Effect of 5-Aminolevulinic Acid and Sodium Fluorescein on the Extent of Resection in High-Grade Gliomas and Brain Metastasis
Abstract
:Simple Summary
Abstract
1. Introduction
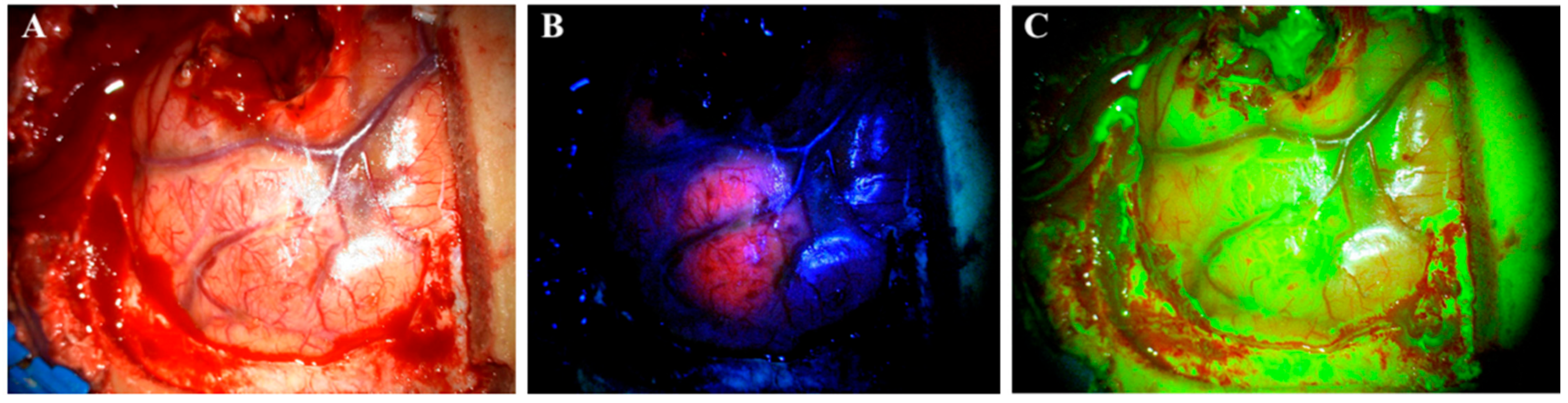
2. Agents for FGS (5-ALA and SF)
3. White Light Surgery Compared to 5-ALA-Guided Surgery in HGGs
4. SF-Guided Surgery Compared to White Light Surgery in HGGs
5. Direct Comparison of 5-ALA and SF
6. SF and 5-ALA in Brain Metastases
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The epidemiology of glioma in adults: A “state of the science” review. Neuro Oncol. 2014, 16, 896–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanai, N.; Polley, M.Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Koshy, M.; Villano, J.L.; Dolecek, T.A.; Howard, A.; Mahmood, U.; Chmura, S.J.; Weichselbaum, R.R.; McCarthy, B.J. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J. Neurooncol. 2012, 107, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Tran, B.; Rosenthal, M.A. Survival comparison between glioblastoma multiforme and other incurable cancers. J. Clin. Neurosci. 2010, 17, 417–421. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Tonn, J.C.; Stupp, R.; Preusser, M.; Cohen-Jonathan-Moyal, E.; Henriksson, R.; Rhun, E.L.; Balana, C.; Chinot, O.; et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017, 18, e315–e329. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection With Survival in Glioblastoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef] [Green Version]
- Glenn, C.A.; Baker, C.M.; Conner, A.K.; Burks, J.D.; Bonney, P.A.; Briggs, R.G.; Smitherman, A.D.; Battiste, J.D.; Sughrue, M.E. An Examination of the Role of Supramaximal Resection of Temporal Lobe Glioblastoma Multiforme. World Neurosurg. 2018, 114, e747–e755. [Google Scholar] [CrossRef]
- Gandhi, S.; Tayebi Meybodi, A.; Belykh, E.; Cavallo, C.; Zhao, X.; Syed, M.P.; Borba Moreira, L.; Lawton, M.T.; Nakaji, P.; Preul, M.C. Survival Outcomes Among Patients With High-Grade Glioma Treated With 5-Aminolevulinic Acid-Guided Surgery: A Systematic Review and Meta-Analysis. Front. Oncol. 2019, 9, 620. [Google Scholar] [CrossRef] [Green Version]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Senders, J.T.; Muskens, I.S.; Schnoor, R.; Karhade, A.V.; Cote, D.J.; Smith, T.R.; Broekman, M.L. Agents for fluorescence-guided glioma surgery: A systematic review of preclinical and clinical results. Acta Neurochir. 2017, 159, 151–167. [Google Scholar] [CrossRef] [Green Version]
- Schipmann, S.; Schwake, M.; Suero Molina, E.; Stummer, W. Markers for Identifying and Targeting Glioblastoma Cells during Surgery. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2019, 80, 475–487. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, S.; Eljamel, M.S. Fluorescence image-guided neurosurgery. Future Oncol. 2017, 13, 2341–2348. [Google Scholar] [CrossRef]
- Kaneko, S.; Suero Molina, E.; Ewelt, C.; Warneke, N.; Stummer, W. Fluorescence-Based Measurement of Real-Time Kinetics of Protoporphyrin IX After 5-Aminolevulinic Acid Administration in Human In Situ Malignant Gliomas. Neurosurgery 2019, 85, E739–E746. [Google Scholar] [CrossRef]
- Maragkos, G.A.; Schüpper, A.J.; Lakomkin, N.; Sideras, P.; Price, G.; Baron, R.; Hamilton, T.; Haider, S.; Lee, I.Y.; Hadjipanayis, C.G.; et al. Fluorescence-Guided High-Grade Glioma Surgery More Than Four Hours After 5-Aminolevulinic Acid Administration. Front. Neurol. 2021, 12, 644804. [Google Scholar] [CrossRef]
- Patel, G.; Armstrong, A.W.; Eisen, D.B. Efficacy of photodynamic therapy vs other interventions in randomized clinical trials for the treatment of actinic keratoses: A systematic review and meta-analysis. JAMA Dermatol. 2014, 150, 1281–1288. [Google Scholar] [CrossRef] [Green Version]
- Hauschild, A.; Stockfleth, E.; Popp, G.; Borrosch, F.; Bruning, H.; Dominicus, R.; Mensing, H.; Reinhold, U.; Reich, K.; Moor, A.C.; et al. Optimization of photodynamic therapy with a novel self-adhesive 5-aminolaevulinic acid patch: Results of two randomized controlled phase III studies. Br. J. Dermatol. 2009, 160, 1066–1074. [Google Scholar] [CrossRef]
- Inoue, K. 5-Aminolevulinic acid-mediated photodynamic therapy for bladder cancer. Int. J. Urol. 2017, 24, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Lakomkin, N.; Hadjipanayis, C.G. Fluorescence-guided surgery for high-grade gliomas. J. Surg. Oncol. 2018, 118, 356–361. [Google Scholar] [CrossRef]
- Tonn, C.J.; Stummer, W. Fluorescence-guided resection of malignant gliomas using 5-aminolevulinic acid: Practical use, risks, and pitfalls. Clin. Neurosurg. 2008, 55, 20–26. [Google Scholar]
- Schupper, A.J.; Rao, M.; Mohammadi, N.; Baron, R.; Lee, J.Y.K.; Acerbi, F.; Hadjipanayis, C.G. Fluorescence-Guided Surgery: A Review on Timing and Use in Brain Tumor Surgery. Front. Neurol. 2021, 12, 682151. [Google Scholar] [CrossRef]
- Bömers, J.P.; Danielsen, M.E.; Schulz, M.K.; Halle, B.; Kristensen, B.W.; Sørensen, M.D.; Poulsen, F.R.; Pedersen, C.B. Sodium fluorescein shows high surgeon-reported usability in glioblastoma surgery. Surgeon 2020, 18, 344–348. [Google Scholar] [CrossRef]
- Schebesch, K.M.; Brawanski, A.; Doenitz, C.; Rosengarth, K.; Proescholdt, M.; Riemenschneider, M.J.; Grosse, J.; Hellwig, D.; Höhne, J. Fluorescence-guidance in non-Gadolinium enhancing, but FET-PET positive gliomas. Clin. Neurol. Neurosurg. 2018, 172, 177–182. [Google Scholar] [CrossRef]
- Falco, J.; Cavallo, C.; Vetrano, I.G.; de Laurentis, C.; Siozos, L.; Schiariti, M.; Broggi, M.; Ferroli, P.; Acerbi, F. Fluorescein Application in Cranial and Spinal Tumors Enhancing at Preoperative MRI and Operated With a Dedicated Filter on the Surgical Microscope: Preliminary Results in 279 Patients Enrolled in the FLUOCERTUM Prospective Study. Front. Surg. 2019, 6, 49. [Google Scholar] [CrossRef] [Green Version]
- Schebesch, K.M.; Brawanski, A.; Hohenberger, C.; Hohne, J. Fluorescein Sodium-Guided Surgery of Malignant Brain Tumors: History, Current Concepts, and Future Project. Turk. Neurosurg. 2016, 26, 185–194. [Google Scholar] [CrossRef]
- Da Silva, C.E.; da Silva, V.D.; da Silva, J.L. Convexity meningiomas enhanced by sodium fluorescein. Surg. Neurol. Int. 2014, 5, 3. [Google Scholar] [CrossRef]
- Schebesch, K.M.; Hoehne, J.; Hohenberger, C.; Acerbi, F.; Broggi, M.; Proescholdt, M.; Wendl, C.; Riemenschneider, M.J.; Brawanski, A. Fluorescein sodium-guided surgery in cerebral lymphoma. Clin. Neurol. Neurosurg. 2015, 139, 125–128. [Google Scholar] [CrossRef]
- Okuda, T.; Kataoka, K.; Kato, A. Effectiveness of intraoperative fluorescence for diagnosis of malignant lymphoma. No Shinkei Geka 2008, 36, 1001–1004. [Google Scholar] [PubMed]
- Romano-Feinholz, S.; Alcocer-Barradas, V.; Benítez-Gasca, A.; Martínez-de la Maza, E.; Valencia-Ramos, C.; Gómez-Amador, J.L. Hybrid fluorescein-guided surgery for pituitary adenoma resection: A pilot study. J. Neurosurg. 2019, 132, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.W.; Donoho, D.A.; Zada, G. Use of optical fluorescence agents during surgery for pituitary adenomas: Current state of the field. J. Neurooncol. 2019, 141, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Göker, B.; Kırış, T. Sodium fluorescein-guided brain tumor surgery under the YELLOW-560-nm surgical microscope filter in pediatric age group: Feasibility and preliminary results. Child’s Nerv. Syst. 2019, 35, 429–435. [Google Scholar] [CrossRef]
- Xue, Z.; Kong, L.; Pan, C.C.; Wu, Z.; Zhang, J.T.; Zhang, L.W. Fluorescein-Guided Surgery for Pediatric Brainstem Gliomas: Preliminary Study and Technical Notes. J. Neurol. Surg. B Skull Base 2018, 79 (Suppl. 4), S340–S346. [Google Scholar] [CrossRef]
- Suero Molina, E.; Stummer, W. Where and When to Cut? Fluorescein Guidance for Brain Stem and Spinal Cord Tumor Surgery-Technical Note. Oper. Neurosurg. 2018, 15, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Acerbi, F.; Cavallo, C.; Schebesch, K.M.; Akçakaya, M.O.; de Laurentis, C.; Hamamcioglu, M.K.; Broggi, M.; Brawanski, A.; Falco, J.; Cordella, R.; et al. Fluorescein-Guided Resection of Intramedullary Spinal Cord Tumors: Results from a Preliminary, Multicentric, Retrospective Study. World Neurosurg. 2017, 108, 603–609. [Google Scholar] [CrossRef]
- Díez Valle, R.; Slof, J.; Galván, J.; Arza, C.; Romariz, C.; Vidal, C. Observational, retrospective study of the effectiveness of 5-aminolevulinic acid in malignant glioma surgery in Spain (The VISIONA study). Neurologia 2014, 29, 131–138. [Google Scholar] [CrossRef]
- Coburger, J.; Hagel, V.; Wirtz, C.R.; König, R. Surgery for Glioblastoma: Impact of the Combined Use of 5-Aminolevulinic Acid and Intraoperative MRI on Extent of Resection and Survival. PLoS ONE 2015, 10, e0131872. [Google Scholar] [CrossRef] [Green Version]
- Della Puppa, A.; Lombardi, G.; Rossetto, M.; Rustemi, O.; Berti, F.; Cecchin, D.; Gardiman, M.P.; Rolma, G.; Persano, L.; Zagonel, V.; et al. Outcome of patients affected by newly diagnosed glioblastoma undergoing surgery assisted by 5-aminolevulinic acid guided resection followed by BCNU wafers implantation: A 3-year follow-up. J. Neurooncol. 2017, 131, 331–340. [Google Scholar] [CrossRef]
- Shinoda, J.; Yano, H.; Yoshimura, S.; Okumura, A.; Kaku, Y.; Iwama, T.; Sakai, N. Fluorescence-guided resection of glioblastoma multiforme by using high-dose fluorescein sodium: Technical note. J. Neurosurg. 2003, 99, 597–603. [Google Scholar] [CrossRef] [Green Version]
- Koc, K.; Anik, I.; Cabuk, B.; Ceylan, S. Fluorescein sodium-guided surgery in glioblastoma multiforme: A prospective evaluation. Br. J. Neurosurg. 2008, 22, 99–103. [Google Scholar] [CrossRef]
- Chen, B.; Wang, H.; Ge, P.; Zhao, J.; Li, W.; Gu, H.; Wang, G.; Luo, Y.; Chen, D. Gross total resection of glioma with the intraoperative fluorescence-guidance of fluorescein sodium. Int. J. Med. Sci. 2012, 9, 708–714. [Google Scholar] [CrossRef] [Green Version]
- Catapano, G.; Sgulò, F.G.; Seneca, V.; Lepore, G.; Columbano, L.; di Nuzzo, G. Fluorescein-Guided Surgery for High-Grade Glioma Resection: An Intraoperative “Contrast-Enhancer”. World Neurosurg. 2017, 104, 239–247. [Google Scholar] [CrossRef]
- Katsevman, G.A.; Turner, R.C.; Urhie, O.; Voelker, J.L.; Bhatia, S. Utility of sodium fluorescein for achieving resection targets in glioblastoma: Increased gross- or near-total resections and prolonged survival. J. Neurosurg. 2019, 132, 1–7. [Google Scholar] [CrossRef]
- Hong, J.; Chen, B.; Yao, X.; Yang, Y. Outcome comparisons of high-grade glioma resection with or without fluorescein sodium-guidance. Curr. Probl. Cancer 2019, 43, 236–244. [Google Scholar] [CrossRef]
- Burns, P.B.; Rohrich, R.J.; Chung, K.C. The levels of evidence and their role in evidence-based medicine. Plast. Reconstr. Surg. 2011, 128, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Hansen, R.W.; Pedersen, C.B.; Halle, B.; Korshoej, A.R.; Schulz, M.K.; Kristensen, B.W.; Poulsen, F.R. Comparison of 5-aminolevulinic acid and sodium fluorescein for intraoperative tumor visualization in patients with high-grade gliomas: A single-center retrospective study. J. Neurosurg. 2019, 133, 1–8. [Google Scholar] [CrossRef]
- Marhold, F.; Mercea, P.A.; Scheichel, F.; Berghoff, A.S.; Heicappell, P.; Kiesel, B.; Mischkulnig, M.; Borkovec, M.; Wolfsberger, S.; Woehrer, A.; et al. Detailed analysis of 5-aminolevulinic acid induced fluorescence in different brain metastases at two specialized neurosurgical centers: Experience in 157 cases. J. Neurosurg. 2019, 133, 1–12. [Google Scholar] [CrossRef]
- Okuda, T.; Kataoka, K.; Yabuuchi, T.; Yugami, H.; Kato, A. Fluorescence-guided surgery of metastatic brain tumors using fluorescein sodium. J. Clin. Neurosci. 2010, 17, 118–121. [Google Scholar] [CrossRef]
- Schebesch, K.M.; Hoehne, J.; Hohenberger, C.; Proescholdt, M.; Riemenschneider, M.J.; Wendl, C.; Brawanski, A. Fluorescein sodium-guided resection of cerebral metastases-experience with the first 30 patients. Acta Neurochir. 2015, 157, 899–904. [Google Scholar] [CrossRef]
- Hohne, J.; Hohenberger, C.; Proescholdt, M.; Riemenschneider, M.J.; Wendl, C.; Brawanski, A.; Schebesch, K.M. Fluorescein sodium-guided resection of cerebral metastases-an update. Acta Neurochir. 2017, 159, 363–367. [Google Scholar] [CrossRef]
- Xiao, S.Y.; Zhang, J.; Zhu, Z.Q.; Li, Y.P.; Zhong, W.Y.; Chen, J.B.; Pan, Z.Y.; Xia, H.C. Application of fluorescein sodium in breast cancer brain-metastasis surgery. Cancer Manag. Res. 2018, 10, 4325–4331. [Google Scholar] [CrossRef] [Green Version]
- Kofoed, M.S.; Pedersen, C.B.; Schulz, M.K.; Kristensen, B.W.; Hansen, R.W.; Markovic, L.; Halle, B.; Poulsen, F.R. Fluorescein-guided resection of cerebral metastases is associated with greater tumor resection. Acta Neurochir. 2021. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, D.G.; Kim, J.W.; Han, J.H.; Kim, Y.H.; Park, C.K.; Kim, C.Y.; Paek, S.H.; Jung, H.W. The role of surgical resection in the management of brain metastasis: A 17-year longitudinal study. Acta Neurochir. 2013, 155, 389–397. [Google Scholar] [CrossRef]
- Stummer, W.; Novotny, A.; Stepp, H.; Goetz, C.; Bise, K.; Reulen, H.J. Fluorescence-guided resection of glioblastoma multiforme utilizing 5-ALA-induced porphyrins: A prospective study in 52 consecutive patients. J. Neurosurg. 2000, 93, 1003. [Google Scholar] [CrossRef]
- Stummer, W.; Tonn, J.C.; Goetz, C.; Ullrich, W.; Stepp, H.; Bink, A.; Pietsch, T.; Pichlmeier, U. 5-Aminolevulinic acid-derived tumor fluorescence: The diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery 2014, 74, 310–320. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.W.; Valdés, P.A.; Harris, B.T.; Fontaine, K.M.; Hartov, A.; Fan, X.; Ji, S.; Lollis, S.S.; Pogue, B.W.; Leblond, F.; et al. Coregistered fluorescence-enhanced tumor resection of malignant glioma: Relationships between δ-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters: Clinical article. J. Neurosurg. 2011, 114, 595–603. [Google Scholar] [CrossRef] [Green Version]
- Suero Molina, E.; Schipmann, S.; Stummer, W. Maximizing safe resections: The roles of 5-aminolevulinic acid and intraoperative MR imaging in glioma surgery-review of the literature. Neurosurg. Rev. 2019, 42, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Kiesel, B.; Mischkulnig, M.; Woehrer, A.; Martinez-Moreno, M.; Millesi, M.; Mallouhi, A.; Czech, T.; Preusser, M.; Hainfellner, J.A.; Wolfsberger, S.; et al. Systematic histopathological analysis of different 5-aminolevulinic acid-induced fluorescence levels in newly diagnosed glioblastomas. J. Neurosurg. 2018, 129, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Promedicin-Sodium Fluorescein. Available online: https://pro.medicin.dk/Medicin/Praeparater/8302 (accessed on 18 January 2022).
- Promedicin-5-Aminolevulinic Acid. Available online: https://pro.medicin.dk/Medicin/Praeparater/4737 (accessed on 18 January 2022).
- Kiesel, B.; Freund, J.; Reichert, D.; Wadiura, L.; Erkkilae, M.T.; Woehrer, A.; Hervey-Jumper, S.; Berger, M.S.; Widhalm, G. 5-ALA in Suspected Low-Grade Gliomas: Current Role, Limitations, and New Approaches. Front. Oncol. 2021, 11, 699301. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Layard Horsfall, H.; Funnell, J.P.; Hanrahan, J.G.; Khan, D.Z.; Muirhead, W.; Stoyanov, D.; Marcus, H.J. Artificial Intelligence in Brain Tumour Surgery-An Emerging Paradigm. Cancers 2021, 13, 5010. [Google Scholar] [CrossRef] [PubMed]
| Study | Patients (N) | Study Design | Definitions (GTR and Postoperative MRI after Surgery) | Results |
|---|---|---|---|---|
| Stummer et al. (2006) | 322 | Prospective randomized controlled trial | GTR = residual tumor < 0.175 cm3 Postoperative MRI: <72 h | GTR: 65% vs. 36% (p < 0.0001) * PFS6: 41% vs. 21.1% (p < 0.0003) * OS: 15.2 months vs. 13.5 months (p < 0.1) |
| Diez Valle et al. (2014) | 251 | Retrospective case–control study | GTR = no contrast-enhancing tumor Postoperative MRI: <28 days | GTR: 67% vs. 45% (p < 0.000) * PFS6: 69% vs. 48% (p < 0.002) * |
| Coburger et al. (2015) | 66 | Retrospective case–control study | GTR ≥ 95% tumor resection Postoperative MRI: <72 h after surgery | GTR: 99.6% vs. 96.0% (p < 0.004) * Volume: 0.1 cc vs. 1.8 cc (p < 0.02) * OS: 18 months vs. 17 months (p < 0.708) PFS: 6 months vs. 6 months (p < 0.309) |
| Della Puppa et al. (2017) | 122 | Retrospective case–control study | GTR = residual tumor < 0.175 cm3 Postoperative MRI: <24 h | GTR: 80% (BCNU wafers + 5-ALA) 47% (5-ALA) 76% (BCNU wafers) OS: 22 months (BCNU wafers + 5-ALA) 18 months (5-ALA) 21 months (BCNU wafers) PFS: 11 months (BCNU wafers + 5-ALA) 10 months (5-ALA) 11 months (BCNU wafers) |
| Study | Patients (N) | Study Design | SF Dose | Yellow 560 nm Filter | Definitions (GTR and Postoperative MRI after Surgery) | Results |
|---|---|---|---|---|---|---|
| Shinoda et al. (2003) | 105 | Retrospective case–control study | 20 mg/kg | None | GTR = no contrast-enhancing tumor Postoperative MRI: 1 month | GTR: 84.4% vs. 30.1% (p = 0.0001) OS: 15 months vs. 13 months (p > 0.05) |
| Koc et al. (2008) | 70 | Nonrandomized prospective study | 20 mg/kg | None | GTR = no contrast-enhancing tumor Postoperative MRI: <24 h | GTR: 83% vs. 55% (p = 0.012) * OS: 44 weeks vs. 42 weeks (p > 0.05) |
| Chen et al. (2012) | 22 | Nonrandomized prospective study | 15–20 mg/kg | None | GTR = no contrast-enhancing tumor Postoperative MRI: <7 days | GTR: 80% vs. 33.3% (p < 0.047) * PFS: 7.2 months vs. 5.4 months (p < 0.033) * |
| Catapano et al. (2017) | 48 | Retrospective case–control study | 5 mg/kg | Yes | GTR = residual tumor < 0.17 cm3 Postoperative MRI: <72 h | GTR: 82.6% vs. 52% (p < 0.05) * |
| Katsevman et al. (2019) | 189 | Retrospective case–control study | 3–3 mg/kg | Yes | GTR = tumor resection > 98% Postoperative MRI: <48 h | GTR: 73.4% vs. 52.5% (p = 0.029) * OS: 78 weeks vs. 60 weeks (p = 0.36) |
| Hong et al. (2019) | 82 | Case-control study | 1.5–2 mg/kg | Yes | GTR = no contrast-enhancing tumor Postoperative MRI: not stated | GTR: 85.7% vs. 62.5% (p = 0.02) * |
| Study | Patients (N) | Study Design | SF Dose | Yellow 560 nm Filter | Definitions (GTR and Postoperative MRI after Surgery) | Results |
|---|---|---|---|---|---|---|
| Okuda et al. (2010) | 36 | Retrospective observational study | 20 mg/kg | None | GTR = total resection Postoperative MRI: not stated | GTR: 86.1% |
| Schebesch et al. (2015) | 30 | Retrospective observational study | 200 mg total | None | GTR = no contrast-enhancing tumor Postoperative MRI: <48 h | GTR: 83.3% |
| Hohne et al. (2017) | 95 | Retrospective observational study | 200 mg total (n = 30) 5 mg/kg (n = 65) | None (n = 30) Yes (n = 65) | GTR = no contrast-enhancing tumor Postoperative MRI: <72 h | GTR: 83% |
| Xiao et al. (2018) | 38 | Retrospective case–control study | 5 mg/kg | Yes | GTR = no contrast-enhancing tumor Postoperative MRI: <72 h | GTR: 94% (SF) vs. 62% (white light) (p = 0.02) * OS: 24.1 months vs. 22.8 months (p > 0.05) |
| Kofoed et al. (2021) | 117 | Retrospective case–control study | 200 mg total | Yes | GTR ≤ 10 mm residual tumor Postoperative MRI: <72 h | GTR: 94% vs. 84% (p = 0.000) * OS (1 year): 44.6% vs. 31.1% (p = 0.0001) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahrens, L.C.; Krabbenhøft, M.G.; Hansen, R.W.; Mikic, N.; Pedersen, C.B.; Poulsen, F.R.; Korshoej, A.R. Effect of 5-Aminolevulinic Acid and Sodium Fluorescein on the Extent of Resection in High-Grade Gliomas and Brain Metastasis. Cancers 2022, 14, 617. https://doi.org/10.3390/cancers14030617
Ahrens LC, Krabbenhøft MG, Hansen RW, Mikic N, Pedersen CB, Poulsen FR, Korshoej AR. Effect of 5-Aminolevulinic Acid and Sodium Fluorescein on the Extent of Resection in High-Grade Gliomas and Brain Metastasis. Cancers. 2022; 14(3):617. https://doi.org/10.3390/cancers14030617
Chicago/Turabian StyleAhrens, Lasse Cramer, Mathias Green Krabbenhøft, Rasmus Würgler Hansen, Nikola Mikic, Christian Bonde Pedersen, Frantz Rom Poulsen, and Anders Rosendal Korshoej. 2022. "Effect of 5-Aminolevulinic Acid and Sodium Fluorescein on the Extent of Resection in High-Grade Gliomas and Brain Metastasis" Cancers 14, no. 3: 617. https://doi.org/10.3390/cancers14030617
APA StyleAhrens, L. C., Krabbenhøft, M. G., Hansen, R. W., Mikic, N., Pedersen, C. B., Poulsen, F. R., & Korshoej, A. R. (2022). Effect of 5-Aminolevulinic Acid and Sodium Fluorescein on the Extent of Resection in High-Grade Gliomas and Brain Metastasis. Cancers, 14(3), 617. https://doi.org/10.3390/cancers14030617






