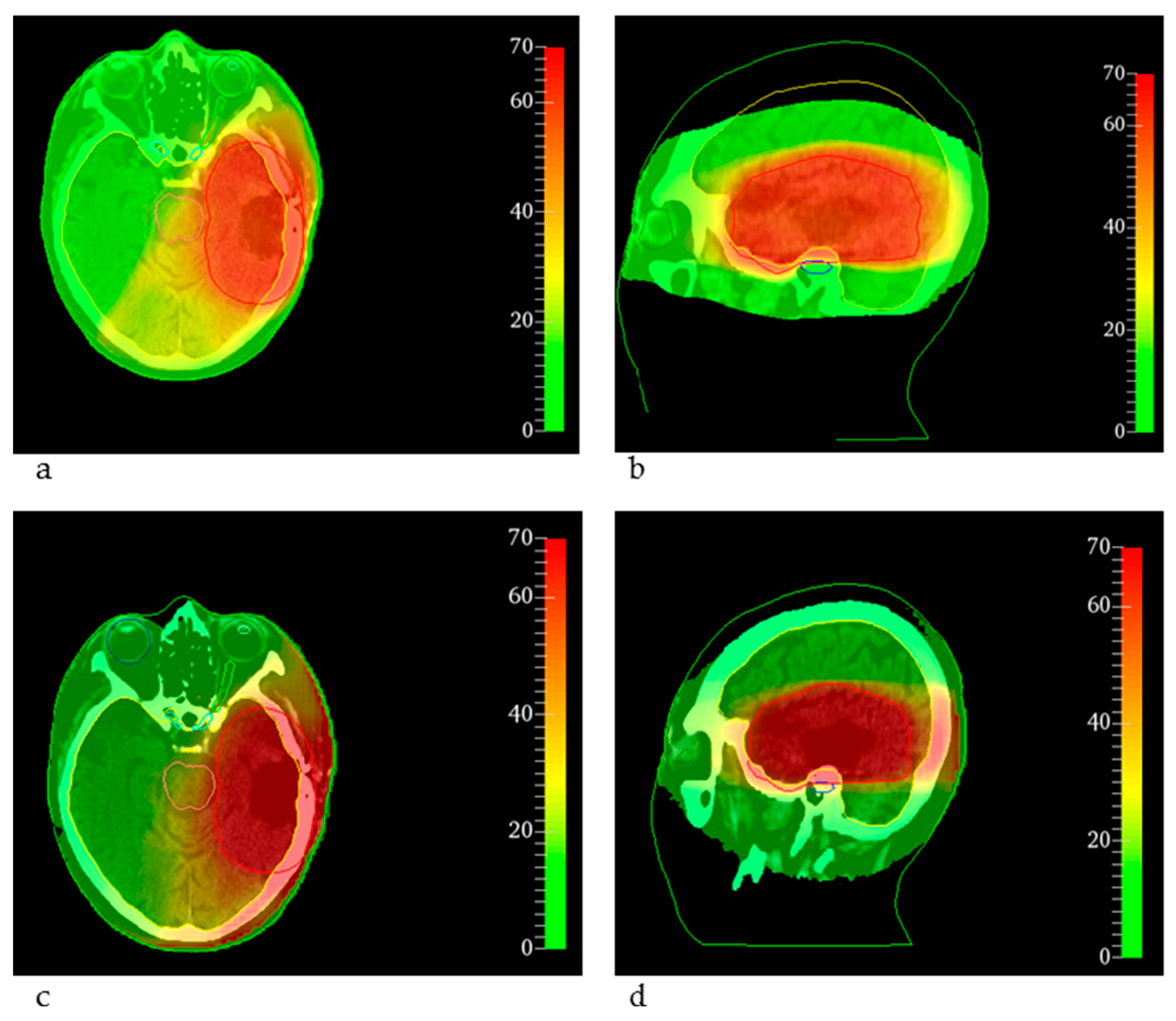
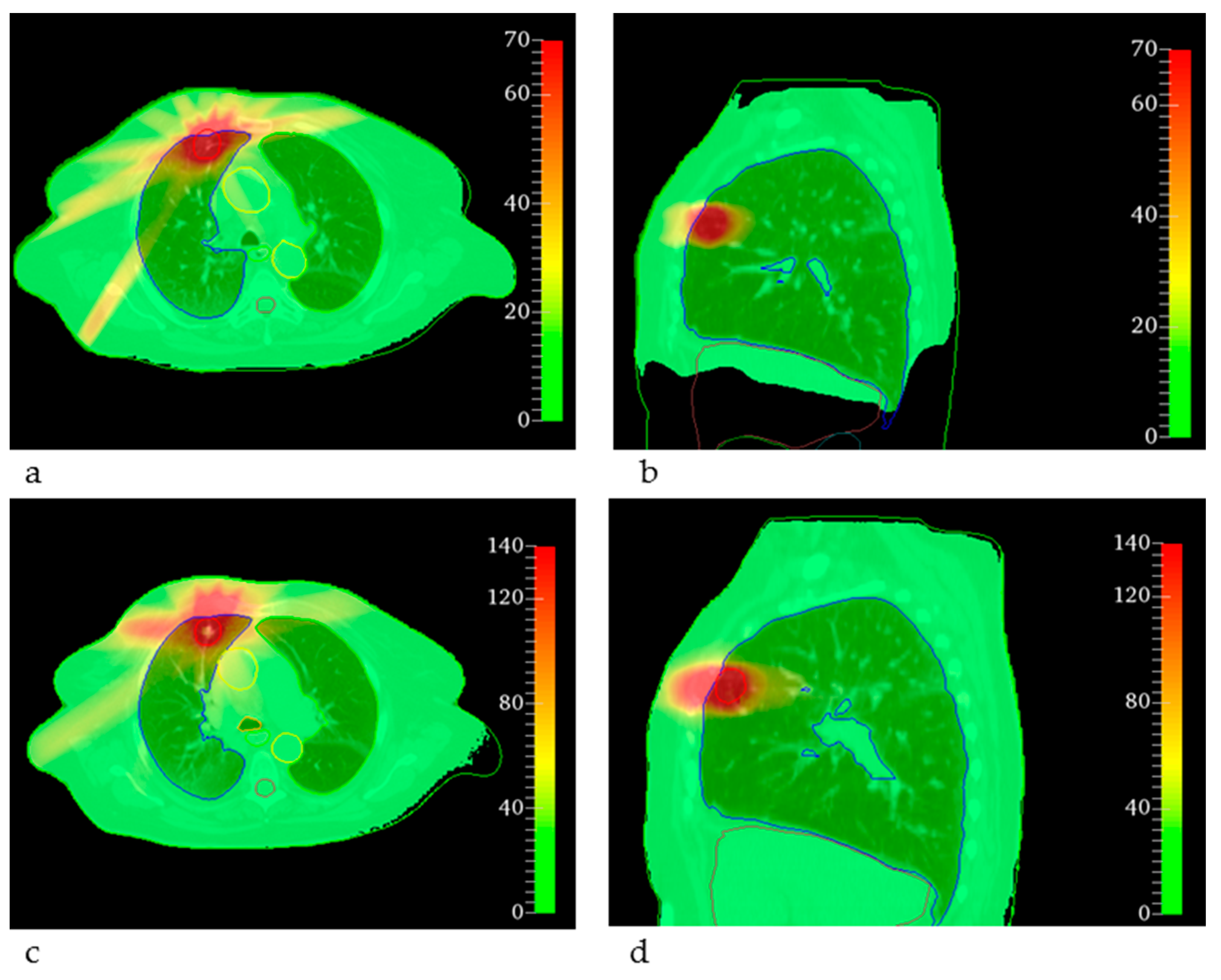
| Plan | Clinical Dprescription | EQD2clinical | EQDMRT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| α/β (Gy) | α (Gy−1) | β (Gy−2) | Dprescription (Gy) | D98% (Gy) | Dmean (Gy) | D2% (Gy) | D98% (Gy) | Dmean (Gy) | D2% (Gy) | ||
| GBM cavity | 2.096 | 0.035 | 0.0167 | 60 Gy in 2 Gy fractions | 60.00 | 53.05 | 59.57 | 63.82 | 50.11 | 74.98 | 115.29 |
| Lung SBRT | 10.0 | 0.3 | 0.03 | 37.5 Gy in 12.5 Gy fractions to 60% isodose | 70.31 | 106.89 | 168.01 | 238.27 | 97.15 | 159.41 | 274.62 |
| Sarcoma bone metastasis | 3.00 | 0.0585 | 0.0195 | 39 Gy in 3 Gy fractions | 46.80 | 38.54 | 47.61 | 51.24 | 38.77 | 82.22 | 120.50 |
| Sarcoma brain metastasis RS | 3.00 | 0.0585 | 0.0195 | 20 Gy in a single fraction | 92.00 | 61.39 | 121.54 | 153.48 | 61.97 | 112.70 | 157.03 |
| Breast tumor hypofractionated | 4.20 | 0.1025 | 0.02631 | 40.05 Gy in 2.67 Gy fractions | 44.38 | 18.04 | 43.71 | 48.87 | 25.46 | 59.41 | 89.93 |
| Plan | OAR | α/β (Gy) | α (Gy−1) | β (Gy−2) | Dose Values (Gy) (%) | ||
|---|---|---|---|---|---|---|---|
| EQD2constraints | EQD2clinical | EQD2MRT | |||||
| GBM cavity | |||||||
| Brain stem | 2.096 | 0.035 | 0.0167 | Dmax < 54 Gy | Dmax = 51.49 Gy Dmean = 21.39 Gy | Dmax = 65.79 Gy Dmean = 16.58 Gy | |
| Cochlea | 2.096 | 0.035 | 0.0167 | Dmax < 45 Gy | Dmax = 39.97 Gy D0.1cm3 = 16.58 Gy | Dmax = 65.92 Gy D0.1cm3 = 20.95 Gy | |
| Chiasm | 2.988 | 0.0251 | 0.0084 | Dmax < 55 Gy | Dmax = 32.51 Gy | Dmax = 30.08 Gy | |
| Optiv nerve r | 2.994 | 0.0497 | 0.0166 | Dmax < 55 Gy | Dmax = 10.40 Gy | Dmax = 10.13 Gy | |
| Optic nerve l | 2.994 | 0.0497 | 0.0166 | Dmax < 55 Gy | Dmax = 22.67 Gy | Dmax = 25.02 Gy | |
| Spinal cord | 2.007 | 0.0307 | 0.0081 | Dmax < 50 Gy | Dmax = 0.88 Gy | Dmax = 2.35 Gy | |
| Pituitary gland | 2.096 | 0.035 | 0.0167 | Dmax < 45 Gy | Dmax = 26.96 Gy | Dmax = 25.62 Gy | |
| Brain without PTV | 2.096 | 0.035 | 0.0167 | Dmean < 30 Gy | Dmean = 10.79 Gy | Dmean = 10.60 Gy | |
| Parotid gland l | 2.991 | 0.0341 | 0.0114 | Dmean < 26 Gy | Dmean = 0.62 Gy | Dmean = 1.29 Gy | |
| Lens l | 1.002 | 0.0544 | 0.0543 | Dmax < 5 Gy | Dmax = 4.68 Gy | Dmax = 4.98 Gy | |
| Lung SBRT | |||||||
| Heart | 1.997 | 0.0579 | 0.029 | Dmax < 26 Gy | Dmax = 1.25 Gy | Dmax = 16.44 Gy D0.1cm3 = 14.94 Gy | |
| Trachea | 2.00 | 0.1 | 0.05 | Dmax < 32 Gy | Dmax = 7.92 Gy | Dmax = 47.32 Gy D0.1cm3 = 19.00 Gy | |
| Aorta | 2.00 | 0.1 | 0.05 | Dmax < 45 Gy | Dmax = 23.21 Gy | Dmax = 59.11 Gy D0.1cm3 = 54.10 Gy | |
| Esophagus | 3.00 | 0.0585 | 0.0195 | Dmean < 34 Gy | Dmean = 0.59 Gy | Dmean = 2.23 Gy | |
| Lung total | 3.79 | 0.0307 | 0.0081 | V20Gy < 20% | V20Gy = 2.38% | V20Gy = 5.12% | |
| Lung ipsilateral | 3.79 | 0.0307 | 0.0081 | Dmean < 7 Gy | Dmean = 3.67 Gy | Dmean = 7.70 Gy | |
| Sarcoma bone metastasis | |||||||
| Lung ipsilateral | 3.79 | 0.0307 | 0.0081 | Dmean < 7 Gy | Dmean = 1.20 Gy | Dmean = 2.36 Gy | |
| Myelon | 2.007 | 0.0307 | 0.0153 | Dmax < 45 Gy | Dmax = 0.03 Gy | Dmax = 0.32 Gy | |
| Lung total | 3.79 | 0.0307 | 0.0081 | V20Gy < 20% | V20Gy = 1.04% | V20Gy = 1.20% | |
| Heart | 1.997 | 0.0579 | 0.029 | Dmean < 26 Gy | Dmean = 0.29 Gy | Dmean = 1.73 Gy | |
| Stomach/ Small bowel | 7.0 | 0.0895 | 0.0128 | 150 cm3 < 30 Gy | 150 cm3 = 0.37 Gy | 150 cm3 = 1.78 Gy | |
| Kidney ipsilateral | 3.0 | 0.0106 | 0.0036 | V50% < 14 Gy | V50% = 0 Gy | V50% = 0 Gy | |
| Esophagus | 3.00 | 0.0585 | 0.0195 | Dmean < 30 Gy | Dmean = 0.03 Gy | Dmean = 0.20 Gy | |
| Brain metastasis RS | |||||||
| Brain stem | 2.096 | 0.035 | 0.0167 | Dmax < 54 Gy | Dmax = 0.01 Gy | Dmax = 0.57 Gy | |
| Optic nerve l | 2.994 | 0.0497 | 0.0166 | Dmax < 55 Gy | Dmax = 0.01 Gy | Dmax = 0.38 Gy | |
| Myelon | 2.007 | 0.0307 | 0.0153 | Dmax < 50 Gy | Dmax = 0.00 Gy | Dmax = 0.14 Gy | |
| Brain-GTV | 2.096 | 0.035 | 0.0167 | Dmax < 60 Gy | Dmax = 155.24 Gy | Dmax = 164.61 Gy | |
| Lens l | 1.002 | 0.0544 | 0.0543 | Dmax < 5 Gy | Dmax = 0.00 Gy | Dmax = 0.14 Gy | |
| Eye l | 2.0 | 0.1 | 0.05 | Dmax < 45 Gy | Dmax = 0.01 Gy | Dmax = 0.22 Gy | |
| Breast tumor hf | |||||||
| Lung total | 3.79 | 0.0307 | 0.0081 | V20Gy < 20% | V20Gy = 4.13% | V20Gy = 3.70% | |
| Lung ipsilateral | 3.79 | 0.0307 | 0.0081 | Dmean < 7 Gy | Dmean = 4.12 Gy | Dmean = 6.35 Gy | |
| Heart | 1.997 | 0.0579 | 0.029 | Dmean < 4 Gy | Dmean = 0.25 Gy | Dmean = 1.28 Gy | |
| Liver | 1.500 | 0.0683 | 0.0455 | Dmean < 30 Gy | Dmean = 3.36 Gy | Dmean = 7.06 Gy | |
| Myelon | 2.007 | 0.0307 | 0.0153 | Dmax < 40 Gy | Dmax = 0.28 Gy | Dmax = 1.06 Gy | |
| Breast contralateral | 3.400 | 0.3 | 0.0882 | Dmax < 2.64 Gy | Dmax = 5.69 Gy | Dmax = 3.64 Gy | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraus, K.M.; Winter, J.; Zhang, Y.; Ahmed, M.; Combs, S.E.; Wilkens, J.J.; Bartzsch, S. Treatment Planning Study for Microbeam Radiotherapy Using Clinical Patient Data. Cancers 2022, 14, 685. https://doi.org/10.3390/cancers14030685
Kraus KM, Winter J, Zhang Y, Ahmed M, Combs SE, Wilkens JJ, Bartzsch S. Treatment Planning Study for Microbeam Radiotherapy Using Clinical Patient Data. Cancers. 2022; 14(3):685. https://doi.org/10.3390/cancers14030685
Chicago/Turabian StyleKraus, Kim Melanie, Johanna Winter, Yating Zhang, Mabroor Ahmed, Stephanie Elisabeth Combs, Jan Jakob Wilkens, and Stefan Bartzsch. 2022. "Treatment Planning Study for Microbeam Radiotherapy Using Clinical Patient Data" Cancers 14, no. 3: 685. https://doi.org/10.3390/cancers14030685
APA StyleKraus, K. M., Winter, J., Zhang, Y., Ahmed, M., Combs, S. E., Wilkens, J. J., & Bartzsch, S. (2022). Treatment Planning Study for Microbeam Radiotherapy Using Clinical Patient Data. Cancers, 14(3), 685. https://doi.org/10.3390/cancers14030685







